Abstract
Although sclerostin (SOST) and Dickkopf-related protein 1 (DKK1) are major regulators in bone metabolism, their associations with osteoporotic fracture (OF) in Asians are inconclusive. Furthermore, there have been no clinical studies separately considering non-vertebral and vertebral fractures in terms of the blood levels of SOST and DKK1. Among 513 consecutive postmenopausal Korean women, we identified 103 cases defined as subjects with OF (i.e., non-vertebral and/or vertebral fractures). The controls were randomly selected from the remaining 410 subjects and matched 1:1 to cases according to both age and body mass index. Non-vertebral and morphological vertebral fractures were identified by an interviewer-assisted questionnaire and lateral thoracolumbar radiographs, respectively. Bone mineral density (BMD) and plasma levels of SOST and DKK1 were measured. Plasma SOST levels were lower in subjects with OF than in the control group. Each standard deviation decrement of plasma SOST concentration was associated with a multivariable-adjusted odds ratio of 1.77 for any prevalent OF type. The odds for OF was 2.97-fold higher in subjects in the lowest SOST tertile compared with subjects in the highest SOST tertile. These associations remained significant when the non-vertebral and vertebral fractures were analyzed separately. However, prevalent OF was not associated with plasma DKK1 levels, regardless of the type of fracture and the adjustment model employed. Consistently, plasma SOST levels were positively related with BMD values at all measured skeletal sites, although this was not observed for DKK1. Circulating SOST but not DKK1 may be a potential biomarker for predicting bone health in Asians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporotic fractures (OFs) have become a worldwide public health issue causing significant morbidity and disability in older people [1]. Particularly, Korea is one of the most rapidly aging countries, and recent epidemiologic study reported that the incidence of hip fractures increased in Korean populations over age 50 between 2008 and 2012 [2]. Therefore, the socioeconomic burden is expected to increase in Korea along with the increased estimated number of OFs [3]. While low bone mineral density (BMD) estimated using dual-energy X-ray absorptiometry (DXA) has remained an important determinant of OF risk, it does not reveal all the risk factors for OF [4]. Prospective cohort studies reported that up to half of incident fractures occurred in individuals with baseline spine or hip BMD T-score > −2.5 assessed by a DXA which is the diagnostic cutoff for osteoporosis defined by the World Health Organization (WHO) [5, 6]. To enhance the ability of predicting OF risk in individuals, fracture risk assessment tool (FRAX) was developed based on relevant clinical risk factors (CRFs) identified by the WHO working group [7]. However, the overall predictability of OF risk in individuals remains suboptimal despite the availability of FRAX [8, 9]. Therefore, additional biomarkers that may predict the risk of OFs independently of or in combination with BMD and CRFs are required.
The Wnt/β-catenin signaling pathway is critically involved in bone remodeling and healing, and has emerged as an important player in skeletal homeostasis [10, 11]. Activation of this pathway expands osteoprogenitor cells while reducing osteoblast apoptosis, resulting in bone anabolism [12, 13]. Binding of the Wnt ligands to a co-receptor complex, composed of a 7-transmembrane domain-spanning frizzled receptor and low-density lipoprotein receptor-related protein (LRP) 5 or 6, stabilizes cytoplasmic β-catenin protein, which translocates into the nucleus and activates the transcription of target genes such as Runx-2 and osteoprotegerin [14, 15]. The major regulators of the canonical Wnt pathway in the bone are sclerostin (SOST) and Dickkopf-related protein 1 (DKK1). SOST and DKK1 are Wnt antagonists that act on bone metabolism by competitive binding to LRP-5 and LRP-6 [16, 17]. As SOST and DKK1 have a secretory feature, several epidemiological studies were performed to assess their role as a predictor for osteoporosis-related phenotypes. However, almost all of these studies only focused on SOST and not DKK1 in Western or Arab populations, and their results were inconsistent [18–21]. Therefore, the associations between these soluble Wnt antagonists and bone health in the Asian population, who may have distinct biological characteristics [22, 23], are rather inconclusive. As yet, there have been no clinical studies separately considering non-vertebral and vertebral fractures in terms of the blood levels of SOST and DKK1. To provide further clarification of these unsolved points, we performed a case–control study in postmenopausal Korean women.
Materials and Methods
Study Subjects and Protocol
All consecutive postmenopausal Korean women who attended the osteoporosis clinic of the Asan Medical Center (AMC; Seoul, Korea) between June 2011 and December 2012 were included in this case–control study. These women were attending the osteoporosis clinic due to concerns regarding osteoporosis, or the subjects had been referred following a diagnosis of osteoporosis during a routine examination. Menopause was defined as the absence of menstruation for at least 1 year and was confirmed by the measurement of serum follicle-stimulating hormone levels. Women who exhibited premature menopause (<40 years of age) and women who had taken drugs that may affect bone metabolism, such as bisphosphonate, systemic glucocorticoids, or hormone-replacement therapy, for more than 6 months or within the previous 12 months were excluded. Subjects with diseases that may affect bone metabolism, such as diabetes, neoplastic diseases, hyperparathyroidism, rheumatoid arthritis, asthma/chronic obstructive pulmonary disease, and major cardiovascular diseases, and subjects who exhibited osteophyte formation that exceeded grade 4 of the Nathan classification and/or severe facet joint osteoarthritis in the lumbar spine, as determined by conventional spine radiographs, were also excluded from the study. Other exclusion criteria included the following: the presence of fever (oral temperature, ≥38.0 °C); low or high leukocyte (<4.0 or >10.0 × 109/L) or platelet (<150 or >350 × 109/L) blood count; and abnormal liver, kidney, or thyroid function. These criteria were imposed to exclude subjects with a systemic illness.
The prevalence of morphological vertebral fracture was determined by obtaining lateral thoracolumbar (T4–L4) radiographs, which were analyzed at the AMC, according to the recommendations of the Working Group on Vertebral Fractures [24], by expert radiologists who were blinded to this study. A vertebral fracture was quantitatively defined as >20 % reduction in any measured vertebral height (anterior, middle, or posterior) [25]. Non-vertebral fractures, namely, those at the forearm, humerus, and hip, which are regarded as major osteoporosis-related fracture sites [7, 26], were assessed by applying an interviewer-assisted questionnaire. Fractures that were considered to be non-osteoporotic, such as fractures arising from major trauma such as motor vehicle accidents or a fall from a height higher than standing, and all fractures of the fingers, face, skull, and toes, were excluded. In this study, the remaining fractures, all of which were at osteoporosis-related sites and had clearly been caused by low trauma at over 50 years of age or after menopause, were regarded as OFs.
The following patient information was obtained by using a self-administered questionnaire: smoking habits (current smoker), alcohol intake (≥3 units/day), regular outdoor exercise (≥30 min/day), history of medication use, previous medical or surgical procedures, and reproductive status (including menstruation). An interviewer-assisted questionnaire was used to assess whether each subject had a parental history of OF.
After adopting the aforementioned exclusion criteria, 513 women were deemed eligible for participation. Among these women, 103 cases were identified as subjects with an OF of any type, such as non-vertebral and/or vertebral fractures. From the remaining 410 subjects, controls were randomly selected and matched (1:1) to cases according to both age (within 2.5 years) and body mass index (BMI; within 1.0 kg/m2).
BMD Measurement
Areal BMD (g/cm2) was measured at the lumbar spine (L1–L4) and proximal femur (total hip, femoral neck, and trochanter) by DXA using Lunar equipment (Prodigy; Madison, WI, USA). In terms of the coefficient of variation (CV), the precision values of the equipment were 0.67 % for the lumbar spine and 1.25 % for the femoral neck, and were determined by measuring 17 volunteers who were not enrolled in this study. The volunteers were required to individually undergo five scans on the same day and to climb on and off the table between examinations.
Biochemical Measurements
Serum calcium concentrations were measured using the cresolphthalein complexone method on a Toshiba 200FR Autoanalyzer (Toshiba Medical Systems Co., Ltd, Tokyo, Japan). The intra- and interassay CVs were 1.24 and 2.06 %, respectively, and the reference interval was 2.07–2.50 mmol/L. Serum phosphorus concentrations were measured using the phosphomolybdate ultraviolet method (Toshiba 200FR instrument). The intra- and inter-assay CVs were 1.28 and 2.54 %, respectively, and the reference interval was 0.81–1.45 mmol/L. To measure biochemical bone turnover markers (BTMs), fasting blood samples were obtained in the morning. Serum bone-specific alkaline phosphatase (BSALP) levels were determined using the MetraTM BAP immunoassay kit (Quidel Corp., San Diego, CA, USA), with inter- and intra-assay CVs of 4.4 and 3.6 %, respectively. The reference interval for postmenopausal women was 14.2–42.7 U/L. The serum C-terminal telopeptide of the type I collagen (CTX) levels were measured using an electrochemical-luminescence immunoassay (Roche Diagnostics GmbH, Mannheim, Germany), with intra- and inter-assay CVs of 1.0–4.6 and 1.6–4.7 %, respectively. The reference mean ± SD value for postmenopausal women was 0.556 ± 0.226 ng/mL.
Measurement of Plasma SOST and DKK1 Concentrations
Fasting venous blood samples were obtained. After centrifugation, the supernatants were carefully collected to exclude cellular components. All samples demonstrating hemolysis or clotting were discarded. The plasma samples were stored at −80 °C prior to determining the concentrations of SOST and DKK1. Plasma levels of SOST were measured using a SOST competitive enzyme-linked immunosorbent assay (ELISA) kit (BIOMEDICA, Vienna, Austria) according to the manufacturer’s instructions. The lower limit of detection for the SOST competitive ELISA kit was 72.7 pg/mL, and the intra- and inter-assay CVs were 7 and 5 %, respectively. Plasma levels of DKK1 were measured using the DKK1 competitive ELISA kit (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. The lower limit of detection for the DKK1 competitive ELISA kit was 4.2 pg/mL, and the intra- and inter-assay CVs were 4.2 and 7.6 %, respectively.
Statistical Analysis
All data are presented as mean ± standard deviation (SD) or numbers and percentages, unless otherwise specified. The cases and controls were compared in terms of baseline characteristics by using Student’s t-tests for continuous variables and Chi squared tests for categorical variables. The multivariable-adjusted least-squares means levels (95 % confidence intervals [CIs]) of potential biomarkers, in terms of OF status, were estimated using analysis of covariance (ANCOVA) after adjusting for well-known demographic and behavioral factors that may affect bone metabolism. These factors were age, years since menopause (YSM), BMI, current smoking status, alcohol intake, regular outdoor exercise, and parental history of OF. Age and BMI were included in the multivariable-adjusted model because although this is an age- and BMI-matched case–control study, age and BMI are very strong determinants of OF and/or bone mass, and we were concerned about the possible residual effects of these factors on bone metabolism, even after matching. We also adjusted for BMD to determine whether SOST or DKK1 has an association with prevalent fracture, independent or not of BMD. To generate the odds ratios (ORs) (95 % CIs) per SD decrement in the plasma biomarker concentration for prevalent OFs, multiple logistic regression analyses were performed before and after adjustment for confounders. Next, all subjects were categorized into three groups according to plasma biomarker concentrations. Multiple logistic regression analyses were then performed to generate ORs (95 % CIs) to demonstrate the association of OF in the subjects in the lower two tertiles relative to the subjects in the highest tertile, both before and after adjusting for confounders. The association of plasma biomarker concentrations with BMD values at various skeletal sites and BTMs was investigated by multiple linear regression analyses before and after adjustment for confounders. Finally, differences in BMD values according to plasma SOST tertiles after adjustment for potential confounders were compared by ANCOVA. All statistical analyses were performed using SPSS statistical software (SPSS Inc., Chicago, IL, USA). A P value less than 0.05 was considered as statistically significant.
Results
Clinical Characteristics of Subjects With and Without OF
The baseline characteristics of the study subjects are shown in Table 1. Of the 103 postmenopausal women with OF, 62 had non-vertebral fractures and 50 had vertebral fractures. In the 62 subjects with non-vertebral fractures, there were 44 forearm fractures, 8 humerus fractures, and 10 hip fractures. The mean age of the 103 OF cases was 62.8 ± 6.1 (range 51–79) years, and the mean age of the 103 controls was 62.8 ± 6.1 (range 50–79) years. The two groups did not differ in terms of YSM, weight, height, BMI, smoking, drinking, exercise habits, BMD values at all measured sites, and the levels of calcium, phosphorus and BTMs. However, the subjects with OF were significantly more likely to have a parental history of OF.
Plasma SOST and DKK1 Levels in OF Cases and Controls
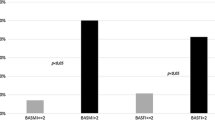
Prior to the adjustment for age, YSM, BMI, current smoking status, alcohol intake, regular outdoor exercise, and parental history of OF, subjects with OF of any type (i.e., non-vertebral and/or vertebral fractures) (mean 1.21 ng/mL, 95 % CI 1.15–1.27) had 11.0 % lower plasma SOST levels compared with subjects without OF (mean 1.36 ng/mL, 95 % CI 1.30–1.42, P = 0.001). Following adjustment for these factors, subjects with OF (mean 1.20 ng/mL, 95 % CI 1.14–1.26) had 11.9 % lower plasma SOST levels compared with subjects without OF (mean 1.37 ng/mL, 95 % CI 1.31–1.43, P < 0.001) (Fig. 1a). When the total hip BMD value was added as a confounding variable in this model, the statistical significance persisted (P = 0.001). When non-vertebral and vertebral fractures were separately analyzed in the multivariable adjustment models, subjects with non-vertebral (mean 1.21 ng/mL, 95 % CI 1.13–1.28) and vertebral fractures (mean 1.19 ng/mL, 95 % CI 1.10–1.28) had 11.6 and 11.9 % lower plasma SOST levels than their matched controls, respectively (mean 1.36 ng/mL, 95 % CI 1.30–1.42, P = 0.002 and mean 1.35 ng/mL, 95 % CI 1.29–1.41, P = 0.002, respectively) (Fig. 1b, c respectively). Following the additional adjustment for total hip and lumbar spine BMD, respectively, the differences of plasma SOST levels, according to non-vertebral and vertebral fracture status, remained significant (P = 0.003 and 0.004, respectively). When we only considered hip fracture status among subtypes of non-vertebral fractures, subjects with hip fracture (mean 1.15 ng/mL, 95 % CI 0.98–1.32) had significantly lower plasma SOST levels compared with their age- and BMI-matched controls (mean 1.37 ng/mL, 95 % CI 1.31–1.43, P = 0.017) after adjustment for all potential confounders including BMD values. However, subjects with and without OF did not differ in terms of plasma DKK1 levels, regardless of the adjustment model used (Supplementary Fig. 1).
Differences in plasma sclerostin levels following adjustment for confounders according to the osteoporotic fracture (OF) status. Estimated means with 95 % confidence intervals were generated and compared using analysis of covariance (ANCOVA). The multivariable adjustment factors were age, YSM, body mass index, current smoking, alcohol intake (≥3 Units/day), regular outdoor exercise (≥30 min/day), and parental history of OF
ORs for OF According to Plasma SOST and DKK1 Concentrations
The ORs per 1 SD decrement in plasma SOST concentration for any prevalent OF type were 1.68 before and 1.77 after multivariable adjustment (Table 2a). Performance of a further adjustment for total hip BMD revealed that the SD decrement of plasma SOST concentration was still associated with a multivariable-adjusted OR of 1.72 for any prevalent OF type. The non-vertebral and vertebral fractures were separately analyzed. Each SD decrement of plasma SOST concentration was significantly associated with 1.81-fold higher ORs for the non-vertebral fractures and 1.95-fold higher ORs for the vertebral fractures, after considering all potential confounders including BMD values (Table 2b, c). By contrast, the ORs for prevalent OF did not differ according to plasma DKK1 concentrations in any of the adjustment models (Supplementary Table 1). Meanwhile, the OR values of all covariates included in the multivariable adjustment model for any prevalent OF type were presented as Supplementary Table 2. A parental history of OF was significantly associated with 3.16-fold higher ORs, whereas the statistical significance for the association between total hip BMD and any prevalent OF was only marginal (P = 0.094).
ORs for OF According to Plasma SOST Tertiles
As plasma SOST was associated with prevalent OF and not DKK1, we performed further analyses after categorizing subjects into three tertiles on the basis of plasma SOST levels. The prevalence of any type of OF in Q1, Q2, and Q3 were 62.3, 51.5, and 36.2 %, respectively. Multiple logistic regression analyses revealed that the ORs for OF increased in a linear fashion with decreasing SOST tertile (P for trend = 0.002 in the multivariable adjustment model): compared to Q3, the odds for OF in the subjects in Q1 were 2.97-fold higher following adjustment for potential confounders (Table 3a). The OR in Q1 remained statistically significant following addition of the adjustment for the total hip BMD value to the multivariable model. When non-vertebral and vertebral fractures were separately analyzed, the odds for prevalent non-vertebral fractures were 2.81-fold higher, and the odds for vertebral fractures were 3.06-fold higher, in subjects in Q1 than in subjects in Q3 following adjustment for potential confounders (Table3b, c). Statistical significance persisted even when adjustment for BMD values was added.
Association of Plasma SOST and DKK1 Concentrations with BMD at Various Skeletal Sites
Plasma SOST levels Positively associated with BMD values at the lumbar spine, total hip, femoral neck, and trochanter before and after adjustment for age, YSM, BMI, current smoking status, alcohol intake, regular outdoor exercise, and parental history of OF (Table 4). Consistently, BMD values at all measured sites were significantly lower in subjects in Q1 than in those in Q3 after considering all potential confounders (Table 5). However, an association of plasma DKK1 levels with BMD values at any site was not found, regardless of the adjustment model applied (Supplementary Table 3). Meanwhile, neither plasma SOST nor plasma DKK1 had no correlation with the levels of serum CTX and BSALP (Table 4 and Supplementary Table 3, respectively).
Discussion
This case–control study demonstrated that postmenopausal women with OF had markedly lower plasma SOST levels than their age- and BMI-matched controls. Furthermore, each SD decrement of plasma SOST concentration was associated with a multivariable-adjusted OR of 1.77 for any prevalent OF type. These associations remained significant when the non-vertebral and vertebral fractures were analyzed separately. However, prevalent OF was not associated with plasma DKK1 levels, regardless of the type of fracture and the adjustment model employed. Consistently, plasma SOST levels were positively related with BMD values, although this was not observed for DKK1. To the best of our knowledge, this is the first clinical study to investigate associations between both plasma SOST and DKK1 levels and osteoporosis-related phenotypes in the Asian population, and to separately analyze non-vertebral and vertebral fractures regarding these circulating Wnt antagonists.
Although both SOST and DKK1 are well-known as important regulators in bone metabolism, little data exist on their direct comparison as a biomarker for osteoporosis-related phenotypes except one cross-sectional study [27]. In the present study, prevalent OF and BMD values were significantly associated with plasma SOST levels but not with plasma DKK1 levels. These results may be partially explained by the widespread expression of DKK1 compared with that of SOST. Both SOST and DKK1 are synthesized by osteocytes. However, SOST is almost exclusively expressed in osteocytes and late osteoblasts in adults [28], whereas DKK1 is expressed in several tissues, such as endothelial cells, neural cells, and platelets, with the latter considered as a major contributor of circulating DKK1 levels [29, 30]. The report by Drake et al. [31] showed strong correlations of SOST between peripheral blood and bone marrow fluid, whereas DKK1 did not have any associations. Collectively, blood SOST levels are presumed to superiorly reflect the status in the bone microenvironment than blood DKK1 levels, and thus circulating SOST may be of clinical relevance and an appropriate biomarker for bone health in humans.
In spite of substantial efforts from researchers, the relationship between blood SOST levels and the risk of fracture are still controversial [18–21]. Importantly, although the risk of fracture is strongly affected by ethnicity [22], no study so far has examined the association between blood SOST levels and OF in an Asian population, with the exception of a cross-sectional study performed in Japan that comprised diabetic patients [32]. However, the Japanese results can be biased by diabetes having the potential impact on the association between fracture and SOST [33], and thus our present study is meaningful in this regard because it is the first to demonstrate a significant association in the Asian population, particularly in the absence of a disease that may affect bone metabolism.
The fact that low SOST levels were associated with a high prevalence of OF and low BMD values appears initially inconsistent with the known functions of this protein: SOST is a secreted Wnt antagonist that plays a fundamental role as a detrimental factor in bone tissue. Although the reasons for this counterintuitive finding are unclear, our results were validated by those from a recent study by Szulc et al [21], who also hypothesized that blood SOST may be a marker of bone strength. Specifically, mechanical loading was associated with higher strains and lower SOST secretion in a dose-dependent manner [34, 35]. Conversely, mechanical unloading was associated with higher SOST secretion [34, 36, 37]. Thus, circulating SOST may reflect the adaptation of the metabolic activity of osteocytes to existing bone strains. Subjects with a higher BMD and better bone microarchitecture may have greater bone strength, lower mechanical strains, and higher SOST levels. Conversely, subjects with a lower BMD and poor bone microarchitecture may have higher mechanical strains in the remaining bone, resulting in the decrease of SOST expression to compensate bone fragility. Meanwhile, in our present study, among the 103 cases we evaluated, the average time between a fracture event and plasma SOST assessment was 1.39 ± 1.28 years and the time elapsed since fracture were positively associated with circulating SOST (β = 0.279, P = 0.021), meaning that subjects with more recent fractures had lower SOST levels. This observation can be explained by quantitative polymerase chain reaction analyses in human bones showing that SOST expression was down-regulated during fracture repair [38]. Taken together, all these results suggest the presence of a compensatory mechanism where SOST expression is decreased to overcome poor bone health.
In the present study, plasma SOST levels had no correlation with the levels of serum CTX and BSALP and had modest associations with BMD values. These results indicate that a potential role of SOST in human bone metabolism could be mediated by various other components consisting of bone strength, such as bone microarchitecture [39, 40], besides bone mass or independently of bone turnover rate.
Some potential limitations should be considered when interpreting our results. First, as this study is a case–control study, we were unable to determine whether a causal relationship exists between plasma SOST concentrations and osteoporosis-related phenotypes. Second, the study population comprised women who attended a referral hospital, and this may have imposed selection bias as this cohort may not be representative of the general population. Third, there is no standardized method for measuring blood Wnt antagonists at present, and thus the results showing the absence of relationship between DKK1 level and fracture and the discrepancies in the literature regarding the association between SOST level and fracture could be caused by technical issues, such as the efficiency of the kit used. Lastly, although we attempted to consider as many confounding factors as possible, we cannot exclude the possibility that the observed association was the result of uncontrolled factors that affect SOST and/or bone variables, such as 25-hydroxyvitamin D levels.
In summary, decreased circulating SOST levels were significantly associated with an increased prevalence of OF, even following division into non-vertebral and vertebral fractures and low BMD values in postmenopausal women. Given the in vitro and animal studies that demonstrated the harmful effects of SOST on bone metabolism, we speculated that subjects with high bone fragility and resultant fractures would have low SOST levels, as this may counteract the deterioration in bone strength and facilitate fracture healing. Importantly, plasma DKK1 was not associated with prevalent fracture and BMD. These observations suggest that blood SOST but not DKK1 may be a potential biomarker for predicting bone health in the Asian population. Further interventional studies are required to confirm this possibility.
References
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733. doi:10.1007/s00198-006-0172-4
Ha YC, Kim TY, Lee A, Lee YK, Kim HY, Kim JH, Park CM, Jang S (2016) Current trends and future projections of hip fracture in South Korea using nationwide claims data. Osteoporos Int. doi:10.1007/s00198-016-3576-9
Yi H, Ha YC, Lee YK, Lim YT (2013) National healthcare budget impact analysis of the treatment for osteoporosis and fractures in Korea. J Bone Metab 20:17–23. doi:10.11005/jbm.2013.20.1.17
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24:23–57. doi:10.1007/s00198-012-2074-y
Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD (2005) Identification of osteopenic women at high risk of fracture: the OFELY study. J bone mineral res 20:1813–1819. doi:10.1359/jbmr.050609
Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JP, Pols HA (2004) Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 34:195–202
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397. doi:10.1007/s00198-007-0543-5
Tremollieres FA, Pouilles JM, Drewniak N, Laparra J, Ribot CA, Dargent-Molina P (2010) Fracture risk prediction using BMD and clinical risk factors in early postmenopausal women: sensitivity of the WHO FRAX tool. J Bone Minera res 25:1002–1009. doi:10.1002/jbmr.12
Bolland MJ, Siu AT, Mason BH, Horne AM, Ames RW, Grey AB, Gamble GD, Reid IR (2011) Evaluation of the FRAX and Garvan fracture risk calculators in older women. J Bone minera res 26:420–427. doi:10.1002/jbmr.215
Huang H, He X (2008) Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol 20:119–125. doi:10.1016/j.ceb.2008.01.009
Rossini M, Gatti D, Adami S (2013) Involvement of WNT/beta-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int 93:121–132. doi:10.1007/s00223-013-9749-z
Gordon MD, Nusse R (2006) Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 281:22429–22433. doi:10.1074/jbc.R600015200
Wagner ER, Zhu G, Zhang BQ, Luo Q, Shi Q, Huang E, Gao Y, Gao JL, Kim SH, Rastegar F, Yang K, He BC, Chen L, Zuo GW, Bi Y, Su Y, Luo J, Luo X, Huang J, Deng ZL, Reid RR, Luu HH, Haydon RC, He TC (2011) The therapeutic potential of the Wnt signaling pathway in bone disorders. Curr Mol Pharmacol 4:14–25
Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB (2005) Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem 280:33132–33140. doi:10.1074/jbc.M500608200
Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA (2005) Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA 102:3324–3329. doi:10.1073/pnas.0408742102
Semenov M, Tamai K, He X (2005) SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 280:26770–26775. doi:10.1074/jbc.M504308200
Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA (2001) Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol 3:683–686. doi:10.1038/35083081
Ardawi MS, Rouzi AA, Al-Sibiani SA, Al-Senani NS, Qari MH, Mousa SA (2012) High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: the Center of Excellence for Osteoporosis Research Study. J Bone Minera Researc 27:2592–2602. doi:10.1002/jbmr.1718
Arasu A, Cawthon PM, Lui LY, Do TP, Arora PS, Cauley JA, Ensrud KE, Cummings SR (2012) Serum sclerostin and risk of hip fracture in older Caucasian women. J Clin Endocrinol Metab 97:2027–2032. doi:10.1210/jc.2011-3419
Garnero P, Sornay-Rendu E, Munoz F, Borel O, Chapurlat RD (2013) Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroid hormones, and fracture risk in postmenopausal women: the OFELY study. Osteoporos Int 24:489–494. doi:10.1007/s00198-012-1978-x
Szulc P, Bertholon C, Borel O, Marchand F, Chapurlat R (2013) Lower fracture risk in older men with higher sclerostin concentration: a prospective analysis from the MINOS study. J Bone Minera Res 28:855–864. doi:10.1002/jbmr.1823
Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM (2005) Osteoporosis and fracture risk in women of different ethnic groups. J Bone Minera Res 20:185–194. doi:10.1359/jbmr.041007
Costa AG, Walker MD, Zhang CA, Cremers S, Dworakowski E, McMahon DJ, Liu G, Bilezikian JP (2013) Circulating sclerostin levels and markers of bone turnover in Chinese-American and white women. J Clini Endocrinol Metabol 98:4736–4743. doi:10.1210/jc.2013-2106
Kiel D (1995) Assessing vertebral fractures. National Osteoporosis Foundation Working Group on Vertebral Fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 10:518–523. doi:10.1002/jbmr.5650100403
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Minera Res 8:1137–1148. doi:10.1002/jbmr.5650080915
Park C, Ha YC, Jang S, Jang S, Yoon HK, Lee YK (2011) The incidence and residual lifetime risk of osteoporosis-related fractures in Korea. J Bone Miner Metab 29:744–751. doi:10.1007/s00774-011-0279-3
Dovjak P, Dorfer S, Foger-Samwald U, Kudlacek S, Marculescu R, Pietschmann P (2014) Serum levels of sclerostin and dickkopf-1: effects of age, gender and fracture status. Gerontology 60:493–501. doi:10.1159/000358303
Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG (2006) Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39:754–766. doi:10.1016/j.bone.2006.03.017
Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, Shaughnessy JD Jr (2009) The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 113:517–525. doi:10.1182/blood-2008-03-145169
Voorzanger-Rousselot N, Goehrig D, Facon T, Clezardin P, Garnero P (2009) Platelet is a major contributor to circulating levels of Dickkopf-1: clinical implications in patients with multiple myeloma. Br J Haematol 145:264–266. doi:10.1111/j.1365-2141.2009.07587.x
Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95:5056–5062. doi:10.1210/jc.2010-0720
Yamamoto M, Yamauchi M, Sugimoto T (2013) Elevated sclerostin levels are associated with vertebral fractures in patients with type 2 diabetes mellitus. The Journal of clinical endocrinology and metabolism 98:4030–4037. doi:10.1210/jc.2013-2143
Heilmeier U, Carpenter DR, Patsch JM, Harnish R, Joseph GB, Burghardt AJ, Baum T, Schwartz AV, Lang TF, Link TM (2015) Volumetric femoral BMD, bone geometry, and serum sclerostin levels differ between type 2 diabetic postmenopausal women with and without fragility fractures. Osteoporos Int 26:1283–1293. doi:10.1007/s00198-014-2988-7
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283:5866–5875. doi:10.1074/jbc.M705092200
Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, Price JS (2012) Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int 23:1225–1234. doi:10.1007/s00198-011-1656-4
Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, Pulvirenti I, Hawa G, Tringali G, Fiore CE (2010) Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab 95:2248–2253. doi:10.1210/jc.2010-0067
Spatz JM, Fields EE, Yu EW, Divieti Pajevic P, Bouxsein ML, Sibonga JD, Zwart SR, Smith SM (2012) Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab 97:E1736–E1740. doi:10.1210/jc.2012-1579
Delgado-Calle J, Arozamena J, Garcia-Renedo R, Garcia-Ibarbia C, Pascual-Carra MA, Gonzalez-Macias J, Riancho JA (2011) Osteocyte deficiency in hip fractures. Calcif Tissue Int 89:327–334. doi:10.1007/s00223-011-9522-0
Modder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ 3rd, Khosla S (2011) Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 26:373–379. doi:10.1002/jbmr.217
Szulc P, Boutroy S, Vilayphiou N, Schoppet M, Rauner M, Chapurlat R, Hamann C, Hofbauer LC (2013) Correlates of bone microarchitectural parameters and serum sclerostin levels in men: the STRAMBO study. J Bone Minera Res 28:1760–1770. doi:10.1002/jbmr.1888
Acknowledgments
This study was supported by grants from the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (project number HI14C2258), and from Takeda Pharmaceuticals Korea Co., Ltd., Seoul, Korea. The sponsor did not participate in the study design, data collection and analysis, writing of the manuscript, or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Yejee Lim, Chong Hwa Kim, Sun-Young Lee, Hyeonmok Kim, Seong Hee Ahn, Seung Hun Lee, Jung-Min Koh, Yumie Rhee, Ki Hyun Baek, Yong-Ki Min, Deog-Yoon Kim, Beom-Jun Kim and Moo-Il Kang declare that they have no conflict of interest.
Human Rights and Informed Consent
This study was approved by the Institutional Review Board of Asan Medical Center. All enrolled subjects provided written informed consent.
Additional information
Yejee Lim and Chong Hwa Kim have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, Y., Kim, C.H., Lee, SY. et al. Decreased Plasma Levels of Sclerostin But Not Dickkopf-1 are Associated with an Increased Prevalence of Osteoporotic Fracture and Lower Bone Mineral Density in Postmenopausal Korean Women. Calcif Tissue Int 99, 350–359 (2016). https://doi.org/10.1007/s00223-016-0160-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0160-4