Abstract
Previous studies suggest that visual information is essential for balance and stability of locomotion. We investigated whether visual deprivation is met with active reactions tending to minimize worsening balance and stability during walking in humans. We evaluated effects of vision on kinetic characteristics of walking on a treadmill-ground reaction forces (GRFs) and shifts in the center of mass (COM). Young adults (n = 10) walked on a treadmill at a comfortable speed. We measured three orthogonal components of GRFs and COM shifts during no-vision (NV) and full-vision (FV) conditions. We also computed the dynamic balance index (DN)—the perpendicular distance from the projection of center of mass (pCOM) to the inter-foot line (IFL) normalized to half of the foot length. Locally weighted regression smoothing with alpha-adjusted serial T tests was used to compare GRFs and DN between two conditions during the entire stance phase. Results showed significant differences in GRFs between FV and NV conditions in vertical and ML directions. Variability of peak forces of all three components of GRF increased in NV condition. We also observed significant increase in DN for NV condition in eight out of ten subjects. The pCOM was kept within BOS during walking, in both conditions, suggesting that body stability was actively controlled by adjusting three components of GRFs during NV walking to minimize stability loss and preserve balance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Locomotion is an everyday behavior in which vision plays an essential role (Gibson 1998; Warren 1998), by influencing balance, stability, body orientation, and gait patterns in different environments (Patla 1997; Jahn et al. 2001; Hallemans et al. 2010; Matthis et al. 2018; Miller et al. 2018). Vision also provides information about objects to avoid or specific targets to reach (Hollands et al. 2002). The loss of visual inputs during walking may affect different kinematic and kinetic characteristics of walking: speed, cadence, step length and width, duration of double support phase, shape of lower limb trajectory, foot clearance and foot placement on the ground (Hollands and Marple-Horvat 1996; Patla 1997; Rietdyk et al. 2005; Reynolds and Day 2005; Rietdyk and Rhea 2006; Rhea and Rietdyk 2007; Hallemans et al. 2009a, 2010; Uematsu et al. 2011; Cho et al. 2013; Collins and Kuo 2013; Maeda et al. 2017).
The influence of visual perturbations on gait stability was usually evaluated by recording kinematic characteristics of gait. Little is known regarding the effects of visual perturbations on kinetic characteristics, such as ground reaction forces (GRFs) that play an essential role in the production of locomotion. Analysis of kinetic effects of visual perturbations might be also important for understanding of gait stability. GRFs are directly related to the acceleration of the body’s center of mass (COM) (Karlsson and Frykberg 2000; Rose and Gamble 2006). Mechanically, locomotion can be defined as resulting from changes in neurophysiological parameters that translate body balance and stability in the environment (Masani et al. 2002; Patla 2003; Feldman et al. 2011). Therefore, GRFs may be essential characteristic of gait stability (Masani et al. 2002; Chockalingam et al. 2004).
To our knowledge, there is only one study (Oliveira et al. 2017) in which only one vertical component of GRF was investigated during gait with and without vision. This GRF component was reduced during walking with eyes closed. It remains unclear whether the medial–lateral (ML) and anterior–posterior (AP) components of GRFs are also affected in blindfolded walking. The deviation of COM within the base of support (BOS) might be another helpful variable in the evaluation of gait stability (You et al. 2001; Lugade et al. 2011; Niiler and Janick 2017). Some gait modifications such as reduced speed, and step length, increased step width and limited movement of the hip and ankle may occur to keep the COM within the BOS and maintain gait stability (Redfern and Schumann 1994; Patla 1997; Hallemans et al. 2010). Condition-dependent changes in the GRFs could be responsible for these adjustments. For example, Hsiang and Chang (2002) suggested that changes in the COM displacement are responsible for an increase in body stability by keeping the COM within the BOS. Hsiang and Chang (2002) did not quantify the COM displacement. Studies which investigated the COM motion in NV condition are limited by measuring the acceleration of upper body segments and of the pelvis (Iosa et al. 2012) without any relation to the BOS (Hallemans et al. 2009b, 2010).
In the present study, we measured the COM motion using normalized dynamic balance index (DN) that evaluates variability of the COM with respect to a dynamic reference (the feet) or a fixed reference (laboratory frame) as suggested by Niiler et al., (Niiler and Janick 2017; Niiler 2018a, b). We measured DN as the deviation of COM relative to the line between the midpoints of the feet, which is approximately passes through the center of BOS (see “Methods”). Furthermore, DN is a variable that might be more sensitive to changes in experimental conditions (e.g. due to perturbation) than the medial–lateral/vertical displacements of the COM in the detection of changes in balance (Niiler 2020).
The goal of this study was to examine whether the three orthogonal GRF components changed during walking in no-vision (NV) compared to full-vision (FV) condition. In addition, we determined the COM motion to better understand gait balance and stability control. Step length and step width were measured as general kinematic outcome parameters. Although the anticipated effect of NV is worsening balance and stability, we addressed the question of whether or not the nervous system has the capacity to actively minimize destabilizing effects in NV conditions.
Methods
Participants
Ten subjects (4 males and 6 females, 31.5 ± 3.8 years) participated in the present study. Participants were asked to sign informed consent forms approved by the ethics committee of the Centre for Interdisciplinary Research in Rehabilitation (CRIR). After filling out a general medical history questionnaire, they reported to be in generally good health, with no history of neurophysiological and musculoskeletal problems.
Experimental procedure
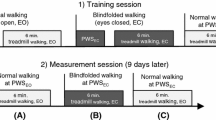
Prior to the experiment, participants were given enough time to warm up and familiarize with walking on a motorized treadmill (Bertec’s Fully Split-Belt Instrumented Treadmill, Bertec Corp., USA). They walked at their comfortable speed during the actual tests. Comfortable speed was determined by gradually increasing the belt speed until a comfortable speed was reported by the participant. The belt speed was then increased beyond the reported comfortable speed until it was found to be uncomfortable and subsequently was gradually decreased until a comfortable walking speed was reported again, and the latter speed was used in test trials. The average of comfortable speed for ten subjects was 1.095 ± 0.183 m/s. During the actual test, subject wore portable liquid crystal glasses that can be made transparent or opaque by an electrical signal. To familiarize with visual deprivation, a trial with three perturbations was performed by participants. To avoid adaptation, participants received gradually increasing vision occlusion during 1, 3 and 5 s. Two trials were recorded: (1) 1-min walk, FV, (2) NV walking, until participants approached the edges of the treadmill and the experimenter deactivated the glasses to prevent falling, which was also ensured by overhead safety harness worn by participants. The average duration of NV walking was 15.82 ± 3.52 s.
Data collection
The GRFs were sampled at 600 Hz recorded from the force plates embedded under the belt of the treadmill. The force platforms were able to measure the orthogonal forces according to the three components (Fx, Fy, and Fz). Kinematic data were recorded using a three-dimensional motion analysis system (Optotrak Certus, Northern Digital Inc., Waterloo, ON, Canada). Three to six infrared markers positioned bilaterally on each body part which were captured by four Optotrak acquisition cameras (two sides, one front and one back) at a frequency of 30 Hz. All markers installed on the lateral aspect of the extremities and back of the trunk (Fig. 1). Therefore, their movements were easily tracked by cameras. Because the markers do not determine specifically the spatial relationship between segments, we completed the definition of each segment by adding some anatomical points (Fig. 1). In this way, a digitizing probe was applied to determine anatomical points relative to the markers. The relative position of markers to the joint axes is used to make a rigid body. Consequently, a 3D-link chain model is built which determines the position of each segment in a series of joints. Furthermore, anthropometric measures (body weight and height, lengths and circumference of body segments) were used to modify this biomechanical model to estimate COM motion. Finally, using regression equations, which take into account the length, diameter and, total weight of the body, a software (homemade) calculated the segmental and global position of COM (Winter 2009; Dubreucq et al. 2017).
Data analysis
The fourth-order Butterworth filter (6 Hz cut-off frequency) was used to filter kinematic data. The force data were low-pass filtered (10 Hz, fourth-order, zero-lag Butterworth) and normalized to the body weight. For data alignment, the force and kinematic data re-sampled to 60 Hz. The walking cycles were defined from the vertical GRF using 10 N threshold for detecting heel contact (HC) and toe-off (TO). Stance phase was normalized from 0% as HC to 100% as TO. Nine cycles were selected for each subject to further analysis.
Step width and length
Step width was defined as the ML distance, and step length was calculated as the AP distance between right and left heel markers during consecutive heel contacts.
Ground reaction force
For assessment of difference between two conditions, we used the method of LOESS Alpha-Adjusted Serial T test introduced by Niiler (2017). Data of three GRF components were modeled by locally weighted regression smoothing (LOESS), and then the modeled mean and standard deviation curves of Fy, Fz and Fx were compared at each 100 points by paired sample t test with adjusted alpha. The adjusted alpha was calculated using the correlation coefficient between one point time-shifted of adjacent P values time series and itself (Niiler 2017; Nicholson et al. 2018; Kruger et al. 2019). Based on correlation, a new adjusted alpha was introduced:
where N is the number of comparisons and \(\rho\) is the correlation coefficient in adjacent P values. When adjacent P value curve falls below the new adjusted alpha, it means significant difference between two conditions.
In addition, variability of the peaks of three components were calculated as the percentage coefficient of variation (%CV) across nine cycles. The CV was defined as
where SD is the standard deviation and \(\overline{X}\) is the mean of distribution of peak values. For the vertical direction, first peak or weight-acceptance peak (Fz1), valley (Fz2) and second peak or push-off peak (Fz3) were calculated. For the anterior–posterior direction, the magnitude of deceleration peak (Fy1) and acceleration peak (Fy2) were measured. From the medial–lateral component, the magnitude of negative peak (Fx1), the first positive peak (Fx2) and second positive peak (Fx3) were calculated for CV analysis.
Dynamic stability index, DN
Dynamic base of support was defined as the inter-foot-line (IFL)—the line extending through the midpoint of heel and toe markers of each foot. Then, the shortest distance between projection of the COM (pCOM) and the IFL was defined as DIFL. The DIFL was normalized to half of the foot length to account for different foot size and different base of support:
In which DN represents a normalized gravitational moment arm (Fig. 2). It should be noted that DN is positive when the moment arm is in the front of the IFL and negative if the moment arm is located behind the IFL (Niiler 2020). The zero DN indicates a complete balance and when the DN is greater than 1 or − 1 it means that the COM does not lie within the base of support. The LAAST method was also used to compare entire DN curve between two conditions.
Statistical analysis
Statistical analyses were conducted on GRFs and DN using LAAST method. CV for each component were quantified by multivariate analysis of variance (MANOVA). A paired t test was also used to analyze SL and SW data to identify differences between conditions.
Results
Step length and width
Step length significantly increased under the NV condition (t = 5.741, P = 0.000). Similarly, participants walked with wider steps in the NV condition (t = − 6.650, P = 0.000), (Fig. 3).
Ground reaction force
No significant differences were identified between two conditions for AP direction (Fig. 4). For vertical direction, only points of 97–99 fell below the adjusted alpha line (Fig. 5). However, ML direction was significantly different between FV and NV conditions in more regions across the stance phase (10–13 and 30–66%) (Fig. 6). The CV was greater for Fy2 (P = 0.012) for NV condition. For the vertical GRF, the CV was greater for Fz1 (P = 0.035), Fz2 (P = 0.021) and Fz3 (P = 0.000) in NV condition. The CV was greater for Fx1 (P = 0.033), Fx2 (P = 0.000) and Fx3 (P = 0.048) for NV condition. Table 1 shows the CVs for each condition at the three axes.
Anterior–posterior component of GRF. Nine cycles from illustrative subject for FV (a), and NV (b) conditions. Mean (± SD) AP direction for FV and NV conditions across all ten subjects (c). Adjacent P values between FV and NV conditions (d), red line shows a log (P value) of -5.7365, which is equal to adjusted alpha of 0.0032. There was no significant difference between two conditions
Vertical component of GRF. Nine cycles from illustrative subject for FV (a), and NV (b) conditions. Mean (± SD) vertical direction for FV and NV conditions across all ten subjects (c). Adjacent P values between FV and NV conditions (d), red line shows a log (P value) of − 5.6607, which is equal to adjusted alpha of 0.0035. There was no difference between NV and FV conditions (except for those between 97 and 99% of stance)
Medial–lateral component of GRF. Nine cycles from illustrative subject for FV (a), and NV (b) conditions. Mean (± SD) ML direction for FV and NV conditions across all ten subjects (c). Adjacent P values between FV and NV conditions (d), red line shows a log (P value) of − 6.8252, which is equal to adjusted alpha of 0.0011. The regions between 10–13 and 30–66 percent of stance phase were significant
Dynamic stability index, DN
Figure 7 shows mean and SD of DN curve and adjacent P values curve for ten subjects in two conditions. P value curve shows that DN is similar entire the stance phase between FV and NV conditions. Since DN curves are asymmetrical and subjects could be affected in different area of the stance phase, it may wash out any effects while we average the curves. Therefore, we also used the LAAST method for data in each subject. Value of DN increased during NV condition compared to FV condition in eight out of ten subjects (Fig. 8, panel a).
Discussion
The aim of present study was to investigate the effects of visual deprivation on three orthogonal components of the GRF and dynamic stability index, DN, in healthy adults during walking on the treadmill. The results revealed that walking with eyes closed increased step width, decreased step length and changed kinetic gait variables. There was no difference between NV and FV conditions in Fy and Fz. (except for those between 97 and 99% of stance phase), However, for Fx, we found significant difference in more areas of stance phase compared to Fy and Fz (11–12% and 31–66%). The CVs of magnitudes for all peaks (except Fy1) increased in the NV condition. These data may confirm that the loss of visual information changed the GRF in all three directions.
Our result showed that the GRFs were affected by visual deprivation in three directions. It has been shown that local, and lower level sensory feedback and spinal reflexes are sufficient for stabilizing walking in the AP direction (Grillner and Wallen 1985). However, in the ML direction, the active control is necessary to integrate visual, vestibular, proprioceptive and other sensory inputs by higher levels of the nervous system mediated, in particular, by the brain stem and cerebellum (Forssberg 1982). Previous studies suggest that the sensitivity of COP to visual perturbations in different directions is different. For example, O’Connor and Kuo (2009) showed that the variability of COP displacement in ML direction, in contrast to AP direction was affected by virtual loss of vision. In our study, the variability of GRF displacement in all three directions was affected in NV condition. One explanation for the differences in results is that the control of stability along AP and vertical directions is more challenging when vision is completely blocked (Iosa et al. 2012). Conversely, reactions to perturbations in the virtual reality environment (O’Connor and Kuo 2009; McAndrew et al. 2010, 2011), can still rely on visual information, especially about the direction of walking that can only be obtained from (Hollands et al. 2002). Reflex control might be sufficient to neutralize effects of small perturbations without involving higher centers of the nervous system (Donelan et al. 2004). However, our findings suggest that the CNS must actively adjust GRF parameters to control balance along AP and ML, or even vertical directions, and to reduce the negative effects of restricted vision and facilitate balance when encountering large perturbations resulting from visual deprivation. Nevertheless, our results confirm the hypothesis that the ML direction is more sensitive to visual perturbation than the AP axis. Because our finding showed that not only the CVs but also the magnitude of Fx in more regions of stance phase affected by vision loss. Consistent with our findings, Iosa et al. (2012) showed that although three directions are affected by visual deprivation, however, the acceleration of body in the latero-lateral axis was the only variable influenced by the interaction between vision and environment.
In terms of variability, according to the Fitts and Posner (1967) three-stage model, high variability of performance is one of the specific characteristics of the first stage of learning a new task, indicating no consistency from one trial to the other. During the second stage, performance becomes more consistent due to the practice and features of consecutive attempts become more similar. Therefore, it seems that in our study, the lack of participant’s experience in the task, may explain the higher variability of the GRFs during eyes closed condition compared to normal walking (eyes open).
Regarding dynamical stability analysis, this study used a novel method for quantification of the COM motion. During normal walking, the pCOM fluctuates between the feet over the whole gait cycle (Winter 1995). As a result, the nervous system is needed to ensure using an appropriate method to measure the COM deviations, which takes into account the position of the pCOM relative to the feet (Niiler 2020; Niiler 2018a). Results showed significant increase in DN for NV condition for eight of ten subjects, indicating that blindfolded walking may challenge postural stability. DIFL has a larger lateral component than anterior component for the majority of the gait cycle. This may explain why previous studies mainly focused on this component in predicting instability during locomotion (Niiler 2020; Niiler 2018b). Results of DN and medial–lateral GRF analysis may support each other, confirming that ML direction is more sensitive to perturbation than other components (O’Connor and Kuo 2009; McAndrew et al. 2010, 2011). Note that DN values were between 1 and − 1, for both NV and FV conditions (Figs. 7 and 8, panel a), implying that subjects were able to keep the pCOM within the BOS. It is clear that adults can preserve stability when there is no visual information. The goal of locomotion is to translate the COM forward stably in space (Masani et al. 2002; Patla 2003). To achieve this goal, and maintaining the COM constantly, individuals adjust some gait characteristics, like step length and width, GRFs or movement speed to keep the COM within the BOS. For example, Iosa et al. (2012) suggested that individuals restricted the upper body acceleration by reducing the speed of walking to control dynamic stability. Other studies (Hallemans et al. 2009b, 2010) also found that visual deprivation cause individuals to walk at a slower speed, lower cadence and restricted movements of the hip and the ankle and limited pelvis movement compared to gait in FV condition. The present results are consistent with these studies implying that adult use conservative strategies to keep the COM within the base of support, allowing them to reduce uncertainty and overcome the fear of falling. According to the uncontrolled manifold hypothesis (UCM) (Scholz and Schöner 1999; Latash et al. 2007), the CNS may employ a variety of different solutions to meet the task demands. Consistent with this assumption, some research on gait investigated the COM stabilization during load carriage and fatigue (Qu 2012), among preadolescents with and without Down syndrome (Black, Smith, Wu, & Ulrich, 2007), and among adults with and without neuropathology (Papi et al. 2015). The results showed that the COM is the variable controlled by the CNS (performance variable) using various combinations of lower and upper body joint angles (elemental variables) that together stabilize the COM in a certain position (Papi et al. 2015). Therefore, it is possible that in the present experiment, subjects used the variability of GRF components as elemental variables to stabilize the COM as a performance variable under no-vision condition. Moreover, individuals with no experience in the task employ considerable number of solutions and body configurations to stabilize a task-related variable. As learning progresses, they can achieve the goal efficiently by fewer solutions (Black et al. 2007).
Conclusion
In summary, we measured the three components of GRF and the COM motion during walking on the treadmill with eyes closed. The variability of GRFs in the three directions increased in no-vision condition and the ML direction was more sensitive to visual deprivation. Normalized gravitational moment arm, DN, increased during NV condition. However, individuals were able to control balance and keep the COM within the base of support during visual deprivation. Our results suggest that reduced visual information involves control reactions to minimize the influence of visual deprivation on balance and stability of walking.
References
Black DP, Smith BA, Wu J, Ulrich BD (2007) Uncontrolled manifold analysis of segmental angle variability during walking: preadolescents with and without down syndrome. Exp Brain Res 183:511–521. https://doi.org/10.1007/s00221-007-1066-1
Cho S-Y, Ryu Y-U, Je HD et al (2013) Effects of illumination on toe clearance and gait parameters of older adults when stepping over an obstacle: a pilot study. J Phys Ther Sci 25:229–232. https://doi.org/10.1589/jpts.24.229
Chockalingam N, Dangerfield PH, Rahmatalla A et al (2004) Assessment of ground reaction force during scoliotic gait. Eur Spine J 13:750–754. https://doi.org/10.1007/s00586-004-0762-9
Collins SH, Kuo AD (2013) Two independent contributions to step variability during over-ground human walking. PLoS One. https://doi.org/10.1371/journal.pone.0073597
Donelan JM, Shipman DW, Kram R, Kuo AD (2004) Mechanical and metabolic requirements for active lateral stabilization in human walking. J Biomech 37:827–835. https://doi.org/10.1016/j.jbiomech.2003.06.002
Dubreucq L, Mereu A, Blanc G et al (2017) Introducing a psychological postural threat alters gait and balance parameters among young participants but not among most older participants. Exp Brain Res 235:1429–1438. https://doi.org/10.1007/s00221-017-4902-y
Feldman AG, Krasovsky T, Baniña MC et al (2011) Changes in the referent body location and configuration may underlie human gait, as confirmed by findings of multi-muscle activity minimizations and phase resetting. Exp Brain Res 210:91–115. https://doi.org/10.1007/s00221-011-2608-0
Fitts PM, Posner MI (1967) Human performance. Brooks/Cole, Oxford
Forssberg H (1982) Spinal locomotor functions and descending control. In: Brainstem control of spinal mechanisms. Elsevier Biomedical, Amsterdam, pp 253–271
Gibson JJ (1998) Visually controlled locomotion and visual orientation in animals (reprinted from British Journal of Psychology, vol 49, pg 182–194, 1958). Ecol Psychol 10:161–176. https://doi.org/10.1207/s15326969eco103&4_2
Grillner S, Wallen P (1985) Central pattern generators for locomotion, with special reference to vertebrates. Annu Rev Neurosci 8:233–261. https://doi.org/10.1146/annurev.ne.08.030185.001313
Hallemans A, Beccu S, Van Loock K et al (2009a) Visual deprivation leads to gait adaptations that are age- and context-specific: II. Kinematic parameters. Gait Posture 30:307–311. https://doi.org/10.1016/j.gaitpost.2009.05.017
Hallemans A, Beccu S, Van Loock K et al (2009b) Visual deprivation leads to gait adaptations that are age- and context-specific: I. Step-time parameters. Gait Posture 30:55–59. https://doi.org/10.1016/j.gaitpost.2009.02.018
Hallemans A, Ortibus E, Meire F, Aerts P (2010) Low vision affects dynamic stability of gait. Gait Posture 32:547–551. https://doi.org/10.1016/j.gaitpost.2010.07.018
Hollands MA, Marple-Horvat DE (1996) Visually guided stepping under conditions of step cycle-related denial of visual information. Exp Brain Res 109:343–356. https://doi.org/10.1007/BF00231792
Hollands MA, Patla AE, Vickers JN (2002) “Look where you’re going!”: gaze behaviour associated with maintaining and changing the direction of locomotion. Exp Brain Res 143:221–230. https://doi.org/10.1007/s00221-001-0983-7
Hsiang SM, Chang C (2002) The effect of gait speed and load carrying on the reliability of ground reaction forces. Saf Sci 40:639–657. https://doi.org/10.1016/S0925-7535(01)00064-9
Iosa M, Fusco A, Morone G, Paolucci S (2012) Effects of visual deprivation on gait dynamic stability. Sci World J 2012:1–7. https://doi.org/10.1100/2012/974560
Jahn K, Strupp M, Schneider E et al (2001) Visually induced gait deviations during different locomotion speeds. Exp Brain Res 141:370–374. https://doi.org/10.1007/s002210100884
Karlsson A, Frykberg G (2000) Correlations between force plate measures for assessment of balance. Clin Biomech 15:365–369. https://doi.org/10.1016/S0268-0033(99)00096-0
Kruger KM, Graf A, Flanagan A et al (2019) Segmental foot and ankle kinematic differences between rectus, planus, and cavus foot types. J Biomech 94:180–186. https://doi.org/10.1016/j.jbiomech.2019.07.032
Latash ML, Scholz JP, Schöner G (2007) Toward a new theory of motor synergies. Mot Control 11:276–308
Lugade V, Lin V, Chou LS (2011) Center of mass and base of support interaction during gait. Gait Posture 33:406–411. https://doi.org/10.1016/j.gaitpost.2010.12.013
Maeda RS, O’Connor SM, Donelan JM, Marigold DS (2017) Foot placement relies on state estimation during visually guided walking. J Neurophysiol 117:480–491. https://doi.org/10.1152/jn.00015.2016
Masani K, Kouzaki M, Fukunaga T (2002) Variability of ground reaction forces during treadmill walking. J Appl Physiol 92:1885–1890. https://doi.org/10.1152/japplphysiol.00969.2000
Matthis JS, Yates JL, Hayhoe MM (2018) Gaze and the control of foot placement when walking in natural terrain. Curr Biol 28:1224–1233.e5. https://doi.org/10.1016/j.cub.2018.03.008
McAndrew PM, Dingwell JB, Wilken JM (2010) Walking variability during continuous pseudo-random oscillations of the support surface and visual field. J Biomech 43:1470–1475. https://doi.org/10.1016/j.jbiomech.2010.02.003
McAndrew PM, Wilken JM, Dingwell JB (2011) Dynamic stability of human walking in visually and mechanically destabilizing environments. J Biomech 44:644–649. https://doi.org/10.1016/j.jbiomech.2010.11.007
Miller AB, Lajoie K, Strath RA et al (2018) Coordination of gaze behavior and foot placement during walking in persons with glaucoma. J Glaucoma 27:55–63. https://doi.org/10.1097/IJG.0000000000000819
Nicholson K, Church C, Takata C et al (2018) Comparison of three-dimensional multi-segmental foot models used in clinical gait laboratories. Gait Posture 63:236–241. https://doi.org/10.1016/j.gaitpost.2018.05.013
Niiler T (2017) The problem of multiple comparisons between groups of time dependent data. In: Gait Clin. Mov. Anal. Soc. Annu. Meet
Niiler T (2018a) Assessing dynamic balance in children with cerebral palsy. In: Miller F, Bachrach S, Lennon N, O’Neil M (eds) Cerebral palsy. Springer International Publishing, Cham, pp 1–32
Niiler T (2018b) Measures to determine dynamic balance. In: Müller B, Wolf S (eds) Handbook of human motion. Springer International Publishing, Cham, pp 887–913
Niiler T (2020) Comparing groups of time dependent data using locally weighted scatterplot smoothing alpha-adjusted serial T-tests. Gait Posture 76:58–63. https://doi.org/10.1016/j.gaitpost.2019.10.028
Niiler T, Janick T (2017) The utility of passive dynamic walkers as a proxy for humans in dynamic balance studies. Gait Posture 57:356–357. https://doi.org/10.1016/j.gaitpost.2017.06.465
O’Connor SM, Kuo AD (2009) Direction-dependent control of balance during walking and standing. J Neurophysiol 102:1411–1419. https://doi.org/10.1152/jn.00131.2009
Oliveira AS, Schlink BR, Hairston WD et al (2017) Restricted vision increases sensorimotor cortex involvement in human walking. J Neurophysiol 118:1943–1951. https://doi.org/10.1152/jn.00926.2016
Papi E, Rowe PJ, Pomeroy VM (2015) Analysis of gait within the uncontrolled manifold hypothesis: stabilisation of the centre of mass during gait. J Biomech 48:324–331. https://doi.org/10.1016/j.jbiomech.2014.11.024
Patla AE (1997) Understanding the roles of vision in the control of human locomotion. Gait Posture 5:54–69
Patla AE (2003) Strategies for dynamic stability during adaptive human locomotion. IEEE Eng Med Biol Mag 22:48–52
Qu X (2012) Uncontrolled manifold analysis of gait variability: effects of load carriage and fatigue. Gait Posture 36:325–329. https://doi.org/10.1016/j.gaitpost.2012.03.004
Redfern MS, Schumann T (1994) A model of foot placement during gait. J Biomech 27:1339–1346. https://doi.org/10.1016/0021-9290(94)90043-4
Reynolds RF, Day BL (2005) Visual guidance of the human foot during a step. J Physiol 569:677–684. https://doi.org/10.1113/jphysiol.2005.095869
Rhea CK, Rietdyk S (2007) Visual exteroceptive information provided during obstacle crossing did not modify the lower limb trajectory. Neurosci Lett 418:60–65. https://doi.org/10.1016/j.neulet.2007.02.063
Rietdyk S, Rhea CK (2006) Control of adaptive locomotion: effect of visual obstruction and visual cues in the environment. Exp Brain Res 169:272–278. https://doi.org/10.1007/s00221-005-0345-y
Rietdyk S, McGlothlin JD, Williams JL, Baria AT (2005) Proactive stability control while carrying loads and negotiating an elevated surface. Exp Brain Res 165:44–53. https://doi.org/10.1007/s00221-005-2279-9
Rose J, Gamble JG (2006) Human walking, 3rd edn. Lippincott Williams & Wilkins, Philadelphia
Scholz JP, Schöner G (1999) The uncontrolled manifold concept: Identifying control variables for a functional task. Exp Brain Res 126:289–306. https://doi.org/10.1007/s002210050738
Uematsu A, Inoue K, Hobara H et al (2011) Preferred step frequency minimizes veering during natural human walking. Neurosci Lett 505:291–293. https://doi.org/10.1016/j.neulet.2011.10.057
Warren WH Jr (1998) Visually controlled locomotion: 40 years Later. Ecol Psychol 10:177–219. https://doi.org/10.1080/10407413.1998.9652682
Winter DA (1995) Human balance and posture control during standing and walking. Gait Posture 3:193–214
Winter DA (2009) Biomechanics and motor control of human movement, 4th edn. Wiley, Hoboken
You JY, Chou YL, Lin CJ, Su FC (2001) Effect of slip on movement of body center of mass relative to base of support. Clin Biomech 16:167–173. https://doi.org/10.1016/S0268-0033(00)00076-0
Acknowledgements
We thank Philippe Gourdou for help in data collection and analysis.
Funding
This study was supported by the National Science Engineering Research of Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Communicated by Carlo Alberto Marzi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shoja, O., Farsi, A., Towhidkhah, F. et al. Visual deprivation is met with active changes in ground reaction forces to minimize worsening balance and stability during walking. Exp Brain Res 238, 369–379 (2020). https://doi.org/10.1007/s00221-020-05722-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-020-05722-0