Abstract
Exercise has a profound impact on one’s health, and it is becoming increasingly accepted that exercise also benefits cognitive functioning. Yet, the neural mechanism for which cognitive enhancement occurs is less understood. Therefore, the purpose of our study was to experimentally test whether an acute exercise activity was able to increase theta power and behavioral performance during an executive functioning attentional control task. Participants were randomly assigned to either a stationary-bike exercise or a resting control condition. Thereafter, they completed the Eriksen flanker task, and most participants completed this while EEG data were recorded. From the flanker task data, we demonstrated an interaction effect from both accuracy and reaction time measurements. Importantly, the exercise group was more accurate than the control group in incongruent trials. From the EEG data, theta power was overall higher in the exercise group, especially during the congruent trials, compared to controls. Our results add to the limited but growing body of research that suggests acute exercise produces a general increase in theta power, which in turn may play a role in enhancing cognitive performance. These results, combined with previous research, could have widespread implications in multiple settings such as in the investigation of a biomarker of physical fitness, neurorehabilitation, and in education.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that exercise has profound benefits to the body, and recently there has been a surge of interest in understanding how exercise may also benefit the mind and brain. Historically, it is well documented that chronic aerobic exercise can increase cognitive performance (Kramer et al. 1999). For example, research shows that adults who are more physically active perform better during executive function tasks than those who are inactive (Archer 2012; Guiney and Machado 2013; Luque-Casado et al. 2020). Regular exercise is now generally accepted as neuroprotective and even therapeutic in reducing the risk of dementia and general cognitive decline in older adults (Ahlskog et al. 2011; Colcombe et al. 2004; Cotman and Engesser-Cesar 2002; Marques-Aleixo et al. 2021). More recently, there is evidence that in addition to chronic exercise, acute exercise may also increase cognitive abilities (Chang et al 2015, 2017; Ludyga et al. 2016; Waters et al. 2020; Won et al. 2019). Yet, our understanding of what types of cognitive abilities as well as the underlying mechanisms of this enhancement from acute exercise is more limited. As brain-based biomarkers of fitness continue to be pursued and attempts of acute exercise to be used for neuro-rehabilitation are increasingly taking place, our understanding of this connection needs to be better elucidated.
Executive functioning, which encompasses our most complex cognitive abilities, continues to develop throughout young adulthood with the maturation of the prefrontal cortex. These executive abilities largely involve those related to social and goal-oriented behaviors. For example, working memory, cognitive flexibility, inhibitory control, and the ability to plan, make decisions and ascertain potential consequences are all examples of executive functions (Barkley 1997, 2012; Diamond 2013; Lezak 1995; Norman and Shallice 1986). Poor development of executive function may be associated with increased risk taking, maladaptive emotional regulation, negative decision-making, reduced regulation of inhibitory control, and decreased selective attention (Moeller et al. 2001; Martin and Potts 2009; Moustafa et al. 2017; Sweitzer et al. 2008; Sudikoff et al. 2015; Leung et al. 2009). A recent research direction that has gained much interest is the neurocognitive connection between exercise and executive functioning, especially in the domain of attentional control.
Attentional control describes the ability to sustain top-down selectivity of attention and focus on relevant information while ignoring distractors (Eysenck and Derakshan 2011). Attentional control can be assessed with psychological paradigms such as the Eriksen flanker (Eriksen and Eriksen 1974) and Stroop tasks (Stroop 1935). Both tasks involve response competition, selective attention and inhibitory control, which are primary subcomponents of attentional control. The neural mechanisms for attentional control include multiple brain regions, particularly between the frontoparietal connections associated with the executive control network (Petersen and Posner 2012). fMRI research has also identified subcortical involvement during attentional conflict tasks, such as the anterior cingulate cortex (Botvinick et al. 2001; Fan et al. 2003). Although fMRI is a valuable tool for understanding brain regions underlying selective attention, interest has piqued in the field of neuroscience with the utility of electroencephalogram (EEG) and the contributions this method offer in our understanding of neural mechanisms involving executive function.
EEG allows researchers to investigate the online temporal dynamics of large groups of neurons as they fire synchronously in response to either endogenous or exogenous events, which is not possible with fMRI. The electrical activity that is recorded by EEG is assumed to arise from postsynaptic potentials of the cortical pyramidal neurons (Britton et al. 2016), thus providing a direct link between neural activity and cognition. Much of what we know from EEG research has stemmed from event-related potentials (ERPs), which provide valuable information about the latency and amplitude of averaged waveforms associated with cognition. Additionally, frequency-specific oscillations can be quantified using EEG to examine changes in the synchrony of firing neurons relative to an event (Pfurtscheller and Lopes da Silva 1999). Using spectral analyses, the power of these frequencies within specified band ranges is derived and evaluated by increases or decreases in band power. An event-related synchronization (ERS) occurs when there is an increase in band power in response to an event, whereas an event-related desynchronization (ERD) is described when a decrease is observed (Pfurtscheller and Lopes da Silva 1999).
Specific to exercise and EEG, ERPs have been more commonly examined than spectral power band changes. P3 in general is one of the most widely examined event-related potential components involved in our understanding of attention and higher-level cognitive function. P3 is marked by its positive peak approximately 300 ms after an endogenous reaction to a stimulus. In the exercise literature, it is commonly reported that P3 waveform amplitudes increase in response to exercise (Luque-Casado et al. 2016, 2020). Higher-level attentional control tasks result in increased P3 amplitudes in response to acute exercise as well, such as during the Stroop, Eriksen flanker, and go/no-go tasks (Chang et al. 2017; Drollette et al. 2014; Kamijo et al. 2009). Interestingly, recent evidence suggests ERPs may be affected or possibly even derived from oscillatory frequency dynamics (Klimesch et al. 2007), and P3 in particular may stem from frontal theta waves (Luque-Casado et al. 2020). This presents the need for an increased and ongoing investigation of oscillatory-based analyses, and especially pertaining to theta.
Research suggests that frontal midline theta may be involved in executive function (Begus and Bonawitz 2020; Klimesch 1999). Theta in the human brain has been examined within the frequency range commonly found between 4 and 8 Hz, although there is research to suggest that the upper range cutoff may be closer to 7.5 Hz (Klimesch 1999). However, this cutoff between the upper band of theta with the next higher-frequency band, specifically alpha waves (8–12 Hz), may not be exact and instead there may be more overlap and individual variability than previously assumed (Klimesch 1999). Despite this, theta waves are considered slower waves and are commonly dominant during meditative and sleepy states. They also appear related to tasks involving working memory (Xie et al. 2021; Yamagishi et al. 2008) and executive attention (Clayton et al. 2015). Research in attention has led some to interpret theta activity as indexing active control of attention and cognitive effort, as it has been suggested that theta oscillations reflect the individual's allocation of attention and investment of cognitive effort in what is most relevant to their environment. Theta may also reflect active learning (Begus and Bonawitz 2020), which may be in part related to its noted connection with the hippocampus (Klimesch 1999). Increased frontal theta power has been demonstrated post-executive function training in those with severe mental disorders, suggesting a neural therapeutic effect from power changes in this frequency band (Best et al. 2019).
Despite the relevance of theta with cognition, this relationship has only been minimally investigated in the context of exercise. The scarce literature that exists has either reported no change (Chaire et al. 2020) or an overall increase in theta band spectral power (Lardon and Polich 1996; Luque-Casado et al. 2020). Possibly, the contradiction in results between these studies is due to critical methodological differences. Specifically, Chaire et al. (2020) examined theta power during a working memory and visual search task before and after a 4-month exercise program in a sedentary group of participants. Increased theta power on the other hand was shown during resting state in those who intensively exercise (e.g., for competitive training) on a regular basis compared to those who only exercise some (Lardon and Polich 1996). Similarly, Luque-Casado et al. (2020) found increased theta power in those defined as a highly fit group compared to those less fit (less than 2 h per week of exercise) during a sustained attention task, consequently suggesting theta power as an underlying neural connection in this group’s enhanced sustained attention. Yet, this suggestion is still weak as the limited research isn’t conclusive regarding the causal relationship between exercise and theta power. Further, it is unknown whether theta power, if enhanced by exercise, is generalizable to both acute and chronic exercise, or if the association with enhanced cognitive performance is broadly related to basic information processing or more specifically encompasses executive attention (e.g., attentional control).
The purpose of our study is to address some of these gaps and extend upon the above literature by conducting an experimental design to test whether an acute exercise activity results in increased theta power and behavioral performance during an executive function attentional control task, specifically the flanker task. If theta is enhanced by acute exercise, this could suggest a more generic underlying neural connection between the effects of exercise that is not limited to highly fit individuals. Given the previously mentioned research, we hypothesized that theta power would be higher in the exercise group compared to the control group, and that this would be highest during the incongruent trials and thus reflecting specificity to executive functioning. As the flanker task is used to measure executive functioning, specifically attentional control during the incongruent trials, the second goal of our study was to examine behavioral performance between groups during this task by comparing accuracy and reaction time to the congruent and incongruent trials. Again based on prior research, we hypothesized that accuracy would be greater and that reaction time would be faster in the exercise group to incongruent trials, reflecting better attentional control performance.
Method
Participants
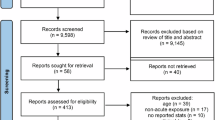
A G*Power analysis (Faul et al. 2009) conducted prior to data collection indicated 42 participants were needed for sufficient power (α = 0.05, 1 – β = 0.80, partial η2 = 0.09) to reach a medium–large effect size (Cohen 1988; Field 2018) for a mixed model ANOVA testing for a within–between interaction using SPSS. Participants were students enrolled at a small public liberal arts university in the southeast USA. Exclusion criteria for enrollment pertained to safety precautions involving exercise and being under the influence of alcohol or psychoactive drugs at the time of participation. Specific to EEG, participants were excluded from this portion of the experiment if they suffered a traumatic brain injury within the past year (due to the timeline of neural plasticity within this time frame) or had a diagnosis of epilepsy, which excluded two participants from this portion of the study. We did not adopt any other exclusionary criteria, such as brain injury prior to one year or psychological disorders (e.g., major depressive disorder or attention-deficit/hyperactivity disorder), to allow our data to be the most generalizable to the population. Those in our sample with prior brain injury were from concussion, and the proportion of those in our study with prior concussion or psychological disorder matched the proportion of that found within the general population of the USA. Fifty-nine participants in total were recruited for this study (see Table 1), from which clean EEG data from 45 participants were further analyzed. All participants were provided a letter of invitation and consented to participate per IRB approval from the University of South Carolina.
Materials
The physical activity readiness questionnaire (PAR-Q+ ; Warburton et al. 2021)
The PAR-Q + is a standardized screening form to assess the possible health risks prior to beginning an exercise activity, such as cardiovascular risk. All participants in both groups (for consistency) completed the PAR-Q+ before beginning the experiment to assess for exclusionary criteria. No participants were found to be at risk from exercising.
Demographic survey
Participants completed a self-report demographic survey to collect data for several reasons. The first was to acquire data about the group for generalizability purposes (sex, ethnicity/race, diagnosis of psychological disorders, history of brain trauma/neurological disorders). If brain trauma was noted, further inquiry regarding age and type occurred. Again, participants were excluded if trauma was within the past year. Last, we collected data about age and weekly exercise frequency to control for these known influences on attentional control and electrophysiological activity (Klimesch 1999; Ludyga et al. 2016; Reuters et al. 2019).
Exercise condition
Participants were randomly assigned to either the exercise or control condition. When participants in the control condition arrived, they were allowed to simply rest before the EEG portion of the experiment began. All participants were instructed to not exercise for 24 h prior to their arrival. Participants that self-reported exercising within this time frame were rescheduled.
The exercise group completed 30 min of a moderate–vigorous level intensity exercise on a Monark Ergomedic 328 stationary cycle ergometer (Monark Exercise AB, Vansbro, Sweden). Participants had to reach and maintain at least a moderate-intensity activity level for a designated 30 min, as research shows that moderate to vigorous exercise over 20 min in duration is most effective at increasing cognitive functioning after an acute session (see Erickson et al. 2019). Moderate activity was obtained by reaching a heart rate at 64–76% of the participant’s age-predicted maximal heart rate (APMHR), which was calculated using the formula 220-age in years (ACSM 2021; Liguori 2022). Heart rate was monitored using a Polar H1 heart rate sensor (Polar Electro, Kempele, Finland). The intensity was set individually by adjusting the pedal revolutions and resistance applied to the flywheel to achieve a HR in the moderate-to-low-vigorous intensity range.
Participants were fitted with the heart rate monitor, with the sensor positioned on the chest at the xiphoid process, and resting HR was recorded. The seat of the cycle ergometer was set to a height at which the knee was at a slight bend at the bottom of the pedal stroke with the ankle in a neutral position. The exercise session began by pedaling at a self-selected cadence and the resistance was adjusted until the HR was within 64–76% of the individually calculated APMHR. Once this level was reached, the timer was set for 30 min and resistance was adjusted throughout to maintain a consistent HR for the duration of the exercise. At the end of the 30 min, the resistance was lowered so the participants could cool down and return to a resting heart rate before moving into the EEG portion of the experiment. Water was provided throughout the session, and during the cool-down period a fan in the room allowed participants to physically cool off and a towel was used to dry any perspiration from the skin. At the completion of the exercise activity, participants were then moved to the EEG Lab located in the neighboring room, where they were prepped for the EEG recording.
EEG procedures
If in the experimental group, the participant’s head was sized prior to the exercise session. The cap was then prepped by a research assistant during the exercise session to minimize the time delay between exercise and the flanker task/EEG recording. Putting the cap on the participant’s head and gelling of the electrodes occurred post exercise, after the participant was completely dried off and cooled down. The duration between when the participant ended their exercise session to when acceptable impedance levels were reached was approximately 20 min. If we were unable to reach acceptable impedance levels and begin the flanker task within 25 min post exercise, then the EEG portion was aborted and we proceeded immediately with the flanker task (n = 5). It was still expected that the exercise session would have an effect on executive functioning within this time duration, as the decay of this transient effect is moderated by both exercise intensity and duration. Specifically, these effects are still shown after 20 min post-exercise when the exercise is at least 20 min in duration, and at least moderate in intensity (Erickson et al. 2019).
After the cap size was selected, 32 active Ag/AgCl electrodes were placed in the EEG cap (EasyCap, Brain Products GmbH, Munich, Germany) using the International 10/20 system sites (Klem et al. 1999), and then prepared with high viscosity gel when placed on the participant’s head to reach impedance levels below fifteen kΩ (M = 7.84, SD = 4.57). Two horizontal and two vertical electrooculograms (EOGs) were placed on cleaned and abraded skin on either side of the right and left canthi, and above and below the right eye to record horizontal movement and eye blinks, respectively, for later artifact rejection. FPz was used as the ground electrode, and data were referenced online to the linked average mastoid electrodes.
Data were continuously recorded from all electrodes at a sample rate of 500 Hz using BrainVision PyCorder and no predetermined online filtering (although the ActiChamp amplifier is automatically preset with an anti-aliasing filter at ¼ the sampling rate). Online triggers indicating the onset of each trial were sent from the presentation computer to PyCorder Recording Software via a parallel port connection. To reduce environmental noise, participants sat 143 cm away from the monitor (Clayson and Larson 2012; Luck and Kappenman 2012), and the overhead DC lights were dimmed to the same brightness for each participant. The recording occurred at room temperature at or below 72 °F for each participant.
EEG analyses
Data were analyzed offline using BrainVision Analyzer (Brain Products GmbH, Germany; www.brainproducts.com). First, bipolar channels were created for later identification of vertical and horizontal eye movements. Channels were individually examined and four participants each demonstrated a bad channel. These four channels were interpolated (Oz, O3, T8, and P8) using the triangulation method. To account for non-task-specific frequencies, a Butterworth zero-phase band-pass filter was applied at 0.1–30 Hz cut-offs. For both task and baseline, brainwaves from each trial were segmented surrounding each respective trial type. Baseline segments included the data 300–0 ms prior to stimulus onset, and trial segments included data between 0–1200 ms post-onset. This resulted in 112 segments for congruent and incongruent trials each and 112 segments for each corresponding condition’s baseline. Ocular correction was applied (Gratton et al. 1983), and then data were inspected and bad segments manually removed during the artifact rejection stage using a semi-automated process beginning with a cut-off of 150 μV amplitude (Smulders et al. 2018). Theta spectral power (μV2) between the 4.0–8.0 Hz range, following in line with recent exercise research by Luque-Casado et al. (2020), was calculated on average for 85.71 + − 25.60 remaining segments from each condition using a Fast Fourier Transformation (FFT) with a 10% Hanning window. These segments were then averaged and power values from baseline and task for incongruent and congruent segments were extracted from anterior electrodes F3, Fz, and F4 per participant. The ratio between task and baseline was calculated for each participant at each of these electrode sites, which was then averaged and finally entered into SPSS v. 28 for further statistical analyses.
Eriksen flanker task (Eriksen and Eriksen 1974)
During the EEG portion of the experiment, all participants completed the Eriksen flanker task (note: some participants did not have EEG data collected, see Results section below). Again, this task was used to measure attentional control, and specifically response inhibition. The task was presented on a 29-inch LCD Dell monitor using Presentation Software. At the onset of each trial, a gray fixation point was presented in the center of the screen for 150 ms. The target screen with flankers then appeared for 200 ms. The goal of this task was to indicate if the central target arrow pointed to the left ( <) or to the right ( >) by pressing a left- or right-arrow key on a keyboard. This target arrow was accompanied by two flanking stimuli on each side that were either congruent (i.e., < < < < < or > > > > >) or incongruent (i.e., < < > < < or > > < > >).
Each flanker and target stimuli were aligned horizontally and spaced 0.20 degrees of visual angle apart (center to center) and presented directly in the participant's line of view on the monitor at 0 degrees visual angle using an electronically adjustable table. Flankers and the target arrow were presented in black font on a white background. Participants were instructed to respond as quickly and as accurately as possible (Clayson and Larson 2012). A blank screen appeared after the stimuli and remained until the participant made a response. Participants used their right hand to respond on the keyboard and selected the left arrow with their index finger and the right arrow with their middle finger. To decrease neural habituation as well as reduce the predictability of stimulus onset, an inter-stimulus-interval jitter between 1300 and 1700 ms (sampled randomly) was applied immediately following the participant’s response (Kóbor et al. 2014; Clayson and Larson 2012).
Participants completed a practice block of 10 trials before they began the actual task. When the participant completed these practice trials, the examiner was present to check for comprehension of the task directions (Clayson and Larson 2012). For the actual task, participants completed eight blocks of testing. Each block contained 28 trials, with 7 right and left congruent trials and 7 right and left incongruent trials each all randomly presented within the block, resulting in 224 total trials per experimental session. A self-paced rest period occurred after each block in which participants could relax, blink, and/or stretch for as long as they needed before proceeding, following in line with EEG best practices suggested by Kappenman and Luck (2016). Online, Presentation calculated the error rate of each block. If less than10% errors were made, the Presentation software prompted the participant to speed up on the upcoming block. If more than 20% errors were made, the participant was prompted to slow down for the subsequent block. Error rates were not shown or indicated to participants other than these prompts, which were displayed on the computer screen during this rest period. The task itself took approximately 7 min to complete, not including the self-paced break between the blocks.
Hypothesis testing
Our primary analyses incorporated mixed model ANCOVAs. In these models, group (exercise or control) was the between-subjects independent variable, task (congruent and incongruent) was the within-subjects independent variable, and age and exercise frequency were covariates. Three primary models were conducted to assess the main and interaction effects for each dependent variable, specifically reaction time, accuracy, and theta power. Effect sizes (e.g., small, medium, large) are reported for partial eta2 using conventional effect size cut-offs (Cohen 1988; Field 2018).
Results
All 59 participants completed the flanker task and attempted the EEG. From this group, EEG data was not included in the final analyses for the following reasons: five participants were unable to complete the EEG portion due to inability to access the scalp well enough to obtain acceptable impedance levels, seven participants were removed due to excessive artifacts in the data (four that excessively blinked, and three with excessive channel noise), and two more were removed due to experimenter/technical error (incorrect configuration file loaded). The final EEG data set consisted of data from 45 participants (exercise = 24, control = 21). The demographics for our full sample are displayed in Table 1.
For participants in the exercise condition, pedal revolutions, resistance, and heart rate was recorded every 10 min after they reached a HR within the target HR range, which began the 30-min exercise session. The mean HR (beats*min−1) and power output (W) were used to quantify the intensity. See Table 2 for descriptive data.
Flanker task
Thirty participants from the exercise condition and 28 from the control condition completed the flanker task (from which 24 in the exercise group and 21 from the control group also completed while EEG data was simultaneously collected). Data were also checked for parametric assumptions and extreme outliers. Data met these assumptions and therefore statistical models can be generalized. One extreme outlier beyond a z-score of − 3.5 was identified from the incongruent accuracy score and removed from further analyses.
In the flanker task, responses to incongruent trials should be slower and less accurate when compared to congruent trials. This is referred to as the flanker compatibility effect (Ridderinkhof et al 1995; Servant and Logan 2019) and is due to the effortful inhibition of response competition triggered by the incongruent flankers. To confirm our data followed this effect, paired sample t-tests were conducted to compare the mean difference between congruent and incongruent trials for both accuracy and reaction time. Accuracy was significantly higher in the congruent trials (M = 96.06, SD = 6.18) compared to the incongruent trials (M = 80.66, SD = 15.71), t(57) = 8.63, p < 0.001, d = 1.13. Reaction time was significantly faster during the congruent trials (M = 384 ms, SD = 62.15) compared to the incongruent trials (M = 439 ms, SD = 68.57), t(57) = 14.29, p < 0.001, d = 1.86. Thus, our behavioral data demonstrated the expected flanker effect.
Hypothesis testing: flanker task accuracy and reaction time between groups
The first goal of our study was to examine the differences between accuracy and reaction time between congruent and incongruent flanker trials. A repeated-measures mixed model ANCOVA was conducted to test the main effects and interaction between groups and trial type. In this model, group (control vs. exercise) was the between-subjects variable, and mean accuracy to the congruent and incongruent trials was the within-subjects variable (task). Again, age and weekly exercise frequency were included in the model as covariates to control for their known influence on the dependent variables. This model supported our hypothesis. Specifically, our model demonstrated a significant medium-large interaction effect (see Fig. 1) between task and group, F(1, 54) = 4.75, p = 0.03, partial η2 = 0.08. This interaction was driven by the incongruent trials, as post hoc least significant difference (LSD) tests showed a medium effect of higher accuracy in the exercise group than in the control group, t(57) = 1.97, p = 0.05, partial η2 = 0.07. There was not a significant difference between groups for the congruent trials, t(56) = 0.23, p = 0.82, partial η2 < 0.01, as these averages were fairly equal (see Table 3). From the ANCOVA, there was also a large significant main effect for task, F(1, 54) = 13.02, p < 0.001, partial η2 = 0.19. There was not a main effect for group, F(1, 54) = 2.66, p = 0.11, partial η2 = 0.05, as accuracy means between groups were similar for the congruent trials. For our covariates, age had a medium effect on accuracy, F(1, 54) = 3.86, p = 0.06, partial η2 = 0.07, which was also driven by the incongruent trials as age had an effect on these means (p = 0.04) but not congruent trials (p = 0.34). Exercise frequency was not related to accuracy, p = 0.94.
Reaction time was examined for an interaction effect in a separate ANCOVA, which also showed a significant medium-large interaction effect between task and group, F(1, 54) = 4.72, p = 0.03, partial η2 = 0.08 (see Fig. 2). This interaction appeared to be driven by task and not group, as there was a significant main effect for task, F(1, 54) = 5.07, p = 0.03, partial η2 = 0.08 but not for group, F(1, 54) = 0.31, p = 0.58, partial η2 = 0.01. Group differences from LSD tests within each task type did not emerge either for the incongruent, t(57) = 0.97, p = 0.34, partial η2 = 0.02, or congruent trials, t(57) = 0.08, p = 0.94, partial η2 < 0.01. The significant interaction appears to have been driven by the within-groups difference between congruent and incongruent trials, as the mean difference between the control group was − 62.54, p < 0.001 95% CI [52.12, 72.96], and − 46.37 for the exercise group, p < 0.001, 95% CI [35.76, 56.97]. Neither covariate had a significant influence on reaction time (age p = 0.47; exercise p = 0.31). See Table 3 for descriptive statistics from this model.
Hypothesis testing: EEG data
From the original sample, EEG data was collected from 24 participants in the exercise condition and 21 in the control condition. Our EEG analysis aimed to examine the influence of acute exercise on theta power during the flanker task. A repeated-measures mixed model ANCOVA was conducted to examine the interaction between group (exercise and control) and task (incongruent and congruent) on theta power over frontal electrodes. This model did not show a significant interaction effect of task x group, F(1, 41) = 0.06, p = 0.80, partial η2 < 0.01. There was however a significant medium-large overall main effect for task (see Fig. 3), F(1, 41) = 4.29, p = 0.045, partial η2 = 0.10. Also, a medium near-significant effect between-group difference emerged, F(1, 41) = 3.26, p = 0.08, partial η2 = 0.07. Examining this further, the exercise group had significantly greater theta power to the congruent trials (Madj = 0.34, SE = 0.02) than the control group (Madj = 0.29, SE = 0.02), F(1, 41) = 4.27, p = 0.045, partial η2 = 0.09. There was however no difference in theta power to incongruent trials between the exercise (Madj = 0.39, SE = 0.02) and control groups (Madj = 0.36, SE = 0.02), F(1, 41) = 1.89, p = 0.18, partial η2 = 0.04. Examining the covariates, exercise frequency had a significant effect on theta between groups, F(1, 41) = 9.44, p = 0.004, partial η2 = 0.19, but age did not F(1, 41) = 0.19, p = 0.66, partial η2 < 0.01
Exploratory alpha analysis
Although we were specifically interested in theta power changes from exercise, these reported differences could have resulted from a more generic increase in neural activity post-exercise across other frequency bands and brain regions (Ciria et al. 2018, 2019; Crabbe and Dishman 2004). Alpha (8–12 Hz) is another frequency band that is commonly examined as a proxy for attention and executive functions. Unlike theta, event-related desynchronization (ERD) of alpha occurs during engagement of the attentional networks (Pfurtscheller and Lopes da Silva 1999), thus decreased alpha power typically represents increased neural engagement. For the purpose of consistency to compare with theta power, an FFT was applied and alpha was extracted and normalized by calculating task over baseline using the same procedures we used to calculate theta, and then was exported between 8 and 12 Hz from the same electrodes. A repeated-measures ANCOVA did not indicate an interaction effect between task and group, F(1, 41) = 0.38, p = 0.54, partial η2 = 0.01. There also wasn’t a within-groups difference in alpha for task, F(1, 41) = 0.02, p = 0.90, partial η2 < 0.01, or a between-groups difference in alpha, F(1, 41) = 1.61, p = 0.21, partial η2 = 0.04.
Discussion
Despite widespread evidence that exercise may benefit cognitive performance, the neurophysiological underpinnings of this benefit are still not fully understood and questions remain regarding the specificity of this association. We aimed to address some of these gaps by experimentally manipulating an acute exercise and no-exercise condition between participants to test for changes in theta power and behavioral performance during an executive function attentional control task. There are several key findings from our study. First, we demonstrated an interaction effect from the flanker task with both accuracy and reaction time measurements. Importantly, the exercise group was more accurate than the control group to incongruent trials. Last, theta power was higher in the exercise group, particularly during the congruent trials, compared to controls.
Increased accuracy performance during the flanker task in the exercise group provides further evidence for a possible causal effect exercise may have on executive functioning. Specifically, we demonstrate this effect with increased inhibitory control in our exercise group, which is indicated by greater accuracy to the incongruent trials when compared to the control group. This effect on the flanker task in young adults does not corroborate previous research (Ciria et al. 2018). Specifically, Ciria et al. (2018) did not report differences in accuracy to incongruent trials between groups who either engaged in a light- or moderate-intensity exercise manipulation. A possible explanation for the difference in results between our studies is that both groups in Ciria et al. (2018) engaged in an acute bout of exercise, which was differentiated by intensity level. Examining this closer, the average accuracy in each group was similar to the accuracy level of our exercise group, and about 10% more accurate than our control (non-exercise) group. It is possible therefore that a cognitive benefit could be produced regardless of exercise intensity. This is supported by related research, albeit in older individuals. For example, there is recent evidence that even light exercise, operationally defined as insufficiently active by engaging in less than 599 metabolic equivalent (MET) minutes per week, can reduce the risk of dementia in older adults (Yoon et al. 2021). Additionally, there are multiple reports of increased performance to incongruent trials during the flanker task within varying degrees of exercise contexts, such as in those who are physically fit compared to those who are not (Colcombe et al. 2004), after exercise training programs (Liu-Ambrose et al. 2012), and also after acute moderate-exercise activities in older adults (Kamijo et al. 2009; Won et al. 2019). Relative to our sample, other cognitive benefits have been reported in young adults after an acute exercise session, such as increased short-term memory performance (Waters et al. 2020). Taken together, the surrounding literature supports an increase in executive functioning, at least temporarily, as a result of exercise.
As expected, overall reaction time was shorter for congruent trials compared to incongruent trials in both groups. Interestingly though, reaction time was less affected by exercise specifically. Although there was an interaction effect between groups and trial types, no between group differences emerged. Rather, this interaction reflected a general trend of shorter responses to congruent trials in both groups, and slightly faster responses to incongruent trials in the exercise group compared to the control group, albeit insignificantly different. Previous research has previously found though that exercise can shorten reaction times to incongruent stimuli (Chang et al. 2015). However, a reaction time effect may be contingent on multiple factors, as a recent meta-analysis examining response speed during executive functioning tasks after exercise noted this is not a consistent effect (Chang et al. 2014). The authors from this meta-analysis suggests that increased speed of responding could be due to an inverted-U hypothesis of performance based on intensity level of the exercise. Specifically, performance peaks mostly during moderate-level exercise, but is less during light and vigorous levels. Given that our average age-predicted HRmax level was in the vigorous activity range, we may have demonstrated support for this hypothesis. Chang and colleagues also indicated more recently that reaction time effects may be negligible if the exercise session is less or greater than 20 min in duration (Chang et al. 2019).
Results from the EEG data indicated that the exercise group had increased theta power compared to the control group. Interestingly, disparity between groups was greatest in the congruent trials. This result supports the notion that exercise may produce a more generalized increase in arousal of the nervous system, which may globally impact neural oscillatory patterns (Ciria et al. 2018). Our results support this generalized response particularly regarding theta, as we did not see differences in alpha power. Although we are unable to make comparisons between our neural and behavioral data due to different sample sizes, increased theta power during congruent trials seen in our study could bridge with previous research in which an overall general facilitation of cognitive functions and neural activity was found. For example, Chang and colleagues found in several studies that acute exercise increased performance in both congruent and incongruent trials in a Stroop task (Change et al. 2014, 2015, 2017), along with increased neural recruitment involved in allocation of attentional resources (Change et al. 2015, 2017). Collectively, these studies support a general improvement hypothesis in which exercise appears to benefit neural functioning related to cognition more broadly, rather than a selective improvement hypothesis where increased theta is specific to only executive function.
Collectively, our results add to the limited but growing body of research that suggests that even an acute bout of exercise can result in more accurate executive functioning performance. This enhancement may be related to an underlying mechanism involving a general increase in theta power. These results, combined with previous research, have widespread implications in multiple settings. In the form of neurorehabilitation, physical and cognitive exercise coupled together could potentially compound the beneficial effect of therapy following stroke (see Han et al. 2017; Pang et al. 2018). In another related example, adults with mild cognitive impairment (MCI) assigned to either a physical or cognitive therapy condition were both noted to have an increase in memory performance after treatment (Fonte et al. 2019). In contrast, patients in this study with Alzheimer’s disease and MCI, who did not engage in either physical or cognitive therapy, demonstrated an overall decline in mental functioning over the span of the study’s 9-month duration. In the education setting, exercise may be an important part of the curriculum for the purpose of enhanced learning. This should be further examined as many schools in the USA have continued a trend of replacing movement-based activities, both structured (e.g., physical education) and unstructured (e.g., playground recess), with increased formal lessons and academic curriculum (Society of Health and Physical Educators; Youth Sports Trust). This trend is taking place despite reports by the Centers for Disease Control (CDC) and the 2018 Physical Guidelines for Americans that reduced physical activity can hinder learning. A decrease in youth physical activity has been further exacerbated by the COVID-19 pandemic, as physical activity amongst youth has decreased by nearly half from pre-pandemic levels (Grimes et al. 2022). We suggest that research further investigates the potential consequences of this trend, including the neural consequences of increased sedentary behaviors in children and students in general.
Limitations and future directions
For being an exercise-based study, there was lack of important anthropometrical characteristics and cardiorespiratory fitness level measurements in our sample. For example, we did not collect weight or height information, which limited our ability to compare to previous research as well as account for these possible influences on the data. Additionally, our data on exercise habits were limited to self-report of weekly exercise, and not specific to which type of exercise. This variability in exercise types could have inadvertently confounded our covariate of exercise habits. For example, someone who engages in vigorous high-intensity exercise (e.g., marathon training) six times a week would have been quantified the same as someone who engages in low-intensity (e.g., yoga) exercise six times a week. Because recent evidence suggests that all exercise can result in better health and cognition, we chose to not quantify the difference in exercise types. However, further research would benefit from differentiating exercise habits to better control for them, as well as potentially help address some of the inconsistencies in the literature.
Specific to EEG, a major limitation that needs to be addressed is the decrease in our EEG sample compared to the behavioral sample as a result of several issues (e.g., difficulty in accessing the scalp or obtaining acceptable impedance levels, poor data quality, and experimenter error). Overall, this presents an issue of generalizability in our study as well as an overarching limitation in being able to compare theta activity to the flanker task performance in our participants.
In regard to our interpretation of the data, it is acknowledged that potential confounds may have played a role in the results found. For example, we did not statistically control for exercise-induced changes in our participants, such as body temperature changes or perspiration levels between groups that could have influenced theta power differences observed. Also, we did not complete a pre-exercise flanker task to more accurately determine the change (if present) in response inhibition as a result of acute exercise. Future research would benefit from a baseline measure of theta power and response inhibition performance to directly compare to results post-exercise manipulation. Additionally, there is evidence that this enhancement is temporary (Ciria et al. 2018); therefore, continued research is warranted to examine the duration of cognitive benefits post-acute exercise.
Importantly, several of our results are only marginally significant with medium effect sizes. It is possible that we encountered a Type I error in our results. Therefore, further replication and continuation of this research is warranted before solid conclusions can be made from our data. Overall, we suggest our results be interpreted with caution.
Conclusion
Our results add to the limited but growing body of research that suggests acute exercise produces a general increase in theta power, which was especially observed during congruent trials in our study. This effect may play a role in the enhanced executive function performance seen after exercise. Further examination of this relationship could shed light on the neural connection between exercise and cognition via theta oscillatory dynamics, again since theta power appears important in the context of learning and executive function. Even if this enhancement is only temporary, it remains important in the realm of education and neurorehabilitation, since even a temporary enhancement could still result in permanent learning and neuroplasticity over time. Broadly, these results support the further evaluation of identifying the role theta power may serve as a brain-based biomarker of fitness.
Data availability
All data are available upon request.
References
Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC (2011) Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc 86(9):876–884. https://doi.org/10.4065/mcp.2011.0252
American College of Sports Medicine & Fountaine-Walker C (2021) Acsm’s guidelines for exercise testing and prescription (11ème). Wolters Kluwer, Berlin
Archer T (2012) Influence of physical exercise on traumatic brain injury deficits: Scaffolding effect. Neurotoxic Res 21(4):418–434. https://doi.org/10.1007/s12640-011-9297-0
Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121(1):65–94. https://doi.org/10.1037/0033-2909.121.1.65
Barkley RA (2012) Executive functions: what they are, how they work, and why they evolved. Guilford Press
Begus K, Bonawitz E (2020) The rhythm of learning: Theta oscillations as an index of active learning in infancy. Dev Cognit Neurosci 45:100810. https://doi.org/10.1016/j.dcn.2020.100810
Best MW, Gale D, Tran T, Haque MK, Bowie CR (2019) Brief executive function training for individuals with severe mental illness: effects on EEG synchronization and executive functioning. Schizophr Res 203:32–40. https://doi.org/10.1016/j.schres.2017.08.052
Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001) Conflict monitoring and cognitive control. Psychol Rev 108(3):624–652. https://doi.org/10.1037/0033-295X.108.3.624
Britton JW, Frey LC, Hopp JL et al (2016) Electroencephalography (EEG): an introductory text and atlas of normal and abnormal findings in adults, children, and infants. American Epilepsy Society, Chicago
Chaire A, Becke A, Düzel E (2020) Effects of physical exercise on working memory and attention-related neural oscillations. Front Neurosci. https://doi.org/10.3389/fnins.2020.00239
Chang YK, Chi L, Etnier JL, Wang CC, Chu CH, Zhou CL (2014) Effect of acute aerobic exercise on cognitive performance: role of cardiovascular fitness. Psychol Sport Exerc 15(5):464–470. https://doi.org/10.1016/j.psychsport.2014.04.007
Chang YK, Chu CH, Wang CC, Song TF, Wei GX (2015) Effect of acute exercise and cardiovascular fitness on cognitive function: an event-related cortical desynchronization study. Psychophysiology 52(3):342–351. https://doi.org/10.1111/psyp.12364
Chang Y-K, Alderman BL, Chu C-H, Wang C-C, Song T-F, Chen F-T (2017) Acute exercise has a general facilitative effect on cognitive function: a combined ERP temporal dynamics and BDNF study. Psychophysiology 54(2):289–300. https://doi.org/10.1111/psyp.12784
Chang YK, Chen FT, Kuan G, Wei GX, Chu CH, Yan J, Chen AG, Hung TM (2019) Effects of acute exercise duration on the inhibition aspect of executive function in late middle-aged adults. Front Aging Neurosci 11:227. https://doi.org/10.3389/fnagi.2019.00227
Ciria LF, Perakakis P, Luque-Casado A, Sanabria D (2018) Physical exercise increases overall brain oscillatory activity but does not influence inhibitory control in young adults. Neuroimage 181:203–210. https://doi.org/10.1016/j.neuroimage.2018.07.009
Ciria LF, Luque-Casado A, Sanabria D, Holgado D, Ivanov PCh, Perakakis P (2019) Oscillatory brain activity during acute exercise: tonic and transient neural response to an oddball task. Psychophysiology. https://doi.org/10.1111/psyp.13326
Clayson PE, Larson MJ (2012) Cognitive performance and electrophysiological indices of cognitive control: a validation study of conflict adaptation. Psychophysiology 49(5):627–637. https://doi.org/10.1111/j.1469-8986.2011.01345.x
Clayton MS, Yeung N, Cohen Kadosh R (2015) The roles of cortical oscillations in sustained attention. Trends Cogn Sci 19(4):188–195. https://doi.org/10.1016/j.tics.2015.02.004
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Academic Press, New York
Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S (2004) Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci 101(9):3316–3321. https://doi.org/10.1073/pnas.0400266101
Cotman CW, Engesser-Cesar C (2002) Exercise enhances and protects brain function. Exerc Sport Sci Rev 30(2):75–79
Crabbe JB, Dishman RK (2004) Brain electrocortical activity during and after exercise: a quantitative synthesis. Psychophysiology 41(4):563–574. https://doi.org/10.1111/j.1469-8986.2004.00176.x
Diamond A (2013) Executive functions. Annu Rev Psychol 64(1):135–168. https://doi.org/10.1146/annurev-psych-113011-143750
Drollette ES, Scudder MR, Raine LB, Moore RD, Saliba BJ, Pontifex MB, Hillman CH (2014) Acute exercise facilitates brain function and cognition in children who need it most: an ERP study of individual differences in inhibitory control capacity. Dev Cogn Neurosci 7:53–64. https://doi.org/10.1016/j.dcn.2013.11.001
Eriksen BA, Eriksen CW (1974) Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys 16(1):143–149. https://doi.org/10.3758/BF03203267
Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE, FOR 2018 PHYSICAL ACTIVITY GUIDELINES ADVISORY COMMITTEE* (2019) Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc 51(6):1242–1251. https://doi.org/10.1249/MSS.0000000000001936. (PMID: 31095081; PMCID: PMC6527141)
Eysenck MW, Derakshan N (2011) New perspectives in attentional control theory. Pers Individ Differ 50(7):955–960. https://doi.org/10.1016/j.paid.2010.08.019
Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI (2003) Cognitive and brain consequences of conflict. Neuroimage 18(1):42–57. https://doi.org/10.1006/nimg.2002.1319
Faul F, Erdfelder E, Buchner A, Lang AG (2009) Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41:1149–1160
Field AP (2018) Discovering statistics using Ibm Spss statistics, 5th edn. SAGE Publications Ltd
Fonte C, Smania N, Pedrinolla A, Munari D, Gandolfi M, Picelli A, Varalta V, Benetti MV, Brugnera A, Federico A, Muti E, Tamburin S, Schena F, Venturelli M (2019) Comparison between physical and cognitive treatment in patients with MCI and Alzheimer's disease. Aging 11(10):3138–3155. https://doi.org/10.18632/aging.101970
Gratton G, Coles MG, Donchin E (1983) A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 55(4):468–484. https://doi.org/10.1016/0013-4694(83)90135-9
Grimes A, Lightner JS, Pina K, Donis de Miranda ES, Meissen-Sebelius E, Shook RP, Hurley EA (2022) Designing an adaptive adolescent physical activity and nutrition intervention for COVID-19-related health challenges: formative research study. JMIR Form Res 6(1):e33322. https://doi.org/10.2196/33322
Guiney H, Machado L (2013) Benefits of regular aerobic exercise for executive functioning in healthy populations. Psychon Bull Rev 20(1):73–86. https://doi.org/10.3758/s13423-012-0345-4
Han P, Zhang W, Kang L, Ma Y, Fu L, Jia L, Yu H, Chen X, Hou L, Wang L, Yu X, Kohzuki M, Guo Q (2017). Clinical evidence of exercise benefits for stroke, pp 131–151. https://doi.org/10.1007/978-981-10-4304-8_9
Kamijo K, Hayashi Y, Sakai T, Yahiro T, Tanaka K, Nishihira Y (2009) Acute effects of aerobic exercise on cognitive function in older adults. J Gerontol Ser B Psychol Sci Social Sci 64(3):356–363. https://doi.org/10.1093/geronb/gbp030
Kappenman ES, Luck SJ (2016) Best practices for event-related potential research in clinical populations. Biol Psychiatry Cognit Neurosci Neuroimaging 1(2):110–115. https://doi.org/10.1016/j.bpsc.2015.11.007
Klimesch W (1999) EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev 29:169–195. https://doi.org/10.1016/S0165-0173(98)00056-3
Klimesch W, Sauseng P, Hanslmayr S, Gruber W, Freunberger R (2007) Event-related phase reorganization may explain evoked neural dynamics. Neurosci Biobehav Rev 31(7):1003–1016. https://doi.org/10.1016/j.neubiorev.2007.03.005
Kóbor A, Takács Á, Honbolygó F, Csépe V (2014) Generalized lapse of responding in trait impulsivity indicated by ERPs: The role of energetic factors in inhibitory control. Int J Psychophysiol 92(1):16–25. https://doi.org/10.1016/j.ijpsycho.2014.01.008
Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A (1999) Ageing, fitness and neurocognitive function. Nature 400(6743):418–419. https://doi.org/10.1038/22682
Lardon MT, Polich J (1996) EEG changes from long-term physical exercise. Biol Psychol 44(1):19–30. https://doi.org/10.1016/S0301-0511(96)05198-8
Leung K-K, Lee TMC, Yip P, Li LSW, Wong MMC (2009) Selective attention biases of people with depression: Positive and negative priming of depression-related information. Psychiatry Res 165(3):241–251. https://doi.org/10.1016/j.psychres.2007.10.022
Lezak MD (1995) Neuropsychological assessment, 3rd edn. Oxford University Press, New York
Liguori G (ed) (2022) ACSM’s guidelines for exercise testing and prescription, Eleventh. Wolters Kluwer, Philadelphia
Liu-Ambrose T, Nagamatsu LS, Voss MW, Khan KM, Handy TC (2012) Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging 33(8):1690–1698. https://doi.org/10.1016/j.neurobiolaging.2011.05.010
Luck SJ, Kappenman ES (2012) The Oxford handbook of event-related potential components. Oxford University Press
Luque-Casado A, Perakakis P, Hillman CH, Kao SC, Llorens F, Guerra P, Sanabria D (2016) Differences in sustained attention capacity as a function of aerobic fitness. Med Sci Sports Exerc 48(5):887–895.https://doi.org/10.1249/MSS.0000000000000857
Luque-Casado A, Ciria LF, Sanabria D, Perakakis P (2020) Exercise practice associates with different brain rhythmic patterns during vigilance. Physiol Behav 224:113033. https://doi.org/10.1016/j.physbeh.2020.113033
Marques-Aleixo I, Beleza J, Sampaio A, Stevanović J, Coxito P, Gonçalves I, Ascensão A, Magalhães J (2021) Preventive and therapeutic potential of physical exercise in neurodegenerative diseases. Antioxid Redox Signal 34(8):674–693. https://doi.org/10.1089/ars.2020.8075
Martin LE, Potts GF (2009) Impulsivity in decision-making: an event-related potential investigation. Pers Individ Differ 46(3):303–308. https://doi.org/10.1016/j.paid.2008.10.019
Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC (2001) Psychiatric aspects of impulsivity. Am J Psychiatry 158(11):1783–1793. https://doi.org/10.1176/appi.ajp.158.11.1783
Moustafa AA, Tindle R, Frydecka D, Misiak B (2017) Impulsivity and its relationship with anxiety, depression and stress. Compr Psychiatry 74:173–179. https://doi.org/10.1016/j.comppsych.2017.01.013
Norman DA, Shallice T (1986) Attention to action. In: Davidson RJ, Schwartz GE, Shapiro D (eds) Consciousness and self-regulation. Springer, Boston, MA. pp 1–18. https://doi.org/10.1007/978-1-4757-0629-1_1
Pang MYC, Yang L, Ouyang H, Lam FMH, Huang M, Jehu DA (2018) Dual-task exercise reduces cognitive-motor interference in walking and falls after stroke. Stroke 49(12):2990–2998. https://doi.org/10.1161/STROKEAHA.118.022157
Petersen SE, Posner MI (2012) The attention system of the human brain: 20 years after. Annu Rev Neurosci 35(1):73–89. https://doi.org/10.1146/annurev-neuro-062111-150525
Pfurtscheller G, Lopes da Silva FH (1999) Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110(11):1842–1857. https://doi.org/10.1016/s1388-2457(99)00141-8
Reuters EM, Vieluf S, Koutsandreou F, Hübner L, Budde H, Godde B, Voelcker-Rehage C (2019) A Non-linear relationship between selective attention and associated ERP markers across the lifespan. Front Psychol 10:30. https://doi.org/10.3389/fpsyg.2019.00030
Ridderinkhof KR, van der Molen MW, Bashore TR (1995) Limits on the application of additive factors logic: violations of stage robustness suggest a dual-process architecture to explain flanker effects on target processing. Acta Physiol (oxf) 90(1–3):29–48. https://doi.org/10.1016/0001-6918(95)00031-O
Ludyga S, Gerber M, Brand S, Holsboer‐Trachsler E, Pühse U (2016) Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: a meta-analysis. Psychophysiology 53(11):1611–1626. https://doi.org/10.1111/psyp.2016.53
Servant M, Logan GD (2019) Dynamics of attentional focusing in the Eriksen flanker task. Atten Percept Psychophys 81(8):2710–2721. https://doi.org/10.3758/s13414-019-01796-3
Smulders FTY, ten Oever S, Donkers FCL, Quaedflieg CWEM, van de Ven V (2018) Single-trial log transformation is optimal in frequency analysis of resting EEG alpha. Eur J Neurosci 48(7):2585–2598. https://doi.org/10.1111/ejn.13854
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18(6):643–662. https://doi.org/10.1037/h0054651
Sudikoff EL, Bertolin M, Lordo DN, Kaufman DA (2015) Relationships between executive function and emotional regulation in healthy children. J Neurol Psychol S(2):8
Sweitzer MM, Allen PA, Kaut KP (2008) Relation of individual differences in impulsivity to nonclinical emotional decision making. J Int Neuropsychol Soc 14(5):878–882. https://doi.org/10.1017/S1355617708080934
Warburton D, Jamnik V, Bredin S, Shephard R, Gledhill N (2021) The 2021 Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and electronic Physical Activity Readiness Medical Examination (ePARmed-X+). Health Fitness J Can 14(1):83–87. https://doi.org/10.14288/hfjc.v14i1.351
Waters A, Zou L, Jung M, Yu Q, Lin J, Liu S, Loprinzi PD (2020) Acute exercise and sustained attention on memory function. Am J Health Behav 44(3):326–332. https://doi.org/10.5993/AJHB.44.3.5. (PMID: 32295680)
Won J, Alfini AJ, Weiss LR, Callow DD, Smith JC (2019) Brain activation during executive control after acute exercise in older adults. Int J Psychophysiol 146:240–248. https://doi.org/10.1016/j.ijpsycho.2019.10.002
Xie Y, Li Y, Duan H, Xu X, Zhang W, Fang P (2021) Theta oscillations and source connectivity during complex audiovisual object encoding in working memory. Front Hum Neurosci 15:614950. https://doi.org/10.3389/fnhum.2021.614950
Yamagishi N, Callan DE, Anderson SJ, Kawato M (2008) Attentional changes in pre-stimulus oscillatory activity within early visual cortex are predictive of human visual performance. Brain Res 1197:115–122. https://doi.org/10.1016/j.brainres.2007.12.063
Yoon M, Yang P-S, Jin M-N, Yu HT, Kim T-H, Jang E, Uhm J-S, Pak H-N, Lee M-H, Joung B (2021) Association of physical activity level with risk of dementia in a nationwide cohort in Korea. JAMA Netw Open 4(12):e2138526. https://doi.org/10.1001/jamanetworkopen.2021.38526
Acknowledgements
We would like to thank all our participants for donating their time to this study. We would also like to thank Lauren Jackson, Rachel Eubanks, Emma Spurling, Jefferson Bass, Harshi Lodha Jain, Kaia McMullen, Maggie Zheng, Chayse Stevens, Laney Dezanet, Jayla Jackson, and McKenzie Guest for helping assist with data collection.
Funding
This work was supported by a Magellan grant received through the Advanced Support for Innovative Research Excellence at the University of South Carolina and the University of South Carolina Aiken’s Summer Scholars Institute.
Author information
Authors and Affiliations
Contributions
MAG: conceptualization, formal analysis, funding acquisition, investigation, methodology, software, writing—review and editing, project management. BP: conceptualization, supervision, resources. NSV: formal analysis. LJ-S: data curation, formal analysis, supervision, resources, writing—original draft/review and editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Additional information
Communicated by Matthew Heath.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Griggs, M.A., Parr, B., Vandegrift, N.S. et al. The effect of acute exercise on attentional control and theta power in young adults. Exp Brain Res 241, 2509–2520 (2023). https://doi.org/10.1007/s00221-023-06660-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-023-06660-3