Abstract
A northward trend of declining abundance of sea urchins (Strongylocentrotus droebachiensis) and increasing kelp recovery along the coast of Norway has provided an opportunity to explore whether the decline of sea urchins can be explained by systematic variations in demographic or fitness-related traits. The population density of sea urchins in mid-Norway (65°70′N) close to the boundary between barren grounds and recently recovered kelp forests was only 26 % of that on barren grounds in northern Norway (70°70′N). Populations were dominated by young (2–4 years) individuals and age-specific numbers decreased nearly exponentially with age. The variation in most demographic variables was high among replicate sampling sites and test size. Mean age and growth rates did not differ consistently among sites in mid- and northern Norway nor between barren ground and kelp sites. In contrast, mortality rates in mid-Norway were 50 % higher than in northern Norway. We suggest that increasing predation pressure on sea urchins partly explains the higher mortality in mid-Norway. Recent studies have shown that recruitment of sea urchins is significantly lower in mid-Norway than in northern Norway. The lower mean density of sea urchins, and hence the progressing recovery of the kelp forests in mid-Norway, may thus result from a combination of recruitment failure and increasing mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
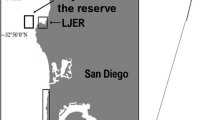
Community phase shifts from kelp forests to barren grounds as a result of overgrazing by sea urchins have been observed worldwide over the last five decades (Lawrence 1975; Johnson and Mann 1988; Sivertsen 1997; Gagnon et al. 2004; Fujita 2010). While kelp forests are among the most productive ecosystems worldwide (Dayton 1985; Mann 2000), sea urchin-dominated barrens are structurally simple. The loss of kelp can affect organisms that depend on kelp for habitat and food (Steneck and Johnson 2014). Overgrazing of kelp and shift to barren grounds was first observed along the west and north coast of Norway in the early 1970s and was correlated with extensive population outbreaks of the green sea urchin, Strongylocentrotus droebachiensis (O.F. Müller) (Sivertsen 1997; Norderhaug and Christie 2009). The overgrazed barren ground was largest in the late 1980s (Fig. 1), covering an estimated 2000 km2 (Norderhaug and Christie 2009) from Trøndelag (63°N) in mid-Norway, northwards along the whole Norwegian coast and further eastwards into Russia at 71°N (Skadesheim et al. 1995; Sivertsen 1997). Along this part of the coastline, kelp forests only remained in areas with high wave exposure (Sivertsen 1997). However, kelp forests have recently recovered in the southernmost part of the overgrazed area and recovery is currently progressing northwards (Norderhaug and Christie 2009). The southern limit of the barren ground area was located south of the Vega archipelago in mid-Norway (65°N, Fig. 1) in 2007 (Norderhaug and Christie 2009). More recently, kelp forest recovery has been observed north of Vega, where patches of kelp forests seem to have re-established within barren ground areas maintained by sea urchins (Rinde et al. 2014). Extensive barren grounds still remain further north (Norderhaug and Christie 2009).
Study sites in North Norway (Hammerfest, 70°70′N) and mid-Norway (Vega, 65°70′N). Filled circle—barren ground sampling sites, filled triangle—kelp forest sampling sites. Black dashed line marks the extent of the remaining barren ground area, while light grey dashed line marks the kelp recovery area. The progressive northward movement of the interface between barrens and recovered kelp forests is indicated by years and lines (from Norderhaug and Christie 2009)
Phase shifts from kelp-dominated states to barren grounds and vice versa are generally caused by changes in sea urchin grazing intensity as a result of marked changes in the abundance of sea urchins (Filbee-Dexter and Scheibling 2014 and references therein). Fluctuations in the abundance of sea urchins can be caused by changes in recruitment (Hart and Scheibling 1988) and mortality rates (Estes et al. 1998). The recent recovery of kelp forests in mid-Norway appears to be correlated with a substantial, yet undocumented decrease in sea urchin abundance (Norderhaug and Christie 2009). The decreasing abundance of sea urchins in mid-Norway seems partly correlated with low larval settlement, which may have been caused by recent ocean warming (Fagerli et al. 2013).
The green sea urchin is an opportunistic feeder, which can tolerate food deprivation for prolonged periods (Russell 1998). On barren grounds, sea urchins acquire energy from various food sources, including drift and encrusting algae and other invertebrates (Levitan 1988; Russell et al. 1998; Scheibling and Hatcher 2001; Krumhansl and Scheibling 2012). Once a barren ground state has formed, it may persist for decades (Steneck et al. 2002). Longevity in S. droebachiensis can be high, e.g. 30–45 years (Vadas et al. 2002; Ebert and Southon 2003). Ebert (1983), Sivertsen (1997) and Vadas et al. (2002) suggested that a long reproductive lifespan, abundant gamete production and occasional successful recruitment events are sufficient to repopulate and maintain sea urchin populations in barrens (cf. bet-hedging theory proposed by Ebert 1982). If true, urchin populations should be dominated by large individuals. However, limited food supply is expected to result in small size, low gonad index and possibly short lifespans (Himmelmann 1978; Himmelman 1986; Sivertsen and Hopkins 1995; Stien et al. 1998; Konar 2001). Christie and Rueness (1998) argued that sea urchin populations in barrens must depend on regular recruitment to compensate for high adult mortality. If true, this should result in population structures showing a steady decline in numbers with increasing age. Comparative studies on differences in population density and demographic structure between remaining sea urchin populations in mid-Norway and those in the north may provide information about the ongoing phase shift in the southern part of the overgrazed area.
In the present study, we investigate whether sea urchin populations in mid-Norway have a lower density than populations further north, as suggested by Norderhaug and Christie (2009). We also test whether populations from mid- and northern Norway differ in demographic traits (i.e. age structure, overall mortality rate) and fitness-related traits (i.e. size, individual growth rate). Population (age) structure and demographic characteristics are studied in sea urchin populations and in kelp forests and on barren grounds from mid-Norway around the island of Vega, and in northern Norway near Hammerfest.
Materials and methods
Study area and sampling
Two study areas were selected for this study (Fig. 1). One area was located north of the island Vega at 65°70′N (mid-Norway) and one in the area around Hammerfest, at 70°70′N (northern Norway). Kelp forests have recovered south of Vega, while a mosaic of barren grounds and recovered kelp forests are found on the northern side of the island. Hammerfest is located in the northernmost part of the overgrazed area, where sea urchins dominate and no signs of kelp recovery have been reported. Three replicate barren ground and kelp sites were selected in each area (Vega and Hammerfest) for a total of 12 sampling sites. All sampling sites were at a depth of ~5 m and consisted of bedrock with varying cover of boulders and gravel. Kelp sites were dominated by Laminaria hyperborea (Gunnerus) Foslie, while Saccharina latissima (Linnaeus) C. E. Lane, C. Mayes, Druehl & G. W. Saunders and Alaria esculenta (Linnaeus) Greville were present in low abundances. The nature of kelp sites differed substantially between the two areas. Kelp sites in mid-Norway were characterized by large, more or less continuous forests extending over 100–1000 m, while kelp sites in the north consisted of small and scattered patches of kelp extending over 10–100 m.
The population density of sea urchins was quantified at all sampling sites by visual counts in May 2008. Ten 0.25-m2 quadrats were randomly dropped from 1 to 2 m above the sea floor, and all visible sea urchins within each quadrat were counted. Crevices and loose substrate were carefully searched to ensure that small sea urchins were counted.
Sea urchins were collected at each site for ageing in May 2010, with the exception of site V3 north of Vega (Fig. 1), which was sampled in October 2009. Quadrats (0.25 m2) were haphazardly dropped from 1 to 2 m above the sea floor, and all visible sea urchins including those hiding in crevices and among loose substrate were collected. This procedure was repeated until at least 200 individuals were obtained per site, with the exception of kelp sites near Vega, where no sea urchins were found. Crevices and loose substrate were carefully searched to ensure that small sea urchins were also collected. All individuals were measured to the nearest millimetre with callipers.
Age determination
Natural growth zones form in the ossicles of sea urchins during growth and appear as light and dark zones on their test plates (Pearse and Pearse 1975). Pale, opaque rings reflect periods of fast growth, whereas dark, translucent rings represent periods of slow growth (Pearse and Pearse 1975). The growth zones are assumed to form seasonally, adding one opaque ring after a period of fast summer growth and one translucent ring after slower winter growth. By using fluorescent tags and comparing the position of the marks relative to formation of growth zones, Robinson and MacIntyre (1997) validated the annual banding pattern in S. droebachiensis.
Age of sea urchins was determined by counting growth zones in the interambulacral plates (Jensen 1969). Individuals were cut in half and rinsed with ethanol. The cleaned halves were dried at 60 °C for 1–7 days until the zones became visible. The number of growth zones in the interambulacral plates was counted under a dissecting microscope, adding vegetable oil to make the growth lines clearly visible. Some plates exhibited weakly pigmented lines, probably representing periods of food deprivation (Russell and Meredith 2000), and were excluded for growth analysis (approximately 367 out of a total of 1544). This method is considered reliable for individuals of S. droebachiensis between 20 and 50 mm in diameter where growth lines are distinct and clearly spaced (Robinson and MacIntyre 1997; Meidel and Scheibling 1998; Vadas et al. 2002). However, the growth rate of sea urchins eventually slows and indistinct growth zones may lead to an underestimation of the age of larger individuals (Russell and Meredith 2000).
Size, growth rate and mortality rate estimation
Maximum test diameter and individual growth rate (increase in diameter) of S. droebachiensis was estimated from data on individual test diameter at age. The Gompertz function (Gompertz 1825) was fitted to data of test diameter versus age (Winsor 1932):
where TDx is the test diameter at age x, TD∞ is the asymptotic test diameter (in mm), k is the maximum relative growth rate during the growth phase, and M is the age (in years) at which maximum growth rate occurs.
The instantaneous mortality rate (Z) for each of the nine populations sampled was estimated from the frequencies of individuals in each age class under the assumption that mortality rates are approximately the same for all ages included (Beverton and Holt 1957). Mortality rate, Z, was estimated by fitting an exponential function to the right hand (i.e. to the right of the mode, typically leaving out data for age classes 0–1 and 1–2 years), descending part of the abundance versus age curve (Ricker 1975):
where N t is the abundance of sea urchins at age t, N 0 is the density at age 0, and t is age (in years). In cases where the abundance versus age curve was bimodal (e.g. sites H3, H6 and V3—see “Results”), the exponential function was only fitted to one of the two descending parts, typically the oldest one, which often included most age classes.
Statistical analyses
The density of sea urchins was compared across area and habitat using two-way nested (permutational) ANOVA with the factors Area (two levels: mid-Norway and northern Norway) and Habitat (two levels: barren and kelp) considered fixed factors and site (random) nested into Area and Habitat. No urchins were found in any of the three kelp sites in mid-Norway near Vega so the orthogonal design used to analyse density data could not be used to analyse the remaining response variables. Instead, one-factor (permutational) ANOVA was used to compare mean test diameter, age, growth parameters and mortality rates across the remaining three combinations of Area and Habitat (i.e. mid-barren, north barren and north kelp). We used nested analysis (sites nested into each of the three combinations of Area and Habitat) for comparing test diameter and age, since we had multiple observations for these variables from each site (ten samples, each with multiple observations per site). The remaining variables (growth parameters and mortality rates) were obtained from fitting procedures including all sea urchins collected from a site, yielding only one value for each variable per site. Growth parameters and mortality rates were therefore compared across the three combinations of Area and Habitat using one-factor (permutational) ANOVA. We compared the relative abundance of different age groups (i.e. young, middle aged and old) across each combination of Area and Habitat using two-way (permutational) ANOVA with Area/Habitat and age group as fixed factors. Permutational ANOVA analyses were conducted using PERMANOVA+ for PRIMER v. 6 (Anderson et al. 2008). The analyses were followed by multiple comparisons test when ANOVA indicated significant effects of one or more of the main factors. Analyses were carried out using type III sum of squares on geometric (Euclidean) distances and unrestricted permutation of raw data using a α-level of 0.05. Equality of variances was tested a priori using the PERMDISP routine in PRIMER+. We used a robust version of Levenes test that uses deviations from medians since most data sets were not normally distributed (Brown and Forsythe 1974). Data were log x + 1-transformed prior to analysis when necessary to obtain homoscedasticity.
In cases where ANOVA provided p values larger than 0.05, we performed power analysis to estimate the power (i.e. 1−β, where β is the probability of making a type two error) of that test and to estimate the needed number of (replicates) sites to be sampled to obtain a power of 0.8 (Zar 1999).
The Gompertz function (Eq. 1) was fitted to test diameter at age data from each individual site, and the derived parameters (asymptotic test diameter, maximum growth rate and age at maximum growth) were estimated using nonlinear (least square) regressions. Instantaneous mortality rates (Z) were estimated for each site using nonlinear (least square) regression on abundance at age data (Eq. 2). All regression analyses were carried out using SYSTAT v. 13.
Results
The mean density of S. droebachiensis ranged from 0 m−2 at the kelp sites in mid-Norway to (mean ± SE) 42.9 ± 9.6 m−2 at barren ground sites in northern Norway (Fig. 2). Overall, northern sites (both barrens and kelp) had a higher mean density than sites in mid-Norway (33.9 ± 8.5 vs. 5.5 ± 2.9 m−2; p = 0.007, Table 1), while barren sites had a higher density of sea urchins than kelp sites (27.0 ± 8.5 vs. 12.4 ± 8.3 m−2; p = 0.012, Table 1). There was no significant Area × Habitat interaction (p = 0.362, Table 1). There was a highly significant variation in density among replicate sites within each combination of Area and Habitat (p < 0.001, Table 1).
The size distributions of S. droebachiensis varied among the nine sampling sites (Fig. 3). The size distributions were almost uni-modal at most sites, but tended to be bimodal at some sites (H3, H6 and V3). The mean test diameter of S. droebachiensis ranged from 34.3 ± 5.4 mm on barren ground sites in mid-Norway to 47.1 ± 10.1 mm at barren ground sites in northern Norway (Table 2), but variation among replicate sites was large (p = 0.001, Table 1) and differences in mean test diameter between combinations of Area and Habitat were not significant (p = 0.745).
Size distributions of S. droebachiensis at each sampling site and average size distribution (right panels of figures, mean ± 1SE) for barren ground populations in Hammerfest (H1–H3), for kelp populations in Hammerfest (H4–H6) and for barren ground populations around Vega (V1–V3). No sea urchins are found within kelp forests at Vega
With the exception of one study site (H3), the age distributions of S. droebachiensis (Fig. 4) followed the typical pattern with a high abundance of young (1–4 years) and a decreasing abundance of individuals with increasing age. The youngest age classes (0–1 years) seemed under-represented at all sites. There were only a few indications of certain cohorts being significantly over- or under-represented (i.e. deviating markedly from the expected pattern), but see, for example, age classes 6–8 years at H3, 3–6 years at H6 and 5–9 years at V3, which seemed over-represented (Fig. 4). Mean age ranged from 2.78 ± 1.34 years (site H5) to 6.54 ± 3.11 years (site H3) across all sampling sites. Mean age tended to be higher in the north (i.e. north barren 4.88 ± 1.63 years vs. mid-barren 4.31 ± 1.03 years) and higher in populations from barrens than in populations from kelp forests (i.e. north barren 4.88 ± 1.63 years vs. north kelp 3.69 ± 1.08 years), but all differences across combinations of Area and Habitat were statistically non-significant (p = 0.683, Table 1). Mean age of the populations varied considerably among replicate sites within each combination of Area and Habitat (p = 0.001, Table 1).
Age distributions of S. droebachiensis at each sampling site and average age distribution (right panels of figures, mean ± 1SE) for barren ground populations in Hammerfest (H1–H3), for kelp populations in Hammerfest (H4–H6) and for barren ground populations around Vega (V1–V3). No sea urchins are found within kelp forests at Vega
Urchin populations were generally dominated by young (age classes 2–4 years) individuals that represented up to 78 % of the sea urchins (Fig. 5). Young and middle-aged individuals were significantly more abundant than older individuals (p = 0.013, Table 1; Fig. 5). Populations from kelp sites in northern Norway had slightly more young and fast-growing individuals than populations from barren ground sites in both areas (Fig. 5). The proportion of individuals of medium age was similar among habitats and areas, while barren ground sites tended to have a larger proportion of older, reproductive, individuals with very slow or no growth (Figs. 5, 6). However, the proportion of different age groups did not vary significantly among combinations of Area and Habitat, as shown by the non-significant interaction between Area/Habitat and age group (p = 0.734, Table 1). The oldest individuals found were 14 years old.
Mean proportion of S. droebachiensis individuals belonging to the youngest age classes (recruits aged 2–4 years), medium age classes (young reproductive individuals aged 5–7 years) and older age classes (older reproductive individuals aged 8–14 years) on barren grounds and in kelp forests in northern (Hammerfest, 70°70′N) and mid-Norway (Vega, 65°70′N). Mean values ±1 SE across replicate sites (n = 3). No sea urchins are found within kelp forests at Vega. Bars not sharing the same letters are statistically different
Age-specific mean test diameter (±1 SE) for S. droebachiensis from kelp forest and barren ground sites in northern (Hammerfest, 70°70′N) and mid-Norway (Vega, 65°70′N). No sea urchins are found within kelp forests near Vega. Curves are fits of the Gompertz function. Mean test diameters are based on site means (n = 1–3 sites for each age group)
Test diameter increased with age in a sigmoid manner and the Gompertz growth function provided good fits for sea urchin test diameter against age (r 2 = 0.949–0.995). Parameter estimates are provided in Table 3, while growth curves are shown in Fig. 6. The average (across replicate sites) asymptotic test diameter (TD∞) ranged from 60.2 ± 14.2 mm (mid-Norway barrens) to 65.3 ± 3.6 mm (northern Norway kelp), which corresponded to the size of the largest sea urchins found. Maximum test diameter was generally reached when sea urchins approached an age of ca. 7 years. The average growth rate of sea urchins from kelp sites in northern Norway (0.45 ± 0.09 years−1) was similar to those from barren ground sites in mid-Norway (0.42 ± 0.20 years−1) and slightly higher than for sea urchins from barren ground sites in northern Norway (0.35 ± 0.07 years−1). Fast growth occurred at approximately the same age in all habitats and areas (M ranging from 1.78 ± 0.48 to 1.98 ± 0.19 years). None of these trends were, however, statistically significant (p always >0.684, Table 1). Average mortality rates (across replicate sites) of sea urchin populations from barren ground and kelp sites in northern Norway were almost identical (0.45 ± 0.08 vs. 0.41 ± 0.12 years−1), while the mortality rates of barren ground populations in mid-Norway were ca. 50 % higher (0.62 ± 0.02 years−1; p = 0.026) than those in the north (p = 0.026).
Discussion
Demographic and fitness-related traits provide new insight of the variability in sea urchin population performance between the two study areas. The relatively low density of sea urchins around Vega in mid-Norway may explain why the kelp forest is recovering in this area. The mean density of sea urchins observed on barren ground sites in mid-Norway was only about 26 % of that observed in the north. The stability properties of barren grounds have previously been tested experimentally in mid-Norway (Vega) by manipulating the density of sea urchins (Leinaas and Christie 1996). These experiments showed that a reduction in sea urchin density to less than 5–10 m−2 lead to regrowth of algae and a rapid recovery of kelp. The average density of sea urchins on barren ground sites in mid-Norway ranged from 4.8 to 17.2 m−2 with a mean density of 11.1 m−2 across sites. This indicates that densities of sea urchins in mid-Norway are close to the threshold at which a shift towards kelp recovery and dominance is to be expected, at least at some sites. We have been visiting these study sites regularly over the last 5 years (last time during late summer 2013), but there has been no sign of kelp recovery, suggesting that some factors are delaying the process. One explanation may be that biological succession following an abrupt, experimental reduction in sea urchin density (cf. Leinaas and Christie 1996) differs from the prolonged and gradual reduction driven by nature. However, factors such as a long distance to source populations of kelp, limitations in the dispersal of kelp spores and the presence of other herbivores may contribute to a delayed recovery of kelp (Gaylord et al. 2004; Konar 2013).
Previous studies have demonstrated that somatic growth rates of sea urchins are directly related to the quality and availability of food (e.g. Himmelmann and Steele 1971; Vadas 1977; Minor and Scheibling 1997) and that high-density populations on barren grounds are characterized by small individuals with slow somatic growth (Wharton and Mann 1981, Himmelmann 1978, Sivertsen and Hopkins 1995, Stien et al. 1998). Such density-related differences were not found in our study, both in terms of size distributions and growth. We found no significant differences in growth rate, average size or asymptotic size when comparing sea urchins from mid-Norway and northern Norway or kelp and barren ground populations. The variability among replicate sites was considerable, and local variation is probably better explained by local site- or habitat-specific differences in food availability and quality (Wharton and Mann 1981; Himmelman 1986; Stien et al. 1998) than by sea urchin density. S. droebachiensis seems to prefer macroalgae, which is also the best food source to sustain growth (Vadas 1977; Himmelman 1986). Supply of algal detritus transported from productive kelp forests to adjacent, low-productive areas (Krumhansl and Scheibling 2012, Kelly et al. 2012) may explain high somatic growth rates in barren populations where the density of sea urchins is high.
The age structures of the nine populations were rather variable and did not differ systematically between groups of sites (i.e. between area and habitat). We found relatively few newly settled sea urchins (<1 year old) at all sites. This could indicate recent recruitment failure, but may also have been caused by the cryptic behaviour of juveniles, making them hard to find and sample efficiently (see Himmelmann 1986; Russel et al. 1998). Despite the large, site-specific variation in size and age of the sea urchins and despite potential under-representation of the youngest age class, all populations studied were dominated by young individuals (<4 years). This indicates that the populations in both study areas are maintained by regular recruitment rather than by extreme longevity and/or rare successful recruitment events as suggested by Ebert (1983); Sivertsen (1997) and Vadas et al. (2002).
The ageing technique based on growth zones has been criticized because it may underestimate the age of individuals >50 mm (Russel and Meredith 2000). The growth rate of S. droebachiensis is assumed to slow with age and will eventually result in indistinct or incomplete annual growth zone formation. In the present study, the oldest sea urchins were estimated to be 14 years of age, which is relatively young compared to the maximum age of S. droebachiensis reported elsewhere (Russell et al. 1998; Blicher et al. 2007). However, since large individuals constituted a small proportion of the populations and the bias was similar for all populations, a potential underestimation of the age of these individuals is likely unimportant for the overall population structures.
Most of the age structures followed an exponential decrease over time, suggesting a relatively constant recruitment and mortality over several successive years (at least past 5–6 years). However, Fagerli et al. (2013) found that the recruitment of green sea urchins was significantly lower around Vega in mid-Norway than in northern Norway in 2008, 2009 and 2010. Assuming that this difference occurred over many years, a constant but lower recruitment relative to the northern sites would help explain the lower urchin density observed around Vega.
Although most of the age structures suggested that recruitment was relatively constant over several successive years, three of the nine study sites (H3, H6 and V3) exhibited bimodal age structure. Bimodal age structures may indicate periods of high and low recruitment, but could also reflect mortality caused by size-specific predation (Tegner and Dayton 1981; Scheibling 1996) or coexistence of two growth morphs (Vadas et al. 2002). The three sites with bimodal age structures belong, however, to groups representing mid-Norway barrens, northern Norway barrens and northern Norway kelp. The approximate age of older, strong cohorts also differed between sites (i.e. ca. 7, 4 and 9 years ago at sites H3, H6 and V3, respectively). Abrupt changes in recruitment patterns at these sites are therefore not systematically related to the explanatory factors (area and habitat) or to certain years. Recruitment success likely depends on local (i.e. site-specific) conditions making it difficult to identify large-scale differences based on age-structure analyses unless a large number of replicate sites for each area and habitat are included.
In mid-Norway, sea urchins were only found at barren ground sites. This pattern confirmed what we have observed during multiple dives at these sites over the last 4–5 years; we have never observed green sea urchins within the kelp forests around Vega. The among-site variability in density of sea urchins was, in contrast, not related to the presence or absence of kelp in northern Norway. Adult sea urchins are rarely found within dense kelp forests (Hjorleifsson et al. 1995, Skadsheim et al. 1995, C. Fagerli, unpublished data), but they were rather abundant in sections of kelp forest in northern Norway, although with a lower density than in the barren ground sites (ca. 27 vs. 43 m−2). The presence of sea urchins in kelp forests in northern Norway may be explained by the small size of the kelp forests in this area. The kelp forests around Hammerfest were mainly made up of scattered patches of kelp at the scale of 10–100 s of metres, whereas those in the shallow areas around Vega (mid-Norway) were made up of larger forests with a spatial extent at the scale of 100–1000 s of metres. Large forests may harbour a higher diversity of fauna and larger populations of predators that are able to prey on sea urchins, e.g. micro-predators feeding on juveniles (Rowley 1989, Scheibling and Robinson 2008), crabs (Fagerli et al. 2014) and large predatory fish such as wolfish and cod (Keats et al. 2011; Steneck et al. 2002), compared with small, scattered forests of kelp. Low predation pressure may therefore be the most obvious explanation for the presence of sea urchins in the relatively small kelp forests or patches of kelp near Hammerfest in northern Norway.
The density of sea urchins at any site results from the long-term balance between recruitment and mortality (Balch and Scheibling 2001). The average instantaneous mortality rates (Z) of the sea urchin populations in mid-Norway were almost 50 % higher than those in the northern populations. Systematic variations in mortality rates may therefore help explain the lower density of sea urchin in mid-Norway and hence the progressive kelp forest recovery in this area. We have presently no explanation for the high mortality rates in mid-Norway. Disease and parasites can reduce sea urchin populations, but there have been no reports of outbreaks of diseases or high parasite prevalence in Norway lately (Christie et al. 1995; Stien et al. 1998). Increasing predation on sea urchins in mid-Norway may also lead to higher mortality, but it is unclear if predation pressure on sea urchins has increased over the last two decades. The edible crab, Cancer pagurus Linnaeus, has extended its distribution range northwards over the last decades and is now abundant in mid-Norway (Woll et al. 2006). Experiments have shown that C. pagurus is an efficient predator on sea urchins (Fagerli et al. 2014), but top-down control on sea urchin populations has not been documented. However, population growth of related crab species have had a major impact on mortality of juvenile and adult sea urchins in the NW Atlantic, where cohorts of sea urchins have been decimated (Steneck et al. 2013). Increased predation may in part explain why the mortality rates are higher in mid-Norway than in northern Norway.
Conclusion
In contrast to disease and overfishing leading to rapid depletion of sea urchins (Steneck et al. 2004; Scheibling and Lauzon-Guay 2010), relatively high mortality rates in mid-Norway appear to have been operating over some time as age structures suggest no abrupt shifts caused by sudden mass mortality. The changes required to cause large differences in density over many years may simply be too small to be identified easily from studies of age structures and demographic traits, which are highly variable across sites. Small area-specific (e.g. south vs. north) differences in mortality rates resulting from higher predation pressure, together with reduced settlement success (cf. Fagerli et al. 2013), may have been sufficient to reduce sea urchin density in mid-Norway. A negative ratio between sea urchin recruitment and mortality in subsequent years may finally result in further kelp forest recovery in mid-Norway. The large variation in sea urchin populations (density, size, age, growth, etc.) may be due to local variation in oceanographic processes that may affect larval dispersal and settlement, the physical environment and predation pressure (Azzarello et al. 2014; Hereu et al. 2004, 2005). The large variation in demographic traits across replicate sampling sites underscores the importance of including multiple replicates and using large sample sizes when studying demographic patterns of sea urchins.
References
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. Primer-E Ltd, Plymouth
Azzarello J, Smale DA, Lanlois TJ, Håkansson E (2014) Linking habitat characteristics to abundance patterns of canopy forming macroalgae and sea urchins in southwest Australia. Mar Biol Res 10:682–693
Balch T, Scheibling RE (2001) Larval supply, settlement and recruitment in echinoderms. In: Lawrence JM, Jangoux M (eds) Echinoderm studies, vol 6. Balkema, Lisse, pp 1–83
Beverton RJH, Holt SJ (1957) On the dynamic of exploited fish populations. Fish Invest II 19:5–533
Blicher ME, Rysgaard S, Sejr MK (2007) Growth and production of sea urchin Strongylocentrotus droebachiensis in a high-Arctic fjord, and growth along a climatic gradient (64–77°N). Mar Ecol Prog Ser 341:89–102
Brown MB, Forsythe AB (1974) Robust tests for the equality of variances. J Am Stat Assoc 69:364–367
Christie H, Rueness J (1998) Tareskog. In: Rinde E, Bjørge A, Eggereide A, Tufteland G (eds) Kystøkologi, den ressursrike norskekysten. Universitetsforlaget, Oslo, pp 164–169
Dayton PK (1985) Ecology of kelp communities. Annu Rev Ecol Syst 16:215–245
Ebert TA (1982) Longevity, life-history, and relative body wall size in sea urchins. Ecol Monogr 52:353–394
Ebert TA (1983) Recruitment in echinoderms. In: Jangoux M, Lawrence JM (eds) Echinoderm studies, vol 1. Balkema, Rotterdam, pp 169–201
Ebert TA, Southon JR (2003) Red sea urchins (Strongylocentrotus franciscanus) can live over 100 years: confirmation with A-bomb 14 carbon. Fish Bull 101:915–922
Estes JA, Tinker MT, Williams TM (1998) Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282:473–476
Fagerli CW, Norderhaug KM, Christie H (2013) Lack of sea urchin settlement may explain kelp forest recovery in overgrazed areas in Norway. Mar Ecol Prog Ser 488:119–132
Fagerli CW, Norderhaug KM, Christie H, Pedersen MF, Fredriksen S (2014) Predators of the destructive sea urchin grazer Strongylocentrotus droebachiensis on the Norwegian coast. Mar Ecol Prog Ser 502:207–218
Filbee-Dexter K, Scheibling RE (2014) Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar Ecol Prog Ser 495:1–25
Fujita D (2010) Current status and problems of isoyake in Japan. Bull Fish Res Agency 32:33–42
Gagnon P, Himmelman JH, Johnson LE (2004) Temporal variation in community interfaces: kelp-bed boundary dynamics adjacent to persistent urchin barrens. Mar Biol 144:1191–1203
Gaylord B, Reed DC, Washburn L, Raimondi PT (2004) Physical-biological coupling in spore dispersal of kelp forest macroalgae. J Mar Syst 49:19–39
Gompertz B (1825) On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philos Trans R Soc Lond 115:513–585
Hart MW, Scheibling RE (1988) Heat waves, baby booms, and the destruction of kelp beds by sea urchins. Mar Biol 99:167–176
Hereu B, Zabala M, Linares C, Sala E (2004) Temporal and spatial variability in settlement of the sea urchin Paracentrotus lividus in the NW Mediterranean. Mar Biol 144:1011–1018
Hereu B, Zabala M, Linares C, Sala E (2005) The effects of predator abundance and habitat structural complexity on survival of juvenile sea urchins. Mar Biol 146:293–299
Himmelman JH (1986) Population biology of green sea urchins on rocky barrens. Mar Ecol Prog Ser 33:295–306
Himmelmann JH (1978) Reproductive cycle of the green sea urchin (Strongylocentrotus droebachiensis). Can J Zool 56:1828–1836
Himmelmann JH, Steel DH (1971) Foods and predators of the green sea urchin Strongylocentrotus droebachiensis in Newfoundland waters. Mar Biol 9:315–322
Hjorleifsson E, Kaasa O, Gunnarsson K (1995) Grazing of kelp by green sea urchin in Eyjafjordur, North Iceland. In: Skjoldal HR, Hopkins C, Erikstad KE, Leinaas HP (eds) Ecology of fjords and coastal waters. Elsevier, Amsterdam, pp 593–597
Jensen M (1969) Age determination of echinoids. Sarsia 37(1):41–44
Johnson CR, Mann KH (1988) Diversity, patterns of adaptation, and stability of Nova Scotian kelp beds. Ecol Monogr 58:129–154
Keats DW, Steele DH, South GR (2011) Atlantic wolffish (Anarhichas lupus L.; Pisces: Anarhichidae) predation on green sea urchins (Strongylocentrotus droebachiensis (O.F. Mull.); Echinodermata: Echinoidea) in eastern Newfoundland. Can J Zool 64(9):1920–1925
Kelly JR, Kira AK, Scheibling RE (2012) Drift algal subsidies to sea urchins in low-productivity habitats. Mar Ecol Prog Ser 452:145–157
Konar B (2001) Seasonal changes in subarctic sea urchin populations from different habitats. Polar Biol 24:754–763
Konar B (2013) Lack of recovery from disturbance in high-arctic boulder communities. Polar Biol 36:1205–1214
Krumhansl KA, Scheibling RE (2012) Production and fate of kelp detritus. Mar Ecol Prog Ser 467:281–302
Lawrence JM (1975) On the relationships between marine plants and sea urchins. Oceanogr Mar Biol Annu Rev 13:213–286
Leinaas HP, Christie H (1996) Effects of removing sea urchins (Strongylocentrotus droebachiensis): stability of the barren state and succession of kelp forest recovery in the east Atlantic. Oecologia 105:524–536
Levitan DR (1988) Density-dependent size regulation and negative growth in the sea urchin Diadema antillarum Philippi. Oecologia 76:627–629
Mann KH (2000) Ecology of coastal waters. With implications for management. Blackwell Science, Oxford
Meidel SK, Scheibling RE (1998) Annual reproductive cycle of the green sea urchin, Strongylocentrotus droebachiensis, in differing habitats in Nova Scotia, Canada. Mar Biol 131:461–478
Minor MA, Scheibling RE (1997) Effects of food ration and feeding regime on growth and reproduction of the sea urchin Strongylocentrotus droebachiensis. Mar Biol 129:159–167
Norderhaug KM, Christie HC (2009) Sea urchin grazing and kelp re-vegetation in the NE Atlantic. Mar Biol Res 5:515–528
Pearse JS, Pearse VB (1975) Growth zones in the echinoid skeleton. Am Zool 15:731–753
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Fish Res Board Can Bull 191:1–382
Rinde E, Christie H, Fagerli CW, Bekkby T, Gundersen H, Norderhaug KM, Hjermann DØ (2014) The influence of physical factors on kelp and sea urchin distribution in previous and still grazed areas in the NE Atlantic. PLoS One. doi:10.1371/journal.pone.0100222
Robinson SMC, MacIntyre AD (1997) Aging and growth of the green sea urchin. Bull Aquac Assoc Can 97:56–60
Rowley MP (1989) Settlement and recruitment of sea urchins (Strongylocentrotus spp.) in a sea urchin barren ground and a kelp bed: are populations regulated by settlement or post-settlement processes? Mar Biol 100:485–495
Russell MP (1998) Resource allocation plasticity in sea urchins: rapid, diet induced, phenotypic changes in the green sea urchin, Strongylocentrotus droebachiensis (Muller). J Exp Mar Biol Ecol 220:1–14
Russell MP, Meredith RW (2000) Natural growth lines in echinoid ossicles are not reliable indicators of age: a test using Strongylocentrotus droebachiensis. Invertebr Biol 119:410–420
Russell MP, Ebert TA, Petraitis PS (1998) Field estimates of growth and mortality of the green sea urchin, Strongylocentrotus droebachiensis. Ophelia 48:137–153
Scheibling RE (1986) Increased macroalgal abundance following mass mortalities of sea urchins (Strongylocentrotus droebachiensis) along the Atlantic coast of Nova Scotia. Oecologia 68:186–198
Scheibling RE (1996) The role of predation in regulating sea urchin populations in eastern Canada. Oceanol Acta 19:421–430
Scheibling RE, Hatcher BG (2001) The ecology of Strongylocentrotus droebachiensis. In: Lawrence JM (ed) Edible sea urchins: biology and ecology. Elsevier Science B.V, Amsterdam, pp 271–306
Scheibling RE, Lauzon-Guay JS (2010) Killer storms: North Atlantic hurricanes and disease outbreaks in sea urchins. Limnol Oceanogr 55:2331–2338
Scheibling RE, Robinson MC (2008) Settlement behaviour and early post-settlement predation of the sea urchin Strongylocentrotus droebachiensis. J Exp Mar Biol Ecol 365:59–66
Sivertsen K (1997) Geographic and environmental factors affecting the distribution of kelp beds and barren grounds and changes in biota associated with kelp reduction at sites along the Norwegian coast. Can J Fish Aquat Sci 54:2872–2887
Sivertsen K, Hopkins CCE (1995) Demography of the echinoid Strongylocentrotus droebachiensis related to biotope in the northern Norway. In: Skjoldal HR, Hopkins C, Erikstad KE, Leinaas HP (eds) Ecology of fjords and coastal waters. Elsevier, Amsterdam, pp 549–571
Skadsheim A, Christie H, Leinaas HP (1995) Population reductions of Strongylocentrotus droebachiensis (Echinodermata) in Norway and the distribution of its endoparasite Echinomermella matsi (Nematoda). Mar Ecol Prog Ser 119:199–209
Steneck RS, Johnson C (2014) Kelp forests: dynamic patterns, processes and feedbacks. In: Bertness MD, Bruno JF, Silliman BR, Stachowicz JJ (eds) Marine community ecology and conservation. Sinauer Associates, USA, pp 315–366
Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459
Steneck RS, Vavrinec J, Leland AV (2004) Accelerating trophic-level dysfunction in kelp forest ecosystems of the western North Atlantic. Ecosystems 7:323–332
Steneck RS, Leland A, McNaught DC, Vavrinec J (2013) Ecosystem flips, locks, and feedbacks: the lasting effects of fisheries on Maine’s kelp forest ecosystem. Bull Mar Sci 89:1–25
Stien A, Leinaas HP, Halvorsen O, Christie H (1998) Population dynamics of the Echinomermella matsi (Nematoda)—Strongylocentrotus droebachiensis (Echinoida) system: effects on host fecundity. Mar Ecol Prog Ser 163:193–201
Tegner MJ, Dayton PK (1981) Population structure, recruitment and mortality of two sea urchins (Strongylocentrotus franciscanus and S. purpuratus)in a kelp forest. Mar Ecol Prog Ser 2:255–268
Vadas RL (1977) Preferential feeding: an optimization strategy in sea urchins. Ecol Monogr 47:337–371
Vadas RL, Smith BD, Beal B, Dowling T (2002) Sympatric growth morphs and size bimodality in the green sea urchin (Strongylocentrotus droebachiensis). Ecol Monogr 72:113–132
Wharton WG, Mann KH (1981) Relationship between destructive grazing by the sea urchin, Strongylocentrotus droebachiensis, and the abundance of American lobster, Homarus americanus, on the Atlantic coast of Nova Scotia. Can J Fish Aquat Sci 38:1339–1349
Winsor CP (1932) The Gompertz curve as a growth curve. Proc Natl Acad Sci USA 18:1–8
Woll AK, van der Meeren GI, Fossen I (2006) Spatial variation in abundance and catch composition of Cancer pagurus in Norwegian waters: biological reasoning and implications for assessment. ICES J Mar Sci 63:421–433
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Upper Saddle River 663 pp
Acknowledgments
This study was funded by the Norwegian Research Council (NFR) and Norwegian Institute for Water Research (NIVA) as part of the project “Habitat restoration in overgrazed areas on the northern Norwegian coast”. The authors would like to thank four anonymous reviewers for their helpful and constructive comments that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Gagnon.
Rights and permissions
About this article
Cite this article
Fagerli, C.W., Stadniczeñko, S.G., Pedersen, M.F. et al. Population dynamics of Strongylocentrotus droebachiensis in kelp forests and barren grounds in Norway. Mar Biol 162, 1215–1226 (2015). https://doi.org/10.1007/s00227-015-2663-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2663-3