Abstract
Grazing sea urchins can reduce kelp abundance and therefore strongly affect kelp forest community structure. Despite the ecological importance of sea urchins, direct field studies on the role that urchin predators play in shaping urchin populations are rare for southern California. We conducted surveys and manipulative experiments within kelp forests near San Diego, CA, (32–51′28″N, 117–16′00″W) from 2006 to 2009 to determine whether predators such as sheephead (Semicossyphus pulcher) and spiny lobsters (Panulirus interruptus) may be linked to purple urchin (Strongylocentrotus purpuratus) and red urchin (Strongylocentrotus franciscanus) distribution and habitat use, as well as purple urchin density-dependent mortality. Purple urchins were less dense and more cryptic inside a local marine protected area (MPA) that contained high predator abundance than in nearby heavily fished areas, whereas red urchins rarely were found outside the MPA. Urchin proportional mortality was inversely density dependent during the day when sheephead were active, despite fish aggregations in plots of high urchin density, but was density independent during the night when lobsters were active. Urchin mortality was reduced under understory algal cover during the day, but not during the night. Examining whether urchin mortality from predation is density dependent and how habitat complexity influences this relationship is imperative because behavioral changes and increases in urchin populations can have vast ecological and economic consequences in kelp forest communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Canopy-forming kelps, such as the giant kelp Macrocystis pyrifera, form important habitats for a variety of vertebrate and invertebrate fauna in temperate latitudes worldwide. Sea urchins typically are an abundant member of kelp forest communities, and as herbivores that consume kelp they may play key roles in dictating kelp abundance and spatial patterns of kelp forest habitat structure (Ling et al. 2015). Large urchin “barrens” may form where urchins move out of their crevices and overgraze macroalgae, leading to greatly reduced species diversity. Urchin-induced deforestation has been increasing over the past several centuries (Steneck et al. 2002; Ling et al. 2015), at least in part due to reduced abundance of key predators including sea otters, lobsters, and large fishes (Wharton and Mann 1981; Tegner and Dayton 1981; Tegner and Levin 1983; Shears and Babcock 2002; Lafferty 2004). For instance, in Alaska, removal of the sea otter Enhydra lutris led to an increase in their urchin prey, which resulted in increased herbivory and a loss of kelp forest habitat (Estes and Palmisano 1974; Estes et al. 1998).
Predator abundance and type (Shears and Babcock 2002; Sala et al. 1998; Pederson and Johnson 2006; Ling et al. 2009; Clemente et al. 2013), urchin density and group size (Bernstein et al. 1981), urchin body size (Tegner and Levin 1983; Shears and Babcock 2002; Pederson and Johnson 2006; Guidetti 2006; Clemente et al. 2013), disease (Sala et al. 1998), and the amount of spatial refuge available to urchins (Hereu et al. 2005; Farina et al. 2009) all may dictate the potential for predators to control sea urchin populations. Urchin mortality rates typically are higher within marine protected areas that house larger predators than in nearby fished areas, which can be depleted of predators (Shears and Babcock 2002; Pederson and Johnson 2006). Whereas predatory fishes often are the primary predators of urchins, nocturnally foraging lobsters may cause more predator-induced urchin mortality in areas where they are locally abundant (Lafferty 2004; Pederson and Johnson 2006).
To avoid predators, urchins typically remain cryptic on rocky reefs, inhabiting crevices or the undersides of rocks and feeding on drift algae. Small urchins can also avoid predation by sheltering in the spine canopy of conspecific adults (Clemente et al. 2013). Urchins can emerge from shelters to actively graze on live macroalgae when predator densities are low or when drift algae becomes scarce (Harrold and Reed 1985). Sheltering behavior can deter predation by fishes and invertebrates, and mortality of urchins often is highest for larger juvenile or adult urchins that can no longer inhabit small crevices (Shears and Babcock 2002; Pederson and Johnson 2006). Very large urchins, however, may have a size refuge from many predators (Tegner and Levin 1983; Guidetti 2006). Though rocky crevices and similar “hard” shelters may provide the best refuges for urchins, algae and marine plants also may offer protection from predators; in the Northwest Mediterranean Sea, predation on urchins by fishes decreased with increasing algal (Hereu et al. 2005) and seagrass (Farina et al. 2009) structural complexity.
Urchin barrens are not prevalent in the Point Loma kelp forest (San Diego, CA, USA), the largest kelp forest in California. Though sea otters once likely regulated urchin abundance in this area (Dayton et al. 1998), they have been locally extinct for over a century. Instead, densities of the two most abundant urchins, the purple urchin (Strongylocentrotus purpuratus) and the red urchin (S. franciscanus), may be effectively controlled by California spiny lobster (Panulirus interruptus), a nocturnal predator, and fishes such as the California sheephead (Semicossyphus pulcher), a diurnal predator (Tegner and Levin 1983; Lafferty 2004). Red urchins support a large fishery in southern California, and their low density also may contribute to the lack of urchin barrens in this region. Fishing influences urchin predators as well: lobsters and sheephead are heavily fished and resulting reductions in the densities and sizes of these predators may reduce their ability to control urchin populations (Tegner and Levin 1983; Hamilton et al. 2011). Fishery-induced alterations in the size distribution of predators such as lobsters (Barsky 2001) can have community-wide impacts because only large predators are able to consume large urchins (Tegner and Levin 1983; Clemente et al. 2013). Despite the increasing number of studies focusing on urchin mortality and habitat use, direct field studies on urchin population regulation are relatively rare. Evidence that top kelp forest predators regulate urchin populations primarily comes from inverse correlations between urchin and predator densities (Mayfield and Branch 2000; Lafferty 2004; Carter et al. 2007). Our study is one of the few direct manipulative experiments within kelp forests conducted in southern California.
Our objective in this study was to determine the effects of predator abundance and habitat structure on the proportional mortality and habitat use of sea urchins in southern California kelp forests. Specifically, we conducted surveys and experiments within an MPA and two nearby heavily fished areas to determine (1) how habitat structure (understory algal cover), time of day (day vs. night), and urchin density interact to affect urchin proportional mortality, and (2) whether red and purple urchin abundance and habitat use vary with the abundance of their potential predators.
Methods
Study sites and study species
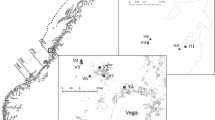
Our work took place at three sites within the kelp forests along the coast of San Diego, California: (1) inside the La Jolla Ecological Reserve (LJER), a 2.16 km2 no-take MPA established in 1971; (2) adjacent to the reserve (ATR), ca. 500 m outside the LJER, and (3) 10 km to the south in the Point Loma kelp forest (PL) (Fig. 1). We selected these three sites to represent differences in predator abundance, largely caused by fishing pressure. The LJER contains high abundances of lobsters and large fishes (Parnell et al. 2005; Loflen 2007), whereas we expected recreationally and commercially fished areas outside the reserve (ATR and PL) to have lower predator abundance. The kelp forest near Point Loma is the largest contiguous kelp forest in California and is one of the most heavily fished areas for lobsters, sheephead and other large fishes in the state, which may have dramatically reduced their predatory influence in this ecosystem (Dayton et al. 1998; Tegner and Dayton 2000). Dominant algae at each site include the surface canopy-forming giant kelp, Macrocystis pyrifera, and understory kelps Pterygophora californica and Laminaria farlowii. Other commonly encountered algae include Egregia menziesii, Eisenia arborea, and Stephanocystis osmundacea.
In southern California, purple and red sea urchins are prominent herbivores that consume a variety of brown and red algae but prefer the kelp M. pyrifera (Morris et al. 1980). California spiny lobsters range from Monterey Bay, California, to Magdalena Bay in Baja California, Mexico, where they inhabit shelters during the day and emerge from shelters at dusk to actively forage on benthic invertebrates, including mussels (Robles et al. 1990) and both red and purple urchins (Tegner and Levin 1983) during the night (Lindberg 1955; Withy-Allen and Hovel 2013). The California sheephead is a protogynous sequential hermaphrodite that occurs in temperate rocky reefs and kelp beds from Monterey Bay to the Gulf of California. Sheephead feed on macroinvertebrates including echinoids, bryozoans, molluscs, brachyurans, and polychaetes. Sheephead are diurnally active and return to home shelters at night (Topping et al. 2005).
Urchin proportional mortality
To determine how urchin density and algal cover interact to affect urchin mortality, we exposed purple urchins to predators inside the LJER where predator densities are high. We performed urchin mortality experiments during the day and at night to compare effects of major predators, sheephead (diurnal foragers) and lobsters (nocturnal foragers).
We exposed purple urchins to predators within standardized, artificial urchin habitat units (UHUs). UHUs allow urchins to mimic natural sheltering behavior and avoid enhanced predation as a result of tethering (Peterson and Black 1994). Each 43 cm long × 38 cm wide UHU consisted of six, 10-cm-diameter PVC pipes anchored vertically in cement, creating artificial crevices 6–7 cm deep. We placed UHUs side by side to create each plot, with each plot holding a maximum of 36 urchins (for additional detail see Fig. 1A, a photograph of the plot in the Appendix). We used only purple urchins with test diameters of 6–8 cm in this experiment due to their high abundance in surveys and because they do not have a size refuge from predators such as lobsters (Tegner and Levin 1983). Urchin proportional mortality experiments were conducted from July to October 2008 and June to September 2009. Three weeks before trials began, we placed two plots on rocky substrate in an open area with no macroalgal cover and two plots in an area of high macroalgal cover at a depth of approximately 10 m. For each trial, each plot received either 4, 8, 16, 24, or 36 urchins to represent a gradient of low to high urchin density, chosen randomly.
To conduct daytime urchin predation trials, between 0900 and 1000 hours, we placed urchins in artificial crevices and covered each urchin with a small piece of M. pyrifera to aid in acclimation to the plot. We ensured urchins remained in artificial crevices for 1 min and then returned to plots to count the number of live urchins remaining after 20 min, 1, 2, 24, and 48 h. Predators generally found urchins quickly (see “Results” section), and 1 h was sufficient time to see differences among treatments. Trials were identical for nocturnal urchin exposures, except that exposures began at ca. 2000–2100 hours when diurnal predators were inactive, and we returned to quantify urchin proportional mortality shortly before dawn (ca. 8-h exposures). We conducted a total of 3–7 replicate trials for each combination of density × cover × time of day. Urchin mortality rates were standardized to 1 h so that we could statistically compare daytime versus nighttime data.
To analyze urchin proportional mortality data, we used an analysis of covariance (ANCOVA) to test how urchin proportional mortality varied with urchin density (covariate), algal cover, and time of day. Our daytime and nighttime data are unbalanced, which can reduce power and increase the possibility of overlooking an effect when the effect truly exists (Shaw and Mitchell-Olds 1993). In light of this, we chose to minimize our type II error by adopting a P level of 0.1 as suggested by Dayton et al. (1999). In highly variable coastal habitats with considerable natural variation, this level of probability is sufficient to suggest a meaningful relationship (Dayton et al. 1999). After running the full ANCOVA model with all interaction terms present, we used post hoc pooling to eliminate highly non-significant interactions from our model (Underwood 1997). Though Underwood (1997) suggests post hoc pooling is appropriate when P ≥ 0.25, all terms removed from our model had P values ≥0.65.
We used video to determine whether predators exhibit an aggregative response to increased urchin densities. One Sony HDR HC-9 camera in an underwater housing was used to record the number and species of predators visiting plots over a 1-h period during the day. The camera was placed at an oblique angle approximately 2.5 m from the plots. One plot was recorded for each level of urchin density. Upon replaying the video, predators were counted by pausing the tape every 15 s to identify and quantify predators (N = 1430 frames). We used linear regression to determine whether mean fish abundance (average number of fishes observed per frame) varied with urchin density.
Missing urchins may have been removed by predators or may have moved from plots on their own. We determined whether predators had likely removed missing urchins by (1) monitoring daytime plots with underwater video and (2) tagging a subset of urchins with small, inconspicuous pieces of plastic sleeve insulation from electrical wire (Hereu 2005). Three haphazardly selected plots were recorded for 90 min after divers completed urchin placement with the video camera. Upon reviewing the video, no urchins were seen moving from the plots. We tagged twenty urchins in plots by gluing two pieces of sleeve insulation onto the largest ventral spines. When we returned to plots to count surviving urchins, we extensively searched surrounding areas for tagged and untagged purple urchins. Though we often found broken tests and partially consumed urchins, we found no live tagged urchins outside of plots.
Urchin, lobster, and fish surveys
We conducted transect surveys (March 1 to July 31, 2008) to quantify the density of red and purple urchins and their chief predators, spiny lobsters, and large fishes in LJER, ATR, and PL. To sample for urchins and lobsters, we swam 20 m × 4 m transects in haphazardly chosen locations designed to encompass a variety of habitats in each of the three sites. We conducted a total of 19, 17, and 20 transects in the LJER, ATR, and PL, respectively, in eight unique locations on average per site. In order to effectively represent the diversity of habitats in each of the three sites, each transect performed during a dive was swum in a haphazard direction originating from the same starting point. Sampling began 5 m away from the starting point to avoid over sampling at the center of the radial transects. During transect surveys, a team of two divers searched extensively for lobsters and urchins by flipping boulders and exploring all shelters and crevices. Using rulers, lobster carapace length (CL) was recorded to the nearest 0.5 cm.
During surveys, we also recorded the habitat status for all urchins. Based on preliminary surveys, we categorized each urchin into one of four categories, ranging from least exposed to most exposed: concealed (the urchin was completely concealed under a boulder that needed to be flipped to expose urchins), burrowed (the urchin was visible but tightly embedded into a deep cavity within the rock), ledge (the urchin was found under a ledge that was accessible to at least some predators), or fully exposed (the urchin was out in the open with no concealment or cover). Concealed and burrowed urchins are extremely difficult for predators to find or remove, whereas urchins under ledges and exposed on rocks are comparatively vulnerable to predators. Therefore, for analysis, we combined the concealed and burrowed categories to form a “protected” category and combined the ledge and exposed categories to form an “exposed” category.
We used separate one-way ANOVAs to test for differences in lobster and urchin density and lobster size among the three sites. Data were visually inspected for normality, and we tested for homogeneity of variances using Cochran’s test in this and all subsequent ANOVAs. We evaluated differences in means using Student–Newman–Keuls (SNK) multiple comparisons. To test whether purple and red urchin habitat status differed among sites, we used separate Pearson’s Chi-square tests.
To estimate fish abundance, a team of two divers conducted 25 × 4 × 4 m transects and identified each fish encountered (Parnell et al. 2005). There are numerous kelp forest fishes known to feed on juvenile or adult urchins including black surfperch, Embiotoca jacksoni, rubberlip surfperch, Rhacochilus toxotes, and señorita, Oxyjulis californica (Kenner 1992). Other fishes including kelp bass, Paralabrax clathratus, and rock wrasse, Halichoeres semicinctus, are known to scavenge urchins after sheephead attacks (Cailliet 2000; KDN personal observation). Some of the fishes observed in the area are not only urchin predators, but also known predators of spiny lobsters. Giant black sea bass, Stereolepis gigas, one important spiny lobster predator, were seen regularly during the course of this research but were not observed on transects. We conducted transects between the hours of 0700–1300 from June 26 to October 1, 2008, for a total of 20 haphazardly located transects at each site (for total counts see Table 3). We used separate one-way ANOVAs to analyze differences in fish abundances and Simpson’s index of diversity (D s) among the three sites.
Habitat surveys: rugosity
In order to infer whether available refuge habitat for urchins differs among sites, we estimated substratum complexity by quantifying rugosity along urchin and lobster transects. We quantified rugosity as the ratio l/L, where L is the actual distance between two points and l is the linear distance between such points (Luckhurst and Luckhurst 1978). At 12 haphazardly chosen points along 3–4 transects per site we laid a small linked, 3-m brass-plated twist chain (Campbell® #200) directly along the contours of the substrate (L) and then compared that measurement to the total linear distance (l). We tested for differences in rugosity among the three sites using a one-way ANOVA.
Results
Urchin proportional mortality
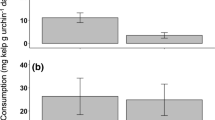
There was a significant interactive effect of urchin density and time of day, and a significant interactive effect of cover and time of day on purple urchin proportional mortality (Table 1). Urchin proportional mortality decreased with urchin density during the day but was density independent during the night (Fig. 2), and algal cover reduced purple urchin proportional mortality during the day, but not at night (Fig. 3).
Proportional mortality for purple urchins (Strongylocentrotus purpuratus) exposed to predators in the La Jolla Ecological Reserve during the day and during the night, either under cover of understory algae or in the open (no cover). Asterisk denotes significant difference in proportional mortality between cover and no-cover treatments
The main predator observed removing urchins from the plots during daytime trials was the California sheephead. Video recordings showed a marginally significant increase in sheephead abundance with urchin density (Linear regression, r 2 = 0.71, F 1,4 = 7.2, P = 0.07; Fig. 4). A distinctive behavioral pattern was observed, with a male sheephead removing an urchin from the plot, which then attracted female sheephead, kelp bass, señoritas, and rock wrasses to scavenge the remains.
We observed several species of predators removing urchins from plots during the night. Numerous spiny lobsters were observed near plots consuming removed urchins. Additionally, a horn shark, Heterodontus francisci, was observed consuming a sea urchin during nighttime experimental trials though not observed during daytime fish transects.
Urchin, lobster and fish surveys
We found strong evidence for a difference in the density and habitat use of purple urchins and red urchins among the three sites (Table 2). Purple urchins were significantly denser in PL than in LJER, but densities did not differ between ATR and LJER and ATR and PL (Fig. 5). For red urchins, densities in PL were significantly lower than densities at the other two sites (Fig. 5). We found only six individuals on 20 transects in PL, but found 42 individuals in LJER and 39 individuals in ATR. For both species, most urchins found on transects were in rock burrows (64.7 %). Fewer were under ledges (22.5 %) sheltered under boulders (11.3 %), or exposed (1.3 %), with exposed urchins found only at PL. Purple urchin habitat status (protected vs. exposed) did not differ among sites (Pearson’s Chi square, χ 2 = 1.36, P = 0.50). For red urchins, we found a significant difference in habitat status between LJER and ATR (PL was not included in red urchin analyses due to low sample size). Most red urchins (74 %) were exposed in LJER, whereas only 33 % of red urchins were exposed to predators in ATR (Pearson’s Chi square, χ 2 = 13.4, P = 0.003).
Mean density of a red urchins (Strongylocentrotus franciscanus) and purple urchins (Strongylocentrotus purpuratus), and b California spiny lobsters (Panulirus interruptus) and predatory fishes from transect surveys conducted in the La Jolla Ecological Reserve (LJER), adjacent to the reserve (ATR), and Point Loma (PL). Unlike letters above bars denote treatments that differed significantly at P < 0.05 in post hoc tests conducted separately for each species or group
For lobsters, we found significantly higher lobster density in LJER than in PL, but lobster density in LJER and ATR, and in PL and ATR did not differ significantly (Table 2; Fig. 5). Mean lobster CL was greater in LJER than in ATR and PL (ANOVA, F(2152) = 16.89, P < 0.001). LJER housed some very large lobsters that were absent from PL and ATR where lobster fishing is prevalent (Fig. 6).
We observed a total of 13 fish species in our surveys, and the abundance of fish predators did not precisely follow trends of fishing intensity among our three sites (Table 3; Fig. 5). PL had significantly lower fish abundance than the other two sites, but we counted a high number of sheephead and kelp bass in ATR, which had a slightly (but not significantly) higher abundance of predators than in LJER (ANOVA, F(2,57) = 3.35, P = 0.04). Simpson’s index of diversity (D s) was significantly lower in PL than in ATR or LJER (ANOVA, F(2,57) = 4.59, P = 0.01).
Habitat surveys: rugosity
Rugosity varied substantially among the three sites and was significantly higher in LJER (0.43 ± SE = 0.05) than in ATR (0.22 ± SE = 0.06) and PL (0.27 ± SE = 0.08; ANOVA, F(2,34) = 3.67, P = 0.04). Most of the locations we sampled in PL tended to be flat with very few boulders. Shelters at this site were typically long contiguous ledges, whereas in LJER, there are more large boulders and a greater diversity of substrate types. ATR infrequently had large boulders but did not contain as much substrate diversity as within LJER.
Discussion
Determining the degree of density dependence associated with urchin mortality from predation, and how habitat structure influences predation and urchin behavior are essential to understanding the effects of urchin grazing on algae and the implications this has for kelp forest dynamics and stability. In our experiment, per capita mortality rates of urchins exposed to predators did not increase with increasing urchin density (i.e., a potentially regulating predator response was not observed). This was surprising given the high density and high average sizes of major urchin predators in LJER; sheephead and spiny lobsters. In our experiments, sheephead appeared to aggregate on plots with high urchin density, but videos of sheephead feeding revealed that a common pattern of behavior was for a large male sheephead to begin consuming an urchin, after which smaller female sheephead and other species such as kelp bass would come in to devour smaller urchin pieces, resulting from the crushing of the urchin by the large male. Fish size and type influence their ability to consume urchins as only larger fish may be able to break the urchin test (Clemente et al. 2010). The high numbers of fish observed during the day on high urchin density plots did not often result in a large proportion of urchins being consumed over periods of 1 h. We also did not observe an increase in purple urchin proportional mortality with urchin density at night, when lobsters are actively foraging. Similar to our results, in Nova Scotia, Canada, urchin Strongylocentrotus droebachiensis aggregations reduced the number of attacks by the predatory crab Cancer irroratus (Bernstein et al. 1981).
Though our predation experiment results suggest that predators such as lobsters and large fishes may not be able to substantially reduce urchin density when urchins achieve very high densities, our results and those of others suggest that predators may effectively control urchin density and behavior at relatively low urchin densities (Hereu et al. 2012; Clemente et al. 2011). The fact that urchin proportional mortality was lowest at our highest urchin density suggests that dense patches of urchins experience low per capita urchin mortality. Predators may not be able to achieve high enough foraging rates to substantially reduce urchin densities when urchins are found in dense aggregations. In future experiments including higher urchin densities may be advisable, however, urchin proportional mortality in our experiments was high at low urchin densities, which are akin to densities normally found in healthy kelp forests. In our surveys, purple urchins were least dense in the LJER where lobsters and fishes were abundant (and lobsters were larger), and were most dense in PL where lobsters and fishes were less abundant (and lobsters were smaller). Several studies have shown correlations between predator abundance and urchin behavior in marine reserves, with predators being more abundant and effective and urchins, exhibiting more cryptic behavior within reserves as compared to non reserve sites (Sala and Zabala 1996; Sala et al. 1998; Clemente et al. 2011). This pattern also exists among sites in kelp forests of the Channel Islands in southern California (Lafferty 2004).
A dramatic increase in spiny lobster Jasus lalandii abundance was correlated with strong reductions in sea urchin Parechinus angulosus abundance in South Africa (Mayfield and Branch 2000), though in contrast to our results, laboratory experiments with these species revealed that the number of sea urchins consumed by spiny lobsters was proportional to their density (Mayfield et al. 2001). Laboratory feeding experiments with California spiny lobster, purple urchins, and red urchins led Tegner and Levin (1983) to propose that spiny lobster predation in southern California kelp forests was sufficient to dictate urchin size-frequency distributions. Additionally, Cowen (1983) compared sea urchin abundance between sheephead removal and non-removal sites of southern California kelp forests and concluded that sheephead strongly control red urchin abundance and sheltering behavior. It is also important to note that predation may synergistically act with other factors influencing urchin abundance, such as food availability, larval supply, recruitment, and disease (Tegner and Dayton 1977; Watanabe and Harrold 1991; Pearse 2006, Foster and Schiel 2010).
Shelter is a key factor in determining predation rates on sea urchins (Cowen 1983; Sala et al. 1998; Hereu et al. 2005). Though urchins commonly seek shelter in crevices or under boulders, they also may use understory algae as a refuge. We hypothesized that macroalgal cover would reduce urchin mortality during the day by hiding urchins from visually oriented predators, but might increase urchin mortality at night because spiny lobsters, a primary nocturnal predator of urchins, often seek cover under macroalgae (Mai and Hovel 2007; Hovel and Lowe, unpublished data) or even seek out macroalgae after emerging from shelter to forage on attached invertebrates (Withy-Allen and Hovel 2013). Daytime predation by fishes was reduced when plots contained high macroalgal cover, but there was no difference in urchin mortality between cover and no-cover treatments at night. Similarly, Hereu et al. (2005) found that predation by fishes on juvenile urchins (Paracentrotus lividus) was reduced under algae and in rocky crevices in a Mediterranean Sea marine reserve.
An interesting result from our experiment was that the presence of habitat structure had no influence on the relationship between urchin proportional mortality and urchin density. Urchin proportional mortality decreased with urchin density at the same rate with and without understory algae present. In contrast, many other studies have shown that habitat structure can influence whether prey mortality rates are density dependent. For example, kelp perch Brachyistius frenatus proportional mortality was inversely density dependent when kelp habitat structure was low, but was density independent when moderate or high amounts of kelp were added to laboratory mesocosms (Anderson 2001). Similar results were found in field experiments on the small coral reef goby Coryphopterus glaucofraenum, in which limited shelter caused density-dependent mortality, but proportional mortality was density independent in plots with abundant refuges (Forrester and Steele 2004). A difference between our experiment and Forrester and Steele (2004) was that gobies used discrete rocky crevices in their study, but urchins were provided with more diffuse algal cover in our study, preventing the “musical chairs” type of shelter limitation in which particular individuals are without shelter. Similar to our study, proportional mortality of the invasive Asian mussel Musculista senhousia was inversely density dependent at all levels of eelgrass Zostera marina habitat structure in southern California, even though their primary predator (the gastropod Pteropurpura festiva) aggregated in plots with high mussel density and displayed different functional responses among different levels of seagrass habitat structure (Kushner and Hovel 2006).
In our surveys, we saw no difference in purple urchin habitat use among sites. Most urchins we found were under rocks or embedded within small crevices, and nearly all remaining urchins were found under ledges. However, our low-predator-density site (PL) was the only site at which urchins were found completely exposed on top of rocks (2.5 % of purple urchins found in PL). Kelp forest urchins typically remain cryptic and feed on drift algae and may only leave hiding places when drift algae becomes scarce (Harrold and Reed 1985). However, there is evidence that predator abundance may influence urchin behavior (Sala et al. 1998). On southern California rocky reefs, red urchins Strongylocentrotus franciscanus were less cryptic in areas where sheephead Semicossyphus pulcher were removed and where sheephead densities were low (Cowen 1983). Sheephead removal also resulted in increased urchin abundance (Cowen 1983). In the northwest Mediterranean Sea, daily and monthly movement of the urchin Paracentrotus lividus was reduced by high predator abundance (Hereu 2005). Lobster predation is limited on large urchins (Tegner and Levin 1983; Andrew and MacDiarmid 1991; Mayfield et al. 2001). The red urchins we observed in both the LJER and ATR sites were typically large and not very cryptic, which suggests a possible size refuge for this species. The proportion of red urchins found exposed was higher in LJER than in the ATR, even though predator density and urchin density were similar between these two sites, and rugosity was higher in the LJER, potentially providing more crevices.
A caveat of our study is that there are a variety of factors that may vary between our sites, which may influence purple and red urchin habitat use and behavior (e.g., predation, habitat structure, recruitment, food supply, and competition). Since both predator density and rugosity varied among the three sites, we cannot explicitly tease apart the effect of fishing pressure and rugosity on urchin abundance and habitat use, as rugosity is positively correlated with reef fish abundance and species richness (Luckhurst and Luckhurst 1978, Friedlander and Parish 1998) and urchin density is correlated with habitat structure (Guenther et al. 2012). For red urchins, fishing strongly influences abundance, and red urchins were almost completely absent in PL. Fishers also may select exposed urchins over cryptic urchins, leading to fewer exposed urchins in ATR than in LJER. Thus, in areas outside of marine reserves, the commercial fishery for red urchins may be partly filling the ecological role of large lobsters and fishes (Dayton et al. 1998). Ideally, our study would have encompassed a larger geographic area and multiple marine reserves allowing us to explicitly tease out the effects fishing pressure may have on this system. While we believe the no-take LJER established in 1971 has been effective, particularly in increasing local diversity and predator abundances in the area, replicating our study in multiple reserves would strengthen our understanding of the relationships we observed.
Though the role of sheephead and lobsters in regulating urchin population abundance has come into question (Foster and Schiel 2010), their ecological role as predators reflects the complexity of trophic interactions that sustain the kelp forest food web (Tegner and Levin 1983; Cowen 1986; Hamilton et al. 2011, Guenther et al. 2012). The kelp forests in southern California alone support more than 200 species of algae, invertebrates, fishes, and mammals (Graham 2004). The distributions of many of these organisms are known to be linked tightly to the presence of M. pyrifera, due to a variety of trophic and habitat associations. This habitat also supports a broad array of extractive and non-extractive industries, including fisheries, aquaculture, and tourism. The factors influencing the ecological stable state of a kelp forest community are numerous (Steneck et al. 2002); understanding which factors impact urchin communities most significantly can help to avoid undesirable urchin barrens and promote ecologically resilient communities.
Studying urchin population regulation and potential density dependence over large scales is important to understand potential global regime shifts (Ling et al. 2015). Though predation is one of many factors influencing urchin populations, the fishing of predators can have dramatic effects on urchin behavior (Sala et al. 1998) and the influence of fishing pressure on top predators may be particularly important in kelp forest systems (Halpern et al. 2006). Ecological data on top predators and the impacts they have on the community are important in kelp forests not only to foster an understanding of basic ecological processes but also to promote practical management schemes, particularly those dealing with fisheries management and reserve design (Parnell et al. 2006). Our results help to elucidate the processes behind urchin population regulation, which is critical due to the possible community-wide impacts an increase in urchin abundance may have.
References
Anderson TW (2001) Predator responses, prey refuges, and density-dependent mortality of a marine fish. Ecology 82:245–257
Andrew NL, MacDiarmid AB (1991) Interrelations between sea urchins and spiny lobsters in northeastern New Zealand. Mar Ecol Prog Ser 70:211–222
Barsky KC (2001) California spiny lobster. In: Leet WS, Dewees CM, Klingbeil K, Larson EJ (eds) California’s living marine resources: a status report. Agric Nat Resour Commun Serv, Richmond, pp 98–100
Bernstein BB, Williams BE, Mann KH (1981) The role of behavioral responses to predators in modifying urchin (Strongylocentrotus droebachiensis) destructive grazing and seasonal foraging patterns. Mar Biol 63:39–49
Cailliet GM (2000) Final report: biological characteristics of nearshore fishes of California: a review of existing knowledge and proposed additional studies for the Pacific Ocean Interjurisdictional Fisheries Management Plan Coordination and Development Project. CA Department of Fish and Game, pp 1–9
Carter SK, VanBlaricom GR, Allen BL (2007) Testing the generality of the trophic cascade paradigm for sea otters: a case study with kelp forests in northern Washington, USA. Hydrobiol 579:233–249
Clemente S, Hernández JC, Rodríguez A, Brito A (2010) Identifying keystone predators and the importance of preserving functional diversity in sublittoral rocky-bottom areas. Mar Ecol Prog Ser 413:55–67
Clemente S, Hernández JC, Brito A (2011) Context-dependent effects of marine protected areas on predatory interactions. Mar Ecol Prog Ser 437:119–133
Clemente S, Hernández JC, Montaño-Monctezuma G, Russell MP, Ebert TA (2013) Predators of juvenile sea urchins and the effect of habitat refuges. Mar Biol 437:579–590
Cowen RK (1983) The effect of sheephead (Semicossyphus pulcher) predation on red-sea urchin (Strongylocentrotus franciscanus) populations an experimental analysis. Oecologia 58:249–255
Dayton PK, Tegner MJ, Edwards PB, Riser KL (1998) Sliding baselines, ghosts and reduced expectations in kelp forest communities. Ecol Appl 8:309–322
Dayton PK, Tegner MJ, Edwards PB, Riser KL (1999) Temporal and spatial scales of kelp demography: the role of oceanographic climate. Ecol Monogr 69:219–250
Estes JA, Palmisano J (1974) Sea otters: their role in structuring nearshore communities. Science 185:1058–1060
Estes JA, Tinker MT, Williams TM, Doak DF (1998) Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282:473–476
Farina S, Tomas F, Prado P, Romero J, Alcoverro T (2009) Seagrass meadow structure alters interactions between the sea urchin Paracentrotus lividus and its predators. Mar Ecol Prog Ser 377:131–137
Forrester GE, Steele MA (2004) Predators, prey refuges, and the spatial scaling of density-dependent prey mortality. Ecology 85:1332–1342
Foster MS, Schiel DR (2010) Loss of predators and the collapse of southern California kelp forests: alternatives, explanations and generalizations. J Exp Mar Biol Ecol 393:59–70
Friedlander AM, Parish JD (1998) Habitat characteristics affecting fish assemblages on a Hawaiian coral reef. J Expl Mar Bio Ecol 224:1–30
Graham MH (2004) Effects of local deforestation on the diversity and structure of Southern California giant kelp forest food webs. Ecosys 7:341–357
Guenther CM, Lenihan HS, Le Grant et al (2012) Trophic cascades influenced by lobster fishing are not ubiquitous in southern California kelp forests. PLoS One 7(11):e49396
Guidetti P (2006) Marine reestablish lost predatory interactions and cause community changes in rocky reefs. Ecol Appl 16(3):963–976
Halpern BS, Cottenie K, Broitman BR (2006) Strong top-down control in southern California kelp forest ecosystem. Science 312(5777):1230–1232
Hamilton SL, Caselle JE, Lantz CA et al (2011) Extensive geographic and ontogenetic variation characterizes the trophic ecology of a temperate reef fish on southern California (USA) rocky reefs. Mar Ecol Prog Ser 429:227–244
Harrold C, Reed DC (1985) Food availability, sea urchin grazing, and kelp forest community structure. Ecology 66:1160–1169
Hereu B (2005) Movement patterns of the sea urchin Paracentrotus lividus in a marine reserve and an unprotected area in the NW Mediterranean. Mar Ecol 26:54–62
Hereu B, Zabala M, Linares C, Sala E (2005) The effects of predator abundance and habitat structural complexity on survival of juvenile sea urchins. Mar Biol 146:293–299
Hereu B, Linares C, Sala E, Garrabou J, Garcia-Rubies A, Diaz D, Zabala M (2012) Multiple processes regulate long-term population dynamics of sea urchins on Mediterranean rocky reefs. PLoS One 7(5):e36901. doi:10.1371/journal.pone.0036901
Kenner MC (1992) Population dynamics of the sea urchin Strongylocentrotus purpuratus in a Central California kelp forest: recruitment, mortality, growth and diet. Mar Biol 112:107–118
Kushner RB, Hovel KA (2006) Effects of native predators and eelgrass habitat structure on the introduced Asian mussel Musculista senhousia (Benson in Cantor) in southern California. J Exp Mar Biol Ecol 332:166–177
Lafferty KD (2004) Fishing for lobsters indirectly increases epidemics in sea urchins. Ecol Appl 14:1566–1573
Lindberg RG (1955) Growth, population dynamics and field behavior of the spiny lobster, Panulirus interruptus. Univ of CA Pub Zool 59:157–247
Ling SD, Johnson CR, Frusher SD, Ridgway KR (2009) Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc Natl Acad Sci 106(52):22341–22345
Ling SD, Scheibling RE, Rassweiler A, Johnson CR, Shears N, Connell SD, Salomon AK, Norderhaug KM, Pérez-Matus A, Hernández JC, Clemente S, Blamey LK, Hereu B, Ballesteros E, Sala E, Garrabou J, Cerbrian E, Zabala M, Fujita D, Johnson LE (2015) Global regime shift dynamics of catastrophic sea urchin overgrazing. Phil Trans B 370:20130269
Loflen CL (2007) Behavioral responses by the California spiny lobster (Panulirus interruptus) to predation inside and outside a marine protected area. Master thesis, San Diego State University
Luckhurst BE, Luckhurst K (1978) Analysis of the influence of substrate variables on coral reef fish communities. Mar Biol 49:317–323
Mai TT, Hovel KA (2007) Influence of local-scale and landscape-scale habitat characteristics on California spiny lobster (Panulirus interruptus) abundance and survival. Mar Freshw Res 58:419–428
Mayfield S, Branch GM (2000) Interrelations among rock lobsters, sea urchins, and juvenile abalone: implications for community management. Can J Fish Aquat Sci 57:2175–2185
Mayfield S, de Beer E, Branch GM (2001) Prey preference and the consumption of sea urchins and juvenile abalone by captive rock lobsters (Jasus lalandii). Mar Freshw Res 52:773–780
Morris RH, Abott DP, Haderlie EC (1980) Intertidal invertebrates of California. Stanford Univ Press, Stanford
Parnell PE, Lennert-Cody CE, Geelen L, Stanley LD, Dayton PK (2005) Effectiveness of a small marine reserve in southern California. Mar Ecol Prog Ser 296:39–52
Parnell PE, Dayton PK, Lennert-Cody CE, Rasmussen LL, Leichter JJ (2006) Marine reserve design: optimal size, habitats, species affinities, diversity and ocean microclimate. Ecol Appl 16:945–962
Pearse JS (2006) Ecological role of purple sea urchins. Science 314:940–941
Pederson HG, Johnson GR (2006) Predation of the sea urchin Helicidaris erythrogramma by rock lobsters (Jasus edwardsii) in no-take marine reserves. J Exp Mar Biol Ecol 336:120–134
Peterson CH, Black R (1994) An experimentalist’s challenge: when artifacts of intervention interact with treatments. Mar Ecol Prog Ser 111:289–297
Robles CD, Sweetnam DA, Eminike J (1990) Lobster predation on mussels: shore-level differences in prey vulnerability and predator preference. Ecology 71:1564–1577
Sala E, Zabala M (1996) Fish predation and the structure of the sea urchin Paracentrotus lividus populations in the NW Mediterranean. Mar Ecol Prog Ser 140:71–81
Sala E, Boudouresque CF, Harmelin-Vivien M (1998) Fishing, trophic cascades, and the structure of algal assemblages: evaluation of an old but untested paradigm. Oikos 82:425–439
Shaw RG, Mitchell-Olds T (1993) Anova for unbalanced data: an overview. Ecology 74:1638–1645
Shears NT, Babcock BC (2002) Marine Reserves demonstrate top-down control of community structure on temperate reefs. Oecol 132:131–142
Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459
Tegner MJ, Dayton PK (1977) Sea urchin recruitment patterns and implications of commercial fishing. Science 196:324–326
Tegner MJ, Dayton PK (2000) Ecosystem effects of fishing in kelp forest communities. ICES J Mar Sci 57:579–589
Tegner MJ, Levin LA (1983) Spiny lobsters and sea urchins: analysis of a predator-prey interaction. J Exp Mar Biol Ecol 73:125–150
Topping DT, Lowe CG, Caselle JE (2005) Home range and habitat utilization of adult California sheephead Semicossyphus pulcher (Labridae), in a temperate no take marine reserve. Mar Biol 147:301–311
Underwood AJ (1997) Experiments in ecology. Cambridge Univ Press, Cambridge
Watanabe JM, Harrold C (1991) Destructive grazing by sea urchins Strongylocentrotus spp. in a central California USA kelp forest: potential roles of recruitment depth and predation. Mar Ecol Prog Ser 71:125–141
Wharton WG, Mann KH (1981) Relationship between destructive grazing by the sea urchin, Strongylocentrotus droebachiensis, and the abundance of American lobster, Homarus americanus, on the Atlantic coast of Nova Scotia. Can J Fish Aquat Sci 38:1339–1349
Withy-Allen KRY, Hovel KA (2013) California spiny lobster (Panulirus interruptus) movement behavior and habitat use: implications for the effectiveness of marine protected areas. Mar Freshw Res 64:359–371
Acknowledgments
This research was supported by grants from the Edna Bailey Sussman Foundation, San Diego State University and California Sea Grant. Thanks to K. Withy-Allen, E. Moore, B. Cheng, J. Coates, K. Tait, R. Jenkinson, C. Loflen and M. Castorani for their assistance in the field and lab. Special thanks also to B. Hembrough for all of his enthusiastic help in the field. We also thank M. Edwards and K. Farley for comments on the study. We thank the San Diego State University diving and boating program for boat usage and provision of tanks. This is contribution 39 from the Coastal and Marine Institute at San Diego State University.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Sean Connell.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nichols, K.D., Segui, L. & Hovel, K.A. Effects of predators on sea urchin density and habitat use in a southern California kelp forest. Mar Biol 162, 1227–1237 (2015). https://doi.org/10.1007/s00227-015-2664-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2664-2