Abstract
Autosomal Dominant Osteopetrosis type II (ADO2) is a rare bone disease of impaired osteoclastic bone resorption that usually results from heterozygous missense mutations in the chloride channel 7 (CLCN7) gene. We previously created mouse models of ADO2 (p.G213R) with one of the most common mutations (G215R) as found in humans and demonstrated that this mutation in mice phenocopies the human disease of ADO2. Previous studies have shown that roflumilast (RF), a selective phosphodiesterase 4 (PDE4) inhibitor that regulates the cAMP pathway, can increase osteoclast activity. We also observed that RF increased bone resorption in both wild-type and ADO2 heterozygous osteoclasts in vitro, suggesting it might rescue bone phenotypes in ADO2 mice. To test this hypothesis, we administered RF-treated diets (0, 20 and 100 mg/kg) to 8-week-old ADO2 mice for 6 months. We evaluated bone mineral density and bone micro-architecture using longitudinal in-vivo DXA and micro-CT at baseline, and 6-, 12-, 18-, and 24-week post-baseline time points. Additionally, we analyzed serum bone biomarkers (CTX, TRAP, and P1NP) at baseline, 12-, and 24-week post-baseline. Our findings revealed that RF treatment did not improve aBMD (whole body, femur, and spine) and trabecular BV/TV (distal femur) in ADO2 mice compared to the control group treated with a normal diet. Furthermore, we did not observe any significant changes in serum levels of bone biomarkers due to RF treatment in these mice. Overall, our results indicate that RF does not rescue the osteopetrotic bone phenotypes in ADO2 heterozygous mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autosomal Dominant Osteopetrosis type II (ADO2) is a rare bone disease of impaired osteoclastic bone resorption that usually results from heterozygous missense mutations in the chloride channel 7 (CLCN7) gene [1,2,3,4,5]. To understand the pathophysiology of ADO2 and to identify novel treatment strategies of this disease, we developed mouse models of ADO2 by introducing a knock-in (p.G213R) mutation in the Clcn7 gene, which is analogous to one of the common mutations (G215R) found in humans [6]. We found that the homozygous mice (ADO2−/−) carrying the mutation exhibited severe osteopetrosis and these mice usually die within a month after birth [6]. On the other hand, the heterozygous mice (ADO2+/−) displayed mild-to-moderate osteopetrosis and the phenotypic variability depends on their genetic backgrounds [6].
Previously, we demonstrated that osteoclasts derived from ADO2 heterozygous mice formed larger multinucleated cells with attenuated bone resorption capacity compared to osteoclasts from wild-type (WT) control mice [6]. Similarly, in-vitro studies using human osteoclasts derived from peripheral blood of ADO2 patients revealed that these osteoclasts exhibited reduced bone resorption compared to normal individuals (or asymptomatic carrier) [7]. These findings indicate that osteoclast dysfunction, rather than the bone microenvironment, is primarily responsible for the ADO2. Consequently, the identification of drugs or molecules that can increase osteoclast activity, restore osteoclast function, and promote bone resorption may be targeted as potential therapies for ADO2.
Roflumilast (RF), a selective phosphodiesterase 4 (PDE4) inhibitor and an FDA-approved drug, is currently used for treating chronic obstructive pulmonary disease (COPD) in people [8, 9]. RF increases intracellular cyclic adenosine monophosphate (cAMP) levels by inhibiting the PDE4 isoenzyme, and cAMP activation has been shown to play an important role in lysosomal acidification in osteoclasts [10, 11] and to enhance osteoclastogenesis and bone resorption [12, 13]. Additionally, studies have reported that disease-causing mutations in CLCN7 interfere with the process of endosomal–lysosomal transport and impede the acidification of osteoclasts, resulting in a reduction of bone resorption [14,15,16,17]. Our in-vitro results also demonstrated that RF promoted the differentiation and bone resorption activity of both WT and ADO2 osteoclasts, which was described in detail in a separate manuscript submitted in the same journal [18, 19]. Therefore, we hypothesized that RF might enhance ADO2 osteoclast differentiation and function in vivo, and thereby rescue the bone phenotypes in ADO2 heterozygous mice. To test this hypothesis, we administered RF in 8-week-old male and female ADO2+/− mice via diet at two different doses (20 mg/kg; low dose and 100 mg/kg; high dose) for a duration of 6 months. We analyzed bone mineral density and bone micro-architecture using longitudinal in-vivo DXA and micro-CT, while serum bone biomarkers were measured by ELISA. The results indicate that despite in-vitro data demonstrating that treatment of ADO2 osteoclasts with RF significantly increased osteoclast bone resorption activity, the administration of RF in vivo did not improve the osteopetrotic phenotype in ADO2 heterozygous mice.
Materials and Methods
Experimental Animals
For the bone phenotype study, we used a total of 66 mice, consisting of 35 male and 31 female (10 WT and 56 ADO2 heterozygous) mice. Mice were randomly assigned to different groups: WT control male (n = 5), ADO2 control male (n = 10), ADO2 RF dose 1 (20 mg/kg) male (n = 10), ADO2 RF dose 2 (100 mg/kg) male (n = 10), WT control female (n = 5), ADO2 control female (n = 8), ADO2 RF dose 1 (20 mg/kg) female (n = 8), and ADO2 RF dose 2 (100 mg/kg) female (n = 10). At 8 weeks of age, both male and female WT control mice were fed a regular diet, whereas ADO2 heterozygous mice in both sexes received either a regular diet as control or two different doses (20 and 100 mg/kg) of RF-treated diet for the duration of up to 6 months. The doses were calculated based on the mice’s approximate body weight of 25 g and their daily food intake of 4 g per mouse. The amount of RF (Sigma-Aldrich, St. Louis, MO) required for the low- and high-dose groups for a total of 20 mice comprising 10 male and 10 female for each dose for the duration of 6 months was calculated (288 and 1440 mg for low dose and high dose, respectively). In addition, the amount of food necessary for 20 mice for the entire study duration per dose was determined (14.4 kg). Subsequently, RF was mixed with 14.4 kg diet (Teklad custom diet, Envigo, Indianapolis, IN) separately for each dose and stored at a controlled temperature of 4 °C. Mice in each dose and sex groups were housed separately in individual cages and provided with fresh regular diet or RF-treated diet weekly. In addition, to determine the levels of roflumilast (RF) in both serum and bone tissue, we performed a small pharmacokinetic study in female WT mice at 8 weeks of age. Mice were fed with normal diet without RF as well as RF dose 1 (20 mg/kg) and RF dose 2 (100 mg/kg) diets (n = 4/group) for 1 week followed by the collection of serum and femur bone tissues on day 8 from these mice. All mice were generated and maintained at Indiana University. Mice were housed in polycarbonate cages in a vivarium maintained on a 12-h light and 12-h dark cycle and were fed a regular or RF-treated diet and water ad libitum. The procedures performed throughout the experiment followed the guidelines of the Indiana University Animal Care and Use committee (IACUC).
Euthanasia and Specimen Collection
Mice were euthanized at 32 weeks of age, which corresponds to 24 weeks of post-baseline age. Subsequently, the lower limbs were dissected from these animals, and the femora were stripped of muscle tissue. The femora were then transferred to 70% ethyl alcohol and stored at 4 °C for densitometry analyses. Furthermore, to monitor longitudinal bone biomarkers, we collected serum from peripheral blood (facial vein) at baseline, and 12- and 24-week post-baseline time points from these mice. In addition, we obtained serum from cardiac blood at sacrifice for serum biochemistry analysis. All collected sera were promptly stored at −80 °C until further analysis. For the pharmacokinetic study, after 1 week of administration of diet we collected serum and femur bone tissues and measured the levels of RF and its metabolite RF N-oxide in these samples using HPLC–MS/MS (ABSciex 5500 MS/MS).
In-vivo Dual-energy X-ray Absorptiometry (DXA) Analysis
The whole body, femur, and lumbar vertebrae 3 through 5 (L3-5) of both WT and ADO2 heterozygous mice were scanned using DXA (PIXImus II mouse densitometer; Lunar Corp., Madison, WI, USA) with ultra-high resolution (0.18 × 0.18 mm/pixel) as described previously [6]. These scans were performed at baseline (8 weeks) and at 6-, 12-, 18-, and 24-week post-baseline time points. The global window for the whole body was defined as the whole-body image minus calvarium, mandible, and teeth. After completion of the scan of each bone, mutually exclusive region of interest (ROI) boxes were drawn around the femur and spine (L3-5). Finally, aBMD (g/cm2) and BMC (g) measurements were obtained from these scans.
In-vivo Micro-computer Tomography (μCT) Analysis
The femora of both WT and ADO2 heterozygous mice at baseline (8 weeks) and at 6-, 12-, 18-, and 24-week post-baseline were scanned using μCT scanner (Skyscan 1176, Bruker, MA) with an isotropic voxel size of 9 μm3 as described previously [6]. In brief, using cross-sectional images within CT analyzer software, the growth plate location was identified and trabecular bone measurements consisting of 222 slices (2 mm) were determined from about 0.5 mm proximal to the growth plate. Finally, 3D and 2D morphometric evaluations were performed for the trabecular bone from each scan, and bone volume over total volume (BV/TV) and structural parameters (trabecular number, Tb.N; trabecular thickness Tb.Th; trabecular separation, Tb.Sp) were determined. The use of the nomenclature, symbols, and units were followed as described in Dempster DW et al. [20].
Serum Biochemistry and Bone Biomarker Analysis
Serum levels of mouse pro-collagen 1 intact N-terminal (P1NP), carboxy-terminal collagen crosslinks (CTX), and tartrate-resistant acid phosphatase 5, isoform b (TRAcP5b) of WT and ADO2 heterozygous mice at baseline (8 weeks) and at 12- and 24-week post-baseline were measured by Enzyme-Linked ImmunoSorbent Assay (ELISA) kits (Immunodiagnostics Systems, Mountain Lakes, NJ, USA and Biomedical Technologies Inc., MA, USA) according to the manufacturers’ instructions. Serum calcium (Ca), phosphorus (Phos), creatinine (CREA), and alkaline phosphatase (ALP) were measured at 24-week post-baseline using the Randox Rx kit (Daytona Analyzer, WV, USA).
Statistical Analysis
Quantitative data were expressed as mean ± SD unless otherwise indicated. One-way analysis of variance (ANOVA) was used to identify mean differences for variables among control and experimental groups at baseline, 6-, 12-, 18-, and 24-week post-baseline. The Fisher’s protected least significant difference (PLSD) was used for all pairwise post hoc comparisons. All statistical analysis was performed using the statistical software package StatView (Abacus Concepts, Inc., Berkeley, CA). The level of significance was set at p < 0.05.
Results
Concentrations of Roflumilast (RF) and Its Metabolite RF N-oxide in Serum and Bone Tissue in Mice
The concentrations of RF in the serum were 0.7 ± 0.2 and 11.1 ± 7.8 ng/mL for the low and high doses (20 and 100 mg/kg) of RF groups, respectively. In addition, the concentrations of RF N-oxide for the low and high doses of RF groups in the serum were 5.9 ± 0.7 and 57.6 ± 38.2 ng/mL, respectively. As expected, the control group had no detectable levels of RF and RF N-oxide in the serum. In addition, the concentrations of both RF and RF N-oxide in bone tissue (femur) were below the detectable threshold (0.1 ng/mL) for all groups—control, low, and high RF doses.
Roflumilast Treatment Had No Influence on the Body Weight in ADO2 Mice
Mice body weights were recorded at baseline and every 6 weeks thereafter up to 6 months. Throughout the study, body weights of both WT and ADO2 heterozygous mice fed with either a normal diet or RF-treated diet gradually increased from baseline values in both male and female mice (Table 1). No significant differences in body weight were observed between WT and ADO2 control mice fed a normal diet for both sexes up to the duration of 6 months. In addition, ADO2 mice fed with either a normal diet or RF-treated diet at both low and high doses showed no significant differences in body weight compared to the control group at 6-, 12-, 18-, and 24-week post-baseline (Table 1). These findings suggest that RF treatment had no influence on body mass in ADO2 heterozygous mice in both male and female.
Roflumilast Treatment Did Not Improve the Whole Body, Femur, and Spinal aBMD Phenotypes in ADO2 Mice
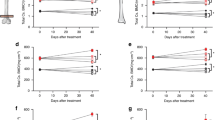
As expected, the whole-body areal BMD values were significantly (p < 0.05) higher in ADO2 control group compared to the WT control mice at baseline and at 6-, 12-, 18-, and 24-week post-baseline time points in male mice (Fig. 1a). In contrast, the male ADO2 mice treated with low-dose (20 mg/kg) RF diet showed no significant differences in whole-body aBMD values compared to the ADO2 control mice at baseline and all post-baseline time points (Fig. 1a). Similarly, the male ADO2 mice administered with the high dose of RF (100 mg/kg) did not exhibit significant differences in whole-body aBMD compared to the ADO2 control mice at baseline, and at 12- and 18-week post-baseline time points (Fig. 1a). However, high-dose RF-treated male ADO2 mice displayed significantly higher (p < 0.05) aBMD values at 6 and 24 weeks of post-baseline age compared to the ADO2 control group (Fig. 1a).
Whole body, femur, and spine (L3-5) aBMD measured by in-vivo DXA in male and female mice. WT and ADO2 mice treated with a normal diet (WT and ADO2 control, respectively) and ADO2 mice treated with 2 doses of RF, low dose—20 mg/kg (ADO2 RF20) and high dose—100 mg/kg (ADO2 RF100), via diet for the period of 6 months in both sexes. Measurements of aBMD were performed in vivo at baseline (8 weeks), and at 6-, 12-, 18-, and 24-week post-baseline time points. Values are given as mean ± SD *p < 0.05 vs. WT control and ¶p < 0.05 vs. ADO2 control. aBMD areal bone mineral density, BL baseline, 6W 6 weeks, 12W 12 weeks, 18W 18 weeks, 24W 24 weeks
In female, the whole-body aBMD were significantly (p < 0.05) higher in ADO2 control group compared to WT control mice at 6-, 12-, 18-, and 24-week post-baseline time points (Fig. 1d). In contrast, female ADO2 mice treated with both low-dose and high-dose RF diets did not exhibit significant differences in whole-body aBMD values compared to the ADO2 control group at any post-baseline ages (Fig. 1d). These data indicate that both low and high doses of RF treatment in ADO2 mice for up to 6 months from baseline age did not prevent the gain of whole-body bone mineral density in both male and female mice.
Femur aBMD values were slightly higher in ADO2 control mice compared to WT control group at baseline and all post-baseline periods in both male and female mice. However, this difference was not significant except for significantly higher (p < 0.05) aBMD values at 6- and 24-week post-baseline in ADO2 control mice compared to the WT control group in female mice (Figs. 1b and e). Furthermore, compared to ADO2 control mice, low-dose treated ADO2 mice exhibited no significant differences in femur aBMD values at baseline and all post-baseline time points in both sexes (Figs. 1b and e). For ADO2 mice treated with a high dose of RF, femur aBMD values were similar to those of ADO2 control mice at all post-baseline time points in both male and female mice except femur aBMD was significantly higher (p < 0.05) in RF-treated mice at 6- and 12-week post-baseline periods in male mice (Figs. 1b and e).
Spine aBMD values were significantly higher (p < 0.05) in the ADO2 control mice at baseline and all post-baseline time points compared to WT control group in both male and female mice except aBMD at this site was similar for both WT and ADO2 control mice at baseline in female mice (Figs. 1c and f). No significant differences of spine aBMD were observed between low-dose RF-treated ADO2 mice and normal diet-treated ADO2 control mice in both sexes at all time points tested in this study (Figs. 1c and f). Similarly, spine aBMD values were similar between ADO2 control and high-dose RF-treated mice at 12-, 18-, and 24-week post-baseline periods in male mice and all post-baseline periods in female mice (Figs. 1c and f). In contrast, ADO2 mice treated with high-dose RF showed significant higher (p < 0.05) values for spine aBMD at 6 weeks of post-baseline in male compared to the ADO2 control group (Fig. 1c). Spine aBMD was significantly lower (p < 0.05) in low-dose RF-treated female ADO2 mice compared to the ADO2 control group only at 24 weeks of post-baseline period (Fig. 1f). All together, these data indicate that both low and high doses of RF treatment up to 6 months from baseline age did not mitigate the gain of femur and spine bone mineral density in ADO2 mice in either sex.
Roflumilast Treatment Did Not Prevent the Trabecular Bone Mass Gain at Distal Femur in ADO2 Mice
To identify whether RF has any effect on trabecular bone gain in ADO2 mice, we conducted longitudinal assessments of bone mass and micro-architectural parameters at the distal femur, which is predominantly composed of trabecular bone. As expected, trabecular BV/TV and Tb.N values were significantly higher (p < 0.005 and p < 0.05 in male and female, respectively) in ADO2 control group compared to WT control mice at baseline as well as at 6-, 12-, 18-, and 24-week post-baseline time points (Figs. 2a and b; Figs. 3a and b). However, no significant differences of BV/TV and Tb.N were observed between ADO2 mice treated with a normal diet and those treated with a low-dose (20 mg/kg) RF diet at baseline and any post-baseline periods throughout the 6-month study period in both sexes (Figs. 2a and b; Figs. 3a and b). High-dose RF-treated ADO2 mice displayed significantly higher BV/TV and Tb.N compared to the ADO2 control mice only at 6- and 12-week post-baseline periods in male mice (Figs. 2a and b). Similarly, as expected Tb.Th values were significantly higher (p < 0.005 in both sexes), whereas Tb.Sp values were significantly lower (p < 0.005 and p < 0.05, in male and female, respectively) in ADO2 control mice compared to WT control group at baseline and all post-baseline time points (Figs. 2c and d; Figs. 3c and d). No significant differences of Tb.Th were observed between ADO2 control and RF-treated mice for low and high doses in both sexes at baseline and all post-baseline time points, except the high-dose RF-treated ADO2 mice exhibited significantly lower (p < 0.05) Tb.Th at 12-week post-baseline in female mice only, compared to the ADO2 control mice (Figs. 2c and 3c). Additionally, high-dose RF-treated ADO2 mice showed significantly lower (p < 0.05) Tb.Sp at 6-week post-baseline in male mice and at 18-week post-baseline in female mice, compared to ADO2 mice treated with a normal diet (Figs. 2d and 3d). Overall, these data indicate that treatment of ADO2 heterozygous mice with either low or high doses of RF diet for up to 6 months did not attenuate trabecular bone mass gain and did not significantly alter micro-architectural parameters in both male and female mice.
Trabecular bone morphometry at distal femur measured by in-vivo micro-CT in male mice. WT and ADO2 mice treated with a normal diet (WT and ADO2 control, respectively) and ADO2 mice treated with 2 doses of RF, low dose—20 mg/kg (ADO2 RF20) and high dose—100 mg/kg (ADO2 RF100), via diet for the period of 6 months in male mice. Measurement of trabecular BV/TV, Tb.N, Tb.Th, and Tb.Sp at distal femur were performed in vivo at baseline (8 weeks), and at 6-, 12-, 18-, and 24-week post-baseline time points. Values are given as mean ± SD **p < 0.005 vs. WT control and ¶p < 0.05 vs. ADO2 control. BV/TV bone volume over tissue volume, Tb.N trabecular number, Tb.Th trabecular thickness, Tb.Sp trabecular separation, BL baseline, 6W 6 weeks, 12W 12 weeks, 18W 18 weeks, 24W 24 weeks
Trabecular bone morphometry at distal femur measured by in-vivo micro-CT in female mice. WT and ADO2 mice treated with a normal diet (WT and ADO2 control, respectively) and ADO2 mice treated with 2 doses of RF, low dose—20 mg/kg (ADO2 RF20), and high dose—100 mg/kg (ADO2 RF100), via diet for the period of 6 months in female mice. Measurement of trabecular BV/TV, Tb.N, Tb.Th, and Tb.Sp at distal femur were performed in vivo at baseline (8 weeks), and at 6-, 12-, 18-, and 24-week post-baseline time points. Values are given as mean ± SD *p < 0.05 and **p < 0.005 vs. WT control and ¶p < 0.05 vs. ADO2 control. BV/TV bone volume over tissue volume, Tb.N trabecular number, Tb.Th trabecular thickness, Tb.Sp trabecular separation, BL baseline, 6W 6 weeks, 12W 12 weeks, 18W 18 weeks, 24W 24 weeks
Serum Levels of Bone Biomarkers Were Similar Between Normal Diet and Roflumilast-Treated ADO2 Mice
At baseline, the level of serum CTX was significantly higher compared to those values at 12- and 24-week post-baseline for all groups (Figs. 4a and e). In addition, serum CTX levels in ADO2 mice treated with both low and high doses of RF diet were comparable to those in ADO2 mice on a normal diet at 12- and 24-week post-baseline in both sexes, except CTX level was significantly higher (p < 0.05) in low-dose RF-treated ADO2 mice compared to the control ADO2 group in male mice (Figs. 4a and e).
Bone turnover markers measured by ELISA in male and female mice. WT and ADO2 mice treated with a normal diet (WT and ADO2 control, respectively) and ADO2 mice treated with 2 doses of RF, low dose—20 mg/kg (ADO2 RF20), and high dose—100 mg/kg (ADO2 RF100), via diet for the period of 6 months in both sexes. Measurement of serum CTX, TRAP, CTX/TRAP ratios, and P1NP levels were performed in vivo at baseline (8 weeks), and 12- and 24-week post-baseline time points. Values are given as mean ± SD *p < 0.05 vs. WT control and ¶p < 0.05 vs. ADO2 control. CTX carboxy-terminal collagen, TRAP tartrate-resistant acid phosphatase 5, isoform b, P1NP pro-collagen 1 intact N-terminal, BL baseline, 12W 12 weeks, 24W 24 weeks
As expected, serum TRAP level at baseline was slightly higher in male ADO2 mice and significantly higher (p < 0.05) in female ADO2 mice, compared to the WT control mice. However, surprisingly, serum TRAP level was significantly higher (p < 0.05) in high-dose RF group in male mice and significantly lower (p < 0.05) in low-dose RF group in female mice at baseline compared to those of sex-matched ADO2 control mice (Figs. 4b and f). In addition, serum TRAP values were significantly higher (p < 0.05) at 12- and 24-week post-baseline periods in ADO2 control mice compared to the WT control mice in both sexes, however, RF treatment did not cause any significant changes in TRAP levels at these post-baseline time points in either sex (Figs. 4b and f). Serum CTX/TRAP ratios were mostly similar among different groups at baseline except ADO2 control group had a significantly higher ratio (p < 0.05) compared to high-dose RF group in male mice and significantly lower ratio (p < 0.05) compared to WT control group in female mice (Figs. 4c and g). Additionally, the ADO2 control group displayed significantly lower CTX/TRAP ratio at 12- and 24-week post-baseline compared to WT control group in male mice. However, the serum CTX/TRAP ratios remained similar between ADO2 control mice and RF-treated ADO2 mice (both low and high doses) at 12- and 24-week post-baseline periods in both male and female mice (Figs. 4c and g). At baseline, serum P1NP values were higher (non-significant) in ADO2 groups compared to WT control mice in both sexes (Figs. 4d and h). However, serum P1NP levels were not significantly affected by RF treatment for both low and high doses in ADO2 mice compared to ADO2 mice on a normal diet at all post-baseline time points (Figs. 4d and h). Together, these data indicate that RF treatment did not exert a significant influence on bone formation and resorption markers up to 6 months of post-baseline periods in ADO2 mice.
Serum Biochemistry Was Mostly Similar Between Control and Roflumilast-Treated ADO2 Mice
Serum levels of calcium (Ca), phosphorus (P), creatinine (CREA), and alkaline phosphatase (ALP) were similar in all WT and ADO2 control mice and RF-treated ADO2 mice for both low and high doses at 24-week post-baseline period in male mice. Serum Ca, P, CREA, and ALP levels were also similar in the female WT and ADO2 control mice and RF-treated ADO2 mice for low dose, however, Ca and CREA levels were significantly higher in the ADO2 mice treated with high dose of RF compared to the ADO2 control mice in female mice (Table 2).
Discussion
In this study, we investigated the effect of roflumilast on bone phenotypes in both male and female ADO2 heterozygous mice for the duration of 6 months. Our findings revealed that RF treatment did not lead to any improvements in bone mineral density in the whole body, spine, and femur of ADO2 mice in both sexes. In addition, RF treatment did not attenuate the gain of trabecular bone mass at the distal femur in ADO2 mice. Furthermore, we did not observe any significant influences of RF on serum bone turnover markers in both male and female ADO2 mice. Overall, these data indicate that RF, at the doses examined in this study, does not rescue the osteopetrotic bone phenotypes in ADO2 heterozygous mice.
Roflumilast is commonly prescribed for treating COPD in humans [8, 9], and it is also used for the treatment of asthma, psoriasis, and inflammatory bowel diseases [9, 21, 22]. The principal mechanism of action of RF involves increasing intracellular cAMP concentration by inhibiting PDE4, the isoenzyme that catalyzes the breakdown of cAMP to AMP [21]. cAMP plays a crucial role as a secondary messenger for intracellular signal transduction in many biological processes. In lung tissues, RF has been shown to restore cystic fibrosis transmembrane conductance regulator activity, inhibit the production of cytokines and release of inflammatory mediators, reduce cell proliferation, migration, and chemotaxis, thereby improving lung function [9]. In bone cells, cAMP influences a wide range of intracellular processes, mainly through activation of cAMP-dependent protein kinase (PKA) [10, 23, 24]. For instance, the effect of parathyroid hormone (PTH) on bone cells through the cAMP-PKA pathway can produce two distinct effects: transient increases in cAMP have a positive effect on osteoblasts and increase bone density, whereas chronic increases in cAMP, as observed in continuous PTH treatment, promote osteoclastogenesis and, consequently, bone loss [23,24,25,26].
Several in-vitro studies have provided evidence that cAMP activation leads to augmented osteoclastogenesis by up-regulating the nuclear factor of activated T cells 1 (NFATc1), a master regulator of osteoclastogenesis [12, 13, 27, 28]. This process is mediated through the cAMP > CREB pathway as well as cAMP-mediated regulation of cathepsin K processing in osteoclasts [12, 28]. Our cell culture studies, detailed in a separate manuscript submitted in the same journal, involving both wild-type and ADO2 osteoclasts also revealed that RF treatment increased osteoclast formation at the concentration of 50–100 nM [18]. Furthermore, we found that RF treatment even at lower concentration (10 and 25 nM) as well as up to as high as 150 nM concentration significantly enhanced the pit resorption of ADO2 osteoclasts, which prompted us to test this drug in our ADO2 mouse model. For the experiments in mice, we selected a low dose of RF at 20 mg/kg, based on the clinical dose of RF (500 µg per day) used for COPD patients in adults and its mouse equivalent dose published in previous studies [29,30,31]. The high dose was selected to be five times higher than the low dose, considering the higher metabolic rate and body surface area in mice compared to humans.
Previously, several studies have reported the pharmacokinetic (PK) profile of RF in both humans and rodents. For humans, the time to reach peak plasma concentration of RF when given orally is approximately one hour (ranging from 0.5 to 2 h) with an average elimination half-life of 17 h (ranging between 7 and 25 h) [32, 33]. In addition, steady-state concentration of RF is typically achieved about 3–4 days following once-daily dosing in humans. In rodents, the time it takes to achieve peak concentration of RF is similar to that in humans, whereas the half-life of RF ranges between 6 and 10 h before reaching baseline levels within about 24 h [34]. The administration of RF via diet in the present study was designed based on this PK profile in rodents.
As for the effects of PDE4 inhibitors on bone and mineral metabolism, previously published studies yielded somewhat controversial results. The only human clinical trial (clinicaltrials.gov identifier: NCT01745848) examining the effect of RF on markers of bone metabolism in 20 adults, with the drug administered 500 µg per day for 30 days, found negligible changes in serum CTX and P1NP levels from baseline. Conversely, several in-vitro and in-vivo animal studies demonstrated that osteoclastogenesis was decreased and bone density was increased or bone loss was prevented due to the treatment of various PDE4 inhibitors including RF [27, 35,36,37,38]. In contrast, other studies showed that RF increased osteoclast differentiation [28], regulated cathepsin K maturation in osteoclasts [12], and promoted osteoclast formation [13]. Similar effects have been observed with other PDE4 inhibitors such as rolipram and pentoxifylline, which have shown both increased bone density [38] and stimulated TRANCE-mediated osteoclast formation [39, 40]. These varying results could be attributed to differences in dose, duration, frequency, and route of drug administration, as well as species-specific differences related to these studies.
In our in-vitro experiments, we observed that RF treatment significantly increased osteoclast bone resorption activity. However, when administered in vivo, RF did not improve the osteopetrotic phenotype in ADO2 heterozygous mice. Several factors could explain these contrasting outcomes. The high dose of RF used in-vivo study, although five times higher than the clinical dose for COPD patients, might not have been optimal to produce substantial changes in bone phenotypes in ADO2 mice. Additionally, we found the concentrations of the RF and its metabolite RF N-oxide vary significantly between serum and bone tissue in mice. We identified the serum RF concentrations of 0.7 ± 0.2 and 11.1 ± 7.8 ng/mL for mice treated with low and high doses (20 and 100 mg/kg) of RF via diet, respectively. Based on the molecular weight of RF (403.2), these concentrations are equivalent to 1.74 and 27.53 nM for the low and high doses, respectively. Our cell culture studies showed higher osteoclast activity for both WT and ADO2 osteoclasts at concentration as low as 10 nM of RF. While this level of RF was not achieved in the serum with the low dose of RF diet in vivo, the high-dose regimen yielded a serum concentration of approximately 2.5 times higher than the minimum RF concentration necessary for improved osteoclast function in vitro. In contrast, the concentration of RF in the bone tissue, even for the high dose of RF diet group, was below the detection threshold of 0.1 ng/mL, indicating very low drug exposure in skeletal tissue. The very low RF concentration in bone tissue likely accounts for the absence of any significant changes of bone phenotypes over the 6 months of study period in ADO2 mice. Further investigation is thus needed to determine the effective dose and mode of administration for RF in vivo.
Efficient delivery of pharmaceutical drugs in the skeletal tissue is a major challenge for the treatment of bone and mineral disorders. This is due to lower blood flow in bone compared to other tissues, coupled with the sequestration and utilization of drugs by other organs before their accessibility to the skeleton. In addition, a substantial proportion of drugs undergo excretion from the body prior to reaching the bone tissue. Consequently, often higher or more frequent doses are required to achieve the requisite drug concentration in the bone for optimal effectiveness, however, this leads to adverse cytotoxic effects in non-target tissues. To overcome these challenges, adopting a bone-targeting drug delivery using various strategies could be undertaken for higher efficacy with reduced toxicity in the future studies.
To the best of our knowledge, the current study is the first study to investigate the effect of RF on bone density and micro-architecture when administered in mice for a period of up to 6 months. To assess the overall effect of RF on bone mineral density, we conducted whole-body DXA analysis. Further, femur and spine DXA analysis was performed to determine site-specific effects of RF in ADO2 mice. However, both low and high doses of RF treatment up to 6 months from baseline age did not rescue the whole body, femur, and spine bone density in ADO2 mice in both sexes (Fig. 1). To minimize radiation effects on bone phenotypes in mice, we measured micro-CT at baseline (8 weeks) and every 6-week post-baseline thereafter, up to 6 months. Our data indicate that treatment of ADO2 heterozygous mice with either low or high doses of RF diet for up to 6 months did not attenuate trabecular bone mass gain and did not significantly alter micro-architectural parameters in both male and female mice (Figs. 2 and 3). In addition, we collected serum from facial vein every 12-week post-baseline to longitudinally monitor changes in bone resorption markers. However, throughout the 12- and 24-week post-baseline periods, RF treatment did not significantly alter CTX, TRAP, CTX/TRAP ratios, and P1NP levels in ADO2 mice (Fig. 4). The total duration of RF treatment for 6 months was selected as sufficient time for the drug to produce any noticeable changes in bone phenotypes considering the average life span of mice is about 2 years. Overall, our findings indicate that RF at both low and high doses did not ameliorate the osteopetrotic bone phenotypes in ADO2 mice.
There are several limitations in this study. We only administered 2 doses (low and high) of RF via diet in ADO2 mice. Further investigations with different doses and administration routes are necessary to determine if these would lead to different outcomes for ADO2 bone phenotypes. Additionally, we estimated in-vivo doses based on previous published studies in these mice. Moreover, we measured serum biochemistry for all control and RF-treated groups at 6-month post-baseline only, which limits the detection of temporal changes in bone metabolism markers.
In conclusion, our results indicate that RF, at the doses tested in this study, was unable to improve bone phenotypes in ADO2 heterozygous mice. Further studies are necessary to determine the mechanism of increased ADO2 osteoclast activity due to RF treatment in vitro, as this could potentially lead to the development of novel treatment strategies for ADO2 patients.
References
Johnston CC Jr, Lavy N, Lord T, Vellios F, Merritt AD, Deiss WP Jr (1968) Osteopetrosis. a clinical, genetic, metabolic, and morphologic study of the dominantly inherited, benign form. Medicine 47:149–167
Cleiren E, Bénichou O, Van Hul E, Gram J, Bollerslev J, Singer FR, Beaverson K, Aledo A, Whyte MP, Yoneyama T, deVernejoul MC, Van Hul W (2001) Albers-Schonberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum Mol Genet 10(25):2861–2867
Waguespack SG, Koller DL, White KE, Fishburn T, Carn G, Buckwalter KA, Johnson M, Kocisko M, Evans WE, Foroud T, Econs MJ (2003) Chloride channel 7 (CICN7) gene mutations and autosomal dominant osteopetrosis, Type II. J Bone Miner Res 18(8):1513–1518
Waguespack SG, Hui SL, DiMeglio L, Econs MJ (2007) Autosomal dominant osteopetrosis: clinical severity and natural history of 94 subjects with chloride channel 7 (C1CN7) gene mutations. J Clin Endocrinol Metab 92(3):771–778
Weber DR, Econs MJ, Levine MA (2014) Osteopetrosis: pathogenesis, management and future directions for research. IBMS BoneKey 11:520
Alam I, Gray AK, Chu K, Ichikawa S, Mohammad KS, Capannolo M, Capulli M, Maurizi A, Muraca M, Teti A, Econs MJ, Del Fattore A (2014) Generation of the first autosomal dominant osteopetrosis type II (ADO2) disease models. Bone 59:66–75
Chu K, Snyder R, Econs MJ (2006) Disease status in autosomal dominant osteopetrosis type 2 is determined by osteoclastic properties. J Bone Miner Res 21(7):1089–1097
Giembycz MA, Field SK (2010) Roflumilast: first phosphodiesterase 4 inhibitor approved for treatment of COPD. Drug Des Devel Ther 4:147–58
Wedzicha JA, Calverley PM, Rabe KF (2016) Roflumilast: a review of its use in the treatment of COPD. Int J Chron Obstruct Pulmon Dis 11:81–90
Rahman N, Ramos-Espiritu L, Milner TA, Buck J, Levin LR (2016) Soluble adenylyl cyclase is essential for proper lysosomal acidification. J Gen Physiol 148(4):325–339
Henriksen K, Sorensen MG, Nielsen RH, Gram J, Schaller S, Dziegiel MH, Everts V, Bollerslev J, Karsdal MA (2006) Degradation of the organic phase of bone by osteoclasts: a secondary role for lysosomal acidification. J Bone Miner Res 21(1):58–66
Park YG, Kim YH, Kang SK, Kim CH (2006) cAMP-PKA signaling pathway regulates bone resorption mediated by processing of cathepsin K in cultured mouse osteoclasts. Int Immunopharmacol 6(6):947–56
Mediero A, Perez-Aso M, Cronstein BN (2014) Activation of EPAC1/2 is essential for osteoclast formation by modulating NFκB nuclear translocation and actin cytoskeleton rearrangements. FASEB J 28(11):4901–13
Schulz P, Werner J, Stauber T, Henriksen K, Fendler K (2010) The G215R mutation in the Cl-/H+-antiporter ClC-7 found in ADO II osteopetrosis does not abolish function but causes a severe trafficking defect. PLoS ONE 5(9):e12585
Weinert S, Jabs S, Hohensee S, Chan WL, Kornak U, Jentsch TJ (2014) Transport activity and presence of ClC-7/Ostm1 complex account for different cellular functions. EMBO Rep 15(7):784–791
Henriksen K, Sørensen MG, Jensen VK, Dziegiel MH, Nosjean O, Karsdal MA (2008) Ion transporters involved in acidification of the resorption lacuna in osteoclasts. Calcif Tissue Int 83(3):230–242
Stauber T, Weinert S, Jentsch TJ (2012) Cell biology and physiology of CLC chloride channels and transporters. Compr Physiol 2(3):1701–1744
Hong JM, Gerard-O’Riley RL, Acton D, Alam I, Econs MJ, Bruzzaniti A. The PDE4 inhibitors Roflumilast and Rolipram rescue ADO2 osteoclast resorption dysfunction (Abstract accepted for publication in Journal of Bone and Mineral Research, supplement 38, 2023)
Hong JM, Rita L. Gerard-O’Riley RL, Acton D, Patel V, Lavu N, Alam I, Econs MJ and Angela Bruzzaniti A (2023) The PDE4 inhibitors Roflumilast and Rolipram Rescue ADO2 Osteoclast Resorption Dysfunction. Calcif Tissue Int (In Revision)
Dempster DW, Compston JE, Drezner MK et al (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res 28(1):2–17
Li H, Zuo J, Tang W (2018) Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol 9:1048
Kawamatawong T (2021) Phosphodiesterase-4 Inhibitors for Non-COPD Respiratory Diseases. Front Pharmacol 12:518345
Ifegwu OC, Awale G, Rajpura K, Lo KW, Laurencin CT (2017) Harnessing cAMP signaling in musculoskeletal regenerative engineering. Drug Discov Today 22(7):1027–1044
Hertz AL, Beavo JA (2011) Cyclic nucleotides and phosphodiesterases in monocytic differentiation. Handb Exp Pharmacol 204:365–390. https://doi.org/10.1007/978-3-642-17969-3_16
Kalinkovich A, Livshits G (2021) Biased and allosteric modulation of bone cell-expressing G protein-coupled receptors as a novel approach to osteoporosistherapy. Pharmacol Res 171:105794
Chen T, Wang Y, Hao Z, Hu Y, Li J (2021) Parathyroid hormone and its related peptides in bone metabolism. Biochem Pharmacol 192:114669
Liu Q, Sun Y, Chen D, Chen K, Huang B, Chen Z (2021) Inhibitory effect of roflumilast on experimental periodontitis. J Periodontol. https://doi.org/10.1002/JPER.20-0858
Koga Y, Tsurumaki H, Aoki-Saito H, Sato M, Yatomi M, Takehara K, Hisada T (2019) Roles of cyclic AMP response element binding activation in the ERK1/2 and p38 MAPK signaling pathway in central nervous system, cardiovascular system, osteoclast differentiation and mucin and cytokine production. Int J Mol Sci 20(6):1346
Möllmann J, Kahles F, Lebherz C, Kappel B, Baeck C, Tacke F, Werner C, Federici M, Marx N, Lehrke M (2017) The PDE4 inhibitor roflumilast reduces weight gain by increasing energy expenditure and leads to improved glucose metabolism. Diabetes Obes Metab 19(4):496–508
Cortijo J, Iranzo A, Milara X, Mata M, Cerdá-Nicolás M, Ruiz-Saurí A, Tenor H, Hatzelmann A, Morcillo EJ (2009) Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury. Br J Pharmacol 156(3):534–44
Martorana PA, Beume R, Lucattelli M, Wollin L, Lungarella G (2005) Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am J Respir Crit Care Med 172(7):848–853
Bethke TD, Böhmer GM, Hermann R, Hauns B, Fux R, Mörike K, David M, Knoerzer D, Wurst W, Gleiter CH (2007) Dose-proportional intraindividual single- and repeated-dose pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor. J Clin Pharmacol 47(1):26–36
Neville KA, Szefler SJ, Abdel-Rahman SM, Lahu G, Zech K, Herzog R, Bethke TD, Gleason MC, Kearns GL (2008) Single-dose pharmacokinetics of roflumilast in children and adolescents. J Clin Pharmacol 48(8):978–85
Moussa BA, El-Zaher AA, El-Ashrey MK, Fouad MA (2019) Roflumilast analogs with improved metabolic stability, plasma protein binding, and pharmacokinetic profile. Drug Test Anal 11(6):886–897
Waki Y, Horita T, Miyamoto K, Ohya K, Kasugai S (1999) Effects of XT-44, a phosphodiesterase 4 inhibitor, in osteoblastgenesis and osteoclastgenesis in culture and its therapeutic effects in rat osteopenia models. Jpn J Pharmacol 79(4):477–83
Yao W, Tian XY, Chen J, Setterberg RB, Lundy MW, Chmielzwski P, Froman CA, Jee WS (2007) Rolipram, a phosphodiesterase 4 inhibitor, prevented cancellous and cortical bone loss by inhibiting endosteal bone resorption and maintaining the elevated periosteal bone formation in adult ovariectomized rats. J Musculoskelet Neuronal Interact 7(2):119–30
Munisso MC, Kang JH, Tsurufuji M, Yamaoka T (2012) Cilomilast enhances osteoblast differentiation of mesenchymal stem cells and bone formation induced by bone morphogenetic protein 2. Biochimie 94(11):2360–5
Kinoshita T, Kobayashi S, Ebara S, Yoshimura Y, Horiuchi H, Tsutsumimoto T, Wakabayashi S, Takaoka K (2000) Phosphodiesterase inhibitors, pentoxifylline and rolipram, increase bone mass mainly by promoting bone formation in normal mice. Bone 27(6):811–7
Cho ES, Yu JH, Kim MS, Yim M (2004) Rolipram, a phosphodiesterase 4 inhibitor, stimulates osteoclast formation by inducing TRANCE expression in mouse calvarial cells. Arch Pharm Res 27(12):1258–62
Takami M, Cho ES, Lee SY, Kamijo R, Yim M (2005) Phosphodiesterase inhibitors stimulate osteoclast formation via TRANCE/RANKL expression in osteoblasts: possible involvement of ERK and p38 MAPK pathways. FEBS Lett 579(3):832–838
Funding
This work was supported by the US National Institutes of Health grant AG069583.
Author information
Authors and Affiliations
Contributions
Study design: IA, AB, and MJE. Study conduct: IA, SLH, RLG, DA, RSP, AB, and MJE. Data analysis: IA, SLH, RLG, AB, and MJE. Data interpretation: IA, SLH, RLG, DA, RSP, JMH, AB, and MJE. Drafting manuscript: IA, RLG, AB, and MJE. Revising manuscript content: IA, RLG, AB, and MJE. Approval of final version of manuscript: IA, SLH, RLG, DA, RSP, JMH, AB, and MJE.
Corresponding author
Ethics declarations
Conflict of interests
Imranul Alam, Sara L. Hardman, Rita L. Gerard‑O’Riley, Dena Acton, Reginald S. Parker, Jung Min Hong, Angela Bruzzaniti, and Michael J. Econs declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures involving animals performed in the study followed the guidelines of the Indiana University Animal Care and Use Committee (IACUC Protocol No. 11378). This study does not involve research in humans.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alam, I., Hardman, S.L., Gerard-O’Riley, R.L. et al. Effect of Roflumilast, a Selective PDE4 Inhibitor, on Bone Phenotypes in ADO2 Mice. Calcif Tissue Int 114, 419–429 (2024). https://doi.org/10.1007/s00223-023-01180-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01180-2