Abstract
We studied the short-latency (SL) effects of postural perturbations produced by impulses applied over the spine of the C7 vertebra or the sternum (“axial impulses”) in 12 healthy subjects. EMG recordings were made bilaterally from the triceps brachii, biceps brachii, soleus, and tibialis anterior muscles, and unilaterally from the deltoid, forearm flexors, forearm extensors, and first dorsal interosseous (FDI) muscles. Sternal impulses evoked short-latency responses in the biceps when subjects leaned posteriorly to support approximately 12% of their body weight with the arms, but these responses were only modestly larger than for isometric contraction of the arms (26.3 vs. 14.7%). In contrast, clear excitatory responses could be evoked in the deltoid, triceps, forearm muscles, and FDI when leaning anteriorly to support similar amounts of body weight. These responses were significantly larger than during isometric contraction. The deltoid (42.5%) and triceps (44.7%) had the largest responses in supported anterior lean and onset latencies increased distally in this condition (mean 31.8 ms in deltoid to 53.7 ms in FDI). There was a disproportionate delay between the forearm muscles and FDI. For both directions of lean, postural reflex responses normally present in the legs were severely attenuated. SL upper limb excitatory responses were bigger in proximal muscles as well as larger and more widespread for anterior axial perturbations compared to posterior axial perturbations when using the arms to support body weight. Our findings also provide further evidence of a role for reticulospinal pathways in mediating these rapid postural responses to accelerations of the trunk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Short-latency (SL) postural reflexes are rapid, corrective reflex responses to external perturbations that affect posture. Characteristically, they are modulated by postural task (Britton et al. 1993) and are directed to posturally relevant muscles. The vestibular-spinal reflex, usually evoked by electrical (galvanic) vestibular stimulation (GVS), is one such example which has been studied extensively (e.g., Britton et al. 1993; Fitzpatrick et al. 1994). Britton et al. (1993) showed that SL vestibular-spinal responses could be elicited in the triceps brachii when the arms were used to maintain posture and these occurred at latencies shorter than the responses recorded in soleus during upright stance (mean: 41 vs. 62 ms). This allowed the authors to estimate central conduction velocity and led them to propose a rapidly conducting efferent pathway distinct from the corticospinal tract. They suggested that the responses might be mediated by the vestibulospinal and reticulospinal tracts (Britton et al. 1993; Rothwell 2006).
Head taps can activate vestibular receptors (Halmagyi et al. 1995), and Bötzel et al. (2001) showed that tendon hammer taps applied to both the sternum and forehead during upright stance could evoke SL responses in the legs that counteracted the perturbation. Because similar responses were obtained with both stimuli, despite opposite effects on vestibular receptors and their presence in patients with vestibular impairment, they concluded that the effects were not primarily due to vestibular afferent activation. Considerable evidence already existed that truncal perturbations could evoke rapid postural reflexes possibly originating in muscle receptors of the trunk, independently of vestibular or ankle afferents (Gurfinkel et al. 1981; Do et al. 1988; Bloem et al. 2000). A small impulsive stimulus (“axial impulse”) applied over the sternum or over the spine of C7 using a mini-shaker has been used in subsequent investigations and has been shown to evoke SL postural reflexes in leg muscles (Govender et al. 2015). Responses were largest in soleus and tibialis anterior (TA) during upright stance, and were attenuated by sitting (Graus et al. 2013). During kneeling, the responses became attenuated in soleus and TA but were evident in more proximal leg muscles which had become more functionally relevant (Govender et al. 2015).
Recently, Teng et al. (2017) showed that axial impulses could also elicit SL postural reflexes in the triceps brachii when the arms had a postural role. They found that these responses were larger when leaning anteriorly using the arms to support body weight than during similar levels of triceps activity with isometric contraction of the arms during upright stance. The reverse effect was found to occur in soleus and TA, i.e., the normal SL postural responses evoked by axial impulses during upright stance were severely attenuated when the patients supported part of their weight using their arms. Based on the difference in latencies between the responses in triceps and soleus, Teng et al. (2017) estimated that the responsible efferent pathway conducted at approximately half the velocity of the corticospinal tract. They argued that the reticulospinal tract was more likely to be responsible than the vestibulospinal tract as vestibular afferents only had a minor role in axially evoked responses.
The present study investigated the presence of axially evoked postural responses, similar to those reported in the triceps brachii, for other major muscles of the upper limb, when the limb had a postural role. We aimed to characterise the extent and timing of muscle activation to define further the properties of axially evoked reflexes and to seek additional evidence of a role for the reticulospinal tract.
Methods
Participants
Twelve healthy subjects (mean age: 19 ± 1.4 years; 4 females and 8 males) with no history of vertigo, dizziness, inner ear pathology, or neurological illness were recruited from students at the University of New South Wales and Macquarie University. Written consent, in accordance with the Declaration of Helsinki, was obtained from all subjects prior to study commencement and the study was approved by the local ethics committee.
Stimulation techniques
A smoothed impulsive stimulus defined by a third-order gamma distribution (Ross 2007) with a 12-ms rise time was used throughout. It was applied either over the spine of the seventh cervical vertebra (C7) or the upper sternum by a hand-held mini-shaker (model 4810, Bruel and Kjaer P/L, Denmark) via an attached Perspex rod (diameter 2.5 cm and length 9.2 cm). The waveform was generated using customised software through a micro1401 laboratory interface (CED, Cambridge UK) and amplified (type 2718, Bruel and Kjaer P/L, Denmark). Stimuli were delivered at a standard driving voltage of 20 V peak (+ 6 dB compared to our standard 10V peak drive and equivalent to ~ 14 N peak force) at a fixed rate of ~ 2.5 Hz for 200 repetitions. All stimuli were compressive, defined as movement of the Perspex rod towards the subject (Teng et al. 2017).
Recordings
Self-adhesive electrodes (Cleartrace 1700-050, Conmed Corp., NY, USA) were used to record EMG for all 12 subjects from biceps and triceps brachii bilaterally. In six of the subjects, the lower limb muscles (soleus and TA) were recorded bilaterally, and in the other six, additional unilateral (right side) recordings were made from the deltoid, forearm flexors, forearm extensors, and first dorsal interosseous (FDI) muscles. Active electrodes were placed over the muscle bellies of the respective lateral heads of the deltoid and triceps. For the biceps, electrodes were placed over the muscle belly of the short head. For the forearm, electrodes were placed approximately 3–4 cm distal to the antecubital fossa, 1–2 cm medial to the midline for the flexors, and on the dorsal surface of the forearm, approximately 2–3 cm distal to the lateral epicondyle of the humerus, in the midline, for the extensors. For FDI, electrodes were positioned between the first and second metacarpals. For the legs, electrodes were placed 1–2 cm distal to the gastrocnemius musculotendinous junction in the midline for soleus and over the muscle belly for TA, 1–2 cm lateral to the anterior border of the tibia. For all muscle recording sites, the reference electrode was positioned 3–5 cm below the active recording electrode. An earth electrode was positioned on the right forearm, 5 cm distal to the antecubital fossa.
Recordings were made from 50 ms prior to stimulus onset to 250 ms afterwards. EMG was amplified (2000×, D360 amplifier, Digitimer Co, Welwyn Garden City, UK), band-pass- filtered (10–1000 Hz), sampled using a Power1401 (Cambridge Electronic Design, Cambridge, UK), and recorded with Signal software (version 6.04, Cambridge Electronic Design, Cambridge, UK). Averages of both unrectified and rectified EMG were made, the latter after removing any residual DC offset.
Ground reaction forces were measured using a force platform (model 9286A, Kistler Instrumente, Winterthur, Switzerland). All data were sampled at 4 kHz, collected using a CED Power1401 laboratory interface, and recorded using Signal software. Centre of pressure (CoP) was calculated using the force platform manufacturer’s formula and custom Matlab software (Mathworks, MA, USA) and averaged.
Experimental protocols
Participants were asked to stand barefoot on the force platform with their feet approximately 5 cm apart, head straight, and eyes closed, and to adopt three different postures: (1) upright stance with hands clasped in front of the chest, contracting the arm muscles; (2) anterior lean with both hands resting on a table to support their body weight; and (3) posterior lean with arms held out in front and elbows flexed, grasping a metal rod attached to a ceiling-fixed harness to support their body weight. Anterior postural perturbations were achieved by impulses from the motor held by the experimenter and applied over the spine of C7, delivered during upright stance and supported anterior lean. Posterior postural perturbations were achieved by impulses from the motor when applied to the upper sternum and delivered during upright stance and supported posterior lean.
Analysis
For quantitative analysis, we focussed on the earliest (SL) responses evident on averaged rectified EMG traces. The presence of muscle responses was determined using the grand mean of averaged rectified EMG. A response was only considered to be present if the average rectified EMG level rose above the pre-stimulus mean by at least 2.5 times the pre-stimulus standard deviation (SD; Teng et al. 2017). Amplitudes and latencies were subsequently measured in individual subjects using customised software for those muscles which had responses meeting our criteria. The onset latency of a response (SL onset) was taken to be the time when the mean-rectified EMG level first crossed the pre-stimulus mean and consistently remained above prior to crossing the 2.5 SD threshold. The end of a response (SL end) was taken to be the time when the mean-rectified EMG level subsequently returned to the pre-stimulus mean value. Given the number of muscles studied simultaneously, some variation in background activation was to be expected between conditions (Table 1). To correct for any scaling of raw amplitudes due to changes in background activation, response amplitudes were calculated as the change in the mean-rectified EMG and expressed (i.e., normalised) as a percentage of the pre-stimulus mean (Welgampola and Colebatch 2001). In cases where a response was present on the grand average but unclear in a few individuals, measurements were taken at the latencies determined from the grand average for those individuals.
Statistical analysis was performed using SPSS software (Version 24.0, IBM Inc., Chicago, USA). P < 0.05 was considered to be statistically significant. In the bilaterally recorded muscles, EMG responses did not differ significantly between the left and right sides (amplitude: P = 0.200–0.366; latency: P = 0.494–0.848). Thus, responses from both sides were averaged for subsequent analysis. Paired t tests were used to test the effects of weight bearing on normalised response amplitudes. Repeated-measures ANOVAs were performed using muscle as a within-subject factor to compare normalised response amplitudes and onset latencies between muscles in the same condition. Results are given in the text and tables as mean ± SD. Figures illustrate mean ± standard error of the mean (SEM).
Results
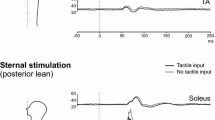
The mean weight of subjects, when standing upright with head straight and isometric contraction of the arms, was 69.0 ± 14.8 kg. In this posture, both C7 and sternal impulses elicited small biphasic responses in the biceps and triceps, but no other upper limb muscles had responses which met our criteria (Fig. 1; Table 1).
Upper and lower limb EMG responses to axial impulses delivered during different postural conditions. The grand means of rectified EMG traces are shown. From left to right, traces are shown for: upright stance with isometric contraction of the arms (C7 stimulation); leaning anteriorly to support body weight with the arms (C7 stimulation); upright stance with isometric contraction of the arms (sternal stimulation); leaning posteriorly to support body weight with the arms (sternal stimulation). Short-latency postural reflexes in the upper limbs became more evident when they were used for support, whereas postural reflexes in the legs were attenuated. *All traces are shown on the same scale (see 20 µV calibration) with the exception of FDI (see 15 µV calibration). F forearm, FDI first dorsal interosseous, TA tibialis anterior
When leaning anteriorly, supported by the arms, subjects’ apparent weight decreased significantly to 56.8 ± 14.6 kg (i.e. by 17.7%, t(11) = 9.0, P < 0.001). In this condition, C7 impulses elicited a large SL excitation in the triceps beginning at 32.7 ± 2.9 ms (Table 2). The normalised amplitude of this response was significantly larger than found in the isometric condition (supported anterior lean: 44.7 ± 13.3%, isometric: 21.7 ± 11.7%, t(11) = 4.5, P = 0.001). Similar responses were demonstrated for the deltoid, forearm flexor, and extensor muscles, and FDI in supported anterior lean (Fig. 1). Responses were not considered to be present in the biceps in this condition as EMG traces were morphologically similar to triceps EMG, but levels of tonic activity were much lower in the biceps than the triceps (Table 1), features which suggested crosstalk. Levels of tonic EMG activity were not significantly different from isometric contraction during anterior lean for the deltoid (12.9 ± 10.4 vs. 16.0 ± 7.2 µV, t(5) = 0.7, P = 0.537) and forearm flexors (22.7 ± 16.0 vs. 37.0 ± 28.5 µV, t(5) = 1.4, P = 0.225) but were significantly higher with supported anterior lean for the other muscles of the upper limb (t(5–11) = 3.0–7.1, P < 0.030, Table 1).
Normalised responses in supported anterior lean were significantly larger for proximal muscles, deltoid, and triceps, than for more distal muscles, forearm flexors, and extensors and FDI (F(4,20) = 22.8, P < 0.001; Fig. 2a and Table 1). Onset latencies were earliest for the deltoid (31.8 ± 4.4 ms) and increased distally (F(4,20) = 57.5, P < 0.001; Fig. 2b and Table 2). The delay in the onset of responses between the forearm extensors and FDI (15.4 ± 4.8 ms) was significantly longer than that between the triceps and forearm extensors (4.8 ± 3.2 ms, t(5) = 3.5, P = 0.017), and the latency difference between deltoid and triceps (1.7 ± 2.1 ms, t(5) = 7.7, P < 0.001).
Mean-normalised response amplitudes (a) and onset latencies (b) of SL responses in the upper limb evoked by C7 impulses during supported anterior lean. Response amplitudes were largest proximally and there was a disproportionate delay to the intrinsic hand muscle, FDI. Error bars represent mean ± standard error of the mean. FDI = first dorsal interosseous muscle. *P < 0.05, **P < 0.01, ***P < 0.001. < = latency difference between distal and proximal muscle groups (FDI—F. extensors, F. extensors—Triceps, and Triceps—Deltoid)
When leaning posteriorly, supported by the arms, subjects’ apparent weight also decreased significantly compared to upright stance (60.6 ± 14.1 kg; equivalent to − 12.2%, t(11) = 3.6, P = 0.004). Sternal impulses in this condition produced SL excitation in the biceps (26.3 ± 23.3%) beginning at 20.1 ± 2.4 ms, but no responses met our criteria for the other muscles of the upper limb (Fig. 1). The large standard deviation of normalised response amplitudes in biceps was explained by a bimodal distribution with four subjects demonstrating responses larger than the mean (34.5–72.7%) and eight subjects demonstrating responses smaller than the mean (5.6–22.0%). In the former group, normalised responses were significantly larger with supported posterior lean (55.2 ± 15.9%) than for isometric contraction (20.5 ± 2.0%, t(3) = 4.6, P = 0.019). For the second group, there was no significant difference in normalised response amplitudes between the two conditions (supported posterior lean: 11.9 ± 5.1%, isometric: 11.9 ± 7.4%, t(7) = 0.01, and P = 0.991). Overall, levels of tonic EMG activity in the biceps were significantly higher in supported posterior lean (44.9 ± 24.0 µV) than for isometric contraction (20.7 ± 12.5 µV, t(11) = 3.4, P = 0.006).
Excitatory effects on leg muscles were largest during upright stance: for soleus (with C7 impulses) 41.1 ± 17.6%; for TA (with sternal impulses): 69.5 ± 30.5%. Both responses began at latencies of 50–60 ms following stimulus onset (Fig. 1; Table 2). Leaning anteriorly to take weight with the arms significantly attenuated the response in soleus to C7 stimulation (12.7 ± 10.2%, t(5) = 3.9, P = 0.011) and a similar effect occurred in TA for sternal stimulation when leaning posteriorly to take weight with the arms (17.1 ± 12.0%, t(5) = 4.7, P = 0.005).
Discussion
We have confirmed that axial (C7) impulses evoke SL responses in the triceps brachii when leaning anteriorly to support body weight with the arms and have also confirmed that supporting body weight with the arms severely attenuates SL responses in the legs compared to upright stance (Teng et al. 2017). In addition, we have shown that similar SL responses occur in the deltoid, forearm muscles, and FDI when leaning anteriorly with the triceps and deltoid having the largest responses. In the case of TA, we further found that a similar attenuation of SL postural responses occurred when leaning posteriorly and taking weight with the arms despite the tonic EMG level actually increasing substantially.
Isometric contraction of the arms produced similar levels of tonic activity to support anterior lean for the deltoid and forearm flexors, but despite this, there were no clear responses to axial impulses when standing upright unsupported, highlighting that a postural role is a key requirement, as previously established for the legs (Graus et al. 2013; Govender et al. 2015) and for triceps brachii (Teng et al. 2017). Matching tonic levels of EMG activity was not always possible, and thus, responses were normalised, because increased tonic activity should increase reflex amplitude (Matthews 1986). The increase in postural reflexes was, however, also seen for muscles in which matching of background activity was successful.
We have previously confirmed that axial perturbations are most effective when potentially destabilising the adopted posture (Bötzel et al. 2001; Govender et al. 2015), and thus, we used stimuli applied over C7 during anterior lean and sternal impulses for posterior lean. When subjects leaned posteriorly to support body weight with the arms, sternal impulses evoked responses in the biceps, but these were only larger than those elicited during isometric contraction of the arms in some of the subjects. Despite this, all subjects showed a greater than 50% attenuation of responses in tibialis anterior (TA) during supported posterior lean and despite the legs still bearing the majority of the subjects’ weight. In the case of the startle reflex, Nonnekes et al. (2013) found attenuation of leg responses when part of the subjects’ weight was supported, leading them to suggest a role for leg afferents. Given the strength of the attenuation despite the increase in TA activity, we feel that this is unlikely to be the mechanism and we prefer a change in gain due to the “subconscious motor system” (Marsden et al. 1981) prompted by the reduced mechanical effectiveness of the leg muscles. This decrease in postural reflexes related to the reduced effectiveness of the leg muscles might also be the basis for the reduction of startle reflexes reported by Nonnekes et al. (2013), given that the two reflexes are likely to share their descending pathway (see below). Postural reflexes appear to be characteristically directed to those muscles most effective in correcting for postural disturbances.
The upper limb responses were better formed for leaning anteriorly (ventral surface down) than posteriorly (dorsal surface down), despite supporting similar levels of body weight. This may be a property of postural reflexes relating to human evolution from quadrupedalism. In addition, crawling and thus using the arms extended in a postural role, analogous to leaning anteriorly and taking weight, is a normal part of human development. Crawling with arms extended remains a physiological means of adult human mobility in certain circumstances. The greater physiological role of postural reflexes with the ventral aspect of the trunk down may explain the better developed and more widespread upper limb postural reflexes for weight bearing with the arms extended.
The acoustic startle reflex, a predominantly flexor protective reflex (Wilkins et al. 1986), originates in the pontomedullary reticular formation and is conducted through the reticulospinal tract (Davis et al. 1982). In humans, the efferent reticulospinal projection mediating acoustic startle is characterised by being having a slow efferent conduction and a disproportionate delay for intrinsic hand muscles (Brown et al. 1991b; Rothwell 2006). In response to C7 impulsive stimulation when leaning anteriorly, we found a proximal-to-distal gradient for both response amplitude and recruitment order of muscles in the upper limb as well as a disproportionately delayed activation of FDI. Both the recruitment order and the larger proximal responses are consistent with the known stronger projections of the reticulospinal neurons to proximal muscle groups (Peterson 1979). Brown et al. (1991b) reported a 22.4 ms difference in latency between biceps and APB for acoustic startle, compared to 10.5 ms difference expected for corticospinal projections. In our case, the latency difference between triceps and FDI was 21.0 ms (Table 2). In hyperekplexia, a condition with pathologically heightened startle, Brown et al. (1991a) reported biceps-TA latency differences of 26–28 ms, similar to the approximately 30 ms difference in our study (Table 2). The similarity of these relative latencies and thus central conduction velocities between our axially evoked responses and those for startle provides further evidence for the previous proposal that the efferent limb of axially evoked reflexes is mediated by the reticulospinal tract (Teng et al. 2017). In addition to their role in mediating startle and postural responses (Stapley and Drew 2009; Deliagina et al. 2014), reticulospinal projections are known also to contribute to locomotion (Drew 1991) and upper limb reaching movements (Davidson et al. 2007).
Despite possibly sharing their efferent pathway, the axial postural reflex clearly is not the same as acoustic startle. The absolute latencies reported for acoustic startle (e.g., 100 ms in TA: Nonnekes et al. 2013) are much longer than the ones which we obtained using axial impulses (e.g., 51.5 ms in TA with sternal impulses during upright stance). Assuming similar peripheral conduction delays, this indicates a substantially shorter central delay for the postural reflex, of the order of 40–50 ms, prior to the generation of the efferent volley. This central delay as well as the characteristic habituation separates the acoustic startle reflex from the postural reflexes shown here. Acoustic startle latencies can be shorter when facilitated by disease and for muscles having a postural role. Brown et al. (1991a) showed latencies of 30 ms in biceps for a hyperekplexic subject when crouching on all fours compared to more typical values of 85–100 ms (Wilkins et al. 1986). Watson and Colebatch (2002) reported a large acoustic startle response in biceps occurring at 42 ms in a patient following a pontine stroke. Posture enhances the normal acoustic startle reflex in leg muscles (Brown et al. 1991a; Nonnekes et al. 2013). Our results suggest that this may be due to upright stance facilitating postural reflexes with acoustic startle reflexes converging upon shared descending pathways.
The efferent reticulospinal pathway mediating startle conducts substantially more slowly than the corticospinal tract (Rothwell 2006). Teng et al. (2017) argued that the efferent pathway mediating axially evoked reflexes also conducted substantially more slowly than the corticospinal tract, at approximately half the velocity. The slower central conduction speed for reticulospinal projections may explain why acoustic startle is better developed for upper limb muscles. While acoustic startle responses occur at shorter than voluntary reaction times in the upper limbs and thus provide the advantage of an earlier, albeit stereotyped, response, any latency advantage would be less for the legs, given the faster conduction of corticospinal projections. Thus, acoustic startle may have a latency of 85 ms for biceps compared to a voluntary reaction time of 110 ms (Thompson et al. 1992), whereas, for TA, the voluntary reaction time (155 ms) is only slightly longer than the typical response to startle (Wilkins et al. 1986). Startle itself can interact and facilitate voluntary movements (Valls-Solé et al. 2008), but any benefit is likely to be more marked for the upper limbs. Teng et al’s (2017) estimate of central conduction speed was based on the value of 25.3 ms which they obtained for the average latency difference between the onset of axially evoked responses in the triceps (during supported anterior lean) and soleus (during upright stance). When compared to the 15.2 ms latency difference between the onset of responses in the biceps and soleus reported for magnetic cortical stimulation, assuming similar peripheral conduction delays, this implied up to 10 ms additional central delay over the corticospinal tract. In the present study, we found the latency difference between the onset of axially evoked responses in the triceps (during supported anterior lean) and soleus (during upright stance) to be 21.3 ms, similar to Teng et al. (2017). However, we obtained a longer value of 31.4 ms for the latency difference between the onset of axially evoked responses in the biceps (during supported posterior lean) and TA (during upright stance). When compared to the 15.3 ms latency difference between these same muscles reported for magnetic cortical stimulation (Rossini et al. 1999), this implies up to 16 ms additional central delay over the corticospinal tract. This is consistent with a slower conducting pathway such as the reticulospinal pathway as suggested by Teng et al. (2017) but indicates that the responsible fibres mediating responses in TA may conduct even more slowly than these authors estimated for soleus. The SL reflexes reported here may be analogous to the spino-bulbar-spinal reflexes which descend from the medial bulbar reticular formation and have an average conduction velocity of 35 m/s (Shimamura and Kogure 1979).
References
Bloem BR, Allum JHJ, Carpenter MG, Honegger F (2000) Is lower leg proprioception essential for triggering human automatic postural responses? Exp Brain Res 130:375–391
Bötzel K, Feise P, Kolev OI, Krafczyk S, Brandt T (2001) Postural reflexes evoked by tapping forehead and chest. Exp Brain Res 138:446–451
Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD (1993) Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res 94:143–151
Brown P, Day BL, Rothwell JC, Thompson PD, Marsden CD (1991a) The effect of posture on the normal and pathological startle reflex. J Neurol Neurosurg Psychiat 54:892–897
Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD (1991b) New observations on the normal auditory startle reflex in man. Brain 114:1891–1902
Davidson AG, Schieber MH, Buford JA (2007) Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci 27:8053–8058
Davis M, Gendelman DS, Tischler MD, Gendelman PM (1982) A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci 2:791–805
Deliagina TG, Beloozerova IN, Orlovsky GN, Zelenin PV (2014) Contribution of supraspinal systems to generation of automatic postural responses. Front Integr Neurosci 8:1–20
Do MC, Brenière Y, Bouisset S (1988) Compensatory reactions to forward fall: are they initiated by stretch receptors? Electroenceph Clin Neurophysiol 69:448–452
Drew T (1991) Functional organization within the medullary reticular formation of the intact unaesthetized cat. III Microstimulation during locomotion. J Neurophysiol 66:919–938
Fitzpatrick RC, Burke D, Gandevia SC (1994) Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol 478:363–372
Govender S, Dennis DL, Colebatch JG (2015) Axially evoked postural reflexes: influence of task. Exp Brain Res 233:215–228
Graus S, Govender S, Colebatch JG (2013) A postural reflex evoked by brief axial accelerations. Exp Brain Res 228:73–85
Gurfinkel VS, Lipshits MI, Mori S, Popov KE (1981) Stabilization of body position as the main task of postural regulation. Hum Physiol 7:155–165
Halmagyi GM, Yavor RA, Colebatch JG (1995) Tapping the head activates the vestibular system: a new use for the clinical tendon hammer. Neurology 45:1927–1929
Marsden CD, Merton PA, Morton HB (1981) Human postural responses. Brain 104:513–534
Matthews PBC (1986) Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol 374:73–90
Nonnekes J, van Geel K, Nijhuis LBO, Bloem BR, Geurts AC, Weerdesteyn V (2013) Loading enhances the occurrence of startle responses in leg muscles. Neuroscience 240:186–190
Peterson BW (1979) Reticulospinal projections to spinal motor nuclei. Ann Rev Physiol 41:127–140
Ross SM (2007) Introduction to probability models, 9th edn. Academic, Amsterdam
Rossini PM, Berardelli A, Deuschl G, Hallett M, de Noordhout AM, Paulus W, Pauri F (1999) Applications of magnetic cortical stimulation. Electroenceph Clin Neurophysiol Suppl 52:171–185
Rothwell JC (2006) The startle reflex, voluntary movement, and the reticulospinal tract. Clin Neurophysiol Suppl 58:223–231
Shimamura M, Kogure I (1979) Reticulospinal tracts involved in the spino-bulbo-spinal reflex in cats. Brain Res 172:13–21
Stapley PJ, Drew T (2009) The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat. J Neurophysiol 101:1334–1350
Teng B, Govender S, Colebatch JG (2017) Postural responses in the upper limbs evoked by axial impulses: a role for reticulospinal projections. Exp Brain Res 235:2235–2242
Thompson PD, Colebatch JG, Brown P, Rothwell JC, Day BL, Obeso JA, Marsden CD (1992) Voluntary stimulus-sensitive jerks and jumps mimicking myoclonus or pathological startle syndromes. Mov Disord 7:257–262
Valls-Solé J, Kumru H, Kofler M (2008) Interaction between startle and voluntary reactions in humans. Exp Brain Res 187:497–507
Watson SRD, Colebatch JG (2002) Focal pathological startle following pontine infarction. Mov Disord 17:212–218
Welgampola M, Colebatch JG (2001) Vestibulospinal reflexes: quantitative effects of sensory feedback and postural task. Exp Brain Res 139:345–353
Wilkins DE, Hallett M, Weiss MM (1986) Audiogenic startle reflex of man and its relationship to startle syndromes. Brain 109:561–573
.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Rights and permissions
About this article
Cite this article
Jeyakumar, N., Govender, S. & Colebatch, J.G. Properties of short-latency responses in the upper limbs evoked by axial impulses during leaning: evidence for reticulospinal projections. Exp Brain Res 236, 2611–2618 (2018). https://doi.org/10.1007/s00221-018-5320-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-5320-5