Abstract
Crack cocaine is the crystal form of cocaine, produced by adding sodium bicarbonate to cocaine base paste. Brazil is the largest consumer of crack cocaine in the world. Users of crack cocaine show important physiological and behavioral alterations, including neuropsychiatric symptoms, such as anxiety-related symptoms. Nevertheless, few pre-clinical studies have been previously performed to understand the neurobiological effects of crack cocaine. The purpose of the present study was to investigate effects of the subchronic treatment (5 days, IP) of rats with crack cocaine in an animal model of anxiety/panic, the elevated T-maze (ETM). The ETM model allows the measurement of two behavioral defensive responses, avoidance and escape, in clinical terms, respectively, associated to generalized anxiety and panic disorder, the two main psychiatric conditions that accompany substance use disorders. Immediately after the ETM model, animals were tested in an open field for locomotor activity assessment. Analysis of delta FosB protein immunoreactivity was used to map areas activated by crack cocaine exposure. Results showed that crack treatment selectively altered escape displayed by rats in the ETM test, inducing either a panicolytic (18 mg/kg IP) or a panicogenic-like effect (25 and 36 mg/kg IP). These effects were followed by the altered functioning of panic-modulating brain regions, i.e., the periaqueductal gray and the dorsal region and lateral wings of the dorsal raphe nucleus. Treatment with 36 mg/kg of crack cocaine also increased locomotor activity. These are the first observations performed with crack cocaine in a rodent model of anxiety/panic and contribute to a better understanding of the behavioral and neurobiological effects of crack cocaine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of addictive drugs has becoming a worldwide trend and represents a major health problem (Ali et al. 2011). Among drugs of abuse, cocaine causes great concern due to its powerful addictive properties and related-health issues (Nutt et al. 2010). According to the World Drug Report (UNODOC 2014) the American continent is the major consumer of cocaine. In particular, in South American developing countries the drug of abuse consumption has dramatically increased (Johnson et al. 2013). In fact, Brazil is considered both a destination and a transit area for cocaine, and the second largest market for consumption, outside the United States of America (United States Department of State Bureau for International Narcotics and Law Enforcement Affair: International Narcotics Control Strategy Report 2013).
The main central mechanism of action of cocaine is the inhibition of dopamine reuptake (Nestler 2005). This seems to be particularly prominent in limbic regions that control emotional responses and memories, and where dopamine terminals are concentrated, such as the amygdala, hippocampus, frontal cortex and the nucleus accumbens (Nestler 2005; Hyman 2001; Kalivas and McFarland 2003). The pleasure of cocaine consumption and the desire to repeat the experience are associated to this mechanism. Nevertheless, higher doses and/or the chronic use of cocaine leads to important physiological and emotional/behavioral changes, and increases the risk for psychiatric-related disturbances, such as paranoia, anxiety and, particularly, panic (Rosen and Kosten 1992; Lacayo 1995; Bolla et al. 1998; Shanti et al. 2013; Pires and Cavaco 2014). Some of these changes have been associated to altered gene-expression due to chronic drug use. The effect of cocaine on one particular product of immediate gene expression, the delta FosB protein, has been the subject of several studies during the last years (Nestler 2008, 2012; Perrotti et al. 2008).
Crack cocaine is produced by adding sodium bicarbonate to cocaine base paste (Falck et al. 2008), resulting in a more alkaline product, which may be injected or inhaled (Lankenau et al. 2004; Butler et al. 2017). Brazil is the largest consumer of crack cocaine in the world (Ribeiro et al. 2019). A national population-based survey, conducted in 2012, showed that approximately two million Brazilians were using crack cocaine (Laranjeira and Mitsuhiro 2012). Crack users show physiological and behavioral alterations that greatly affect their personal lives, also resulting in important economic and social burdens (Vaughn et al. 2010; Riezzo et al. 2012).
A recent literature review, published by our research group, showed that the chronic use of crack cocaine induces, in particular, cognitive and emotional changes, together with neurobiological and molecular alterations (Rosário et al. 2018). Among the psychiatric symptoms that accompany crack cocaine use are depression and anxiety-related symptoms (Zubaran et al. 2010, 2013). Nevertheless, one of the main findings of this literature review was the lack of, in particular, pre-clinical studies, to allow a better understanding of the effects of crack cocaine on brain and behavior (Rosário et al. 2018).
Taking into account that: (1) crack cocaine is a largely abused drug that leads to important behavioral and physiological sequelae, among of which are anxiety-related symptoms, and (2) that few studies have been performed to better understand these behavioral and physiological alterations, the purpose of the present study was to investigate effects of the subchronic treatment (5 days, IP) with crack cocaine in rats submitted to the elevated T-maze (ETM) test (Viana et al. 1994; Teixeira et al. 2000; Poltronieri et al. 2003). This model allows the measurement of two distinct behavioral defensive responses, inhibitory avoidance and one-way escape. According to the ethoexperimental analysis of the defensive repertoire of rodents (Gray and McNaughton 2000; Blanchard et al. 2001), anxiety is mainly associated to behavioral inhibition and risk assessment behavior, reactions observed in situations of potential danger. Thus, avoidance behavior displayed by rats in the ETM test is an example of an anxiety-associated reaction. The main behavioral responses associated to panic, on the other hand, are escape/flight, which are presented when animals are faced with proximal, real danger (Gray and McNaughton 2000; Blanchard et al. 2001), such as escape behavior from the open arms of the ETM test. While anxiolytic drugs, such as benzodiazepines and buspirone, selectively impair avoidance, chronic treatment with antidepressants inhibit escape responses (Viana et al. 1994; Graeff et al. 1998; Teixeira et al. 2000; Graeff and Zangrossi 2002; Poltronieri et al. 2003; Campos et al. 2013). These results are compatible with the view that inhibitory avoidance relates to generalized anxiety and one-way escape to panic disorder (Viana et al. 1994; Teixeira et al. 2000; Graeff and Zangrossi 2002; Poltronieri et al. 2003; Campos et al. 2013). Generalized anxiety and panic disorder are the two main psychiatric conditions associated to substance use disorders (Compton et al. 2007; Smith and Book 2010). Immediately after tests with the ETM, all animals were tested in an open field for locomotor activity assessment.
Additionally, analysis of delta FosB protein immunoreactivity was used to map areas activated by crack cocaine exposure. Previous results suggest that members of the AP1 family, the so-called chronic Fos-related antigens (Fras), mediate the effects of chronic stimulation, such as repeated drug administration (Nestler 2005; Nankova et al. 2000). The chronic Fras are truncated splice variant of the FosB gene-delta FosB isoforms and remain stable for longer periods of time when compared to other markers of neuronal activation such as c-Fos.
Methods
Subjects
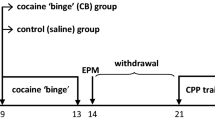
Forty-eight male Wistar rats, weighing approximately 300 g in weight at the time of the experiments (Universidade Federal de São Paulo, CEDEME, Brazil), were used. Animals were housed in groups of four in polypropylene boxes covered with stainless steel grids, under a 12-h light–dark cycle (lights on from 7:00 am to 7:00 pm) and room-controlled temperature (22 ± 1 °C). Throughout the experiments, animals had free access to food and water.
The animals remained in the laboratory, for habituation, for a period of 10 days, until the beginning of the experiments.
The project was approved by the Ethical Committee for Animals Research of the Federal University of São Paulo (CEUA, UNIFESP), under the number 2117061017. All procedures were conducted in conformity with the Brazilian Society of Neuroscience and Behavior Guidelines for Care and Use of Laboratory Animals, which are also in compliance with international laws and policies. All efforts were made to minimize animal suffering.
Chemical analysis
The drug was donated as a courtesy by the Criminal Department of Limeira City, São Paulo State, Brazil, for research purposes. The drug used in all experiments was from a same lot obtained from trade seizing. The quantification of cocaine in the samples used in the present work was verified and the results obtained have already been published in previous articles (Maranhão et al. 2017; Moretti et al. 2016).
Briefly, a 100 mg aliquot of crack cocaine was evaluated by Liquid Chromatography–Mass Spectrometry (LCMS/MS) for the quantification of cocaine. Samples were analyzed by High Performance Liquid Chromatography Agilent 1260 (Agilent Technologies, USA) combined with a 3200 QTRAP hybrid triple quadrupole/linear ion trap (LIT) mass spectrometer (Sciex, Canada) (Shihomatsu et al. 2017; Pereira et al. 2016). Cocaine samples (FE07271503, Cerilliant, USA) were used as analytical reference standards. Injection volumes of 10 μL were analyzed by an Agilent Eclipse XDB-C18 4.6 × 50 mm, 1.8 μm column at 25 °C. The eluent flow rate was 0.7 ml/min−1. The mobile phase for positive mode analysis was 0.1% formic acid (LC–MS Grade, Sigma Aldrich, USA) in water (solvent A) and acetonitrile (LC–MS Grade, JT Baker, Brazil) (solvent B). A linear gradient of 0.7 ml/min was adopted, beginning with a 95% solvent A and 5% solvent B mixture. A linear decrease from 95 to 5%, over 5 min, was acquired for solvent A, which was maintained for 1 min. The mixture was then returned to the original condition, for 2 min. Cocaine was detected and quantified using Electrospray Ionization (ESI) and Multiple Reaction Monitoring (MRM) mode, with the selection of a precursor ion and two ion products to quantify and qualify the compound. Data were recorded and processed using Analyst® 1.5.2 (Sciex, Canada). MRM parameters, limit of detection (LOD) and limit of quantification (LOQ). Results showed that the aliquot of crack cocaine analyzed by LC–MS/MS contained 37.99% of cocaine and that the drug was the main constituent of the sample (Maranhão et al. 2017; Moretti et al. 2016). Apart from cocaine, there were two additional substances identified in the sample: methlylecgonine cinnamate and truxilines. Methylecgonine cinnamate is a natural occurring tropane alkaloid. It is structurally similar to cocaine and considered pharmacologically inactive, if not smoked (Plowman and Rivier 1983). Truxilines are a group of 11 stereoisomers present in coca leaves, which are extracted along with cocaine and other alkaloids during the refining process (Lurie et al. 1990). The detailed spectrometry description of the aliquot was presented in a previous study performed by our research group (Moretti et al. 2016).
Drug administration
Due to its low solubility in water, crack cocaine was first dissolved in dimethylsulfoxide (DMSO). Crack cocaine experimental groups were treated with either 18 (n = 8), 25 (n = 8) or 36 mg/kg (n = 8) of crack cocaine, administered intraperitonially for five consecutive days (in the volume of 1 ml/kg, IP). The 5-day administration period and the doses administered (18, 25 and 36 mg/kg) were chosen from previous reported results (Moretti et al. 2016; Lipaus et al. 2019) and from pilot studies performed in our laboratory. Taking into account the percentage of cocaine present in the sample of crack cocaine used, the doses administered contained, respectively, 6.8, 9.5 and 13.68 mg/kg of cocaine. As controls for each one of the three experimental crack cocaine groups, we simultaneously tested vehicle-treated animals (1 ml/kg, IP; 3 groups of 8 animals each), also administered for a period of five consecutive days. Drug and vehicle administration were performed in the testing room.
Apparatus
The elevated T-maze (ETM) was a device made of wood, with 3 arms of equal dimensions (50 cm × 12 cm). A closed arm, with walls 40-cm high, was located perpendicular to the two opposed open arms. The entire apparatus was elevated 50 cm from the ground. The open arms were surrounded by transparent acrylic (1 cm high), for fall prevention.
The open field used was composed of a round arena (900 mm in diameter), with 45 cm high transparent acrylic walls and a base made of milky acrylic (1000 × 1000 mm), with floor divided into 12 squares.
Luminosity at the center of the ETM and open field was 60 lx. After the experimental sessions, the equipments were cleaned with a 70% ethanol solution.
Procedure
On the morning of the fourth day of treatment, before drug or vehicle administration, the animals were pre-exposed to one of the open arms of the ETM. A wooden barrier mounted on the border of the open arm, between the central area of the maze and the proximal extremity of the ETM arm, isolated this arm from the rest of the maze. It has been shown that this forced pre-exposure potentiates the expression of escape behavior, reducing behavioral reactions to novelty (Teixeira et al. 2000).
On the next day, 30 min after treatment (fifth day of crack or vehicle solution administration), the animals were tested in the ETM. For inhibitory avoidance measurements, each animal was placed at the distal end of the closed arm of the ETM, and the latency to exit this arm with the four paws towards the open space was measured (baseline latency). This same measurement was repeated for two consecutive times (avoidance 1 and avoidance 2), with 30 s intertrial intervals. The animal was then placed at the end of the same open arm, where it had been previously exposed on the day before and the time taken to leave this arm with the four paws was also measured (escape 1). This procedure was repeated for two consecutive times for the acquisition of escape 2 and 3 measurements, again with 30 s intertrial intervals.
Thirty seconds after tests with the ETM, each animal was placed in the center of the open field and allowed to freely explore the apparatus for 5 min, for the measurement of the number of lines crossed and the number of rearings.
Delta FosB immunoreactivity
Immediately after the behavioral tests, six animals from each experimental group were randomly chosen for immunohistochemical analysis. Animals were anesthetized with a mixture of ketamine (75 mg/kg), xylazine (10 mg/kg), fentanil (0.5 mg/kg) and acepromazine (1 m/kg) in the same syringe, and perfused with ≈100 ml of 0.9% saline for approximately 1 min, followed by 500–700 ml of 4% formaldehyde (from paraformaldehyde heated to 60–65 °C) and H2O at 4 °C, pH 9.5, for approximately 25 min. The brains were post-fixed for 1 h in the same fixative, plus 20% sucrose, at 4 °C. Regularly spaced series (5 × 1-in-5) of 30 µm-thick frozen sections were cut in the coronal plane, collected in ethylene glycol-based cryoprotectant solution and stored at − 20 °C for later determination of delta FosB.
Delta FosB immunoreactive neurons were identified using a polyclonal anti-serum raised in rabbit against delta-FosB protein (anti-FosB, 1:1,000; Sigma, St. Louis, MO, USA). Immunohistochemistry was performed using a conventional avidin–biotin immunoperoxidase protocol (Hsu and Raine 1881; Hsu et al. 1981) and Vectastain Elite reagents (Vector Laboratories, Burlingame, CA, USA). Tissue was pretreated with hydrogen peroxide (0.3%; Sigma, St. Louis, MO, USA) before addition of the primary antibody to squelch endogenous peroxidase activity in the tissue. The reaction with diaminobenzidine-DAB (0.05%; Sigma) was amplified using nickel ammonium sulfate. The sections were then mounted on gelatin-coated slides, allowed to dry for approximately 18 h and counterstained with thionin to visualize the labeled cells within the borders of each nucleus. Adjoining series of sections were stained with 0.25% thionin for reference purposes.
Cell counting
Delta FosB cells were quantified in sections, having as reference the following AP coordinates (Paxinos and Watson 2008), using Bregma as a reference: cingulate cortex: + 1.68 mm; hippocampus and septum: + 2.28 mm; amygdala: − 2.92 mm; dorsal columns of the periaqueductal gray matter: − 7.68 mm; median raphe nucleus: − 8.04 mm; dorsal region of the dorsal raphe nucleus: − 8.04 mm, lateral wings of the dorsal raphe nucleus/ventrolateral periaqueductal gray matter: − 8.04 mm under bright-field illumination using the Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA). These regions were selected on the basis of their involvement with the modulation of anxiety/panic responses (Gray and McNaughton 2000; Brandão et al. 2003; Sela et al. 2011; Spiacci et al. 2012; Paul and Lowry 2013). Sections were analyzed by an observer blind to the treatment conditions. Cells were considered delta FosB immunoreactive if their nuclei contained dark, punctate gray-black immunolabeling, and were counted using constant minimum and maximum OD and object size criteria, which were validated with visual counts.
Statistics
Statistical analysis was generated using SPSS software package. All data was analyzed for homogeneity. In case of heterogenous results, data was submitted to a Log10 transform. ETM data were analyzed by two-way ANOVA for repeated measures, with treatment as the independent factor and trials (avoidance or escape latencies) as the dependent factor. In case of significant results of the treatment or of the treatment x trial interaction, between group comparisons in each trial were performed using unpaired Student t-test. Open field and immunohistochemical data were analyzed using unpaired Student t-test. A value of p ≤ 0.05 was considered significant.
Results
Crack cocaine (18 mg/kg) induces panicolytic-like effects and decreases delta FosB immunoreactivity in panic-modulating regions
Figure 1 (upper panel) shows the effects of 18 mg/kg of crack cocaine on avoidance behavior. According to repeated measures ANOVA, there was a significant effect of trials [F (2,24) = 8.67; p = 0.001], but not of treatment [F(1,12) = 0.86; p = 0.77] or of treatment × trials interaction [F(2,24) = 0.83; p = 0.45].
Avoidance (upper panel) and escape latencies (lower panel) (mean ± SEM) in the elevated T-maze performed by vehicle- and 18 mg/kg crack-treated animals (5 days, IP). N = 8 per treatment group. *p < 0.05, compared to control animals in the same trial (ANOVA followed by the independent Student t-test)
Figure 1 (lower panel) shows the effects of the drug on escape behavior. According to repeated measures ANOVA there was a significant effect of treatment [F(1,12) = 13.55; p = 0.003] and of treatment × trials interaction [F (2,24) = 23.84; p = 0.036], but not of trials [F (2,24) = 0.88; p = 0.43]. Animals treated with crack took a longer time to leave the open arm on escape 2 [Student t-test: T(8.13) =− 3.79; p = 0.005] and 3 [Student t-test: T(6.71) = − 3.19; p = 0.016], a panicolytic-like effect.
Table 1 shows the effects of the drug on locomotor activity. According to unpaired Student t-test, there were no differences between vehicle and crack-treated animals, neither with respect to the number of crossings [T (13) = − 0.57; p = 0.58] nor with respect to the number of rearings [T (13) = − 0.87; p = 0.40].
Figure 2 and Table 2 show the effects of treatment with 18 mg/kg of crack on delta FosB immunoreactivity. According to unpaired Student t-test, treatment with crack decreased the number of delta FosB immunoreactive cells in the dorsomedial [T(10) = 6.07; p < 0.002] (2A), dorsolateral [T(10) = 6.23; p < 0.001] (2B) and lateral periaqueductal gray [T(10) = 3.92; p = 0.003] (2C), lateral wings of the dorsal raphe/ventrolateral periaqueductal gray [T(7.34) = 4.67; p = 0.002] (2D) and in the dorsal region of the dorsal raphe [T(10) = 4.31; p = 0.002] (2E). No other significant results were found.
Photomicrographs of delta FosB immunoreactive cells (dark spots) in coronal sections through brain regions with significant decreases in delta FosB immunoreactivity in animals submitted to a 5-day IP treatment with 18 mg/kg of crack, in respect to vehicle-treated animals. N = 6 per treatment group. a dorsolateral column of the periaqueductal gray matter, b lateral column of the periaqueductal gray matter, c dorsal region of the dorsal raphe nucleus, d lateral wings of the dorsal raphe nucleus/ventrolateral column of the periaqueductal gray matter. Magnification: 200 ×. *cerebral aqueduct
Crack cocaine (25 mg/kg) induces panicogenic-like effects and increases delta FosB immunoreactivity in panic-modulating regions
Figure 3 (upper panel) shows the absence of effects of 25 mg/kg of crack cocaine on avoidance behavior. According to repeated measures ANOVA, there was a significant effect of trials [F (2,26) = 7.88; p = 0.002], but not of treatment [F(1,13) = 1.13; p = 0.31] nor of treatment × trials interaction [F(2,26) = 1.23; p = 0.31].
Avoidance (upper panel) and escape latencies (lower panel) (mean ± SEM) in the elevated T-maze performed by vehicle- and 25 mg/kg crack-treated animals (5 days, IP). N = 8 per treatment group. *p < 0.05, compared to control animals in the same trial (ANOVA followed by the independent Student t-test)
Figure 3 (lower panel) shows the panicogenic-like effects of the drug on escape behavior. According to repeated measures ANOVA, there was a significant effect of trials [F (2,26) = 6.48; p = 0.005] and treatment [F(1,13) = 12.17; p = 0.004], but not of treatment × trials interaction [F (2,26) = 1.05; p = 0.37]. Animals treated with crack took a shorter time to leave the open arm on escape 1 [unpaired Student t-test: T (12.77) = 2.32; p = 0.04], 2 [T (13) = 3.17; p = 0.007] and 3 [unpaired Student t-test: T (13) = 2.79; p = 0.015], a panicogenic-like effect.
Table 1 shows the effects of the drug on locomotor activity. According to unpaired Student t-test, there were no significant differences between vehicle and crack-treated animals, neither with respect to the number of crossings [T (13) = 0.62; p = 0.55] nor with respect to the number of rearings [T (13) = 1.21; p = 0.25].
Figure 4 and Table 3 show the effects of treatment with 25 mg/kg of crack on delta FosB immunoreactivity. According to unpaired Student t-test, treatment with crack increased the number of delta FosB immunoreactive cells in the dorsomedial [T(7.13) = − 8.87; p < 0.001] (4A), dorsolateral [T(9.07) = − 11.51; p < 0.001] (4B) and lateral columns of the periaqueductal gray [T(10) = − 11.33; p < 0.001] (4C), in the lateral wings of the dorsal raphe/ventrolateral periaqueductal gray [T(9.73) = − 4.03; p = 0.003] (4D) and in the dorsal region of the dorsal raphe [T(7.76) = − 2.76; p = 0.02] (4E). No other significant results were found.
Photomicrographs of delta FosB immunoreactive cells (dark spots) in coronal sections through brain regions with significant decreases in delta FosB immunoreactivity in animals submitted to a 5-day IP treatment with 25 mg/kg of crack, in respect to vehicle-treated animals. N = 6 per treatment group. a dorsomedial column of the periqueductal gray matter, b dorsolateral column of the periaqueductal gray matter, c lateral column of the periaqueductal gray matter, d dorsal region of the dorsal raphe nucleus, e lateral wings of the dorsal raphe nucleus/ventrolateral column of the periaqueductal gray matter. Magnification: 200 ×. *cerebral aqueduct
Crack cocaine (36 mg/kg) increases panic-like responses, but also alters locomotor activity
Figure 5 (upper panel) shows the absence of effects of treatment with 36 mg/kg of crack cocaine on avoidance behavior. According to repeated measures ANOVA, there was a significant effect of trials [F(2,24) = 14.53; p < 0.001], but not of treatment [F(1,12) = 2.43; p = 0.15] or of trials × treatment interaction [F(2,24) = 1.93; p = 0.17].
Avoidance (upper panel) and escape latencies (lower panel) (mean ± SEM) in the elevated T-maze performed by vehicle- and 36 mg/kg crack-treated animals (5 days, IP). N = 8 per treatment group. *p < 0.05, compared to control animals in the same trial (ANOVA followed by the independent Student t-test)
Figure 5 (lower panel) shows that similar to that observed with 25 mg/kg of crack cocaine, the highest dose of the drug (36 mg/kg) decreased escape latencies. According to repeated measures ANOVA there were no significant effect of trials [F(2,24) = 0.31; p = 0.73] or of trials × treatment interaction [F(2,24) = 0.26; p = 0.78], but there was a significant effect of treatment [F(1,12) = 15.61; p = 0.002]. Escape 1 [unpaired Student t-test: T(12) = 3.08; p = 0.01], 2 [unpaired Student t-test: T(10.37) = 3.29; p = 0.008] and 3 latencies [unpaired Student t-test: T(8.30) = 2.76; p = 0.024] were significantly decreased by treatment with 36 mg/kg.
Nevertheless, Table 2 shows that treatment with 36 mg/kg also induced increases in locomotor activity. According to unpaired Student t-test both the number of crossings [T(5.81) = − 2.72; p = 0.036] and the number of rearings [T(12) = − 2.94; p = 0.012] were increased. Therefore, behavioral results observed with the ETM cannot be attributed to panic solely.
Figure 6 and Table 4 show the effects of treatment with 36 mg/kg of crack on delta FosB immunoreactivity. Again here, according to unpaired Student t-test, treatment with crack increased the number of delta FosB immunoreactive cells in the dorsomedial [T(9.98) = − 15.55; p < 0.001] (6A), dorsolateral [T(10) = − 19.68; p < 0.001] (6B) and lateral columns of the periaqueductal gray [T(8.01) = − 24.18; p < 0.001] (6C), in the lateral wings of the dorsal raphe/ventrolateral periaqueductal gray [T(9.07) = − 3.85; p = 0.004] (6D), and in the dorsal region of the dorsal raphe [T(8.32) = − 2.53; p = 0.03] (6E). No other significant differences were found (p > 0.05).
Photomicrographs of delta FosB immunoreactive cells (dark spots) in coronal sections through brain regions with significant decreases in delta FosB immunoreactivity in animals submitted to a 5-day IP treatment with 36 mg/kg of crack, in respect to vehicle-treated animals. N = 6 per treatment group. a dorsomedial column of the periqueductal gray matter, b dorsolateral column of the periaqueductal gray matter, c lateral column of the periaqueductal gray matter, d dorsal region of the dorsal raphe nucleus, e lateral wings of the dorsal raphe nucleus/ventrolateral column of the periaqueductal gray matter. Magnification: 200 ×. *cerebral aqueduct
Discussion
The findings of the present study showed that: (1) subchronic IP treatment with a lower dose of crack cocaine (18 mg/kg, containing 6.8 mg/kg of cocaine) induces panicolytic-like effects, followed by a decrease in delta FosB protein immunoreactivity in the dorsomedial, dorsolateral and lateral periaqueductal gray, in the dorsal region of the dorsal raphe and in the lateral wings/ventrolateral periaqueductal gray; (2) Increases in the dose administered (25 mg/kg, containing 9.5 mg/kg of cocaine) generate the opposite effects: panicogenic-like results, followed by increases in delta FosB immunoreactivity in the dorsomedial, dorsolateral and lateral aspects of the periaqueductal gray, in the dorsal region of the dorsal raphe and in the lateral wings/ventrolateral periaqueductal gray; (3) Even higher doses (36 mg/kg, containing 13.68 mg/kg of cocaine), apart from increasing panic-related responses and delta FosB immunoreactivity in the same columns of the periaqueductal gray and subregions of the dorsal raphe, also induce increases in locomotor activity.
To our knowledge, this is the first study directed to the evaluation of crack cocaine’s effects in anxiety/panic, using a rodent model. Several pre-clinical and clinical studies, however, have been performed with cocaine. Clinical evidences (Gawin 1988, 1989) show that after the initial use and/or the administration of lower doses of cocaine, users frequently describe feelings of wellbeing and decreases in anxiety-related responses. With animal models of anxiety, most observations performed describe the anxiogenic-like effects of cocaine administration (Rogerio and Takahashi 1992; Yang et al. 1992; Blanchard and Blanchard 1999; Paine et al. 2002; Kohtz et al. 2010). A few studies also describe either no effect or anxiolytic-like effects after the acute IP administration of cocaine (Rogerio and Takahashi 1992; Yang et al. 1992; Blanchard and Blanchard 1999; Paine et al. 2002; Kohtz et al. 2010) or decreases in anxiety followed by the self-administration of the drug (Waters and See 2011). Kohtz et al. (2010) showed that the acute IP injection of 5–20 mg/kg of cocaine increased the time spent by female rodents in the center of an open field, although no significant effects were seen in males. The authors associate these effects to progestogens levels. Using the same dose-interval, Takahashi and Rogerio (1991), showed no effects of cocaine in the elevated plus-maze. Nevertheless, in a subsequent study (Rogerio and Takahashi 1992), the same authors showed that acute cocaine administration (10 mg/kg, IP) either did not alter elevated plus-maze measurements or presented anxiogenic-like effects, depending on the anxious phenotype of the animals (if not anxious or anxious, respectively). Together, these observations indicate that, apart from dose and treatment regimen, gender and anxious phenotype might influence the results observed in rodent models. It would be interesting to investigate the effects of crack cocaine in female rats in future studies.
However, of particular importance is also the fact that in the present work only escape behavior was altered by crack cocaine administration. No effects in avoidance measurements were observed. In this regard, it is interesting to mention that most of the pre-clinical studies, that report the effects of cocaine in anxiety, do not use tests that specifically model panic disorder, but rather tests that have been described as non-selective for the distinct subtypes of anxiety-related disorders found in clinical settings, that is “mixed” animal models (Graeff and Zangrossi 2002). Thus, the use of non-selective animal models might explain some of the discrepant results found in the literature. An important exception to this observation is the study performed by Blanchard and coworkers (Blanchard and Blanchard 1999). These authors showed that the acute IP (10–30 mg/kg) administration of cocaine in mice significantly and dose-dependently enhanced flight and escape behaviors measured in the mouse defense test battery. Similarly, the intravenous administration (4 mg/kg) of cocaine in rats induced well-directed flight responses in a rat runaway test. Although defensive threat/attack and risk assessment behaviors were altered after the administration of higher doses of the drug, the drug’s effect over flight/escape behavior was much clearer, thus indicating that panic-associated responses are the critical reactions altered by cocaine exposure (Blanchard and Blanchard 1999). Our results with crack cocaine go in the same direction. Also, and reinforcing the association between crack cocaine and flight/escape reactions, one of the few previous reports (Areal et al. 2015) performed with the administration of crack cocaine described that, after inhaling the drug, crack-treated mice frequently elicited what the authors called an “escape jumping” behavior, which reminded escape attempts from a restricted environment (Areal et al. 2015).
Although the higher dose of crack cocaine (36 mg/kg, 13.68 mg/kg of cocaine) used in the present study also increased escape behavior, it altered locomotor activity. Similar observations have been reported with the IP administration of doses superior to 10 mg/kg IP of cocaine, although variables such as animal species/strain and the duration of the treatment might interfere with the results (Gulley et al. 2003; Enhardt et al. 2006; Thomsen and Caine 2011).
The effects of crack cocaine on escape behavior were followed by a selective decrease (18 mg/kg, 6.8 mg/kg of cocaine) or increase (25 and 36 mg/kg, 9.5 and 13.68 mg/kg of cocaine, respectively) in delta FosB immunoreactivity in the periaqueductal gray and in two subregions of the dorsal raphe, respectively, related to anxiety and panic modulation, the dorsal region and the lateral wings (Thomsen and Caine 2011; Johnson et al. 2005; Hale et al. 2012; Spiacci et al. 2012; Paul and Lowry 2013). Decreases in delta FosB immunoreactivity accompany a panicolytic-like effect while increases in delta FosB immunoreactivity follow a panicogenic-like effect. No significant effects in delta FosB immunoreactivity were found in other brain regions. Only one previous study measured delta FosB immunoreactivity after crack cocaine treatment (Areal et al. 2015). This study showed that a 5-min long exposure to 5 g of inhaled crack cocaine, twice a day, for 11 days, induced increases in delta FosB immunoreactivity in the nucleus accumbens. Other brain regions, however, were not investigated. Another report, performed with cocaine, showed increases in delta FosB immunoreactivity in different brain areas, including the frontal cortex, lateral septum, hippocampus, amygdala and periaqueductal gray, after a 14-day IP treatment regimen of 15 mg/kg of cocaine, two times a day (Perrotti et al. 2008). The periaqueductal gray, however, was not divided in columns and the dorsal raphe was not analyzed. Differences regarding these results and ours most possibly rely on the dose and duration of drug administration.
Evidences gathered during the last decades show that the electrical or chemical stimulation of the dorsal and lateral aspects of the periaqueductal gray decreases the aversive stimulus intensity necessary to induce an escape/flight reaction and is followed by a series of autonomic reactions, such as tachycardia, increases in blood pressure and exophthalmia (Fernandez-de-Molina and Hunsperger 1959; Brandão et al. 1982; Nogueira and Graeff 1991; Bandler and Shipley 1994; Graeff 2004, 2012). Freezing behavior may also be present, prior to escape/flight, or when escape routes are not available (Roelofs, 2017). Data obtained with human subjects support pre-clinical observations (Nashold et al. 1969; Amano et al. 1978; Young et al. 1992). Also, using the ETM test, it has been previously shown that activation of the dorsal periaqueductal gray by local administration of the benzodiazepine inverse agonist FG 7142 decreases escape displayed by rats in the ETM test, a panicogenic-like effect (Bueno et al. 2005). On the other hand, the chemical inactivation of the structure by intra-dorsal periaqueductal gray administration of either the benzodiazepine midazolam, the GABAA agonist muscimol or the GABAB agonist baclofen, causes the opposite effect: increases escape latencies, a panicolytic-like action (Bueno et al. 2005). In the present study, crack cocaine, dose-dependently, induced neuroadaptations in the dorsal and lateral columns of the periaqueductal gray region what might explain the behavioral effects presently described.
The main subnuclei of dorsal raphe that modulate panic-related reactions are the lateral wings, which work together with the ventrolateral column of the periaqueductal gray (Johnson et al. 2005; Hale et al. 2012; Wscieklica et al. 2017; Paul and Lowry 2013). A previous study performed with the ETM test showed that the exposure of laboratory animals to the escape task significantly increases the activation of these subregions (Spiacci et al. 2012). The reversible chemical inhibition of the lateral wings/ventrolateral column of the periaqueductal gray by cobalt chloride, an inhibitor of synaptic neurotransmission, increases ETM escape latencies, a panicolytic-like effect (Spiacci and Zangrossi, personal communication). Also, we have previously shown that deep brain stimulation, applied to the lateral wings of the dorsal raphe and leading to the functional blockage of the region (through a process called depolarization block), induces similar effects (Wscieklica et al. 2017).
Although the dorsal subregion of the dorsal raphe does not seem to be the main subnucleus involved with panic regulation, it also modulates escape behavior displayed by rats in the ETM test (Spiacci et al. 2012). It is interesting to mention that previous reports show that dopamine neurons are the most abundant group of neurons of the dorsal raphe nucleus, apart from serotonergic cells (Huang et al. 2019). Also, the majority of the dopamine neurons in the dorsal raphe nucleus are located in the rostral pole of the region, particularly in its dorsal subnucleus (Yoshida et al. 1989), where they are intermingled with serotonergic neurons. Remarkably, previous results with L-dopa show that chronic treatment with the drug results in a significant decrease in serotonin in particularly in the dorsal division of the dorsal raphe nucleus (Stansley and Yamamoto 2014), thus confirming dopamine-serotonin interactions in this area of the mesencephalic tegmentum. These interactions might also underlie the panic-related reactions observed after crack cocaine administration. In future studies, it will be important to verify the neurochemical identity of the delta FosB activated cells to allow a better understanding of the neurobiology of crack cocaine addiction and, consequently, to promote therapeutic strategies.
At last, it is important to mention that since crack cocaine is frequently smoked, there are some points that should be taken into account in the interpretation of the current results. The first of them concerns the pharmacokinetics of the drug. Pharmacokinetics play a decisive role in determining behavioral and neurobiological effects of a drug administration. When cocaine is smoked it is promptly absorbed and distributed to the central nervous system (Allain et al. 2015). Therefore, its effects may be more rapidly and intensively experienced. A second point of concern is the presence of different substances in the sample, according to its route of administration. For instance, it has been previously reported that one of the main substances responsible for the toxicity of crack is anhydroecgonine methyl ester (AEME), a cocaine pyrolysis metabolite (Areal et al. 2015; Garcia et al. 2017). In this respect, we are currently validating a protocol to investigate the effects of inhaled crack in male and female rats´ anxiety/panic behavior, which will allow further comparisons with our present results.
To conclude, the results reported herein show, for the first time, that crack cocaine treatment selectively alters a panic-related response, escape behavior elicited by exposure to the open arms of the ETM test, inducing either a panicolytic or a panicogenic-like effect, depending on the dose administered. These behavioral effects are accompanied by the alteration of the functioning of panic-modulating encephalic structures, i.e., the dorsal and lateral aspects of the periaqueductal gray and two subregions of the dorsal raphe nucleus, the dorsal region and the lateral wings/ventrolateral column of the periaqueductal gray. While the panicolytic lower dose decreases delta FosB immunoreactivity in these regions, the higher panicogenic doses increase FosB protein expression. Increases in locomotor activity were also induced by the higher dose administered, thus corroborating literature reports obtained with higher doses of cocaine. These are the first results obtained with crack cocaine in a rodent model of anxiety/panic and contribute to a better understanding of behavioral and neurobiological effects of crack cocaine intake.
References
Ali H, Bushra R, Aslam N (2011) Profile of drug users in Karachi City, Pakistan. E Mediterr Health J 17:41–45. https://doi.org/10.26719/2011.17.1.41
Allain F, Minogianis E-A, Roberts DCS, Samaha A-N (2015) How fast and how often: the pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev 56:166–179. https://doi.org/10.1016/j.neubiorev.2015.06.012
Amano K, Tanikawa T, Iseki H, Kawabatake H, Notani M, Kawamura H, Kitamura K (1978) Single neuron analysis of the human midbrain tegmentum. Rostral mesencephalic reticulotomy for pain relief. Appl Neurophysiol 41:66–78. https://doi.org/10.1159/000102402
Areal LB, Rodrigues LCM, Andrich F, Moraes LS, Cicilini MA, Mendonça JB, Pelição FS, Nakamura-Palacios EM, Martins-Silva C, Pires RGW (2015) Behavioral, biochemical and molecular changes induced by chronic crack-cocaine inhalation in mice: the role of dopaminergic and endocannabinoid systems in the prefrontal cortex. Behav Brain Res 290:8–16. https://doi.org/10.1016/j.bbr.2015.04.036
Bandler R, Shipley MT (1994) Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trend Neurosci 17:379–389. https://doi.org/10.1016/0166-2236(94)90047-7
Blanchard DC, Blanchard RJ (1999) Cocaine potentiates defensive behaviors related to fear and anxiety. Neurosci Biobehav Rev 23:981–991. https://doi.org/10.1016/S0149-7634(99)00031-7
Blanchard DC, Griebel G, Blanchard RJ (2001) Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev 25:205–218. https://doi.org/10.1016/S0149-7634(01)00009-4
Bolla KI, Cadet JL, London ED (1998) The neuropsychiatry of chronic cocaine abuse. J Neuropsychiatry Clin Neurosci 10:280–289. https://doi.org/10.1176/jnp.10.3.280
Brandão ML, Aguiar JC, Graeff FG (1982) GABA mediation of the anti-aversive action of minor tranquilizers. Pharmacol Biochem Behav 16:397–402. https://doi.org/10.1016/0091-3057(82)90441-5
Brandão ML, Viana DM, Masson S (2003) Neural organization of different types of fear: implications for the understanding of anxiety. Braz J Psychiatry 25:36–41. https://doi.org/10.1590/S1516-44462003000600009
Bueno CH Jr, Zangrossi H, Nogueira RL, Soares VP, Viana MB (2005) Panicolytic-like effect induced by the stimulation of GABAA and GABAB receptors in the dorsal periaqueductal gray of rats. Eur J Pharmacol 516:239–346. https://doi.org/10.1016/j.ejphar.2005.04.045
Butler AJ, Rehm J, Fischer B (2017) Health outcomes associated with crack-cocaine use: systematic review and meta-analyses. Drug Alcohol Depend 180:401–416. https://doi.org/10.1016/j.drugalcdep.2017.08.036
Campos AC, Fogaça MV, Aguiar DC, Guimarães FS (2013) Animal models of anxiety disorders and stress. Braz J Psychiatry 35(Suppl 2):S101–S111. https://doi.org/10.1590/1516-4446-2013-1139
Compton WM, Thomas YF, Stinson FS, Grant BF (2007) Prevalence, correlate, disability, and comorbidity of DSM-IV drug abuse and dependence in the united states: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry 64:566–576. https://doi.org/10.1001/archpsyc.64.5.566
Erhardt E, Zibetti LCE, Godinho JM, Bacchieri B, Barros HMT (2006) Behavioral changes induced by cocaine in mice are modified by a hyperlipidic diet or recombinant leptin. Braz J Med Biol Res 39:1625–1635. https://doi.org/10.1590/S0100-879X2006001200014
Falck RS, Wang J, Carlson RG (2008) Among long-term crack smokers, who avoids and who succumbs to cocaine addiction? Drug Alcohol Depend 98:24–29. https://doi.org/10.1016/j.drugalcdep.2008.04.004
Fernandez-de-Molina A, Hunsperger RW (1959) Central representation of affective reactions in forebrain and brain stem: electrical stimulation of amygdala, stria terminalis, and adjacent structures. J Physiol 145:251–265. https://doi.org/10.1113/jphysiol.1959.sp006140
Garcia RCT, Torres LH, Balestrin NT, Andrioli TC, Flório JC, Oliveira CDR, Costa JL, Yonamine M, Sandoval MRL, Camarini R, Marcourakis T (2017) Anhydroecgonine methyl ester, a cocaine pyrolysis product, may contribute to cocaine behavioral sensitization. Toxicology 376:44–50. https://doi.org/10.1016/j.tox.2016.04.009
Gawin FH (1988) Chronic neuropharmacology of cocaine: progress in pharmacotherapy. J Clin Psychiatry 49:11–16
Gawin FH (1989) Cocaine abuse and addiction. J Fam Pract 29:193–197
Graeff FG (2004) Serotonin, the periaqueductal gray and panic. Neurosci Biobehav Rev 28:239–259. https://doi.org/10.1016/j.neubiorev.2003.12.004
Graeff FG, Netto CF, Zangrossi H Jr (1998) The elevated T-maze as an experimental model of anxiety. Neurosci Biobehav Rev 23(2):237–246. https://doi.org/10.1016/S0149-7634(98)00024-4
Graeff FG (2012) New perspective on the pathophysiology of panic: merging serotonin and opioids in the periaqueductal gray. Braz J Med Biol Res 45:366–375. https://doi.org/10.1590/S0100-879X2012007500036
Graeff FG Jr, Zangrossi H (2002) Animal models of anxiety. In: D’Haenen H, Den Boer JA, Willner P (eds) Biological psychiatry. Wiley, London, pp 879–893
Gray JA, McNaughton N (2000) The Neuropsychology of Anxiety: an enquiry into the functions of the septo-hippocampal system. In: Oxford Psychology Series, Oxford University Press, USA 141:634. https://doi.org/10.1017/S000712500011373X
Gulley JM, Hoover BR, Larson GA, Zahniser NR (2003) Individual differences in cocaine-induced locomotor activity in rats: behavioral characteristics, cocaine pharmacokinetics, and the dopamine transporter. Neuropsychopharmacol 28:2089–2101. https://doi.org/10.1038/sj.npp.1300279
Hale MW, Shekhar A, Lowry CA (2012) Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol Neurobiol 32:695–708. https://doi.org/10.1007/s10571-012-9827-1
Hsu SM, Raine L (1881) Protein A, avidin, and biotin in immunohistochemistry. J Histochem Cytochem 29:1349–1353. https://doi.org/10.1177/29.11.6172466
Hsu SM, Raine L, Fanger H (1981) The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol 75:816–821. https://doi.org/10.1093/ajcp/75.6.816
Huang KW, Ochandarena NE, Philson AC, Hyun M, Birnbaum JE, Cicconet M, Sabatini BL (2019) Molecular and anatomical organization of the dorsal raphe nucleus. Elife 14:e46464. https://doi.org/10.7554/eLife.46464
Hyman SE (2001) A 28-year-old man addicted to cocaine. JAMA 286:2586–2594. https://doi.org/10.1001/jama.286.20.2586
Johnson PL, Hollis JH, Moratalla R, Lightman SL, Lowry CA (2005) Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J Psychopharmacol 19:327–341. https://doi.org/10.1177/0269881105053281
Johnson KW, Shamblen SR, Courser MW, Young L, Abadi MH, Browne T (2013) Drug use and treatment success among gang and non-gang members in El Salvador: a prospective cohort study. Subst Abuse Treat Prev Policy 8:20. https://doi.org/10.1186/1747-597X-8-20
Kalivas PW, McFarland K (2003) Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacol 168:44–56. https://doi.org/10.1007/s00213-003-1393-2
Kohtz AS, Paris JJ, Frye CA (2010) Low doses of cocaine decrease, and high doses increase, anxiety-like behavior and brain progesterone levels among intact rats. Horm Behav 57:474–480. https://doi.org/10.1016/j.yhbeh.2010.02.005
Lacayo A (1995) Mirthless laughter in a case of Creutzfeldt-Jakob disease. J Neuropsychiatry Clin Neurosci 7:386–387. https://doi.org/10.1176/jnp.7.3.386
Lankenau SE, Clatts MC, Goldsamt LA, Welle DL (2004) Crack cocaine injection practices and HIV risk: findings from New York and Bridgeport. J Drug Issues 34:319–332. https://doi.org/10.1177/002204260403400204
Laranjeira R, Mitsuhiro SS (2012) Addiction research centres and the nurturing of creativity. National Institute on Alcohol and Drugs Policies, Brazil. Addiction 107:727–732. https://doi.org/10.1111/j.1360-0443.2011.03387.x
Lipaus IFS, Gomes EF, Martins CW, Silva CME, Pires RGW, Malgarin F, Scuck PF, Nakamura-Palacios EM, Rodrigues LCM (2019) Impairment of spatial working memory and oxidative stress induced by repeated crack cocaine inhalation in rats. Behav Brain Res 359:910–917. https://doi.org/10.1016/j.bbr.2018.06.020
Lurie IS, Moore JM, Kram TC, Cooper DA (1990) Isolation, identification and separation of isomeric truxillines in illicit cocaine. J Chromatogr A 504:391–401. https://doi.org/10.1016/S0021-9673(01)89542-X
Maranhão LA, Fontes MK, Kamimura ASS, Nobre CR, Moreno BB, Pusceddu FH, Cortez FS, Lebre DT, Marques JR, Abessa DMS, Ribeiro DA, Pereira CDS (2017) Exposure to crack cocaine causes adverse effects on marine mussels Perna perna. Mar Pollut Bull 123:410–414. https://doi.org/10.1016/j.marpolbul.2017.08.043
Moretti EG, Yujra VQ, Claudio SR, Silva MJ, Vilegas W, Pereira CDS, Oliveira F, Ribeiro DA (2016) Acute crack cocaine exposure induces genetic damage in multiple organs of rats. Environ Sci Pollut Res Int 23:8104–8112. https://doi.org/10.1007/s11356-016-6141-3
Nankova BB, Rivkin M, Kelz M, Nestler EJ, Sabban EL (2000) Fos-related Antigen 2: Potential mediator of the transcriptional activation in rat adrenal medulla evoked by repeated immobilization stress. J Neurosci 20:5647–5653. https://doi.org/10.1523/JNEUROSCI.20-15-05647.2000
Nashold BS, Wilson WP, Slaughter DG (1969) Sensations evoked by stimulation in the midbrain of man. J Neurosurg 30:14–24. https://doi.org/10.3171/jns.1969.30.1.0014
Nestler EJ (2005) Neurobiology of cocaine addiction. Sci Pract Perspect 3:4–10. https://doi.org/10.1151/spp05314
Nestler EJ (2008) Transcriptional mechanisms of addiction: role of delta FosB. Philos Trans R Soc Lond B Biol Sci 363:3245–3255. https://doi.org/10.1098/rstb.2008.0067
Nestler EJ (2012) Transcriptional mechanisms of drug addiction. Clin Psychopharmacol Neurosci 10:136–143. https://doi.org/10.9758/cpn.2012.10.3.136
Nogueira RL, Graeff FG (1991) 5-HT mediation of the antiaversive effect of isamoltane injected into the dorsal periaqueductal gray. Behav Pharmacol 2:73–77. https://doi.org/10.1097/00008877-199102000-00010
Nutt DJ, King LA, Phillips LD (2010) Drug harms in the UK: a multicriteria decision analysis. Lancet 376:1558–1565. https://doi.org/10.1016/S0140-6736(10)61462-6
Paine TA, Jackman SL, Olmstead MC (2002) Cocaine-induced anxiety: alleviation by diazepam, but not buspirone, dimenhydrinate or diphenhydramine. Behav Pharmacol 13:511–523. https://doi.org/10.1097/00008877-200211000-00001
Paul ED, Lowry CA (2013) Functional topography of serotonergic systems supports the Deakin/Graeff hypothesis of anxiety and affective disorders. J Psychopharmacol 27:1090–1106. https://doi.org/10.1177/0269881113490328
Paxinos G, Watson C (2008) The rat brain in stereotaxic coordinates, compact, 6th edn. Academic Press, Sidney
Pereira CDS, Maranhão LA, Cortez FS, Pusceddu FH, Santos AR, Ribeiro DA, Cesar A, Guimarães LL (2016) Occurrence of pharmaceuticals and cocaine in a Brazilian costal zone. Sci Total Environ 548–549:148–154. https://doi.org/10.1016/j.scitotenv.2016.01.051
Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Elmore RG, Knapp DJ, Selley DE, Martin B, Sim-Selley L, Bachtell RK, Self DW, Nestler EJ (2008) Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse 62:358–369. https://doi.org/10.1002/syn.20500
Pires CM, Cavaco AM (2014) Communication between health professionals and patients: review of studies using the RIAS (Roter Interaction Analysis System) Method. Rev Assoc Med Bras 60:156–172. https://doi.org/10.1590/1806-9282.60.02.014
Plowman T, Rivier L (1983) Cocaine and cinnamoylcocaine content of Erythroxylum species. Ann Bot 51(5):641–659. https://doi.org/10.1093/oxfordjournals.aob.a086511
Poltronieri SC Jr, Zangrossi H, Viana MB (2003) Antipanic-like effect of serotonin reuptake inhibitors in the elevated T-maze. Behav Brain Res 147:185–192. https://doi.org/10.1016/S0166-4328(03)00151-7
Ribeiro M, Trevizol AP, Frajzinger R, Robeiro A, Speierl H, Pires L, Andraus M, Tsanaclis L, Alonso ALS, Cordeiro Q, Laranjeira R (2019) Adulterants in crack cocaine in Brazil. Trends Psychiatry Psychother 41:186–190. https://doi.org/10.1590/2237-6089-2017-0143
Riezzo I, Fiore C, Carlo DD, Pascale N, Neri M, Turillazzi E, Fineschi V (2012) Side effects of cocaine abuse: multiorgan toxicity and pathological consequences current. Med Chem 19:5624–5646. https://doi.org/10.2174/092986712803988893
Roelofs K (2017) Freeze for action: neurobiological mechanisms in animal and human freezing. Philos Trans R Soc Lond B Biol Sci 372(1718):20160206. https://doi.org/10.1098/rstb.2016.0206
Rogerio R, Takahashi RN (1992) Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacol Biochem Behav 43:631–633. https://doi.org/10.1016/0091-3057(92)90203-R
Rosário BA, Nazaré MFS, Estadella D, Ribeiro DA, Viana MB (2018) Behavioral and neurobiological alterations induced by chronic use of crack cocaine. Rev Neurosci 31:59–75. https://doi.org/10.1515/revneuro-2018-0118
Rosen MI, Kosten T (1992) Cocaine-associated panic attacks in methadone-maintained patients. Am J Drug Alcohol Abuse 18:57–62. https://doi.org/10.3109/00952999209001611
Sela VR, Biesdorf C, Ramos DH Jr, Zangrossi H, Graeff FG, Audi EA (2011) Serotonin-1A receptors in the dorsal periaqueductal gray matter mediate the panicolytic-like effect of pindolol and paroxetine combination in the elevated T-maze. Neurosci Lett 495:63–66. https://doi.org/10.1016/j.neulet.2011.03.040
Shanti RM, Khan J, Eliav E, Ziccardi VB (2013) Is there a role for a collagen conduit and anti-inflammatory agent in the management of partial peripheral nerve injuries. Int J Oral Maxillofac Surg 71:1119–1125. https://doi.org/10.1016/j.joms.2011.03.054
Shihomatsu HM, Martins EAJ, Cotrim MEB, Lebre DT, Ortiz N, Pires MAF (2017) Development and validation of methodology SPE-LC-MS/MS for pharmaceuticals and illicit drugs determination in Guarapiranga Reservoir Waters-São Paulo/SP, Brazil. J GEP 5:1–17. https://doi.org/10.11606/T.85.2015.tde-28042015-095207
Smith JP, Book SW (2010) Comorbidity of generalized anxiety disorder and alcohol use disorders among individuals seeking outpatient substance abuse treatment. Addict Behav 35:42–45. https://doi.org/10.1016/j.addbeh.2009.07.002
Spiacci A Jr, Coimbra NC Jr, Zangrossi H (2012) Differential involvement of dorsal raphe subnuclei in the regulation of anxiety-and-panic-related defensive behaviors. Neurosci 227:350–360. https://doi.org/10.1016/j.neuroscience.2012.09.061
Stansley BJ, Yamamoto BK (2014) Chronic L-dopa decreases serotonin neurons in a subregion of the dorsal raphe nucleus. J Pharmacol Exp Ther 351:440–447. https://doi.org/10.1124/jpet.114.218966
Takahashi RN, Rogerio R (1991) Nifedipine interacts with cocaine in the elevated plus-maze in rats. Res Comm Subst Abuse 12:19–26
Teixeira RC Jr, Zangrossi H, Graeff FG (2000) Behavioral effects of acute and chronic imipramine in the elevated T maze model of anxiety. Pharmacol Biochem Behav 65:571–576. https://doi.org/10.1016/S0091-3057(99)00261-0
Thomsen M, Caine SB (2011) Psychomotor stimulant effects of cocaine in rats and 15 mouse strains. Exp Clin Psychopharmacol 19:321–341. https://doi.org/10.1037/a0024798
United Nations Office on Drugs and Crime (UNODOC) (2014) World Drug Report 2014, Vienna, Austria
Vaughn MG, Fu Q, Perron BE, Bohnert ASB, Howard MO (2010) Is crack cocaine use associated with greater violence than powdered cocaine use? Results from a national sample. Am J Drug Alcohol Abuse 36:181–186. https://doi.org/10.3109/00952990.2010.491877
Viana MB, Tomaz CA, Graeff FG (1994) The elevated T-maze: a new animal model of anxiety and memory. Pharmacol Biochem Behav 49:549–554. https://doi.org/10.1016/0091-3057(94)90067-1
Waters RP, See RE (2011) Chronic cocaine self-administration attenuates the anxiogenic-like and stress potentiating effects of the benzodiazepine inverse agonist FG 7142. Pharmacol Biochem Behav 99:408–413. https://doi.org/10.1016/j.pbb.2011.05.021
Wscieklica T, Silva MSCF, Lemes JA, Melo-Thomas L, Céspedes IC, Viana MB (2017) Deep brain stimulation of the dorsal raphe inhibits avoidance and escape reactions and activates forebrain regions related to the modulation of anxiety/panic. Behav Brain Res 321:193–200. https://doi.org/10.1016/j.bbr.2016.11.054
Yang XM, Gorman AL, Dunn AJ, Goeders NE (1992) Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav 41:643–650. https://doi.org/10.1016/0091-3057(92)90386-T
Yoshida M, Shirouzu M, Tanaka M, Semba K, Fibiger HC (1989) Dopaminergic neurons in the nucleus raphe dorsalis innervate the prefrontal cortex in the rat: a combined retrograde tracing and immunohistochemical study using anti-dopamine serum. Brain Res 496:373–376. https://doi.org/10.1016/0006-8993(89)91091-3
Young SL, Vosper HJ, Phillips SA (1992) Cocaine: its effects on maternal and child health. Pharmacotherapy 12:2–17. https://doi.org/10.1002/j.1875-9114.1992.tb02664.x
Zubaran C, Foresti K, Thorell MR, Franceschini P, Homero W (2010) Depressive symptoms in crack and inhalant users in southern Brazil. J Ethn Subst Abuse 9:221–236. https://doi.org/10.1080/15332640.2010.501626
Zubaran C, Foresti K, Thorell MR, Franceschini PR (2013) Anxiety symptoms in crack cocaine and inhalant users admitted to a psychiatric hospital in southern Brazil. Rev Assoc Med Bras 59:360–367. https://doi.org/10.1016/j.ramb.2013.01.008
Acknowledgements
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. Bárbara dos Anjos Rosário was the recipient of a fellowship grant from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; grant 001), Brazil. Maria de Fátima Santana Nazaré was the recipient of a fellowship grant from FAPESP, Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Anjos Rosário, B., de Fátima Santana de Nazaré, M., Lemes, J.A. et al. Repeated crack cocaine administration alters panic-related responses and delta FosB immunoreactivity in panic-modulating brain regions. Exp Brain Res 239, 1179–1191 (2021). https://doi.org/10.1007/s00221-020-06031-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-020-06031-2