Abstract
In the present study, we examined the role of the cerebellum in temporal adaptive learning during a coincident timing task, i.e., a baseball-like hitting task involving a moving ball presented on a computer monitor. The subjects were required to change the timing of their responses based on imposed temporal perturbations. Using paired-pulse transcranial magnetic stimulation, we measured cerebellar brain inhibition (CBI) before, during, and after the temporal adaptive learning. Reductions in CBI only occurred during and after the temporal adaptive learning, regardless of the direction of the temporal perturbations. In addition, the changes in CBI were correlated with the magnitude of the adaptation. Here, we showed that the cerebellum is essential for learning about and controlling the timing of movements during temporal adaptation. Furthermore, changes in cerebellar-primary motor cortex connectivity occurred during temporal adaptation, as has been previously reported for spatial adaptation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been reported that the cerebellum plays an essential role in both aspects of timing control, i.e., perception and movement (Ivry et al. 1988; Ivry and Keele 1989; Ivry 1996; Ivry et al. 2002). In a study involving a perceptual prediction task, for example, the posterior cerebellum (lobule VII crus 1) was specifically engaged when the subjects estimated the location of a target based on temporospatial information (O’Reilly et al. 2008). Other studies have revealed that patients with cerebellar disorders displayed worse performance than healthy subjects in an interception task (Bares et al. 2007, 2010, 2011). This task required the subjects to press a button at the optimal time to intercept a moving target presented on a computer screen. In another study involving the same task, baseball players, who are generally well trained in predicting the future state of their surroundings and have refined internal timing-control models, displayed better quantitative performance than the control subjects (Markova et al. 2020). During various daily activities and sports, such as driving a car, playing musical instruments, baseball batting, or playing tennis, timing control is important for performing accurate and smooth movements. To execute such skillful motor tasks, it is necessary to adapt to changes in external circumstances that affect the timing of actions. For instance, hitting a ball in a controlled manner using a tool, such as a baseball bat or tennis racket, requires appropriate context-specific timing adjustment, i.e., coincident timing (CoIT) skill. Previous studies (Bares et al. 2007, 2010, 2011; Markova et al. 2020) have indicated that the cerebellum plays an important role in CoIT (which requires the integration of incoming visual information, such as information regarding the velocity, location, and trajectory of a target, and a timely motor response).
The cerebellum has been shown to play an important role in error-based learning, e.g., it contributed to spatial adaptive learning in a visuomotor reaching task (Schlerf et al. 2012; Spampinato et al. 2017). In these studies, the subjects were required to relearn the relationships between the directions of their reaching movements and the directions in which a cursor shown on a monitor was moving. In addition, changes in cerebellar–primary motor cortex (M1) connectivity have been detected after motor learning in humans using paired-pulse transcranial magnetic stimulation (TMS). In these studies, the cerebellum was stimulated by delivering a conditioning stimulus (CS) to a location 3 cm lateral to the inion, on the line joining the inion and the external auditory meatus (Ugawa et al. 1995; Pinto and Chen 2001). Stimulating this site leads to the activation of Purkinje cells (PCs), probably in the cerebellar lobules V, VII and VIII (Hardwick et al. 2014; Spampinato and Celnik 2020). Compared with the motor evoked potentials (MEPs) elicited by the delivery of a test stimulus (TS) alone, those seen after the delivery of a CS to the cerebellum followed by the delivery of a TS to the contralateral M1 were suppressed. This suppression, i.e., cerebellar brain inhibition (CBI), has been suggested to reflect the activation of cerebellar PCs (Celnik 2015; Fernandez et al. 2018). The CBI technique is useful for assessing how cerebellar–M1 connectivity is affected by spatial adaptive learning (Schlerf et al. 2012), and the changes in CBI found following visuomotor adaptation were found to occur in a somatotopic-specific manner (Spampinato et al. 2017). Furthermore, applying anodal transcranial direct current stimulation (tDCS) over the cerebellum was found to enhance adaptive learning during a visuomotor reaching task and a sequential visual isometric pinch task (SVIPT) (Galea et al. 2011; Hardwick and Celnik 2014; Cantarero et al. 2015). These studies indicated that to perform accurate and smooth movements it is necessary to update internal models or acquire new ones in the cerebellum, as the situation demands. Moreover, Spampinato and Celnik (2018) showed that sequence learning, i.e., when a new sequence of movements on a known sensorimotor map is learnt, also involves changes in cerebellar–M1 connectivity and long-term potentiation-like plasticity in the M1. Penhune and Steele (2012) suggested that the cerebellum plays an essential role in sequence learning, specifically in the acquisition of new internal models, which optimize parameters such as the velocity, force, and timing of movements in a particular context. Another study showed that CBI was only reduced in the region responsible for the muscle involved in the target movement, and this reduction occurred closer to the initiation of movement during a reaction time task (Spampinato et al. 2017). These findings indicate that the cerebellum plays an important role in controlling the timing of movements.
Although previous studies involving the paired-pulse TMS technique indicated that the cerebellum plays an important role in spatial adaptive learning, its role in temporal adaptation has not been examined in detail. Therefore, the aim of the present study was to investigate the role of the cerebellum in temporal adaptive learning. We hypothesized that the cerebellum also plays an important role in temporal adaptive learning, as was reported for various other kinds of adaptation in previous studies (Donchin et al. 2012; Izawa et al. 2012; Morton and Bastian 2006; Prsa and Their 2011; Rabe et al. 2009; Schlerf et al. 2012; Schlerf et al. 2013; Spampinato et al. 2017). To examine this hypothesis, we used the paired-pulse TMS paradigm to assess the changes in cerebellar–M1 connectivity that occur before, during, and after temporal adaptive learning.
Materials and methods
Subjects
Twenty healthy right-handed subjects (two females) with no history of neurological or psychiatric disease participated in the present study. We determined the handedness of each subject with the Edinburgh Handedness Inventory (Oldfield 1971). All of the subjects gave their written informed consent before the experiments. All experimental procedures were conducted in accordance with the Declaration of Helsinki and were approved by the ethics committee of Hiroshima University. After the preliminary experiment, all of the subjects were randomly assigned to one of the two groups described below. Each subject was comfortably seated on a reclined chair and allowed to relax. Then they were instructed to put both of their hands in the prone position on a horizontal plate attached to the chair’s armrests. A computer monitor (ASUSTeK Computer Inc., ROG SWIFT PG258Q, size: 24.5 in., refresh rate: 240 Hz), which was used to present the motor task (see “CoIT task”), was placed ~ 1 m in front of them. All of the subjects stated that they had normal or corrected normal vision.
CoIT task
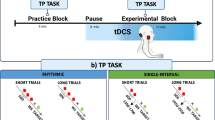
We programmed a custom-made CoIT task, which was based on baseball batting, using LabVIEW (National Instruments Japan, Co.). A schematic illustration of the four phases of the CoIT task (the warning signal, the release of the ball, hitting the ball, and the end of the trial), which were successively represented on the computer monitor, is shown in Fig. 1a. The subjects were instructed to left-click the computer mouse (Razer Inc., DeathAdder Elite, polling rate: 1000 Hz) using their right index finger to trigger a bat swing in the right-handed batter’s box presented on the screen, to hit the ball towards a target area in the center field (located between two red bars shown at the top of the computer monitor). The ball moved straight down the screen from its starting position at a constant speed 300–400 ms after the warning signal, “READY?” When the ball was hit so that it “landed” right in the middle of the area between the two red bars (± 5°), we defined the error as 0 degrees. According to a previous review (Buhusi and Meck 2005), the cerebellum plays an important role in motor control in the sub-second time range. From this point of view, we prepared a CoIT task that required the subjects to respond within 1 s under all of the temporal perturbation conditions described below. Based on this inherent temporal framework and the results of the preliminary experiments, we adopted the following ball velocity and bat swing speed values. The velocity of the ball was set at 24.3 cm/s, and the swing speed of the bat was set at \(\frac{25}{9}\pi \) rad/s in the control (ctrl) conditions. The velocity of the ball did not change throughout the experiment. Under these parameters, the subjects were required to left-click the computer mouse at 520 ms after the ball was released to get it to land in the middle of the area between the two red bars. At the baseline and during the washout stage, the subjects conducted the task using the abovementioned parameters as the baseline conditions. In the learning stage, the bat swing speed was abruptly changed so that it was faster (cond F: \(\frac{25}{3}\pi \) rad/s) or slower (cond S: \(\frac{5}{3}\pi \) rad/s) than in the baseline conditions. Thus, to prevent CoIT errors and hit the ball so that it landed in the middle of the area between the two red bars the subjects had to left-click the mouse 120 ms later/earlier. The subjects were not informed of the optimal response timings for each condition (ctrl: 520 ms, cond F: 640 ms, cond S: 400 ms after the ball was released). In the pseudo-randomized conditions (cond R), the bat swing speed was changed unpredictably to one of the 3 conditions described above, i.e., ctrl, cond F, or cond S, and sequences in which the same conditions were presented repeatedly were avoided where possible.
a Schematic illustration of the CoIT task. Panels 1–4 show each phase of the CoIT task, which was displayed on a computer monitor. A ball was released at a constant speed 300–400 ms after the appearance of a warning signal. The subjects were instructed to hit the ball so that it “landed” in a target area (located between the two red bars), regardless of the swing speed of the bat. b Schematic illustration of the experimental protocol. The upper and central panels show the procedure in cond F (faster swing perturbation) and cond S (slower swing perturbation), respectively. The lower panel illustrates the procedure in cond R (randomized perturbation). The experiment was composed of five stages, which are represented by thick black lines, i.e., the baseline, early learning stage, late learning stage, post 1 washout stage, and post 2 washout stage. We measured CBI at three timepoints, i.e., before, during, and after adaptive learning. The measurements are represented by inverted triangles. During the learning stage, the bat swing speed was changed abruptly so that it became faster (F) or slower (S). In condition R, the bat swing speed changed unpredictably to the speed employed in cond F, cond S, or ctrl. After the practice session, no visual feedback regarding the subjects’ bat swing movements was provided. Each block consisted of 10 task trials
Experimental procedure
Prior to the experiments, the subjects were randomly assigned to one of two groups, the faster swing group (group F: 10 males, mean age: 21.6 ± 2.0 years) and slower swing group (group S: 8 males and 2 females, mean age: 22.3 ± 2.8 years). All subjects participated in two sessions (group F: cond F and cond R, group S: cond S and cond R) in a crossover design across 2 days. Each session occurred at least three days apart, except in the case of one subject who completed the sessions on two successive days. All subjects in both groups were subjected to the same 5-stage experimental protocol (Fig. 1b). At first, the subjects practiced 30 trials (10 trials × 3 blocks) to familiarize themselves with the CoIT task. Visual feedback regarding their bat swing trajectories was only given during this stage. After the practice stage, they performed 150 trials (10 trials × 15 blocks) during the baseline stage, followed by 50 trials (10 trials × 5 blocks) in the early learning stage and 100 trials (10 trials × 10 blocks) in the late learning stage. After the learning stage, another 150 trials were performed as the washout stage (post 1: 10 trials × 5 blocks, post 2: 10 trials × 10 blocks). Thirty-second rest was taken between each block throughout the experiment. While the movement of the ball was shown throughout the experiment, feedback regarding the subjects’ bat swing trajectories was only given during the practice stage. This meant that the subjects had to adapt to the temporal perturbations based solely on trial-by-trial adjustment of the timing of their responses to the ball.
EMG recording
Surface electromyographic (EMG) activity was recorded from the right first dorsal interosseous muscle (FDI) with Ag/AgCl surface electrodes (diameter: 9 mm). Before the start of the experiment, the skin was treated with a mild abrasive gel and then cleaned with isopropyl alcohol to reduce impedance. All EMG recordings were amplified at a bandwidth ranging from 5 Hz to 3 kHz, and all procedures were controlled using a signal processor (NEC San-ei Co. Ltd., Japan, 7S12). The analog outputs from the signal processor were digitized at a sampling rate of 10 kHz and stored on a computer for off-line analysis (PowerLab system, AD Instruments Pty., Ltd., Australia).
TMS application and CBI recording
TMS was delivered as a TS to the left M1 using a Magstim 200 stimulator, connected to a figure-of-eight coil with an external diameter of 90 mm. The coil was placed tangentially to the scalp, with the handle pointed backward at a 45° angle with respect to the anteroposterior axis. The optimal position for evoking MEPs from the right FDI was determined and marked on a nylon mesh swimming cap worn by the subjects with a soft-tip pen to ensure reliable coil placement throughout the experiment. Using a paired-pulse TMS paradigm, we assessed CBI before, during, and after temporal adaptation (Fig. 1b). A double-cone coil with a diameter of 110 mm was centered over the right cerebellar cortex, at 3 cm lateral to the inion, on the line joining the inion and the external auditory meatus, as described in previous studies (Ugawa et al. 1995; Pinto and Chen 2001). The CBI measurements were obtained by performing TMS to deliver a CS over the right cerebellar cortex 5 ms before the TS was delivered over the left M1. The intensity of the TS was adjusted to evoke MEPs with a mean peak-to-peak amplitude of 1–2 mV. In a previous study (Baarbe et al. 2014), the CS intensity was set at a level at which cerebellar–M1 stimulation elicited MEPs that were 50% of the size of the MEPs evoked by the TS alone (CBI50). However, we set the CS intensity at a level that elicited MEPs of 70% of the size of the MEPs evoked by the TS alone (CBI70) in the present experiment to reduce the subjects’ discomfort and make it easy to check for bidirectional CBI modulation. First, we started at a CS intensity of 50% of the maximum stimulator output (MSO). If we could not obtain measurements around CBI70, we increased the CS intensity in 5% steps from 50 to 70% of the MSO and/or changed the inter-stimulus interval (set at 6 ms) between the CS and TS. The maximum CS intensity was set at 70% of the MSO to reduce the probability of cervicomedullary evoked potentials (CMEPs) being elicited. Ten paired-pulse TMS (CS + TS) stimuli and 10 single TMS (TS alone) stimuli were randomly delivered. CBI is expressed as a ratio of the mean MEP amplitude elicited by the CS + TS to the mean MEP amplitude evoked by the TS alone. We measured CBI three times during the experiment, i.e., after the baseline stage, after the early learning stage, and after the late learning stage. Muscle relaxation was monitored based on visual and audio feedback derived from the EMG signals throughout the TMS session. If any involuntary EMG activity was detected in the resting FDI, the associated measurement data were omitted from the data analyses. The root mean square (RMS) values of the background EMG (bEMG) activity seen during the 50-ms period just before the stimulation were determined. Data associated with bEMG activity of > 25 µV were excluded from the off-line analysis.
In a preliminary experiment, we assessed the optimal TMS intensity threshold for brainstem stimulation. We delivered TMS over the inion with a double-cone coil (the same coil as described above), and the stimulator current was directed downward (Ugawa et al. 1995). The subjects pre-activated the FDI using their right index finger by pressing a horizontal plate attached to the chair’s armrests during this session. Then we determined the TMS intensity that evoked CMEPs after 5 of 10 stimuli. We started the stimulation at an intensity of 50% of the MSO, and increased it in 10% steps. If a CS intensity of 80% of the MSO did not elicit CMEPs, a CS intensity of 70% of the MSO, which we employed as the maximum intensity during the main experiment, certainly would not have activated the pyramidal tract neurons.
Measurements and statistical analyses
To assess the subjects’ motor task performance, we evaluated the click time (CT), constant error (CE), and absolute error (AE). The CT was defined as the time interval between the moment at which the ball started moving (the reaction cue) and the moment at which the subject left-clicked the computer mouse to hit the ball. The CT was normalized by subtracting 520 ms (the optimal CT under the ctrl conditions) from the raw data. The CE was defined as the angle between two lines, i.e., the line connecting the contact point with the starting position of the pitch, and the line connecting the contact point with the destination of the ball after it was hit (Fig. 1a, phase 4). If the optimal CT was achieved, and the ball landed right in the middle of the area between the two red bars, we defined the error as 0°. Therefore, negative and positive CE values indicated that the subject had underestimated or overestimated the time when they should have hit the incoming ball, resulting in the ball landing on the left or right side of the field, respectively. In other words, the CE represents the bias in the timing of the subjects’ responses. The AE was defined as the absolute value of CE and indicated the subjects’ accuracy during the CoIT.
We counted the number of trials in which the AE was ≤ 5° and recorded it as the success count. If a subject swung the bat and missed the ball, the CE was incalculable. Thus, we recalculated the CE based on the linear regression equations for the relationships between the CE and CT in each of the temporal perturbation conditions (see Supplementary Fig. 1). The equations were obtained from another 900 trials (300 trials in each of the 3 conditions) performed by one of the authors. The CT, CE, and AE were all calculated by averaging 10 trials for each block. Mean values were calculated by binning the blocks for each learning stage. Fifteen blocks during the baseline stage were averaged to produce the baseline value. The first 5 blocks during the learning stage were averaged to produce the early learning stage value, and the latter 10 blocks were averaged to produce the late learning stage value. The same binning process was applied to the data for the washout stage, with the first 5 blocks averaged to produce the post 1 value and the latter 10 blocks averaged to produce the post 2 value.
In addition, we carried out correlation analysis by calculating Pearson’s product-moment correlation coefficients (r values) for the relationships between CBI and the CT or CE in each of the temporal perturbation conditions. The changes in CBI were calculated by subtracting the baseline values from the late learning stage values. Regarding the CT and CE, in the both cond F and cond S the amounts of adaptation were calculated by subtracting the values for the first block during the early learning stage from those for the last block during the late learning stage (block numbers 16 and 30, respectively; see Fig. 1b upper and central panels and Fig. 2). These calculations were conducted to examine how CBI changed associated with temporal adaptive learning. Unlike the latter analyses, in cond R the changes in the CT and CE were calculated as the differences between the last block during the baseline stage and last block during the late learning stage (block numbers 15 and 30, respectively; see Fig. 1b lower panel and Fig. 2). We selected different blocks for the analyses conducted in cond R because we initially expected that no learning would occur in these pseudo-randomized conditions. Therefore, we examined how CBI and the subjects’ behavior changed before and after they experienced pseudo-randomized temporal perturbations.
Trial to trial changes in the CT (ms) (a, b) and CE (degrees) (c, d) seen in each experimental stage in groups F (left side) and S (right side). The thick black lines represent each stage (the baseline, the early and late learning stages, and the post 1 and post 2 washout stages). The horizontal axes indicate the number of blocks. The mean (lines) and SE (shaded areas) values obtained in the faster (cond F), slower (cond S), and random (cond R) perturbation conditions are represented in green, blue, and red, respectively. Negative CT and CE values indicated an earlier swing at the ball, which would result in the ball being hit to the left. The opposite was true for positive values
All statistical analyses were performed with the software R (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria). Two-way repeated-measures ANOVA was used to compare CoIT task performance, bEMG activity, and the CBI ratio during the temporal adaptive learning in each group, and to compare the miss percentage in cond R between the groups (perturbation conditions × learning stage). If an interaction reached the threshold for significance (p < 0.05), the simple main effects were examined. Bonferroni’s post hoc test for multiple comparisons was used for further analyses. Mauchly’s test was used to test for sphericity before each ANOVA. If the value of epsilon was < 1, the Greenhouse–Geisser correction was used for non-spherical data. All data are shown as the mean ± standard error (SE).
Results
All subjects completed two sessions. The mean miss percentages were as follows: 0.8% in cond F in group F, 2.3% in cond R in group F, 0.1% in cond S in group S, and 3.5% in cond R in group S. An excessively fast swing was the main cause of misses (91%). In fact, only one miss was caused by a slower swing in cond S, and the miss percentage did not decrease during the learning stage in cond R in either group. If a subject did not respond to the target due to a lack of attention or the CT was longer than 850 ms, the associated trials were excluded from the analyses (0.04% of all data).
CoIT task performance
Figure 2 shows the changes in the CT (a, b) and CE (c, d) seen in groups F and S. The horizontal axes indicate the number of blocks. The thick black lines represent each learning stage. In both groups, the subjects apparently adapted to the temporal perturbations during the learning stage, regardless of whether cond F (green line: mean, shaded area: SE) or cond S (blue line: mean, shaded area: SE) was employed. The CT changed abruptly in the early learning phase and gradually approached the optimal CT in the late learning phase in response to each type of temporal perturbation. The CE also changed abruptly in the early phase after the temporal perturbations, and it quickly returned to the baseline level. However, in cond R the CE showed a somewhat negative bias during the learning stage compared with that seen at the baseline in both groups (Fig. 2c, d, red line: mean, shaded area: SE). We also conducted statistical analyses using binned data, as described above. Figure 3 shows the results obtained regarding the CT (A, B), CE (C, D) and the success count (E, F), based on the binned data for each learning stage in groups F and S. The success count represents the degree of accuracy of the timing adjustments performed by the subjects compared with the adjustments that would have resulted in the ball being hit into the target area. The green, blue, and red bars represent cond F, cond S, and cond R, respectively. We performed two-way repeated-measures ANOVA of the CT data for group F (Fig. 3a). As a result, a significant interaction (perturbation conditions × learning stage: F4, 36 = 71.23, p < 0.01) was detected. Therefore, we examined the simple main effects of each of the temporal perturbation conditions, and simple main effects were detected in both conditions (cond F: F4, 36 = 290.37, p < 0.01; cond R: F4, 36 = 4.94, p < 0.01). In addition, significant differences in the CT were detected between the early and late learning stages, and between the post 1 and post 2 washout stages in cond F. In cond R, significant changes in the CT were detected between the baseline and late learning stage. Furthermore, two-way repeated-measures ANOVA of the CT data for group S was also carried out (Fig. 3b). A significant interaction (perturbation conditions × learning stage: F1.43, 12.91 = 43.04, p < 0.01) was detected. A simple main effect of learning stage was also found in cond S (cond S: F2.29, 21.10 = 202.48, p < 0.01). In addition, significant differences in the CT were detected between the early and late learning stages, and between the post 1 and post 2 washout stages. These results indicated that the CT became longer/shorter in response to the temporal perturbations and returned to the baseline during the washout stage in both cond F and cond S, and the changes in the CT seen in cond R in group F were not as marked as those seen in cond F or cond S.
Changes in the mean (± SE) CT (ms) (a, b), CE (degrees) (c, d), and the success count (e, f) in each experimental stage in groups F (left side) and S (right side). Values were obtained by analyzing binned data. The success count represents the number of trials in which the AE was ≤ 5°. Cond F, cond S, and cond R are represented by green, blue, and red bars, respectively. The daggers (cond F and S) and hashes (cond R) indicate significant differences compared with the baseline. The asterisks indicate significant differences between the stages
Next, we conducted two-way repeated-measures ANOVA of the CE data for group F (Fig. 3c). As a result, a significant interaction (perturbation conditions × learning stage: F2.17, 19.55 = 6.6, p < 0.01) was detected. Simple main effects of the temporal perturbation conditions were found in both conditions (cond F: F1.61, 14.50 = 46.01, p < 0.01; cond R: F2.3, 20.7 = 7.04, p < 0.01). In addition, in cond F significant differences in the CE were detected between the early and late learning stages, and between the post 1 and post 2 washout stages, which indicated that the learning and washout stages progressed well after the temporal perturbation. Two-way repeated-measures ANOVA of the CE data for group S was also carried out (Fig. 3d). A significant interaction (perturbation conditions × learning stage: F1.39, 12.53 = 22.31, p < 0.01) was detected. A simple main effect of learning stage (cond S: F2.34, 21.10 = 32.66, p < 0.01; cond R: F1.36, 12.21 = 10.60, p < 0.01) was also found. In addition, significant differences in the CE between the early and late learning stages, and between the post 1 and post 2 washout stages were noted. In cond R, there was no bias in the responses seen in the early learning stage, and no learning occurred during the learning stage in either group.
Figure 3e, f shows the success counts of groups F and S, respectively. Two-way repeated-measures ANOVA of the data for group F (Fig. 3e) showed a significant main effect of learning stage (F2.49, 22.43 = 56.33, p < 0.01), but the perturbation conditions did not have a significant simple main effect (F1, 9 = 5.09, p = 0.051). No interaction (perturbation conditions × learning stage: F1.7, 18.35 = 0.47, p = 0.61) was detected. In group S (Fig. 3f), a significant interaction (perturbation conditions × learning stage: F4, 36 = 31.86, p < 0.01) was seen. Simple main effects of learning stage (F4, 36 = 3.81, p < 0.05) and the perturbation conditions (F4, 36 = 31.70, p < 0.01) were also detected. In addition, the success count, i.e., accuracy, increased significantly between the early and late learning stages. In cond R, the performance seen during the early learning stage was worse than that observed at the baseline, and the subjects’ performance was never better than that seen at the baseline.
Changes in cerebellar–M1 connectivity associated with temporal adaptive learning
We measured cerebellar–M1 connectivity by assessing CBI using the paired-pulse TMS paradigm at the baseline and in the early and late learning stages. We observed no significant differences in bEMG activity between the conditions in either group (Table 1, group F; perturbation condition: F1, 9 = 0.0023, p = 0.96, learning stage: F2, 18 = 0.38, p = 0.69, perturbation conditions × learning stage: F2, 18 = 1.53, p = 0.24, group S; perturbation condition: F1, 9 = 0.38, p = 0.55, learning stage: F2, 18 = 0.45, p = 0.64, perturbation conditions × learning stage: F2, 18 = 1.00, p = 0.39). Figure 4a, b shows typical examples of the averaged MEP waveforms (n = 10) recorded at the baseline and during the early and late learning stages after the delivery of a TS alone (black line) or a CS followed by a TS (green line: cond F, blue line: cond S, red line: cond R) in one subject each from group F and group S, respectively. Figure 4c, d shows the changes in the CBI ratio seen in groups F and S, respectively. In group F, a significant interaction (perturbation conditions × learning stage: F1.73, 15.60 = 4.90, p < 0.05) was detected. A significant simple main effect of time was observed in cond F (F1.27, 11.44 = 13.78, p < 0.01), but not in cond R (F1.88, 16.89 = 2.76, p = 0.09). In group S, a significant interaction (perturbation conditions × learning stage: F2, 18 = 5.38, p < 0.05) was detected. A significant simple main effect of time was also detected in cond S (F2, 18 = 20.52, p < 0.01), but not in cond R (F2, 18 = 1.32, p = 0.29). In addition, the significant differences in the CBI ratio observed between the baseline and the early or late learning stages indicated that prolonged reductions in CBI occurred during and after adaptive learning in the CoIT task in both cond F and cond S. Furthermore, the magnitude of the CBI tended to be lower during the late learning stage than during the early learning stage in cond S (p = 0.07).
a, b Typical examples of averaged MEP waveforms (n = 10) recorded at the baseline or during the early or late learning stages. The MEPs were recorded after the delivery of a TS alone over the left M1 (black line) or after the delivery of a CS over the right cerebellum and a TS over the left M1 (green line: cond F, blue line: cond S, red line: cond R) in one subject each from groups F (a) and S (b). Changes in the mean (± SE) CBI ratio between the baseline and the early or late learning stages in groups F (c) and S (d). Cond F, cond S, and cond R are represented by green, blue, and red bars, respectively. The asterisks indicate significant differences between the stages
The correlations between the amount of adaptive learning and changes in CBI
Figure 5 shows the relationships between the changes in CBI and the changes in the CT (A, C, E) or CE (B, D, F) that occurred during CoIT-related adaptive learning in cond F (green circles), cond S (blue circles), or cond R in group F (red circles). The analyses revealed a correlation between the changes in CoIT task performance and the changes in CBI associated with adaptation. We found that the subjects who exhibited greater CT improvements displayed larger reductions in CBI in both cond F (A: r = 0.64, p < 0.05) and cond S (C: r = − 0.70, p < 0.05). In both groups, we confirmed that the subjects who adapted the most (i.e., those that demonstrated the greatest CT improvements in each of the perturbation conditions throughout the learning stage) exhibited the largest reductions in CBI. Furthermore, the reduction in CBI was correlated with the change in the CE (i.e., the CE plateaued) in cond S (D: r = − 0.70, p < 0.05). However, there was no significant correlation between the changes in the CE and CBI in cond F (B: r = 0.53, p = 0.12). Although the changes in the CT and CE seen after the imposition of temporal perturbations were correlated with the changes in CBI, the subjects could not adapt sufficiently to the temporal perturbations in cond R in group F (E: CT and CBI, r = 0.72, p < 0.05; F: CE and CBI: r = 0.70, p < 0.05).
Discussion
The main finding of the present study was that a reduction in CBI occurred during temporal adaptive learning in a CoIT task, regardless of the temporal perturbation conditions, i.e., whether cond F or cond S was employed, as has been reported for locomotor adaptive learning and spatial adaptive learning (Jayaram et al. 2011; Schlerf et al. 2012; Spampinato et al. 2017). However, the CBI ratio did not change in cond R, in which the subjects’ performance did not improve. Furthermore, in both cond F and cond S, we confirmed that the subjects who adapted the most exhibited the largest reductions in CBI, which was consistent with previous research into locomotor adaptive learning (Jayaram et al. 2011).
Role of the cerebellum in timing control
The cerebellum plays important roles in both motor control and motor perception (Bares et al. 2019). In previous studies, patients with cerebellar disease exhibited poor performance in various motor tasks, such as a task based on the timing of hand opening during overarm throws, a rhythmic finger-tapping task, blinking conditioning, an interception task, and a duration discrimination task (Hore et al. 2002; Schlerf et al. 2007; Gerwig et al. 2005; Bares et al. 2007, 2010, 2011; Ivry et al. 2002). Previous functional magnetic resonance imaging studies detected cerebrocerebellar interactions during temporal information-processing associated with motor or perceptual functions (Aso et al. 2010), and during the prediction of spatiotemporal events (O’Reilly et al. 2008). These findings suggested that feed-forward timing prediction and motor control occur in the cerebellum. In another study, baseball players, who are generally well trained in predicting the future state of their surroundings, displayed better performance than the control subjects in an interception task (Markova et al. 2020). The current study also indicated that the cerebellum is important for integrating visual information with timely motor responses, and updating and refining the internal timing-control model, and it involved the same task as was used in previous studies of patients with cerebellar disorders (Bares et al. 2007, 2010, 2011). In another study, Spampinato and Celnik (2018) showed that sequence learning, which is necessary for learning a new sequence of movements on a known sensorimotor map, also causes changes in cerebellar–M1 connectivity. This indicated that the cerebellum plays an essential role in the acquisition and updating of internal models, including those relating to the timing of movements (Penhune and Steele 2012). Studies using paired-pulse TMS showed that delivering a CS to the cerebellum elicited selective facilitation of excitability in the contralateral M1, and that these changes were related to specific phases during the execution of sequence learning (Torriero et al. 2011). Furthermore, the modulation of CBI was found to occur closer to the initiation of movement during a reaction time task (Kassavetis et al. 2011; Spampinato et al. 2017). These studies indicated that the cerebellum plays an essential role in controlling the timing of movements and adjustments in the execution of movements.
Classification of timing and the CoIT task factors that the subjects needed to learn to improve their task performance
In general, timing is functionally classified into four dimensions, i.e., explicit vs. implicit, and motor vs. perception (Coull and Nobre 2008). Explicit/implicit timing refers to whether subjects are instructed to estimate the duration of a task or part of a task. In other words, the aim of an explicit timing task is to provide an overt estimate of the amount of time that elapses during a particular event, whereas implicit timing tasks have non-temporal goals. In perceptual timing tasks, the subjects are asked to estimate the duration of a particular time period by making a perceptual judgment, e.g., they are asked whether a particular period of time was shorter or longer than another one. In contrast, in motor timing tasks the response to the task involves motor behavior. It has also been suggested that the cerebellum plays an important role in event timing, where temporal information is defined by intervals, but not in continuous cyclic timing, e.g., in a circle-drawing task (Bares et al. 2019). Based on the abovementioned classification, the interception tasks used in previous studies (Bares et al. 2007, 2010, 2011; Markova et al. 2020) and the CoIT task used in the present study are considered to be implicit motor timing tasks. As was the case for the interception tasks used in previous studies, performance in the CoIT task employed in our study was solely determined by the adjustment of the timing of motor responses to the target; therefore, the subjects had to integrate visual information, i.e., the traveling time of the ball, with the time needed to perform the bat swing movement to respond at the optimal time during the learning stage (Fig. 1a, phases 2 and 3). The schematic illustration of the virtual time windows (TWs) in which the subjects had to respond to achieve success in the CoIT task shown in Fig. 6 demonstrates what the subjects had to learn to improve their task performance. The subjects were not informed about the optimal times at which they should respond in each of the temporal perturbation conditions and were not instructed to estimate the optimal time interval between the moment the ball started to move and the moment they tried to hit the ball. Spatial information played an important role in task performance because the goal of the task was to hit the ball back into the target area; however, temporal information was also essential for improving performance due to the inherent temporal framework, i.e., the TW in which the subjects had to respond to achieve success. The TWs, i.e., the temporal ranges in which the subjects had to respond to hit the ball into the target area (TW1) or to not miss it (TW2), became shorter/longer as the swing speed increased/decreased (dark colored areas [TW1]: black: ctrl, within 20 ms; green: cond F, within 6.7 ms; blue: cond S, within 33.3 ms; light colored areas [TW2]: light gray: ctrl, light green: cond F, light blue: cond S). After the temporal perturbations were introduced, the subjects had to realize that TW1 had changed and complete the following two steps to improve their performance: step (1): realize in which direction the TW had shifted during the learning stage and by how much they had to change the timing of their responses, and step (2): time their responses so that they fell within TW1. As the temporal perturbations employed in the CoIT task were consistent and large enough to induce temporal errors, the task required error-based learning, in which the cerebellum is known to play an essential role. Spampinato and Celnik (2020) stated that four processes underlying motor learning, i.e., error-based, reinforcement, use-dependent, and strategy-based forms of learning, and the relative contribution of these processes maybe weighed differently throughout motor learning. Based on the features of our task, we speculated that step (1) needed error-based and strategy-based learning, and step (2) needed error-based and reinforcement learning. It would appeared that the relative contribution of error-based learning was higher than any other process in our CoIT task. Therefore, we hypothesized that the cerebellum also plays an essential role in CoIT task performance.
A schematic illustration of the virtual TWs in which the subjects had to respond to achieve success in the CoIT task. TW1 represents the temporal range in which the subjects had to respond to hit the ball back into the target area (green: cond F, 6.7 ms; blue: cond S, 33.3 ms; black: ctrl, 20 ms). The temporal perturbations made TW1 shorter (cond F) or longer (cond S). If the subjects responded when the ball was outside of the light colored area (TW2, light green: cond F, light blue: cond S, light gray: ctrl), they would miss the ball
Changes in cerebellar–M1 connectivity associated with CoIT task performance
CBI has been attributed to the activation of PCs in the cerebellar cortex, which send inhibitory projections to the deep cerebellar nuclei (DCN) (Celnik 2015). The DCN have excitatory connections with the M1 via the thalamus (cerebro-thalamo-cortical pathways). Therefore, PCs inhibit a facilitatory pathway from the cerebellum to the M1, resulting in the inhibition of M1 excitability. The reduction in the CBI ratio observed during the adaptive motor learning was interpreted to be indicative of an increase in PC inhibitory outputs, i.e., learning-induced long-term depression (LTD), and M1 excitability was consequently inhibited via cerebellar–M1 connectivity (Celnik 2015). In the current study, we found that the subjects’ performance improved during the learning stage, and the CT and CE exhibited opposite trends to those that were expected (Figs. 2, 3a–d) in cond F and cond S. We also found that temporal adaptive learning resulted in a reduction in CBI, which is consistent with the results of previous studies involving spatial adaptive learning, locomotor adaptation, an SVIPT task, or sequence learning (Jayaram et al. 2011; Schlerf et al. 2012; Spampinato et al. 2017; Spampinato and Celnik 2018). This change in cerebellar–M1 connectivity was considered to have resulted from learning-induced LTD (Celnik 2015). This is because the imposed temporal perturbations were consistent and large enough to induce temporal errors in cond F and cond S; therefore, the cerebellum probably played a key role in error-based learning in the CoIT task. In contrast, this learning mechanism, especially spatial aspects of it, would not have been useful in cond R because the temporal perturbations (ctrl, cond F, or cond S) were unpredictable. Thus, no changes in cerebellar–M1 connectivity were observed in cond R in either group, as reported previously in studies in which random perturbation conditions were employed in other kinds of tasks (Jayaram et al. 2011; Schlerf et al. 2012; Spampinato and Celnik 2018). In the present study, CBI decreased during the early learning stage and never returned to its baseline level (Fig. 4c, d). Previous studies reported that a reduction in CBI was only seen in the early learning stage in response to an abrupt temporal perturbation, and increased again in the late learning stage, which corresponded to the attenuation of performance error (Schlerf et al. 2012; Spampinato and Celnik 2018). In the current study, however, a reduction in CBI was even detected in the late learning stage in both temporal perturbation conditions although the CE plateaued during the late learning stage. In fact, the CE plateauing does not mean that the subjects had finished adapting to the temporal perturbations. We speculate that the learning process that occurred in the early learning stage resulted from “step 1” mentioned above, which is to say that the subjects probably realized that they had to respond about 120 ms earlier or later to improve their performance, and hence, the CE was rapidly reduced to 0°.
The changes in the success count (AE: < 5°), which was used as an indicator of accuracy in the CoIT task, that occurred during the study period are shown in Fig. 3e, f. In cond F, performance during the learning stage fell below that seen during the baseline stage and never improved. In contrast, in cond S equivalent performance levels were maintained even after the temporal perturbations were imposed, and the subjects’ performance improved during the late learning stage. These findings indicated that the difficulty of the task was affected by the bat swing speed. We also speculate that “step 2” became more important than “step 1” in the late learning stage, i.e., the subjects started to learn about the TW in which they had to hit the ball in order for it to land in the target area (TW1). As TW1 was shorter in cond F, the prolonged reduction in CBI brought about by adaptive learning could be explained by the difficulty of the task. In fact, in cond S the magnitude of the CBI tended to be lower during the late learning stage than during the early learning stage as accuracy improved (p = 0.07, Fig. 4d), while a reduction in CBI and no improvement in accuracy were seen in cond F. In other words, CBI probably returned to the baseline level when the subjects finished learning about TW1. In previous studies involving a visuomotor reaching task or SVIPT (Galea et al. 2011; Hardwick and Celnik 2014; Cantarero et al. 2015), performing anodal tDCS over the cerebellum caused faster adaptation. Although performing tDCS over the M1 did not affect the subjects’ adaptation, it resulted in a marked increase in their retention of the ability to perform the required task (Galea et al. 2011). These findings indicated that there is clear dissociation between the processes responsible for acquisition and retention during adaptive motor learning and demonstrate that the cerebellum and M1 have distinct functional roles. Hirano et al. (2015) reported that an increase in M1 excitability seen after the learning of a visuomotor tracking task was strongly correlated with the number of task trials required for error correction. In addition, it was also correlated with a positive effect on the retention of adaptive learning. In a study involving the same protocol and total number of trials (Schlerf et al. 2012), Spampinato et al. (2017) detected reductions in CBI in both the early and late learning stages during a visuomotor reaching task. We also speculate that the prolonged reduction in CBI seen in the present study was associated with the difficulty of the task and the time it took for cerebellar function to minimize performance error in both temporal bias and accuracy.
Did the temporal or spatial component of the task drive the observed changes in CBI?
We conducted correlation analyses to examine the relationship between the changes in CBI and the amount of temporal adaptive learning. We confirmed that there was a correlation between the changes in CBI and the changes in CoIT task performance seen during adaptive learning, regardless of the direction of the temporal perturbations (Fig. 5a–d). Under both cond F and cond S, the subjects who showed greater improvements in the CT exhibited less CBI, as reported in a study involving locomotor adaptation (Jayaram et al. 2011). These results, however, were inconsistent with those of another study that reported that there was no relationship between the changes in CBI and the amount of learning that occurred during spatial adaptive learning (Schlerf et al. 2012). They suggested that locomotor adaptive learning takes longer than learning a visuomotor reaching task, and stated that errors that individuals experience while walking can be very costly (i.e., they can result in falls); therefore, error-based learning mechanisms might be more effective than spatial adaptive learning, which was reflected in the correlation observed between changes in cerebellar–M1 connectivity and the amount of locomotor adaptation. However, the temporal perturbations employed in our CoIT task (the subjects had to respond 120 ms earlier/later after the temporal perturbations were introduced) were strong enough to be reflected in the correlation between the changes in cerebellar–M1 connectivity and the amount of temporal learning that occurred. In future research, we hope to explore the differences between people with excellent CoIT skills (e.g., baseball players) and novices that are exposed to the same degree of temporal perturbation. We detected a correlation between the CE and CBI in cond S, but not in cond F (Fig. 5b, d). This was probably caused by bat swing speed-related differences in the difficulty of the task. In terms of both TW1 and TW2 (see Fig. 6), the task became more difficult to perform accurately as the swing speed increased (an improvement in accuracy was seen in cond S, but not in cond F, Fig. 3c, d). If the learning stage had been long enough for the subjects to finish learning about TW1, we might have detected a correlation between the CE and CBI in cond F.
We also carried out correlation analyses in cond R in both groups. Interestingly, the CT was longer in the late learning stage than in the baseline stage in group F (Fig. 3a), and there were correlations between the changes in CBI and CoIT performance in group F (Fig. 5e, f). This learning process probably resulted from attempts to not miss the ball in cond F. Some overlap was seen in TW2 among the various perturbation conditions, and TW1 for the ctrl was located near the edge of TW2 for cond F (see Fig. 6). The subjects in group F probably tried to not miss the ball in cond R, and the desire to not miss the ball would have been stronger in group F than in group S, as the CT only became longer in group F (Fig. 3a). This learning effect, however, was not as marked as those seen in cond F or cond S due to the inconsistency of the temporal perturbations (no improvement in the miss percentage was seen in cond R in either group). Therefore, there were no significant changes in CBI in cond R, but there were correlations between the changes in CBI and the amount of learning that occurred.
Spatial information (the CE) also had an important influence on CoIT task performance because the subjects could use this information to determine whether they should respond earlier/later in the next trial. The CE was more important during “step 1”. In the late learning stage, we speculate that “step 2” became more important than “step 1”. In cond S, we found that CBI tended to return to the baseline level during the late learning stage as the subjects’ task accuracy improved, i.e., once they had completed “step 2”. In addition, we detected a correlation between the changes in cerebellar–M1 connectivity and CoIT performance in cond R in group F. We expected that no learning would occur in cond R because the temporal perturbation conditions were unpredictable so the subjects could not use spatial information. If this were true, the subjects in group F probably used temporal information, i.e., information about the TWs, to avoid missing the ball as much as they could. We also speculate that the changes in the CT, and the correlation between CBI and performance seen in group F probably resulted from learning about overlaps among the TWs. In fact, the CT did not change during the learning stage in group S (Fig. 3b). Moreover, the AE became larger in cond F than in the other conditions, despite the fact that the gap between the optimal CT and the actual CT remained the same. Thus, in group S, the tendency towards more negative CE values seen in cond R was caused by a lack of learning about the temporal aspects of the task. We interpreted these results as meaning that the changes in cerebellar–M1 connectivity that occurred with temporal adaptive learning were mainly caused by the temporal elements of the task. It is important to consider the characteristics of the motor task, and how contributions of distinct motor learning processes and cerebellar–M1 connectivity change throughout acquiring or updating internal models (Spampinato and Celnik 2020; Spampinato et al. 2020). As a limitation of the current study, a further study will be needed to completely disentangle the temporal and spatial aspects of the CoIT task, e.g., a study involving different learning strategies, such as reinforcement learning or error-based learning (Uehara et al. 2018).
In conclusion, we showed that a reduction in CBI, which might have resulted from learning-induced LTD in PCs (Celnik 2015), occurred during temporal adaptive learning, regardless of the direction of the temporal perturbations, as has been found for other types of motor task (Jayaram et al. 2011; Schlerf et al. 2012; Spampinato et al. 2017).
References
Aso K, Hanakawa T, Aso T, Fukuyama H (2010) Cerebro-cerebellar interactions underlying temporal information processing. J Cogn Neurosci 22:2913–2925. https://doi.org/10.1162/jocn.2010.21429
Baarbe J, Yielder P, Daligadu J, Behbahani H, Haavik H, Murphy B (2014) A novel protocol to investigate motor training-induced plasticity and sensorimotor integration in the cerebellum and motor cortex. J Neurophysiol 111:715–721. https://doi.org/10.1152/jn.00661.2013
Bares M, Lungu OV, Liu T, Waechter T, Gomez CM, Ashe J (2007) Impaired predictive motor timing in patients with cerebellar disorders. Exp Brain Res 180:355–365. https://doi.org/10.1007/s00221-007-0857-8
Bares M, Lungu OV, Husarova I, Gescheidt T (2010) Predictive motor timing performance dissociates between early diseases of the cerebellum and Parkinson’s disease. Cerebellum 9:124–135. https://doi.org/10.1007/s12311-009-0133-5
Bares M, Lungu OV, Liu T, Waechter T, Gomez CM, Ashe J (2011) The neural substrate of predictive motor timing in spinocerebellar ataxia. Cerebellum 10:233–244. https://doi.org/10.1007/s12311-010-0237-y
Bares M, Apps R, Avanzino L, Breska A, D’Angelo E, Filip P, Gerwig M, Ivry RB, Lawrenson CL, Louis ED, Lusk NA, Manto M, Meck WH, Mitoma H, Petter EA (2019) Consensus paper: Decoding the contributions of the cerebellum as a time machine. From Neurons to Clinical Applications. Cerebellum 18:266–286. https://doi.org/10.1007/s12311-018-0979-5
Buhusi CV, Meck WH (2005) What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 6:755–765. https://doi.org/10.1038/nrn1764
Cantarero G, Spampinato D, Reis J, Ajagbe L, Thompson T, Kulkarni K, Celnik PA (2015) Cerebellar direct current stimulation enhances on-line motor skill acquisition through an effect on accuracy. J Neurosci 35:3285–3290. https://doi.org/10.1523/JNEUROSCI.2885-14.2015
Celnik PA (2015) Understanding and modulating motor learning with cerebellar stimulation. Cerebellum 14:171–174. https://doi.org/10.1007/s12311-014-0607-y
Coull JT, Nobre AC (2008) Dissociating explicit timing from temporal expectation with fMRI. Curr Opin Neurobiol 18:137–144. https://doi.org/10.1016/j.conb.2008.07.011
Donchin O, Rabe K, Diedrichsen J, Lally N, Schoch B, Gizewski ER, Timmann D (2012) Cerebellar regions involved in adaptation to force field and visuomotor perturbation. J Neurophyiol 107:134–147. https://doi.org/10.1152/jn.00007.2011
Fernandez L, Major BP, Teo WP, Byrne LK, Enticott PG (2018) Assessing cerebellar brain inhibition (CBI) via transcranial magnetic stimulation (TMS): a systematic review. Neurosci Biobehav Rev 86:176–206. https://doi.org/10.1016/j.neubiorev.2017.11.018
Galea JM, Vazquez A, Pasricha N, de Xivry JJ, Celnik PA (2011) Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex 21:1761–1770. https://doi.org/10.1093/cercor/bhq246
Gerwig M, Hajjar K, Dimitrova A, Maschke M, Kolb FP, Frings M, Thilmann AF, Forsting M, Diener HC, Timmann D (2005) Timing of conditioned eyeblink responses is impaired in cerebellar patients. J Neurosci 25:3919–3931. https://doi.org/10.1523/JNEUROSCI.0266-05.2005
Hardwick RM, Celnik PA (2014) Cerebellar direct current stimulation enhances motor learning in older adults. Neurobiol Aging 35:2217–2221. https://doi.org/10.1016/j.neurobiolaging.2014.03.030
Hardwick RM, Lesage E, Miall RC (2014) Cerebellar transcranial magnetic stimulation: the role of coil geometry and tissue depth. Brain Stimul 7:643–649. https://doi.org/10.1016/j.brs.2014.04.009
Hirano M, Kubota S, Tanabe S, Koizume Y, Funase K (2015) Interactions among learning stage, retention, and primary motor cortex excitability in motor skill learning. Brain Stimul 8:1195–1204. https://doi.org/10.1016/j.brs.2015.07.025
Hore J, Timmann D, Watts S (2002) Disorders in timing and force of finger opening in overarm throws made by cerebellar subjects. Ann N Y Acad Sci 978:1–15. https://doi.org/10.1111/j.1749-6632.2002.tb07551.x
Ivry RB (1996) The representation of temporal information in perception and motor control. Curr Opin Neurobilo 6:851–857. https://doi.org/10.1016/S0959-4388(96)80037-7
Ivry RB, Keele SW (1989) Timing functions of the cerebellum. J Cogn Neurosci 1:136–152. https://doi.org/10.1162/jocn.1989.1.2.136
Ivry RB, Keele SW, Diener HC (1988) Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp Brain Res 73:167–180. https://doi.org/10.1007/BF00279670
Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J (2002) The cerebellum and event timing. Ann N Y Acad Sci 978:302–317. https://doi.org/10.1111/j.1749-6632.2002.tb07576.x
Izawa J, Criscimagna-Hemminger SE, Shadmehr R (2012) Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci 32:4230–4239. https://doi.org/10.1523/JNEUROSCI.6353-11.2012
Jayaram G, Galea JM, Bastian AJ, Celnik PA (2011) Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex 21:1901–1909. https://doi.org/10.1093/cercor/bhq263
Kassavetis P, Hoffland BS, Saifee TA, Bhatia KP, Van De Warrenburg BP, Rothwell JC, Edwards MJ (2011) Cerebellar brain inhibition is decreased in active and surround muscles at the onset of voluntary movement. Exp Brain Res 209:437–442. https://doi.org/10.1007/s00221-011-2575-5
Markova L, Bares M, Lungu OV, Filip P (2020) Quantitative but not qualitative performance changes in predictive motor timing as a result of overtraining. Cerebellum 19:201–207. https://doi.org/10.1007/s12311-019-01100-x
Morton SM, Bastian AJ (2006) Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26:9107–9116. https://doi.org/10.1523/JNEUROSCI.2622-06.2006
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. https://doi.org/10.1016/0028-3932(71)90067-4
O’Reilly JX, Mesulam MM, Nobre AC (2008) The Cerebellum predicts the timing of perceptual events. J Neurosci 28:2252–2260. https://doi.org/10.1523/JNEUROSCI.2742-07.2008
Penhune VB, Steele CJ (2012) Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav Brain Res 226:579–591. https://doi.org/10.1016/j.bbr.2011.09.044
Pinto D, Chen R (2001) Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp Brain Res 4:505–510. https://doi.org/10.1007/s002210100862
Prsa M, Their P (2011) The role of the cerebellum in saccadic adaptation as a window into neural mechanisms of motor learning. Eur J Neurosci 33:2114–2128. https://doi.org/10.1111/j.1460-9568.2011.07693.x
Rabe K, Livne O, Gizewski ER, Aurich V, Beck A, Timmann D, Donchin O (2009) Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol 101:1961–1971. https://doi.org/10.1152/jn.91069.2008
Schlerf JE, Spencer RMC, Zelaznik HN, Ivry RB (2007) Timing of rhythmic movements in patients with cerebellar degeneration. Cerebellum 6:221–231. https://doi.org/10.1080/14734220701370643
Schlerf JE, Galea JM, Bastian AJ, Celnik PA (2012) Dynamic modulation of cerebellar excitability for abrupt, but not gradual, visuomotor adaptation. J Neurosci 32:11610–11617. https://doi.org/10.1523/JNEUROSCI.1609-12.2012
Schlerf JE, Xu J, Klemfuss NM, Griffiths TL, Ivry RB (2013) Individuals with cerebellar degeneration show similar adaptation deficits with large and small visuomotor errors. J Neurophyiol 109:1164–1173. https://doi.org/10.1152/jn.00654.2011
Spampinato DA, Celnik PA (2018) Deconstructing skill learning and its physiological mechanisms. Cortex 104:90–102. https://doi.org/10.1016/j.cortex.2018.03.017
Spampinato DA, Celnik PA (2020) Multiple motor learning processes in processes in humans: defining their neurophysiological bases. The Neuroscientist. https://doi.org/10.1177/1073858420939552
Spampinato DA, Block HJ, Celnik PA (2017) Cerebellar-M1 connectivity changes associated with motor learning are somatotopic specific. J Neurosci 37:2377–2386. https://doi.org/10.1523/JNEUROSCI.2511-16.2017
Spampinato DA, Celnik PA, Rothwell JC (2020) Cerebellar-motor cortex connectivity: one or two different networks? J Neurosci 40:4230–4239. https://doi.org/10.1523/JNEUROSCI.2397-19.2020
Torriero S, Oliveri M, Koch G, Lo Gerfo E, Ferlazzo SS, F, Caltagirone C, Petrosini L, (2011) Changes in cerebello-motor connectivity during procedural learning by actual execution and observation. J Cogn Neurosci 23:338–348. https://doi.org/10.1162/jocn.2010.21471
Uehara S, Mawase F, Celnik PA (2018) Learning similar actions by reinforcement sensory-prediction errors rely on distinct physiological mechanisms. Cereb Cortex 28:3478–3490. https://doi.org/10.1093/cercor/bhx214
Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I (1995) Magnetic stimulation over the cerebellum in humans. Ann Neurol 37:703–713. https://doi.org/10.1002/ana.410370603
Acknowledgements
We thank Dr. M. Shin-ya for his helpful comments regarding the custom-made program used for the CoIT task. Dr. M. Hirano was supported as a Research Fellow of the Japan Society for the Promotion of Science (15J03540). The authors thank Medical English Service Co., Ltd., Kyoto, Japan, for their help with the English language editing. This study was supported by Technology of Japan and a Grant-in-aid for scientific research (19K11578) from the Japanese Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
ST, MH, and KF conceived and designed the research; ST performed the experiments; ST analyzed the data; ST, MH, and KF interpreted the results of the experiments; ST prepared the figures; ST and KF drafted the manuscript; and ST, HM, and KF approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Communicated by Winston D Byblow.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
221_2020_5963_MOESM1_ESM.tif
Supplementary file1 Supplementary Fig. 1. The relationships between the CT and CE in each of the temporal perturbation conditions (A: green: cond F, blue: cond S, gray: ctrl). Linear regression lines for the relationships between the CE and CT in each of the conditions are shown. The linear regression lines were obtained from another 900 trials (300 trials conducted in each of the three conditions) performed by one of the authors, and we used them to recalculate the CE based on the CT if the subjects swung and missed the ball. The slope of the regression line became steeper as the rate of change in the swing speed increased (TIF 83 KB)
Rights and permissions
About this article
Cite this article
Tanaka, Sy., Hirano, M. & Funase, K. Modulation of cerebellar brain inhibition during temporal adaptive learning in a coincident timing task. Exp Brain Res 239, 127–139 (2021). https://doi.org/10.1007/s00221-020-05963-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-020-05963-z