Abstract
The aim of this study was to assess the long-term efficacy and safety of i.v. neridronate in the treatment of osteogenesis imperfecta (OI). One hundred and fourteen patients affected by OI were included in the study. Neridronate was administered by i.v. infusion at the dosage of 2 mg/kg, up to a maximum of 100 mg at three-month intervals for 3 years. Dual X-ray absorptiometry of the lumbar spine, hip, and ultradistal and proximal radius were evaluated every 6 months. Blood calcium, phosphate, albumin, fasting urinary calcium/creatinine ratio, total serum alkaline phosphatase, and bone alkaline phosphatase were obtained at baseline and every 3 months. The mean lumbar spine and total hip BMD significantly increased from baseline to any time point (p < 0.001). The mean ultradistal radius BMD significantly increased from baseline only at month 18 (p = 0.026), 30 (p = 0.046), and 36 (p = 0.013), respectively. The mean proximal radius BMD did not change during the whole observation. The levels of bone turnover markers significantly decreased from baseline to any post-baseline observation time. The study was not able to find any statistically significant effect on fracture risk (p = 0.185). The percentage of patients with fractures was unaltered during treatment as compared to the 3-year period before treatment. The most common AEs were fragility fractures, back pain, arthralgia, fever, and joint sprain. An acute phase reaction was reported in 26 (22.8%) patients. None of the reported SAEs were considered as treatment-related. Long-term treatment with i.v. neridronate has positive effects on BMD and bone turnover markers with a good safety profile, although no significant effect on the risk of fracture was observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteogenesis Imperfecta (OI) is a generalized connective tissue disease and represents a fairly common disorder (one in 15–20,000 births). The main features are skeletal fragility and substantial growth deficiency [1–3]. Other relevant findings are often non-skeletal features, including blue sclerae, hearing loss, dentinogenesis imperfecta, impaired pulmonary function, and cardiac valvular regurgitation. Different types of OI have been identified, based on clinical and hereditarily characteristics and on biochemical defects [1–3].
The bone histomorphometric picture is usually characterized by the presence of an excessive number of osteoblasts with impaired activity and a consequent defective bone matrix deposition. The trabecular skeletal turnover is roughly increased, at least up until maturity [4]. These histomorphometric features have been confirmed by the detection of an increase in bone turnover markers, such as serum osteocalcin, collagen peptide cross-links (N-telopeptide), and calcium urinary excretion [5–9].
Several therapies have been suggested for the treatment of OI, including calcitonin [10], growth hormone alone or in combination with other treatments [11, 12], teriparatide [13, 14] and bone marrow transplantation [15]. The increase of bone turnover markers is a hallmark of the disease and represents the rational for therapy with antiresorptive agents such as bisphosphonates or denosumab [16, 17]. To date, bisphosphonates showed to increase bone mineral density (BMD). A positive effect on prevention of fractures both in adults and in children is reported by some studies [17–19], but generally data are inconsistent as shown by a recent metanalysis by Hald et al. [20]. In particular, neridronate has been approved in Italy for the treatment of OI. The aim of this study was to assess the long-term efficacy and safety of i.v. neridronate in the treatment of OI.
Methods
Population
The study sample includes adult patients (aged > 20 years) with OI, enrolled in a single investigational study site. Patients were enrolled from a dedicated outpatient clinic of a national reference Centre for Osteogenesis Imperfecta. All the patients had previously received the diagnosis on the basis of a history of fragility fractures (one or more, especially during youth), concomitant positive family history and/or extraskeletal manifestation specific for the disease such as hearing loss, blue sclerae, or dentinogenesis imperfecta. The study concerned only clinical fractures (both vertebral and non-vertebral). During the study period, we did not routinely perform spine X-rays in order to detect morphometric fractures. Exclusion criteria were a previous treatment with bisphosphonates, serious concomitant comorbidities including cardiovascular, hematological, psychiatric or pulmonary disease, renal failure with serum creatinine >1.5 mg/dl, history of adverse drug reactions to bisphosphonates. Fertile women (except those taking oral contraceptives) were excluded too.
Istituto Gentili (Pisa, Italy), currently Abiogen Pharma, offered to provide the drug free of charge for every enrolled patient and for the entire duration of the study. For the study, the authorization of the Italian ‘Istituto Superiore di Sanità,’ the Italian Ministry of Health, and the reference Independent Ethics Committee (IEC) for the participating center was obtained. Patients signed an informed consent before to be enrolled in the study. Here, we show the data concerning the extension of a previous study in which the data regarding the first 2 years were reported.
Treatment Administered
Neridronate was administered by i.v. infusion at a dosage of 2 mg/kg, up to a maximum of 100 mg in saline solution (NaCl 0.9%) (approximately 10 mg/100 cc), with an infusion time lasting two hours at least. The infusions were scheduled every three months. Supplementation with calcium (1000 mg daily) and Vitamin D (800 Ui daily) was prescribed to the patient.
Efficacy Outcomes
Dual X-ray absorptiometry (DXA) of the lumbar spine, hip, and ultradistal and proximal radius (Hologic 4500, Waltham, US) was evaluated every 6 months. The precision error for BMD at different skeletal sites ranges from 1 to 2.5% in subjects with a wide range of bone mass values.
All the fractures occurred in the last 3 years before the study baseline and the ones occurred during the study were validated through standard X-ray or radiologist validated reports. The serum calcium, phosphate, albumin, and fasting urinary calcium/creatinine ratios were obtained at baseline and every 3 months. For the determination, total alkaline phosphatase (tALP) was used (Roche Diagnostics). Serum bone alkaline phosphatase (BAP, Alkphase–B; Metra Biosystems) was measured by enzyme-linked immunosorbent assay.
Total alkaline phosphatase (tALP: Roche Diagnostics) was measured by an immunoassay analyzer, while serum bone alkaline phosphatase (BAP, Alkphase–B; Metra Biosystems) by an enzyme-linked immunosorbent assay. Both these measurements were performed every 12 months only in a proportion of patients. The inter- and intra-assay variations ranged from 7 to 12%.
Safety Variables
AEs were categorized by System Organ Class (SOC) and Preferred Term (PT) by using the MedDRA dictionary (version 11.0). The occurrence of clinical and biochemical (hematology, blood chemistry, urinalysis) adverse event (AEs) were recorded at all visits.
Statistical Methods
In the statistical analysis, we considered the safety population, defined as all patients who received at least one dose of study medication, and the intention-to-treat population (ITT), defined as all patients who received at least one dose of study medication and with post-baseline data. These two populations coincided in this study.
Demographic and baseline characteristics were summarized by means of descriptive statistics, in form of number of observation, mean, standard deviation, minimum and maximum for continuous variables, and frequency distributions (number and percentages) for categorical variables. Medical history, concomitant diseases (codified using the MedDRA dictionary version 11.0), and previous and concomitant medication (codified with WHO Drug Dictionary) were collected.
The percent changes of bone mineral density (BMD) were analyzed, since the baseline by means of descriptive statistics, and the mean values of percent changes since the baseline were compared with 0 by means of a one-sample Student’s t-test. In order to manage multiple tests, Bonferroni’s correction was applied to the probability associated with each test. The percentage change from baseline of bone markers’ parameters was analyzed as for the densitometric efficacy parameters.
The frequency of fractures was considered as the number of patients with new fractures and number of fractures/year and it was accounted for the 3 years prior the study baseline and during all the treatment period. The distribution of patients’ rates with fractures observed before and during the treatment period was analyzed by means of McNemar’s test. Furthermore, the distributions of the number of fractures in the two periods were compared by means of the Wilcoxon signed-rank test.
Results
The original study sample consisted of 164 patients. Fifty patients were excluded since they were unable to guarantee the appropriate attendance to the scheduled visits. One hundred and fourteen patients were included in the study and started treatment with the study drug. The demographic characteristics and type of OI are shown in Table 1.
Ninety-seven subjects (85.1%) completed the scheduled 36-month treatment period, while 17 (14.9%) of them early discontinued the study. The patients received 11 ± 3 infusions (minimum 1, maximum 13). The summary of patients who completed or discontinued the study and the respective reasons for withdrawal are shown in Table 2. Lack of compliance was the main reason for early discontinuation.
The study population included more females than males (62.3 vs. 37.7%). Most of the patients (76.3%) had type 1 OI. In the medical history of patients, fragility fractures were reported by all the patients leading 90 patients (78.9%) to receive medical support.
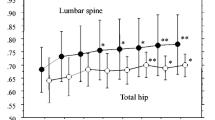
The mean lumbar spine BMD significantly increased from baseline to any time point (p < 0.001). The percentage variations from baseline progressively increased from month 6 to month 36 (Table3). The mean total hip BMD significantly increased from baseline to any time point (p < 0.001 at all times) (Fig. 1). The mean proximal radius BMD did not change (data not shown), while the mean ultradistal radius BMD significantly increased from baseline at month 18 (p = 0.026), 30 (p = 0.046), and 36 (p = 0.013).
As expected, mean ALP and BAP levels significantly decreased from baseline to any post-baseline time point (p < 0.001 at any time point) of about 25–30% (Fig. 2).The study was not able to find any statistically significant effect on fracture risk. The number of patients suffering fractures was unchanged during the 3 years of treatment compared to the 3 years before treatment (p = 0.19) (Table 2).
The mean number of fractures per patient decreased during treatment (Wilcoxon signed-rank test p-value p = 0.003) (Table 2).
The most common AEs were fragility fractures (21.1%), back pain (21.9%), arthralgia (20.2%), fever (3.5%), and joint sprain (6.1%). An acute phase reaction with flu-like illness was reported in 26 (22.8%) patients and was the most frequent adverse event, mainly related to the drug. No fatal events occurred. Serious adverse events (SAEs) were reported in 26 (22.8%) patients. None of the reported SAEs were considered treatment-related, considering the SAE graduation as possibly, probably, or highly probably related with the study treatment. Treatment-related adverse events were reported in 25.4% of patients. No cases of osteonecrosis of the jaw (ONJ) or atypical femur fracture were reported.
The laboratory tests (blood count, general blood chemistry, and urinalysis) did not show substantial changes in the results from baseline up to 36 months. Clinically significant and not treatment-related abnormalities in laboratory parameters were reported in a very small number of patients: 1 patient with anemia, 1 with thrombocytopenia, 1 with AST elevation, 1 with blood creatinine elevation, and 2 with gamma-GT elevation.
Discussion
The aim of this study was to assess the 3-year efficacy and tolerability of neridronate, administered in adult patients with OI by intravenous infusions at the dosage of 2 mg/kg (up to a maximum of 100 mg) once every 3 months for 3 years. The study sample included 114 patients, mostly affected by type 1 OI (type 1 OI 76.3%, type 3 OI 11.4%, or type 4 OI 12.3%).
Neridronate already proved its efficacy since 2003, when the results of a randomized trial involving 46 adults with OI for a study period of 24 months were published. That study reported positive data on BMD, bone turnover markers, and fracture incidence. Two years later, the data of a RCT with a similar design and similar good results on pediatric patients were published. These two positive experiences led to the registration of neridronate for osteogenesis imperfecta in Italy and for both children and adults [18, 19].
The present 3-year observational study represents the extension of the previous 2-year randomized trial (18) and showed that neridronate treatment was associated with marked and statistically significant increase of the BMD at the lumbar spine and total hip from baseline up to 36 months of treatment (Fig. 1). These increases in BMD observed during neridronate therapy are likely caused by suppression of bone resorption and secondary lowering of bone remodeling [21].
Although there is no head-to-head studies, we tried to compare our results with the ones of the trials with alendronate [22] and risedronate [23], on the basis of BMD and bone marker changes induced by the treatment. All these three studies involved adult patients affected by OI. The changes in BMD at 36 months appear in some way similar between alendronate and neridronate (10.1 vs. 8,6% at the spine and 3.3 versus 4.3% at the total hip, respectively) and furthermore both drugs induced a clinically relevant and statistically significant decrease in bone turnover markers. In the study with risedronate 35 mg weekly [23], the changes in BMD were lower (+3.9% at the spine and no change in total hip) and also the bone turnover appeared to be lesser inhibited. So, we estimate that risedronate (35 mg weekly) cannot be considered as equivalent to the treatment with alendronate (10 mg daily) or neridronate in terms of antiresorptive potency. Thus, it is not surprising that the fracture risk did not change in the patients treated with risedronate [23]. In the study with alendronate, the sample size was not powered to show an effect on fracture rate [22], while the present study is not controlled.
Anyway the present study was not able to find any statistically significant effect on fracture risk. The percentage of patients with fractures was unchanged during treatment as compared to the 3 years before treatment (Table 2).
The hallmark of OI is surely the fragility fractures and the patients had an increased fracture rate throughout their life. However, the fracture risk appears to be very high during youth and adolescence and in the elderly, while during the adulthood it is only mildly increased, achieving similar values to the ones of the healthy population of reference [24]. This could be the main reason why in adults with OI it is so difficult to show an anti-fracturative effect of any therapy. When we also consider the 3 years of treatment together versus the 3 years before treatment, most of patients (61.5%) did not have fractures. Thus, we cannot exclude that, for this same reason, the potential positive effect of treatment on the incidence of fractures (expressed as patients incurred in new events during the study as compared to the three years before treatment) could not be detected (Table 2). For these reasons, the observation that the mean amount of fractures observed in the three years of treatment was significantly lower (about 50%) than the one observed in the three years preceding the start of the treatment (Table 2) cannot be considered not relevant.
Besides efficacy, what is essential for a treatment is to be safe and well-tolerated.
Regarding osteoporosis or other bone diseases, we have consistent data on most bisphosphonates and they show good safety profile with a low incidence of serious adverse event such as atypical femur fracture or ONJ. These data cannot be transferred to OI patients mainly due to the different pathophysiology of OI and the different balance between fragility fracture and side effects in this peculiar population. Indeed, in this kind of disease, the risk of ONJ induced by bisphosphonates seems to be very low even though the data were mainly collected in children and adolescents [25, 26]. Furthermore, the pattern of femoral fracture in OI is surely different from the one in osteoporosis. In OI, in fact, the fractures mostly involve the femoral diaphysis, while in osteoporosis the femoral neck is mainly involved and this pattern seems to be changed by bisphosphonate treatment [27].
In any case during the three years of the study, we did not observe any drug-related serious adverse event. No cases of atypical fracture or ONJ were observed, though you should keep in mind that these two events are too rare to be seen in a study of this size.
The most common side effects were the ones related to the acute phase response that sometimes follows an aminobisphosphonate administration, especially if given intravenously. These symptoms are not serious, transient, and usually appear only after the very first infusion. They never led to treatment discontinuation during the study in any patient (Table 1). When patients dropped, they did it mainly because of personal issues which prevented them to guarantee the regular attendance to the visits (Table 1). The trial was monocentric and many patients come from far away with substantial difficulties.
The results of laboratory tests (hematology, blood chemistry, and urinalysis) did not show substantial changes from baseline up to 36 months for any parameter. In particular, there were no alterations in hepatic or renal function.
Differently from the oral one, the i.v. route guarantees a total adherence and bioavailability. Moreover, the i.v. route prevents the well-known gastrointestinal side effects responsible for a significant frequency of discontinuation in the osteoporosis population.
The limits of our study are the sample size and its not controlled design. These features do not allow obtaining conclusive data about the anti-fracturative effect of the treatment.
In conclusion, neridronate in OI adults at the dose of 100 mg i.v. every three months has showed to be safe, well-tolerated, and effective in increasing BMD and decreasing bone turnover. Nevertheless, post-marketing follow-up has a key role to give a complete picture for safety in an even longer-term period. The lack of properly controlled data, necessary to provide adequate evidence on a sustained decrease in fracture risk, makes essential the future design of larger and placebo-controlled trials.
Author contributions
Ombretta Viapiana participated in clinical management of the patients and in writing the paper. Luca Idolazzi participated in clinical management of the patients and in writing the paper. Angelo Fassio participated in data analysis. Giovanni Orsolini participated in data collection. Maurizio Rossini participated in revising the paper and in the study design. Giovanni Adami participated in data collection. Davide Gatti participated in writing and revising the paper, in study design, and supervised the study in every phase.
References
Forlino A, Marini JC (2016) Osteogenesis imperfecta. Lancet Lond Engl 387:1657–1671. doi:10.1016/S0140-6736(15)00728-X
Forlino A, Cabral WA, Barnes AM, Marini JC (2011) New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol 7:540–557. doi:10.1038/nrendo.2011.81
Hoyer-Kuhn H, Netzer C, Semler O (2015) Osteogenesis imperfecta: pathophysiology and treatment. Wien Med Wochenschr 1946 165:278–284. doi:10.1007/s10354-015-0361-x
Baron R, Gertner JM, Lang R, Vignery A (1983) Increased bone turnover with decreased bone formation by osteoblasts in children with osteogenesis imperfecta tarda. Pediatr Res 17:204–207. doi:10.1203/00006450-198303000-00007
Braga V, Gatti D, Rossini M et al (2004) Bone turnover markers in patients with osteogenesis imperfecta. Bone 34:1013–1016. doi:10.1016/j.bone.2004.02.023
Cepollaro C, Gonnelli S, Pondrelli C et al (1999) Osteogenesis imperfecta: bone turnover, bone density, and ultrasound parameters. Calcif Tissue Int 65:129–132
Minisola S, Rosso R, Romagnoli E (1995) Bone resorption assessed by immunoassay of urinary cross-linked collagen peptides in patients with osteogenesis imperfecta. J Bone Miner Res 10:335–339. doi:10.1002/jbmr.5650100222
Brenner RE, Vetter U, Bollen AM et al (1994) Bone resorption assessed by immunoassay of urinary cross-linked collagen peptides in patients with osteogenesis imperfecta. J Bone Miner Res 9:993–997. doi:10.1002/jbmr.5650090706
Iwamoto J, Takeda T, Ichimura S (2002) Increased bone resorption with decreased activity and increased recruitment of osteoblasts in osteogenesis imperfecta type I. J Bone Miner Metab 20:174–179. doi:10.1007/s007740200025
Castells S (1973) New approaches to treatment of osteogenesis imperfecta. Clin Orthop 93:239–249
Marini JC, Bordenick S, Heavner G et al (1993) The growth hormone and somatomedin axis in short children with osteogenesis imperfecta. J Clin Endocrinol Metab 76:251–256. doi:10.1210/jcem.76.1.8421094
Antoniazzi F, Monti E, Venturi G, et al (2010) GH in combination with bisphosphonate treatment in osteogenesis imperfecta. Eur J Endocrinol 163:479–487. doi:10.1530/EJE-10-0208
Gatti D, Rossini M, Viapiana O et al (2013) Clinical development of neridronate: potential for new applications. Ther Clin Risk Manag 9:139–147. doi:10.2147/TCRM.S35788
Orwoll ES, Shapiro J, Veith S et al (2014) Evaluation of teriparatide treatment in adults with osteogenesis imperfecta. J Clin Invest 124:491–498. doi:10.1172/JCI71101
Horwitz EM, Prockop DJ, Gordon PL et al (2001) Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 97:1227–1231
Hoyer-Kuhn H, Semler O, Schoenau E (2014) Effect of denosumab on the growing skeleton in osteogenesis imperfecta. J Clin Endocrinol Metab 99:3954–3955. doi:10.1210/jc.2014-3072
Dwan K, Phillipi CA, Steiner RD, Basel D (2014) Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev 7:CD005088. doi:10.1002/14651858.CD005088.pub3
Adami S, Gatti D, Colapietro F et al (2003) Intravenous neridronate in adults with osteogenesis imperfecta. J Bone Miner Res 18:126–130. doi:10.1359/jbmr.2003.18.1.126
Gatti D, Antoniazzi F, Prizzi R et al (2005) Intravenous neridronate in children with osteogenesis imperfecta: a randomized controlled study. J Bone Miner Res 20:758–763. doi:10.1359/JBMR.041232
Hald JD, Evangelou E, Langdahl BL, Ralston SH (2015) Bisphosphonates for the prevention of fractures in osteogenesis imperfecta: meta-analysis of placebo-controlled trials. J Bone Miner Res 30:929–933. doi:10.1002/jbmr.2410
Adami S, Kanis JA (1995) Assessment of involutional bone loss: methodological and conceptual problems. J Bone Miner Res 10:511–517. doi:10.1002/jbmr.5650100402
Chevrel G, Schott A-M, Fontanges E et al (2006) Effects of oral alendronate on BMD in adult patients with osteogenesis imperfecta: a 3-year randomized placebo-controlled trial. J Bone Miner Res 21:300–306. doi:10.1359/JBMR.051015
Bradbury LA, Barlow S, Geoghegan F, et al (2012) Risedronate in adults with osteogenesis imperfecta type I: increased bone mineral density and decreased bone turnover, but high fracture rate persists. Osteoporos Int 23:285–294. doi:10.1007/s00198-011-1658-2
Folkestad L, Hald JD, Ersbøll AK et al (2016) Fracture rates and fracture sites in patients with osteogenesis imperfecta: A Nationwide Register-Based Cohort Study. J Bone Miner Res. doi:10.1002/jbmr.2920
Hennedige AA, Jayasinghe J, Khajeh J, Macfarlane TV (2013) Systematic review on the incidence of bisphosphonate related osteonecrosis of the jaw in children diagnosed with osteogenesis imperfecta. J Oral Maxillofac Res 4:e1. doi:10.5037/jomr.2013.4401
Maines E, Monti E, Doro F et al (2012) Children and adolescents treated with neridronate for osteogenesis imperfecta show no evidence of any osteonecrosis of the jaw. J Bone Miner Metab 30:434–438. doi:10.1007/s00774-011-0331-3
Nicolaou N, Agrawal Y, Padman M et al (2012) Changing pattern of femoral fractures in osteogenesis imperfecta with prolonged use of bisphosphonates. J Child Orthop 6:21–27. doi:10.1007/s11832-011-0380-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ombretta Viapiana has received speaking fees from Abiogen, Amgen, Merck Sharp & Dohme Corp. Luca Idolazzi has received speaking fees from Eli Lilly, UCB, Abbvie, Novartis. Angelo Fassio, Giovanni Orsolini, and Giovanni Adami declare they have no conflict of interest. Maurizio Rossini has received speaking and consulting fees from Abiogen, Amgen, Eli-Lilly, and Merck Sharp & Dohme Corp. Davide Gatti has received speaking fees from Abiogen, Amgen, Eli-Lilly, and Neopharmed-Gentili.
Human and Animal Rights and Informed Consent
The clinical study was conducted in accordance with the ethics principles of the Declaration of Helsinki and was approved by the local ethics committee. Each enrolled patient signed the informed consent to participate in the study.
Rights and permissions
About this article
Cite this article
Viapiana, O., Idolazzi, L., Fassio, A. et al. Long-term Effects of Neridronate in Adults with Osteogenesis Imperfecta: An Observational Three-Year Italian Study. Calcif Tissue Int 100, 341–347 (2017). https://doi.org/10.1007/s00223-017-0236-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0236-9