Abstract
This study assessed persistence and compliance with anti-osteoporosis therapies, and associations between compliance and clinical outcomes (fracture, fracture-related hospitalization and death), in Hungarian women with postmenopausal osteoporosis. The study used the Hungarian National Health Insurance Fund Administration database and included women with PMO aged at least 50 years, for whom a prescription for anti-osteoporosis medication had been filled between 1 January 2004 and 31 December 2013 (index event). Persistence (prescription refilled within 8 weeks of the end of the previous supply) was evaluated over 2 years; good compliance (medication possession ratio ≥ 80 %) was evaluated at 1 year. Associations between compliance and clinical outcomes (data collected for up to 6 years) were assessed with adjustment for baseline covariates. A total of 296,300 women met the inclusion criteria (524,798 index events). Persistence and compliance were higher for less frequent and parenteral therapies (1- and 2-year persistence: half-yearly [parenteral] vs. daily/weekly/monthly [oral and parenteral], 81 and 38 % vs. 21–34 and 10–18 %, respectively; parenteral vs. oral, 75 and 36 % vs. 32 and 16 %; good compliance: half-yearly vs. daily/weekly/monthly, 70 vs. 24–39 %; parenteral vs. oral 78 vs. 36 %). Good compliance significantly reduced the risks of fracture, fracture-related hospitalization and death (relative risk vs. non-compliance [95 % confidence interval]: 0.77 [0.70–0.84], 0.72 [0.62–0.85] and 0.57 [0.51–0.64], respectively; P < 0.01). Improving compliance through long-interval parenteral therapies may result in clinical benefits for patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic skeletal disease characterized by low bone mass density and bone structure deterioration. The main clinical complication of osteoporosis is an increased susceptibility to fractures due to bone fragility [1]. In Hungary, osteoporosis affects approximately 7–10 % of the population [2–5], with approximately 140,000–150,000 patients treated annually. The economic burden of the disease comprises the cost of fracture-prevention medicine and the cost of treating the incident fractures. In Hungary, annual anti-osteoporosis treatment costs are estimated, on the basis of the 2009 data from the Hungarian National Health Insurance Fund Administration (NHIFA) database, at €22 million [2]. The annual costs associated with fractures (including all medical and health service costs) are even higher, at €24 million. Across Europe, annual osteoporosis-associated costs are expected to increase to €76.7 billion by 2050 [5], owing to a higher incidence of fractures in an ageing population. It is probable that low compliance with fracture-prevention medications increases these costs further, because it results in suboptimal efficacy, thereby increasing the risk of fractures.
Numerous studies have reported low persistence and compliance with anti-osteoporosis therapies in clinical practice. For bisphosphonates, the mainstay of treatment in Europe, 1-year persistence ranges from 25 to 78 %, and average compliance (calculated in terms of medication possession ratio [MPR]) ranges from 25 to 70 % [6–13]. Moreover, a minimum compliance (MPR) level of 50 % has been reported as necessary to achieve any reduction in the risk of fracture, with the benefit increasing exponentially above this level [14]. The variability in the reported levels of persistence and compliance can, to some extent, be attributed to differences in definitions of persistence and compliance, statistical methodology employed and populations studied; therefore, the studies are not necessarily comparable.
The factors that determine treatment persistence and compliance are not fully understood, but include dosing requirements, medication costs, drug-related side effects, the patient–physician relationship and patients’ inability to detect improvements in an asymptomatic disease [7, 8, 15–22]. Research into the relationship between such factors and clinical outcomes is becoming increasingly important. Long dosing intervals and injectable formulations are thought to contribute to better persistence and compliance, which may in turn lead to improved clinical outcomes [12, 14, 23–26]. To date, few studies have followed patients longitudinally to assess the impact of persistence, compliance and other known risk factors on clinical outcomes such as fracture, hospitalization and death.
Several therapies are available for the prevention and treatment of osteoporosis, with the goal of reducing risk of fracture [27]. It is widely recognized that persistence and compliance with these therapies is critical for optimal outcomes, and poor persistence and poor compliance have been reported to increase the risk of fracture, morbidity and mortality significantly [12, 14, 23, 28, 29]. For example, compared with non-persistence, persistence with treatment at 1 year has been reported to reduce risk of fracture by 26–45 %; compared with non-compliance, compliance with treatment at 1 year has been reported to reduce risk of fracture by 19–45 % [23, 29]. Several patient and disease characteristics are known to increase risk of fracture in osteoporosis, including advancing age, previous fractures and long-term use of co-medications [30].
The current study used longitudinal data from the Hungarian NHIFA database to analyse persistence and compliance with anti-osteoporosis treatments retrospectively in a large cohort of women with postmenopausal osteoporosis (PMO). Also, this study assessed the influence of different dosing frequencies and routes on persistence and compliance, specifically whether persistence and compliance were higher with parenteral treatment than with oral therapy. Moreover, we assessed the associations between compliance, baseline covariates, and the risks of fracture, fracture-related hospitalization and death over time.
Methods
Data Source
The study used the longitudinal NHIFA database, which contains records of medical events from 1 January 2004 and is representative of the entire Hungarian population (9,957,731 subjects at the time of the study). Data are available for medications (prescription refill, indication, package information) and outpatient and inpatient care. Every event is identified with one or more International Classification of Diseases (ICD) diagnoses and with codes for procedures and diagnosis-related groups. In Hungary, primary care services are financed on a fixed allowance basis; therefore, no administrative data are available on this level. Basic demographic data (age, gender) are available for every patient and allow longitudinal analysis of the patient pathway. Data to 31 December 2012 were accessed for all analyses except the persistence analysis, which used data to 31 December 2013.
Patients
Eligible individuals were women aged ≥50 years treated with an anti-osteoporosis medication (at least two prescription fills, with no requirement for prescriptions to be for the same specific treatment). Where available, ICD codes were reviewed and women with an ICD-10 diagnosis of osteoporosis (codes M80–M81; Online Resource 1) were included. Women without a recorded ICD code were not excluded from the study if they met all other inclusion criteria. Women were excluded if they had concomitant malignant disease, Paget’s disease, acquired immune deficiency syndrome or any prescription of antineoplastic drugs or cytostatic hormones, or if they died within 3 months of an index date.
Study Design
An index event was defined as initiation of treatment; treatment initiation also defined the index date. Multiple index events were permitted per patient. The persistence and compliance analyses included a 13-month wash-out period before each index date. The analysis periods for persistence and compliance were up to 2 years and 1 year, respectively, after the index event (Online Resource 2a and b).
The risk analysis included a 3-year selection period and index events from 1 January 2007 for up to 6 years, to minimize the potential bias from changes in therapeutic options (Online Resource 2c).
All patients were included in the persistence analysis. Patients with at least 1 year of follow-up were included in the compliance analysis. Patients initiating their first treatment for osteoporosis on or after January 2007 were included in the risk analysis.
Treatment
Anti-osteoporosis treatments were categorized as alendronate and combinations (oral), risedronate and combinations (oral), strontium ranelate (oral), ibandronate (oral), hormone replacement therapy (oral), ibandronate (intravenous [IV]; parenteral), zoledronate (IV; parenteral), denosumab (subcutaneous; parenteral) and teriparatide (1–34 parathyroid hormone) (subcutaneous; parenteral). Treatments were also categorized by administration frequency (daily, weekly, monthly and other), administration route (oral and parenteral), and a combination of frequency and route of administration (oral: daily, weekly and monthly; parenteral: daily, quarterly, half-yearly and yearly). While alendronate and risedronate were available in Hungary from 2004, the other treatments only became available later (strontium ranelate, teriparatide and oral ibandronate in 2006; parenteral ibandronate and zoledronate in 2007; denosumab in 2011) [31].
Outcomes
This study analysed persistence, compliance and the clinical outcomes fracture, fracture-related hospitalization and death.
Persistence
Persistence was estimated at 6, 12, 18 and 24 months as the proportion of patients refilling each subsequent prescription within a specified grace period. For the main analysis, persistence was defined as refilling a prescription within 8 weeks of the end of the previous supply; for the sensitivity analyses, persistence was defined as refilling a prescription within 4 and 12 weeks of the end of the previous supply.
For analysis according to treatment, patients were defined as persistent if their prescription was refilled for the specific treatment within the specified grace period. For analysis according to frequency and route of administration, patients were defined as persistent if their prescription was refilled for a treatment with the same frequency and route. For each analysis period, patients were grouped by their specific treatment at index date and followed until a change in their prescription (i.e. drop-out after the grace period for refilling the prescription).
Compliance
MPR was calculated using all outpatient anti-osteoporosis medications during a 1-year analysis period as the number of days covered by the prescribed number of therapy units in the year divided by 365.
Compliance was assessed in terms of the MPR. For the main analysis, good compliance was defined as MPR ≥80 % (non-compliance: MPR <80 %); for the sensitivity analyses, good compliance was defined as MPR ≥75 and ≥85 %. Non-compliance was used as the reference group. Zoledronate is administered yearly; thus, 1-year compliance for this therapy was assumed to be 100 %.
Clinical Outcomes
Data on fractures, fracture-related hospitalizations and deaths were collected for a maximum of 6 years following the index date. The occurrence of a previous or new osteoporotic fracture was assumed when one of the following ICD-10 codes was documented for diagnosis or reason for hospitalization: S12, S22, S32, S42, S52, S72, S82, T08 and T14.2. Fragility fractures were not considered because these codes are not frequently used in Hungary; therefore, all fractures of unknown severity were included as osteoporotic fractures, except for fractures of fingers, face, mandible, skull, metacarpals, finger phalanges, toe phalanges and those of unknown location. Hospitalizations were based on ICD-10 fracture codes. All deaths, regardless of cause, were included.
Covariates
The following covariates, which are known to influence the risk of fracture, were included in the risk analyses: age (50–59, 60–69, 70–79 and ≥80 years at the first index date); number of previous fracture(s) (0, 1 or ≥2 fractures in the 3-year wash-out period before an index date); co-medication (0, 1 or ≥2 co-medications during the first 3 months after an index date); and new fractures (0 or ≥1 fractures during the 6-year follow-up period). For each covariate, the first category was used as the reference group.
Disease Severity
For the assessment of disease severity, the Hungarian reimbursement protocol requires bone mineral density data to be recorded for patients initiating anti-osteoporosis therapy, with previous therapy determining the patient’s disease severity (Table 1). For each index date in our analyses, the maximum disease severity over the corresponding wash-out period was assessed and included as a static covariate in the risk analyses. Disease severity was also considered as a dynamic covariate, but was excluded during covariate selection because of suboptimal fitting.
Diagnosis Cohorts
Diagnosis cohorts were grouped by the type of prevention (primary or secondary) and were used as static parameters. The primary prevention cohort comprised patients with a diagnosis of osteoporosis with no previous fracture according to ICD-10 codes M81.0, M81.2, M81.5, M81.6, M81.8 and M81.9. The secondary prevention cohort comprised patients with a diagnosis of PMO with pathological fracture according to ICD-10 codes M80.0, M80.2, M80.5, M80.8 and M80.9. The occurrence of a previous or new osteoporotic fracture was assumed when one of the following ICD-10 codes was documented for diagnosis or reason for hospitalization after an index date: S12, S32, S42, S52, S72, S82, T02, T08 and T14.2.
Statistical Analyses
The database analyses in the current study were descriptive. All relevant data were included to minimize missing data bias and potential confounders. Frequency distributions were reported for categorical variables; summary statistics were presented for continuous variables. The sample size was considered adequate to present a 95 % confidence interval (CI) around point estimates with sufficient precision, both overall and by covariates.
Persistence data were summarized according to drug and according to administration frequency and route. Cox proportional hazard models were used to assess differences between drugs and between administration frequencies/routes. The exception to this was for 1-yearly zoledronate, for which 95 % bootstrap CIs were constructed for the differences in persistence between zoledronate and other therapies at 2 years.
Compliance data were summarized by age group and administration frequency and route. Associations between good compliance (MPR ≥80 %) and risks of fracture, fracture-related hospitalization and death were analysed using Cox proportional hazard models. As a regression-type model, this time-to-event approach quantifies the whole structure of dependencies between the therapy characteristics, compliance and fracture, fracture-related hospitalization or death. Additionally, it censors the data for each index event at the end of the analysis period.
For fracture and fracture-related hospitalization, multiple events were considered using the Andersen–Gill method (Cox’s Regression Model for Counting Processes), which includes all events in the calculation of the hazard ratio.
Results
Patients
A total of 296,300 patients (524,789 index events) met the inclusion criteria, all of whom were included in the persistence analyses. The compliance analyses were limited to the 262,236 patients who were followed up for longer than 1 year (369,840 index events). The risk analyses included 185,760 patients (215,377 index events).
Patient characteristics are shown in Table 2. Mean (standard deviation) age was 68.28 (9.67) years. The most frequent administration regimen was weekly (n = 227,373; 77 %). More than half of the patients were administered alendronate, either alone or as a combination oral medication with vitamin D (n = 166,614; 56 %). Approximately 2 % (n = 6483) of patients received zoledronic acid; fewer than 1 % (n = 1104) received denosumab.
Persistence
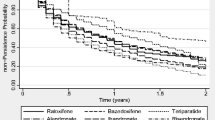
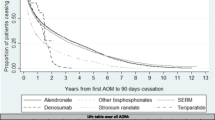
Figure 1 and Table 3 summarize persistence by therapy, and route and frequency of administration, using an 8-week grace period.
Overall persistence (prescription refilled within 8 weeks of the end of the previous supply) was 38.1 % at 1 year and 18.9 % at 2 years (Table 3). Persistence was higher for less frequently administered therapies (1- and 2-year persistence: half-yearly [parenteral] 81.0 and 38.4 % vs. daily/weekly/monthly [oral and parenteral] 22.7–34.2 and 10.0–17.6 %) and parenteral versus oral therapies (1- and 2-year persistence: 74.5 and 35.8 % vs. 31.5 and 15.9 %, respectively) (Table 3; Fig. 1b). Two-year persistence was generally higher for less frequently administered therapies, with the highest persistence observed for half-yearly and yearly therapies administered parentally (38.4 % [95 % CI 34.8–42.2 %] and 42.2 % [95 % CI 32.8–54.3 %], respectively). Similar results were observed for sensitivity analyses that used 4- and 12-week grace periods (data not shown).
Compliance
Table 4 summarizes compliance at 1 year. Overall, 47 % of observations demonstrated good compliance at 1 year. In general, more frequently administered therapies had lower rates of good compliance. Rates of good compliance were higher for parenteral therapies than for oral therapies (78.4 vs. 35.5 %, respectively), and daily oral medications had the lowest level of compliance. Only 24 and 39 % of observations demonstrated good compliance with daily or weekly oral bisphosphonates, respectively. The highest level of compliance at 1 year (other than the 100 % compliance assumed for yearly therapy) was in the half-yearly parenteral group (70 %). Sensitivity analyses defining good compliance as MPR ≥75 % and ≥85 % yielded similar results (data not shown).
Risks of Fracture, Fracture-related Hospitalization and Death
Compared with non-compliance, good compliance (MPR ≥80 %) at 1 year was associated with a statistically significant 23 % reduction in the risk of fracture (relative risk (RR) [95 % CI] 0.77 [0.70–0.84]; P < 0.01) (Fig. 2; Online Resource 3). Age ≥70 years (vs. 50–59 years), secondary prevention (vs. primary prevention), previous fracture (vs. no previous fracture), two or more co-medications (vs. no co-medication) and new fracture (vs. no new fracture) were all associated with a significantly increased risk of fracture (Fig. 2).
Analysis of associations between compliance, covariates and new fractures and risks of fracture, fracture-related hospitalization and death. a Fractures occurring during the 3-year wash-out period before the index date; b co-medications during the 3-month period post index date; c new fractures during the 6-year follow-up period. P values are < 0.01 unless otherwise indicated; † P > 0.01. CI confidence interval, HR hazard ratio
Good compliance was associated with a statistically significant 28 % reduction in the risk of fracture-related hospitalization (RR [95 % CI] 0.72 [0.62–0.85]; P < 0.01) (Fig. 2). Age ≥70 years and new fractures were associated with significantly increased risks of fracture-related hospitalization.
Good compliance was associated with a statistically significant 43 % reduction in the risk of death (RR [95 % CI] 0.57 [0.51–0.64]; P < 0.01) (Fig. 2). Age ≥60 years, secondary prevention, one or more co-medications and new fractures were all associated with a significantly increased risk of death (Fig. 2).
Compliance was the only covariate associated with a significant decrease in the risks of all three clinical outcomes. The occurrence of any new fractures and age ≥70 years were the only covariates associated with a significant increase in the risks of all three clinical outcomes (any new fracture: risk of new fracture, fracture-related hospitalization and mortality increased by 273, 691 and 99 %, respectively; age ≥70 years: risk increased by 37, 109 and 287 %, respectively; Fig. 2).
Discussion
We retrospectively analysed data from a large, longitudinal database, representative of the entire population of women with PMO in Hungary, for persistence and compliance with various anti-osteoporosis therapies. The study also assessed associations between compliance, baseline covariates and the risks of fracture, fracture-related hospitalization and death in this patient cohort over time.
This study found low persistence at 1 year (38 %), particularly for therapies with frequent administration schedules (ranging from 22 to 34 % for daily, weekly and monthly administered therapies). Patients were most likely to persist with less frequent, parenteral therapies (81 % for half-yearly therapy at 1 year). Although persistence decreased across all therapies at 2 years, it remained highest for the less frequent, parenteral therapies; it is worth noting that the low 2-year persistence for once-yearly administered zoledronate (42 %) may reflect physicians’ decisions not to repeat this treatment, perhaps on the basis of evidence which suggests residual anti-fracture benefits following yearly infusions [32, 33]. Similarly, discontinuation of teriparatide may not be indicative of poor medication-taking behaviour as this agent should not be used for more than 24 months over a patient’s lifetime [34]. In contrast, physicians may be more likely to continue prescribing denosumab to avoid the transient increase in bone turnover markers reported following discontinuation [35]. Therefore, data on persistence need to be evaluated in the context of the latest evidence and recommendations for osteoporosis drug use.
Our findings for persistence were generally consistent with other database studies [12, 28, 36]. For example, among 2419 postmenopausal women in France [36], 1-year persistence with orally administered anti-osteoporosis medications was 34 %, compared with 32 % for oral medications in the current study. Similarly to findings from our study, 1-year persistence varied significantly according to the administration frequency; however, compared with weekly therapies, monthly dosing was associated with significantly improved persistence and the daily regimens were associated with significantly poorer persistence [36]. This is in contrast to the 2-year persistence data from our study, in which persistence with weekly therapies was almost double that of monthly therapies. These results may have been influenced by the substantially greater number of patients taking weekly, as opposed to monthly, therapies in this study. For oral therapies, the low 2-year persistence observed is consistent with that reported for oral bisphosphonates in large US and German database studies [12, 29]. Conversely, in the Swedish Adherence Register Analysis (SARA) study [37] investigating 56,586 patients with osteoporosis, persistence with oral therapies at 1 and 2 years (51 and 35 %, respectively) was markedly higher than in the current study (31 and 15 %, respectively). Notably, 1-year persistence of 70 % was reported for the one injectable therapy included in the SARA study (parathyroid hormone). Furthermore, two large observational studies of women with PMO treated in routine practice in North America and Europe have reported 1-year persistence with 6-monthly subcutaneous denosumab of 82 and 87–95 %, respectively [25, 26]. These data support the high 1-year persistence observed in this study for parenteral treatments (74 %), particularly for the less frequently administered parenteral therapies, such as those administered half-yearly (81 %).
Fewer than half of the observations in the current study demonstrated good compliance (MPR ≥80 %) with anti-osteoporosis therapies after 1 year, which is consistent with previous studies [8, 28, 38, 39]. Furthermore, only 24 and 39 % of observations demonstrated good compliance with daily or weekly oral bisphosphonates, respectively. This result is similar to that of a large US database analysis (n = 38,120) in which, over a mean of 1.7 years of follow-up, only one-quarter of women had an MPR ≥80 % [8]. In the current study, the percentage of observations demonstrating good compliance at 1 year was markedly higher for parenteral therapies than for oral therapies. Good compliance was particularly high with less frequently administered, parenteral therapies (70 % for half-yearly therapies).
Several database studies have highlighted the relationship between poor compliance and adverse clinical outcomes [8, 28, 37–39]. Compared with good compliance (MPR ≥80 %), non-compliance has been associated with a 17–45 % increase in risk of fracture and a 37 % increase in risk of all-cause hospitalization [8, 28, 39]. In the SARA study, persistence with treatment over 3 years was associated with a 41 % reduction in 3-year risk of fracture (P < 0.001), with no significant association observed between compliance and risk of fracture [37]. Our data are consistent with these findings, with the risks of fracture and fracture-related hospitalization significantly reduced by 23 and 28 % among patients demonstrating compliance. Furthermore, in our study, the relative risk of death was significantly reduced by 43 % among patients with good compliance. Indeed, compliance was the only covariate associated with a significant decrease in the risks of all three clinical outcomes. Notably, studies have found an association between high-to-moderate compliance levels and significant savings on healthcare costs [8, 38]. Therefore, improving compliance with anti-osteoporosis medications may reduce healthcare costs, possibly through a reduction in the incidence of fractures and the associated costs of hospitalization. The occurrence of any new fracture and older age (≥70 years) were associated with significant increases in the risks of fracture, fracture-related hospitalization and death (any new fracture: 273, 691 and 99 %, respectively; age ≥70 years: 37, 109 and 287 %, respectively), providing further evidence that previous fracture and age are both significant risk factors for future fracture(s). Our data are also supported by the Australian Dubbo Osteoporosis Epidemiology Study, in which fracture was associated with increased mortality for up to 10 years following an initial fracture, particularly in those who had a second fracture within 5 years of their initial fracture [40]. In addition to good compliance, good persistence may also reduce the risk of mortality in patients with osteoporosis. However, this was not evaluated in the current study owing to the risk of immortal time bias.
Whereas information is available on the epidemiology of osteoporosis-related fractures in Hungary [3], to our knowledge, this is the first fully published study to evaluate persistence and compliance with various anti-osteoporosis therapies, and the associations between compliance, baseline covariates and the risks of fracture, fracture-related hospitalization and death over time, using the Andersen–Gill method. The strengths of the current study include the large sample size that was achieved through the use of a country-wide database, rather than one limited to a subset of the population (e.g. managed care, claims or pharmacy databases), and the follow-up of patients to obtain longitudinal data. Baseline data indicate that 6.6 % of this population had previous fractures. Although these data are from the 3 years before treatment initiation, and so may not be generalizable to the entire population of patients with osteoporosis, this figure is broadly consistent with data from other studies. The International Osteoporosis Foundation report a 10-year fracture incidence of 10–15 % [41], and a study in Hungary of the nationwide health insurance database showed that 404,380 women aged ≥50 years had at least one fracture in a 5-year period, corresponding to a yearly mean of 4 % [3].
A limitation of this study is that, because it is based on a public health database, the information on patient and disease characteristics is limited. In Hungary, ICD diagnosis codes are used to determine reimbursement; therefore, for patients with multiple conditions the diagnosis may be biased towards the disease with the highest level of reimbursement. It is thus possible that, for women with osteoporosis and other comorbidities, some fractures and fracture-related hospitalizations may have been coded to a diagnosis other than osteoporosis and hence excluded from our study. Owing to data availability, it was not possible to control for all confounding factors which may be related to compliance, persistence and associated outcomes, such as bone mineral density, socioeconomic variables, comorbidities and lifestyle and self-care behaviours. The effect of these parameters on patient outcomes would be an area of interest for future research. We did, however, control for some variables known to influence compliance and risk of fracture, such as age, previous fracture and co-medication. Disease severity was included as a static covariate and was assessed using prior treatment used, according to the Hungarian reimbursement protocol. Using alternative definitions of disease severity may lead to variations in the results. In addition, administrative data from primary care services were not available. However, in Hungary, all X-rays were carried out in the outpatient or hospital setting; hence, all fractures diagnosed using this method would have been recorded and included. Furthermore, as all osteoporosis medications included in our study are reimbursed in Hungary, the NHIFA database is a comprehensive source for such data.
Another limitation is that newer treatments are likely to be prescribed later in the treatment pathway, after older less expensive alternatives have failed or for secondary fracture prevention. It is possible that compliance may have been overestimated in the current study. Also, because the retrospective calculation of MPR is based on the assumption that patients take all medications for which they have prescriptions filled, and does not take into account whether drugs are taken in accordance with the prescribing instructions, it is possible that compliance may have been overestimated [42]. Nonetheless, MPR is commonly used in this type of study [8, 12, 28, 37, 38], and its use for measuring compliance is recommended by the International Society for Pharmacoeconomics and Outcomes Research [43]. Inclusion criteria in our study required patients to have at least two prescriptions filled for anti-osteoporosis medication, leading to the exclusion of ‘early drop-out’ patients who only had one prescription filled over the entire study period. Epidemiological data from Hungary, however, suggest that this patient subgroup is small and there exclusion would have only a minor effect on the study results [2, 3]. It should also be noted that the exclusion of patients who died within 3 months of the index date may have led to selection bias. Despite these limitations, this study supports previous findings regarding the clinical benefits of compliance with anti-osteoporosis therapies. Moreover, the findings of this study indicate that these benefits are more likely to be achieved through use of long-interval parenteral administration.
The optimal effects of anti-osteoporosis medication depend on long-term compliance and persistence; however, there are few studies designed to investigate persistence patterns after switching therapies. Such studies are important, given that more than 30 % of women switch anti-osteoporosis medication at least once [44, 45]. A study in a cohort of postmenopausal women in the UK who initiated treatment with anti-osteoporosis medication between 1995 and 2008 found that switching medication at least once increased persistence, although persistence with the second and third therapies remained suboptimal [44]. Most women switched from high-frequency medications (e.g. daily) to those with a lower frequency of use (e.g. weekly or monthly), which may have contributed to the improved persistence. In an earlier study by the same authors, persistence was lower in women who switched therapy than in those who did not [45]. While the overview of the relationship between compliance and outcomes reported here highlights the importance of medication-taking behaviour in patients with osteoporosis, it would be useful to explore switching patterns and the effects on persistence in future research in Hungary.
In conclusion, persistence and compliance were highest with parenteral anti-osteoporosis therapies with long dosing intervals. Compliance (MPR ≥80 %) was associated with significantly decreased risk of fracture, fracture-related hospitalization and death. Improving compliance through long-interval parenteral administration may improve clinical benefits for patients.
References
Consensus development conference (1993) Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94:646–650
NHIFA MaCD (2009) Osteoporosis miatti csonttörések primer és szekunder prevenciója, “Finanszírozási protokoll háttéranyaga”. Országos Egészségbiztosítási Pénztár Elemzési, Orvosszakértői és Szakmai Ellenőrzési Főosztály. (Primary and secondary prevention of osteoporosis fractures “Financing-protocol background material”. http://site.oep.hu/prot/Az_osteoporosis_terapia_finanszirozasi_protokolljainak_hatteranyaga2.pdf. Accessed Oct 2015
Pentek M, Horvath C, Boncz I, Falusi Z, Toth E, Sebestyen A, Majer I, Brodszky V, Gulacsi L (2008) Epidemiology of osteoporosis related fractures in Hungary from the nationwide health insurance database, 1999-2003. Osteoporos Int 19(2):243–249. doi:10.1007/s00198-007-0453-6
Delmas PD (2002) Treatment of postmenopausal osteoporosis. Lancet 359(9322):2018–2026. doi:10.1016/S0140-6736(02)08827-X
Kanis JA, Johnell O (2005) Requirements for DXA for the management of osteoporosis in Europe. Osteoporos Int 16(3):229–238. doi:10.1007/s00198-004-1811-2
Silverman SL, Gold DT (2008) Compliance and persistence with osteoporosis therapies. Curr Rheumatol Rep 10(2):118–122
Cramer JA, Amonkar MM, Hebborn A, Altman R (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21(9):1453–1460. doi:10.1185/030079905X61875
Huybrechts KF, Ishak KJ, Caro JJ (2006) Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone 38(6):922–928. doi:10.1016/j.bone.2005.10.022
Solomon DH, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, Brookhart MA (2005) Compliance with osteoporosis medications. Arch Int Med 165(20):2414–2419. doi:10.1001/archinte.165.20.2414
McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J (2004) Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas 48(3):271–287. doi:10.1016/j.maturitas.2004.02.005
Lo JC, Pressman AR, Omar MA, Ettinger B (2006) Persistence with weekly alendronate therapy among postmenopausal women. Osteoporos Int 17(6):922–928. doi:10.1007/s00198-006-0085-2
Hadji P, Claus V, Ziller V, Intorcia M, Kostev K, Steinle T (2012) GRAND: the German retrospective cohort analysis on compliance and persistence and the associated risk of fractures in osteoporotic women treated with oral bisphosphonates. Osteoporos Int 23(1):223–231. doi:10.1007/s00198-011-1535-z
Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Strom O (2015) Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int 26(10):2401–2411. doi:10.1007/s00198-015-3253-4
Siris ES, Selby PL, Saag KG, Borgstrom F, Herings RM, Silverman SL (2009) Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med 122(2 Suppl):S3–S13. doi:10.1016/j.amjmed.2008.12.002
Melton LJ 3rd, Gabriel SE, Crowson CS, Tosteson AN, Johnell O, Kanis JA (2003) Cost-equivalence of different osteoporotic fractures. Osteoporos Int 14(5):383–388. doi:10.1007/s00198-003-1385-4
McCloskey E, De Takats D, Orgee J et al (2013) Characteristics associated with non-persistence during daily therapy. Experience from the placebo wing of a community-based clinical trial. J Bone Min Res 20(1):2025
Lamberg L (2000) Sleep disorders, often unrecognized, complicate many physical illnesses. JAMA 284(17):2173–2175
Eraker SA, Kirscht JP, Becker MH (1984) Understanding and improving patient compliance. Ann Int Med 100(2):258–268
Claxton AJ, Cramer J, Pierce C (2001) A systematic review of the associations between dose regimens and medication compliance. Clin Ther 23(8):1296–1310
Diaz Curiel M, Carrasco de la Pena JL, Honorato Perez J, Perez Cano R, Rapado A, Ruiz Martinez I (1997) Study of bone mineral density in lumbar spine and femoral neck in a Spanish population. Multicentre Research Project on Osteoporosis. Osteoporos Int 7(1):59–64
Unson CG, Siccion E, Gaztambide J, Gaztambide S, Mahoney Trella P, Prestwood K (2003) Nonadherence and osteoporosis treatment preferences of older women: a qualitative study. J Women’s Health 12(10):1037–1045. doi:10.1089/154099903322643965
Nevitt MC, Ettinger B, Black DM, Stone K, Jamal SA, Ensrud K, Segal M, Genant HK, Cummings SR (1998) The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Int Med 128(10):793–800
van den Boogaard CH, Breekveldt-Postma NS, Borggreve SE, Goettsch WG, Herings RM (2006) Persistent bisphosphonate use and the risk of osteoporotic fractures in clinical practice: a database analysis study. Curr Med Res Opin 22(9):1757–1764. doi:10.1185/030079906X132370
Lakatos PT (2013) Persistence & compliance to treatment for osteoporosis in postmenopausal women in Hungary: a retrospective cohort study. Value Health 16:A567–A568
Hadji P, Papaioannou N, Gielen E, Feudjo Tepie M, Zhang E, Frieling I, Geusens P, Makras P, Resch H, Moller G, Kalouche-Khalil L, Fahrleitner-Pammer A (2015) Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12 month results from a European non-interventional study. Osteoporos Int 26:2479–2489. doi:10.1007/s00198-015-3164-4
Silverman SL, Siris E, Kendler DL, Belazi D, Brown JP, Gold DT, Lewiecki EM, Papaioannou A, Simonelli C, Ferreira I, Balasubramanian A, Dakin P, Ho P, Siddhanti S, Stolshek B, Recknor C (2015) Persistence at 12 months with denosumab in postmenopausal women with osteoporosis: interim results from a prospective observational study. Osteoporos Int 26:361–372
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:136. doi:10.1007/s11657-013-0136-1
Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81(8):1013–1022. doi:10.4065/81.8.1013
Hoer A, Seidlitz C, Gothe H, Schiffhorst G, Olson M, Hadji P, Haussler B (2009) Influence on persistence and adherence with oral bisphosphonates on fracture rates in osteoporosis. Patient Prefer Adher 3:25–30
LaFleur J, McAdam-Marx C, Kirkness C, Brixner DI (2008) Clinical risk factors for fracture in postmenopausal osteoporotic women: a review of the recent literature. Ann Pharmacother 42(3):375–386. doi:10.1345/aph.1K203
National Health Insurance Fund of Hungary (Hungarian acronym: OEP) http://www.oep.hu. Accessed Nov 2014
Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, Cummings SR, Hue TF, Lippuner K, Lakatos P, Leung PC, Man Z, Martinez RL, Tan M, Ruzycky ME, Su G, Eastell R (2012) The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 27(2):243–254. doi:10.1002/jbmr.1494
Reid IR, Black DM, Eastell R, Bucci-Rechtweg C, Su G, Hue TF, Mesenbrink P, Lyles KW, Boonen S, Trial HPF, Committees HRFTS (2013) Reduction in the risk of clinical fractures after a single dose of zoledronic Acid 5 milligrams. J Clin Endocrinol Metab 98(2):557–563. doi:10.1210/jc.2012-2868
Eli Lilly (2014) Forsteo (teriparatide) Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000425/WC500027994.pdf. Accessed Sep 2015
Brown JP, Roux C, Torring O, Ho PR, Beck Jensen JE, Gilchrist N, Recknor C, Austin M, Wang A, Grauer A, Wagman RB (2013) Discontinuation of denosumab and associated fracture incidence: analysis from the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial. J Bone Miner Res 28(4):746–752. doi:10.1002/jbmr.1808
Confavreux CB, Canoui-Poitrine F, Schott AM, Ambrosi V, Tainturier V, Chapurlat RD (2012) Persistence at 1 year of oral antiosteoporotic drugs: a prospective study in a comprehensive health insurance database. Eur J Endocrinol 166(4):735–741. doi:10.1530/EJE-11-0959
Landfeldt E, Strom O, Robbins S, Borgstrom F (2012) Adherence to treatment of primary osteoporosis and its association to fractures–the Swedish Adherence Register Analysis (SARA). Osteoporos Int 23(2):433–443. doi:10.1007/s00198-011-1549-6
Briesacher BA, Andrade SE, Yood RA, Kahler KH (2007) Consequences of poor compliance with bisphosphonates. Bone 41(5):882–887. doi:10.1016/j.bone.2007.07.009
Penning-van Beest FJ, Erkens JA, Olson M, Herings RM (2008) Loss of treatment benefit due to low compliance with bisphosphonate therapy. Osteoporos Int 19(4):511–517. doi:10.1007/s00198-007-0466-1
Bliuc D, Nguyen ND, Nguyen TV, Eisman JA, Center JR (2013) Compound risk of high mortality following osteoporotic fracture and refracture in elderly women and men. J Bone Miner Res 28(11):2317–2324. doi:10.1002/jbmr.1968
International Osteoporosis Foundation Fracture risk map. http://www.iofbonehealth.org/facts-and-statistics/frax-map. Accessed Oct 2015
van Mierlo T, Fournier R, Ingham M (2015) Targeting medication non-adherence behavior in selected autoimmune diseases: a systematic approach to digital health program development. PLoS One 10(6):e0129364. doi:10.1371/journal.pone.0129364
Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M (2007) A checklist for medication compliance and persistence studies using retrospective databases. Value Health 10(1):3–12. doi:10.1111/j.1524-4733.2006.00139.x
Li L, Roddam A, Ferguson S, Feudjo-Tepie M, Taylor A, Jick S (2014) Switch patterns of osteoporosis medication and its impact on persistence among postmenopausal women in the U.K. General Practice Research Database. Menopause 21(10):1106–1113. doi:10.1097/GME.0000000000000214
Li L, Roddam A, Gitlin M, Taylor A, Shepherd S, Shearer A, Jick S (2012) Persistence with osteoporosis medications among postmenopausal women in the UK General Practice Research Database. Menopause 19(1):33–40. doi:10.1097/gme.0b013e318221bacd
Acknowledgments
Statistical support was provided by Pál Rakonczai and Tamás Balázs of HealthWare Consulting Ltd (funded by Amgen [Europe] GmbH). Editing support was provided by Claire Desborough, of Amgen (Europe) GmbH, and Oxford PharmaGenesis (funded by Amgen [Europe] GmbH).
Author Contributors
PL designed the study, interpreted the data and prepared early drafts of the paper. PL is guarantor. IT and IM interpreted the data. ET designed the study, and acquired, analysed and interpreted the data. CZ and ZL acquired, analysed and interpreted the data. EP and MI designed the study and interpreted the data. All authors revised the paper critically for intellectual content and approved the final version. All authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly revised.
Funding
The study was funded by Amgen (Europe) GmbH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
PL, IT and IM have received consulting, research and speaker fees and grants from many companies with drugs for bone diseases, including Amgen. ET was an employee of Healthware Tanácsadó Kft when the study was conducted and has been in employment at UCB Biopharma Sprl as a Senior Manager Health Economics & Outcomes Research from December 2013. ZL and CZ are/were employees of Healthware Ltd and conducted this research under contract to Amgen. MI and EP are employees and shareholders of Amgen.
Human and Animal Rights and Informed Consent
For this type of study formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lakatos, P., Takács, I., Marton, I. et al. A Retrospective Longitudinal Database Study of Persistence and Compliance with Treatment of Osteoporosis in Hungary. Calcif Tissue Int 98, 215–225 (2016). https://doi.org/10.1007/s00223-015-0082-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-0082-6