Abstract
Primary motor cortex excitability can be modulated by anodal and cathodal transcranial direct current stimulation (tDCS). These neuromodulatory effects may, in part, be dependent on modulation within gamma-aminobutyric acid (GABA)-mediated inhibitory networks. GABAergic function can be quantified non-invasively using adaptive threshold hunting paired-pulse transcranial magnetic stimulation (TMS). The previous studies have used TMS with posterior–anterior (PA) induced current to assess tDCS effects on inhibition. However, TMS with anterior–posterior (AP) induced current in the brain provides a more robust measure of GABA-mediated inhibition. The aim of the present study was to assess the modulation of corticomotor excitability and inhibition after anodal and cathodal tDCS using TMS with PA- and AP-induced current. In 16 young adults (26 ± 1 years), we investigated the response to anodal, cathodal, and sham tDCS in a repeated-measures double-blinded crossover design. Adaptive threshold hunting paired-pulse TMS with PA- and AP-induced current was used to examine separate interneuronal populations within M1 and their influence on corticomotor excitability and short- and long-interval inhibition (SICI and LICI) for up to 60 min after tDCS. Unexpectedly, cathodal tDCS increased corticomotor excitability assessed with AP (P = 0.047) but not PA stimulation (P = 0.74). SICIAP was reduced after anodal tDCS compared with sham (P = 0.040). Pearson’s correlations indicated that SICIAP and LICIAP modulation was associated with corticomotor excitability after anodal (P = 0.027) and cathodal tDCS (P = 0.042). The after-effects of tDCS on corticomotor excitability may depend on the direction of the TMS-induced current used to make assessments, and on modulation within GABA-mediated inhibitory circuits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-invasive brain stimulation can be used to modulate primary motor cortex (M1) excitability. One such technique is transcranial direct current stimulation (tDCS), which induces plasticity akin to long-term potentiation or depression in an N-methyl-d-aspartate receptor-dependent manner (Nitsche et al. 2003a). For example, M1 excitability increases after tDCS is applied with the anode placed over M1 and cathode over the contralateral supraorbit (anodal tDCS), and decreases if the electrode placement is reversed (cathodal tDCS) (Nitsche and Paulus 2000). However, recent studies have identified a large degree of inter-individual variability in terms of the direction and magnitude of the response to tDCS (Ammann et al. 2017; Fujiyama et al. 2014; Lopez-Alonso et al. 2014; McCambridge et al. 2015; Tremblay et al. 2016; Wiethoff et al. 2014). This variability must be addressed if tDCS is to be used as an effective neuromodulation technique.
Endogeneous levels of the main inhibitory neurotransmitter gamma-aminobutyric acid (GABA) may, in part, mediate tDCS effects on corticomotor excitability. For example, the observed increase in motor evoked potential (MEP) amplitude after anodal tDCS is delayed with prior administration of a GABAA receptor agonist (Nitsche et al. 2004).
Paired-pulse transcranial magnetic stimulation (TMS) protocols are used to examine short- and long-interval intracortical inhibition (SICI and LICI), which are markers of GABAA (Ilić et al. 2002; Ziemann et al. 1996) and GABAB (McDonnell et al. 2006) receptor activity, respectively. The majority of studies utilise the conventional paired-pulse TMS to quantify inhibition, where the conditioned MEP is expressed relative to the nonconditioned MEP (Kujirai et al. 1993; Valls-Sole et al. 1992). However, the modulation of SICI and LICI after tDCS has been inconsistent (Batsikadze et al. 2013; Heise et al. 2014; Kidgell et al. 2013; Lopez-Alonso et al. 2015; Nitsche et al. 2005; Sasaki et al. 2016; Zhang et al. 2014), which may be attributed to the fact MEP amplitude is an inherently variable measure (Darling et al. 2006; Kiers et al. 1993). An alternative approach is adaptive threshold hunting (Awiszus 2003) which eliminates the confound of nonconditioned MEP variability. With threshold hunting, inhibition is measured as the percent increase in test stimulus intensity required to maintain a target threshold MEP amplitude (e.g., 200 µV) in the presence of the conditioning stimulus. Both methods are thought to be similar in their mechanisms of action (Amandusson et al. 2017). Adaptive threshold hunting may be a preferable method for assessing tDCS effects by overcoming MEP amplitude confounds introduced by natural variability.
GABA-mediated inhibition before and after tDCS is usually examined with TMS using a posterior–anterior (PA)-induced current in the brain. PA stimulation preferentially activates early indirect (I)-waves arising from transynaptic activation of pyramidal neurons (Sakai et al. 1997). However, the conditioning stimulus from SICI and LICI supresses later I-waves more so than early (Di Lazzaro et al. 1998; Nakamura et al. 1997). Anterior–posterior (AP) stimulation preferentially activates circuits responsible for late I-waves (Di Lazzaro et al. 2001; Sakai et al. 1997) and more robust SICI and LICI has been shown with AP stimulation than PA (Cirillo and Byblow 2016; Sale et al. 2015; Zoghi et al. 2003). Furthermore, individuals who preferentially recruit late I-waves exhibit greater SICI and LICI than early I-wave recruiters (Cirillo and Byblow 2016) and are less susceptible to tDCS effects on corticomotor excitability (McCambridge et al. 2015; Wiethoff et al. 2014). It is unknown how tDCS after-effects on corticomotor excitability and inhibition differ between PA- and AP-induced current.
The aim of this study was to examine the after-effects of tDCS on M1 corticomotor excitability and GABA-mediated intracortical inhibition (SICI and LICI) using single-pulse and adaptive threshold hunting paired-pulse TMS with PA- and AP-induced current. We hypothesised that the after-effects of tDCS would differ between PA- and AP-induced current due to the preferential recruitment of early and late I-waves, respectively. We also sought to determine whether the modulation of intracortical inhibition was associated with the extent of corticomotor excitability modulation after tDCS.
Methods
Participants
Sixteen neurologically healthy young adults (9 females, mean age 26 ± 1 years, range 21–33 years) participated in this study. All participants were right-handed as assessed by the short version of the Edinburgh Handedness Inventory (Veale 2014), with a mean laterality quotient of 93 ± 3 (range 75–100). Participants were screened for contraindications to TMS before participation using a safety screening questionnaire, developed by our institution based on a previous report (Keel et al. 2001). Each participant provided written informed consent and the study was approved by the University of Auckland Human Participants Research Ethics Committee.
Experimental design
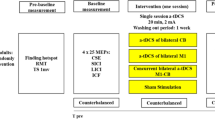
There were three experimental sessions. In each session, participants were pseudorandomly allocated to receive either 13 min of anodal (Nitsche and Paulus 2001), 9 min of cathodal (Nitsche et al. 2003b), or sham tDCS (Gandiga et al. 2006), in a repeated-measures double-blinded crossover design. There were six possible tDCS sequences with either two or three participants assigned to each. Measures of corticomotor excitability and inhibition were investigated at two time points before (B1 and B2; time interval of ~ 5 min) and at four time points after (P0, P20, P40, and P60) tDCS using TMS (Fig. 1). Sessions one and two were separated by a mean of 11 days (range 7–37 days). Sessions two and three were separated by a mean of 16 days (range 5–44 days). All sessions were completed after midday and at the same time of day for each participant, to control for the influence of diurnal variations in cortisol levels on neuroplasticity (Sale et al. 2008).
Schematic illustration of the experimental design. Participants completed three sessions where transcranial magnetic stimulation with posterior–anterior (PA) and anterior–posterior (AP)-induced current was used to assess the threshold hunting target (THT), short-interval intracortical inhibition (SICI), and long-interval intracortical inhibition (LICI) before (B1 and B2) and after (P0–P60) anodal, cathodal, and sham transcranial direct current stimulation
Recording and stimulation procedures
Surface electromyography
Surface electromyography (EMG) was recorded from the first dorsal interosseous (FDI) of the dominant right hand using 10 mm diameter Ag–AgCl recording electrodes (Ambu, Ballerup, Denmark) arranged in a belly-tendon montage, with a 20 mm diameter ground surface electrode (3M, Canada Health Care) positioned on the dorsum of the right hand. EMG signals were amplified (1000×) and band-pass filtered (10–1000 Hz) using a CED1902 amplifier (CED, Cambridge, UK), sampled at 2 kHz using a CED1401 interface (CED, Cambridge, UK) and recorded onto a computer for offline analysis using Signal (Version 5.03, CED, Cambridge, UK) software.
Transcranial magnetic stimulation
A MagPro X100 + option magnetic stimulator (MagVenture, Farum, Denmark) connected to a figure-of-eight coil (MC-B70, outer wing diameter 97 mm) was used to deliver TMS using a monophasic waveform (pulse width = 70 µs). Descending volleys were preferentially activated via early or late I-waves by alternating current flow through M1 (Sakai et al. 1997). PA stimulation (coil ~ 45° to the mid-sagittal line) preferentially elicits early I-waves, whereas AP (coil angle same as PA, but current reversed) preferentially elicits late I-waves. The optimal site to elicit consistent MEPs in the resting right FDI muscle with PA-induced current was marked on the scalp over the left hemisphere. The same scalp position was confirmed as the optimal scalp position for AP-induced current. The current direction order was pseudorandomised between participants and kept constant within participants. TMS was delivered at 0.2 Hz, with 20% variation between trials, and optimal coil position was continually monitored using visual inspection throughout the experiment.
Motor thresholds
A freeware program that employs a maximum-likelihood parameter estimation by sequential testing (PEST) was used for adaptive threshold hunting (Awiszus and Borckardt 2011) to determine rest motor threshold (RMT) and active motor threshold (AMT). The procedure involves adjusting the stimulus intensity up or down as required based on an MEP amplitude criterion for 12 trials (approximate duration of procedure is 1 min) until the target intensity is found within 95% confidence intervals. Two operators were involved in the TMS procedures. One experimenter held the coil, while the other manually entered data into PEST software and adjusted stimulator intensity according to PEST output. For RMT, a trial was deemed successful if the stimulus intensity elicited a MEP of at least 50 µV in amplitude. For AMT, a trial was deemed successful if the stimulus intensity elicited an MEP of at least 100 µV in amplitude, with FDI pre-activated to approximately 10% of the participant’s perceived maximum voluntary contraction. RMT and AMT were only measured at baseline as they were not expected to be modulated by tDCS (Nitsche et al. 2005) and they exhibit very low intraday variability using PEST (Silbert et al. 2013). At all time points, a threshold hunting target (THT) was obtained similar to RMT and AMT, whereby the MEP amplitude criterion for a successful trial was 200 µV (Fig. 2).
Example EMG traces depict motor evoked potentials (MEP) from an individual participant during adaptive threshold hunting. First, the transcranial magnetic stimulation (TMS) intensity required to elicit a fixed MEP amplitude (200 µV) to the single-pulse test stimulus (threshold hunting target, THT) is determined. Dashed lines indicate MEP onset latencies for TMS with posterior–anterior (PA) and anterior–posterior (AP)-induced current. For both short- and long-interval intracortical inhibition (SICI and LICI), adaptive threshold hunting requires an increase in the test stimulus intensity to evoke the target response in the presence of conditioning (grey traces)
Short- and long-interval intracortical inhibition
Adaptive threshold hunting paired-pulse TMS was used to quantify the extent of SICI and LICI within M1 in line with the previous work (Amandusson et al. 2017; Awiszus 2003; Awiszus et al. 1999; Cirillo and Byblow 2016; Mooney et al. 2017). To elicit SICI, a subthreshold conditioning stimulus, set to 90% AMT, was delivered 3 ms prior to a test stimulus (Kujirai et al. 1993). To elicit LICI, a suprathreshold conditioning stimulus set to 120% RMT was delivered 100 ms prior to a test stimulus (Valls-Sole et al. 1992). For both SICI and LICI procedures, PEST was used to determine the test stimulus intensity required to achieve the THT (200 µV MEP) in the presence of the conditioning stimulus. There was approximately 1 min between the end of one estimation of the THT and the start of the next.
Transcranial direct current stimulation
A NeuroConn DC stimulator (NeuroConn, Ilmenau, Germany) was used to administer tDCS through two rubber electrodes inserted into saline soaked sponges. The active electrode (25 cm2) was placed over the left M1 hotspot and the reference electrode (35 cm2) was placed over the right supraorbital ridge. For anodal tDCS (anode over M1) and cathodal tDCS (cathode over M1), the current was ramped up for 30 s and then maintained at a constant 1 mA current for 13 and 9 min, respectively. For sham tDCS, the current was ramped up to 1 mA and immediately ramped down to 0 mA within 30 s. In each session, participants were instructed to sit quietly by the tDCS administrator for 14 min throughout the stimulation. TMS and tDCS were carried out by different operators, with the TMS operator blinded to each tDCS protocol.
MEP latency
At the end of the third session, MEP latencies were assessed, while the participant maintained a low-level voluntary contraction by isometrically abducting the index finger (Hamada et al. 2013; Sakai et al. 1997). Stimulation intensities of 110% of AMTPA and AMTAP were used to target MEP latency from the early and late I-waves, respectively. Lateromedial (LM) stimulation (coil handle 90° to the midline) with a high stimulation intensity (150% of AMTLM) was used to preferentially recruit D-waves. Sixteen MEPs were recorded for each current direction.
Data analysis
During threshold hunting, any trials that were contaminated by pre-stimulus EMG activity (root-mean-squared EMG > 10 µV; 50 ms before stimulation) were rejected online and repeated immediately. The peak-to-peak amplitude of the 16 MEPs elicited by the suprathreshold conditioning stimulus from the LICI protocol was used to index corticomotor excitability at each time point. SICI and LICI induced by the conditioning stimulus were quantified as the ratio of the conditioned and nonconditioned stimulation intensity required to evoke the THT:
where values greater than 1 indicate inhibition. MEP amplitude, THT, SICI, and LICI values obtained at each post-time point were normalised to the baseline values (average of B1 and B2) where values greater than 1 reflect an increase and those less than 1 a decrease.
MEP latency was determined using a semi-automated method and defined as the time point where the rectified EMG after TMS exceeded 2 SD of the mean background EMG (100 ms before the stimulus). The MEP latency differences between PA-LM and AP-LM were used as measures of the early and late I-wave recruitment, respectively (Hamada et al. 2013).
Statistical analysis
Normality was assessed using the Shapiro–Wilk’s test and homoscedasticity of variance using the Levene’s test of equality and Mauchly’s test of sphericity. Non-normal data were log transformed.
Prior to tDCS, baseline RMT and AMT were analysed with a two-way repeated-measures ANOVA with factors SESSION (anodal, cathodal, and sham) and CURRENT DIRECTION (PA and AP). Baseline MEP amplitude, THT, SICI, and LICI were analysed with a three-way repeated-measures ANOVA with factors SESSION, CURRENT DIRECTION, and TIME (B1 and B2). Reliability of threshold hunting TMS was assessed using intraclass correlation coefficients. Intraday reliability (two-way random model, absolute agreement type, and single [2,1] and averaged [2,k] measures) was determined using B1 and B2 data obtained in session 1. Interday reliability (two-way random model, absolute agreement type, and single [3,1] and averaged [3,k] measures) was determined using the average of B1 and B2 data obtained in each session. Reliability estimates were judged as either fair (0.40–0.58), good (0.59–0.74), or excellent (> 0.75) (Cicchetti and Sparrow 1981). Although the intraclass correlation coefficient is an index of how well individuals can be distinguished from one another with repeated testing, it provides no information on the absolute differences between repeated measurements. Therefore, we also calculated the smallest detectable change (SDC), derived from the measurement error, which indicates a true change beyond measurement noise (Schuck and Zwingmann 2003). The SDC for an individual participant was calculated for intraday and interday measures using the following formula (Schuck and Zwingmann 2003):
where the standard error of the measurement (SEMeas) was calculated by taking the square root of the within-subject variance partitioned by a one-way ANOVA with SUBJECT as a factor (Bartlett and Frost 2008), ‘m’ is the number of measurements made per participant, and 1.96 represents a 95% confidence interval. The SDC for the group was then calculated for intraday and interday measures using the following formula (Terwee et al. 2007):
After tDCS, normalised MEP amplitude, THT, SICI, and LICI were analysed with a three-way repeated-measures ANOVA with factors SESSION, CURRENT DIRECTION and TIME (P0, P20, P40, and P60). Pearson’s correlation analyses were used to investigate the relationship between normalised SICI and LICI and MEP amplitude for PA and AP stimulation at each post-time point.
AMT and MEP latency were analysed with a one-way repeated-measures ANOVA with the factor CURRENT DIRECTION (PA, AP, and LM). A paired t test was performed to compare PA-LM and AP-LM latency differences. Pearson’s correlation analyses were used to investigate the relationship between PA-LM and AP-LM latency difference and grand normalised MEP amplitude for PA and AP stimulation in the anodal and cathodal tDCS sessions.
The significance level was set at P < 0.05. Post hoc analyses and Pearson correlation analyses were corrected for multiple comparisons (Rom 1990). Group data are presented as mean ± SEM in the text.
Results
Participants completed all three experimental sessions, with no adverse events.
Baseline
Baseline neurophysiological measures for each session are reported in Table 1 and ANOVA results in Table 2. RMT, AMT, and THT were higher for AP stimulation compared to PA. There were no differences in baseline MEP amplitude or LICI. However, SICI was greater with AP-induced current compared to PA.
Intraclass correlation coefficients and SDC values for RMT, AMT, THT, SICI, and LICI assessed using adaptive threshold hunting are displayed in Table 3. There was good-to-excellent intraday and interday reliability for all measures using both PA and AP stimulation.
Post-tDCS
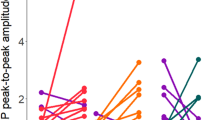
Results of the ANOVAs are displayed in Table 4. After cathodal tDCS, normalised MEP amplitude was greater with AP stimulation than PA stimulation (P = 0.010; Fig. 3c). One-sample t tests indicated that after cathodal tDCS, MEP amplitude was facilitated with AP (P = 0.047) but not PA-induced current (P = 0.74). After anodal tDCS, there was a trend towards an increase in MEP amplitude elicited with PA and AP stimulation (both P > 0.06).
Motor evoked potential (MEP) amplitude at each post-time point (P0–P60) after anodal, cathodal, and sham transcranial direct current stimulation assessed with posterior–anterior (PA; a) and anterior–posterior (AP; b)-induced current. Grand mean MEP amplitude (c) was greater with AP compared with PA after cathodal. Data are presented as mean ± SEM. N = 16. *P < 0.05
Normalised THTPA was greater at P20 after cathodal tDCS compared with sham (P = 0.032; Fig. 4a). Furthermore, normalised THTAP was greater at P20 and P40 after anodal tDCS compared with sham (P = 0.046 and P = 0.013; Fig. 4b).
Threshold hunting target (THT; a, b), short-interval intracortical inhibition (SICI; c, d), and long-interval intracortical inhibition (LICI; e, f) at each post-time point (P0–P60) after anodal, cathodal, and sham transcranial direct current stimulation assessed with posterior–anterior (PA) and anterior–posterior (AP)-induced current. Normalised THT with PA-induced current was greater at P20 after cathodal compared with sham and normalised THT with AP-induced current was greater at P20 and P40 after anodal compared with sham. Normalised SICI with AP-induced current was reduced at P0 after anodal compared with sham. Data are presented as mean ± SEM. N = 16. *P < 0.05
Normalised SICIAP was lower at P0 after anodal tDCS compared with sham (P = 0.04; Fig. 4d). However, there were no differences for normalised SICIPA or LICI (Fig. 4c, e, f).
Anodal tDCS led to a negative correlation between MEP amplitude with PA-induced current and SICIAP at P0 (r = − 0.68, P = 0.016) and P20 (r = − 0.65, P = 0.027; Table 5). Participants with the largest decrease in SICIAP tended to show largest MEP amplitude increases after anodal tDCS. In contrast cathodal tDCS led to a positive correlation between MEP amplitude with PA-induced current and SICIAP and LICIAP at P0 (SICIAP: r = 0.73, P = 0.006; LICIAP: r = 0.62, P = 0.042). Participants with the largest increase in SICIAP or LICIAP exhibited the largest increase in MEP amplitude after cathodal tDCS. There were no other associations between SICI or LICI and MEP amplitude modulation after tDCS (all P > 0.07).
MEP latency
There was a main effect of CURRENT DIRECTION for AMT (F2, 30 = 46.32, P < 0.001). Post hoc comparisons revealed that AMTPA (32.1 ± 1.3% MSO) was lower than AMTLM (36.5 ± 1.5% MSO, P = 0.014) and AMTAP (43.8 ± 1.4% MSO, P < 0.001), and that AMTLM was lower than AMTAP (P < 0.001). There was a main effect of CURRENT DIRECTION for MEP latency (F2, 30 = 353.80, P < 0.001). Post hoc comparisons revealed that PA MEP latency (22.62 ± 0.46 ms) was longer than LM MEP latency (21.36 ± 0.43 ms, P < 0.001), and AP MEP latency (25.20 ± 0.44 ms) was longer than PA (P < 0.001) and LM (P < 0.001). For MEP latency differences indicative of I-wave recruitment, AP-LM (3.83 ± 0.13 ms) was greater than PA-LM (1.25 ± 0.15 ms, P < 0.001). There were no associations between AP-LM or PA-LM latency difference and grand normalised MEP amplitude with PA or AP stimulation in the anodal and cathodal tDCS sessions (all P > 0.11).
Discussion
In support of our hypothesis, tDCS after-effects on corticomotor excitability and intracortical inhibition differed between PA- and AP-induced current. Cathodal tDCS increased corticomotor excitability assessed with AP but not PA stimulation. However, anodal tDCS had no effect on corticomotor excitability with either current direction. GABAA-mediated inhibition was reduced immediately after anodal tDCS and the modulation of GABAA- and GABAB-mediated inhibition was associated with the modulation of corticomotor excitability after anodal and cathodal tDCS. These findings indicate that utilising both PA and AP stimulation activates separate M1 interneuronal populations, which may be advantageous when assessing tDCS effects on corticomotor excitability and inhibition. Furthermore, modulation within GABA-mediated inhibitory circuits may contribute to the variable inter-individual effects of tDCS.
Modulation of corticomotor excitability after tDCS
There was a large degree of inter-individual variability after tDCS which is consistent with recent reports (Fujiyama et al. 2014; Lopez-Alonso et al. 2015; McCambridge et al. 2015; Tremblay et al. 2016; Wiethoff et al. 2014). The mechanism(s) underlying tDCS modulation of MEP amplitude is unclear. In vitro studies have shown that tDCS establishes a polarisation gradient along pyramidal neurons (Kronberg et al. 2017; Radman et al. 2009; Rahman et al. 2013). For example, anodal tDCS depolarises the soma and initial axonal segment in distal compartments, whereas apical dendrites within superficial compartments are hyperpolarised (Kronberg et al. 2017; Rahman et al. 2013). It is conceivable that anodal tDCS may increase the efficacy of early I-wave inputs which synapse close to the soma, but decrease those of late I-wave inputs within superficial compartments (Di Lazzaro and Ziemann 2013). Preferential recruitment of early I-waves has been associated with increased MEP amplitude using PA stimulation after anodal tDCS, whereas preferential recruitment of late I-waves has not (McCambridge et al. 2015; Wiethoff et al. 2014). Interestingly, preferential I-wave recruitment was not associated with MEP modulation after cathodal tDCS (Wiethoff et al. 2014). The implications of I-wave recruitment on MEP amplitude modulation after tDCS remains to be fully elucidated.
To our knowledge, this is the first study that has used both PA- and AP-induced current to examine MEP modulation after anodal and cathodal tDCS. Surprisingly, cathodal tDCS facilitated MEPs obtained with AP but not PA stimulation. This increased MEP amplitude may reflect enhanced synaptic activity from late I-wave inputs to the superficial dendritic compartments of pyramidal neurons that are not detectable with PA-induced current, due to its preferential activation of the early I-wave inputs to distal pyramidal compartments (Di Lazzaro and Ziemann 2013). Batsikadze et al. (2013) also found a paradoxical increase in MEP amplitude after cathodal tDCS. However, they used PA-induced current and facilitation was only evident with a 2 mA (but not 1 mA) intensity, which they suggested may have been due to increased depolarisation within superficial dendritic compartments. These effects are likely also evident after 1 mA cathodal tDCS, but only revealed using AP-induced current through preferential recruitment of late I-wave inputs to these superficial compartments. Comparing tDCS after-effects with PA- and AP-induced currents should be considered in future studies.
The nonconditioned THT was increased after both anodal and cathodal tDCS. The THT corresponds to approximately 110% RMT (Cirillo and Byblow 2016) and tDCS does not normally modulate MEP amplitude at such low stimulation intensities (Nitsche et al. 2005). However, the use of threshold rather than MEP amplitude eliminates the confounding influence of MEP amplitude variability which is often highest at stimulation intensities near threshold (Darling et al. 2006; Kiers et al. 1993). The use of adaptive threshold hunting may also have implications after neurological injury such as stroke, where ipsilesional corticomotor output may be reduced (Stinear et al. 2015), and typical stimulation parameters (i.e., 1 mV MEP) are unattainable. Future studies should consider assessing changes in the THT alongside MEP amplitude-based measures.
Modulation of intracortical inhibition after tDCS
There were no effects of tDCS on SICIPA assessed with adaptive threshold hunting. The previous studies with the conventional TMS have found that anodal tDCS may decrease SICIPA and cathodal tDCS may increase SICIPA, respectively (Batsikadze et al. 2013; Kidgell et al. 2013; Nitsche et al. 2005; Zhang et al. 2014), although these polarity-dependent effects are not always consistent (Lopez-Alonso et al. 2014, 2015; Sasaki et al. 2016). Although a comparison between techniques would be interesting, this was not feasible for the present study due to time constraints. Threshold hunting with PA-induced current may be suboptimal for assessing SICI due to the limited late I-wave recruitment with this induced current direction. MEPs derived from paired-pulse TMS with AP-induced current in the brain may provide a more robust measure of SICI than those derived from PA-induced current (Cirillo and Byblow 2016; Sale et al. 2015; Zoghi et al. 2003). Indeed, we observed a reduction in SICIAP immediately after anodal tDCS, which may reflect decreased activity within GABAA receptor-mediated inhibitory networks within superficial layers of M1 (Di Lazzaro and Ziemann 2013). Alternatively, reduced activity within all synapses in superficial dendritic compartments after anodal tDCS may decrease the size and number of late I-waves, accounting for a reduction in SICIAP (Rahman et al. 2013). However, the latter possibility seems unlikely because epidural recordings have shown that anodal tDCS increases the amplitude of both early and late I-waves (Di Lazzaro et al. 2013). In summary, tDCS effects on SICI may only be evident when threshold hunting with AP and not PA-induced current due to the preferential activation of late I-waves.
With adaptive threshold hunting, we found that that LICIPA and LICIAP were not modulated by tDCS, which corroborates findings from the conventional paired-pulse TMS studies (Antal et al. 2010; Tremblay et al. 2013). Interestingly, Tremblay et al. (2013) observed a reduction in cortical silent period duration after anodal tDCS. However, LICI and cortical silent period duration reflect distinct GABAB receptor-mediated processes (McDonnell et al. 2006). The effect of tDCS may be isolated to GABAB processes which underlie the cortical silent period only.
Association between inhibition and corticomotor excitability after tDCS
There was an association between the modulation of SICI and corticomotor excitability following tDCS. After anodal tDCS, participants with reduced SICIAP had an increase in MEP amplitude with PA-induced current. In contrast, after cathodal tDCS, both those with reduced SICIAP and those with reduced LICIAP exhibited a decrease in MEP amplitude with PA-induced current. These associations were only observed early (0 and 20 min) but not long (40 and 60 min) after tDCS. It is likely that the early disinhibition after tDCS may be necessary for corticomotor excitability modulation. This notion is supported by a pharmacological study, showing that administration of a GABAA agonist prior to anodal tDCS delays the increased MEP amplitude after-effect (Nitsche et al. 2004). Our findings also indicate that GABAB receptor-mediated LICI is associated with MEP amplitude modulation after cathodal tDCS. However, pharmacological interventions targeting GABAB receptors and their influence on tDCS effects remain unknown. Altered GABAA and GABAB receptor-mediated inhibition may contribute to tDCS after-effects on corticomotor excitability.
Modulation of intracortical inhibition may contribute to the inter-individual variability of tDCS effects on MEP amplitude, alongside factors such as basal corticomotor excitability (Tremblay et al. 2016; Wiethoff et al. 2014), I-wave recruitment (McCambridge et al. 2015; Wiethoff et al. 2014), and sensorimotor functional connectivity (Hordacre et al. 2017). For example, Hordacre et al. (2017) observed that participants with high resting-state functional connectivity between M1 and ipsilateral parietal or premotor regions exhibited an expected increase in corticomotor excitability after anodal tDCS, but those with low functional connectivity did not. Functional connectivity between sensory and motor cortical regions is related to GABA-mediated inhibitory tone (Antonenko et al. 2017; Stagg et al. 2014), both of which are susceptible to modulation by tDCS (Antonenko et al. 2017; Bachtiar et al. 2015). It is becoming increasingly evident that multiple neurophysiological factors may contribute to the inter-individual variability of tDCS after-effects.
Limitations
The present study has some limitations. First, a small sample size may lead to false positives from an inflated effect size. Second, the physiological effects of tDCS can be relatively widespread and, therefore, not limited to M1 (Kuo et al. 2013). Third, I-wave recruitment was only assessed in the final session and may account for the absence of association with tDCS-induced effects (McCambridge et al. 2015; Wiethoff et al. 2014), although this is not a great concern, because MEP latency has good intersession reliability (Hamada et al. 2013). Finally, we did not screen female participants for their menstrual cycle which can influence cortical excitability (Smith et al. 1999), nor did we screen all participants for prior exercise history which can influence responsiveness to neuroplasticity paradigms (Cirillo et al. 2009).
Conclusion
In summary, tDCS effects on corticomotor excitability and intracortical inhibition may depend on the direction of the TMS-induced current. Furthermore, modulation of GABAA and GABAB receptor-mediated inhibition may contribute to the inter-individual variability of tDCS after-effects. Future neurorehabilitation studies could consider adopting threshold hunting techniques to examine tDCS after-effects with clinical groups that express high variability.
References
Amandusson Å, Flink R, Axelson HW (2017) Comparison between adaptive and fixed stimulus paired-pulse transcranial magnetic stimulation (ppTMS) in normal subjects. Clin Neurophysiol Pract 2:91–97. https://doi.org/10.1016/j.cnp.2017.04.001
Ammann C, Lindquist MA, Celnik PA (2017) Response variability of different anodal transcranial direct current stimulation intensities across multiple sessions. Brain Stimul 10:757–763. https://doi.org/10.1016/j.brs.2017.04.003
Antal A, Terney D, Kuhnl S, Paulus W (2010) Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manag 39:890–903. https://doi.org/10.1016/j.jpainsymman.2009.09.023
Antonenko D et al (2017) tDCS-induced modulation of GABA levels and resting-state functional connectivity in older adults. J Neurosci 37:4065–4073. https://doi.org/10.1523/JNEUROSCI.0079-17.2017
Awiszus F (2003) TMS and threshold hunting. Suppl Clin Neurophysiol 56:13–23
Awiszus F, Borckardt JJ (2011) TMS motor threshold assessment tool (MTAT 2.0). http://www.clinicalresearcher.org/software.htm
Awiszus F, Feistner H, Urbach D, Bostock H (1999) Characterisation of paired-pulse transcranial magnetic stimulation conditions yielding intracortical inhibition or I-wave facilitation using a threshold-hunting paradigm. Exp Brain Res 129:317–324
Bachtiar V, Near J, Johansen-Berg H, Stagg CJ (2015) Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. Elife. https://doi.org/10.7554/eLife.08789
Bartlett JW, Frost C (2008) Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol 31:466–475. https://doi.org/10.1002/uog.5256
Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA (2013) Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol Lond 591:1987–2000. https://doi.org/10.1113/jphysiol.2012.249730
Cicchetti DV, Sparrow SA (1981) Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic 86:127–137
Cirillo J, Byblow WD (2016) Threshold tracking primary motor cortex inhibition: the influence of current direction. Eur J Neurosci. https://doi.org/10.1111/ejn.13369
Cirillo J, Lavender AP, Ridding MC, Semmler JG (2009) Motor cortex plasticity induced by paired associative stimulation is enhanced in physically active individuals. J Physiol 587:5831–5842. https://doi.org/10.1113/jphysiol.2009.181834
Darling WG, Wolf SL, Butler AJ (2006) Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Exp Brain Res 174:376–385. https://doi.org/10.1007/s00221-006-0468-9
Di Lazzaro V, Ziemann U (2013) The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. Front Neural Circ 7:18. https://doi.org/10.3389/fncir.2013.00018
Di Lazzaro V et al (1998) Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res 119:265–268
Di Lazzaro V et al (2001) The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res 138:268–273
Di Lazzaro V et al (2013) Transcranial direct current stimulation effects on the excitability of corticospinal axons of the human cerebral cortex. Brain Stimul 6:641–643. https://doi.org/10.1016/j.brs.2012.09.006
Fujiyama H, Hyde J, Hinder MR, Kim SJ, McCormack GH, Vickers JC, Summers JJ (2014) Delayed plastic responses to anodal tDCS in older adults. Front Aging Neurosci 6:115. https://doi.org/10.3389/fnagi.2014.00115
Gandiga PC, Hummel FC, Cohen LG (2006) Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117:845–850. https://doi.org/10.1016/j.clinph.2005.12.003
Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC (2013) The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex 23:1593–1605. https://doi.org/10.1093/cercor/bhs147
Heise KF, Niehoff M, Feldheim JF, Liuzzi G, Gerloff C, Hummel FC (2014) Differential behavioral and physiological effects of anodal transcranial direct current stimulation in healthy adults of younger and older age. Front Aging Neurosci 6:146. https://doi.org/10.3389/fnagi.2014.00146
Hordacre B, Moezzi B, Goldsworthy MR, Rogasch NC, Graetz LJ, Ridding MC (2017) Resting state functional connectivity measures correlate with the response to anodal transcranial direct current stimulation. Eur J Neurosci 45:837–845. https://doi.org/10.1111/ejn.13508
Ilić TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U (2002) Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol 545:153–167. https://doi.org/10.1113/jphysiol.2002.030122
Keel JC, Smith MJ, Wassermann EM (2001) A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol 112:720
Kidgell DJ, Goodwill AM, Frazer AK, Daly RM (2013) Induction of cortical plasticity and improved motor performance following unilateral and bilateral transcranial direct current stimulation of the primary motor cortex. BMC Neurosci 14:64. https://doi.org/10.1186/1471-2202-14-64
Kiers L, Cros D, Chiappa KH, Fang J (1993) Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 89:415–423
Kronberg G, Bridi M, Abel T, Bikson M, Parra LC (2017) Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Stimul 10:51–58. https://doi.org/10.1016/j.brs.2016.10.001
Kujirai T et al (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, Nitsche MA (2013) Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimul 6:644–648. https://doi.org/10.1016/j.brs.2012.09.010
Lopez-Alonso V, Cheeran B, Rio-Rodriguez D, Fernandez-Del-Olmo M (2014) Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul 7:372–380. https://doi.org/10.1016/j.brs.2014.02.004
Lopez-Alonso V, Fernandez-Del-Olmo M, Costantini A, Gonzalez-Henriquez JJ, Cheeran B (2015) Intra-individual variability in the response to anodal transcranial direct current stimulation. Clin Neurophysiol 126:2342–2347. https://doi.org/10.1016/j.clinph.2015.03.022
McCambridge AB, Stinear JW, Byblow WD (2015) ‘I-wave’ recruitment determines response to tDCS in the upper limb, but only so far. Brain Stimul 8:1124–1129. https://doi.org/10.1016/j.brs.2015.07.027
McDonnell MN, Orekhov Y, Ziemann U (2006) The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res 173:86–93. https://doi.org/10.1007/s00221-006-0365-2
Mooney RA, Cirillo J, Byblow WD (2017) GABA and primary motor cortex inhibition in young and older adults: a multimodal reliability study. J Neurophysiol 118:425–433. https://doi.org/10.1152/jn.00199.2017
Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H (1997) Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol 498(Pt 3):817–823
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527(Pt 3):633–639
Nitsche MA, Paulus W (2001) Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57:1899–1901
Nitsche MA et al (2003a) Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 553:293–301. https://doi.org/10.1113/jphysiol.2003.049916
Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W (2003b) Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol 114:600–604
Nitsche MA et al (2004) GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur J Neurosci 19:2720–2726. https://doi.org/10.1111/j.0953-816X.2004.03398.x
Nitsche MA et al (2005) Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol 568:291–303. https://doi.org/10.1113/jphysiol.2005.092429
Radman T, Ramos RL, Brumberg JC, Bikson M (2009) Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul 2:215–228, 228e.211–213. https://doi.org/10.1016/j.brs.2009.03.007
Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, Bikson M (2013) Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol 591:2563–2578. https://doi.org/10.1113/jphysiol.2012.247171
Rom DM (1990) A sequentially rejective test procedure based on a modified Bonferroni Inequality. Biometrika 77:663–665. https://doi.org/10.1093/biomet/77.3.663
Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I (1997) Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res 113:24–32
Sale MV, Ridding MC, Nordstrom MA (2008) Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci 28:8285–8293. https://doi.org/10.1523/JNEUROSCI.1963-08.2008
Sale MV, Lavender AP, Opie GM, Nordstrom MA, Semmler JG (2015) Increased intracortical inhibition in elderly adults with anterior–posterior current flow: a TMS study. Clin Neurophysiol. https://doi.org/10.1016/j.clinph.2015.04.062
Sasaki R, Miyaguchi S, Kotan S, Kojima S, Kirimoto H, Onishi H (2016) Modulation of cortical inhibitory circuits after cathodal transcranial direct current stimulation over the primary motor cortex. Front Hum Neurosci 10:30. https://doi.org/10.3389/fnhum.2016.00030
Schuck P, Zwingmann C (2003) The ‘smallest real difference’ as a measure of sensitivity to change: a critical analysis. Int J Rehabil Res 26:85–91. https://doi.org/10.1097/01.mrr.0000070759.63544.65
Silbert BI, Patterson HI, Pevcic DD, Windnagel KA, Thickbroom GW (2013) A comparison of relative-frequency and threshold-hunting methods to determine stimulus intensity in transcranial magnetic stimulation. Clin Neurophysiol 124:708–712. https://doi.org/10.1016/j.clinph.2012.09.018
Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DA, Wassermann EM (1999) Menstrual cycle effects on cortical excitability. Neurology 53:2069–2072
Stagg CJ et al (2014) Local GABA concentration is related to network-level resting functional connectivity. Elife 3:e01465. https://doi.org/10.7554/eLife.01465
Stinear CM, Petoe MA, Byblow WD (2015) Primary motor cortex excitability during recovery after stroke: implications for neuromodulation. Brain Stimul. https://doi.org/10.1016/j.brs.2015.06.015
Terwee CB et al (2007) Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 60:34–42. https://doi.org/10.1016/j.jclinepi.2006.03.012
Tremblay S, Beaule V, Lepage JF, Theoret H (2013) Anodal transcranial direct current stimulation modulates GABAB-related intracortical inhibition in the M1 of healthy individuals. Neuroreport 24:46–50. https://doi.org/10.1097/WNR.0b013e32835c36b8
Tremblay S, Larochelle-Brunet F, Lafleur LP, El Mouderrib S, Lepage JF, Theoret H (2016) Systematic assessment of duration and intensity of anodal transcranial direct current stimulation on primary motor cortex excitability. Eur J Neurosci 44:2184–2190. https://doi.org/10.1111/ejn.13321
Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M (1992) Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85:355–364
Veale JF (2014) Edinburgh Handedness Inventory—short Form: a revised version based on. confirmatory factor analysis. Laterality 19:164–177. https://doi.org/10.1080/1357650X.2013.783045
Wiethoff S, Hamada M, Rothwell JC (2014) Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul 7:468–475. https://doi.org/10.1016/j.brs.2014.02.003
Zhang X, Woolley DG, Swinnen SP, Feys H, Meesen R, Wenderoth N (2014) Changes in corticomotor excitability and intracortical inhibition of the primary motor cortex forearm area induced by anodal tDCS. PLoS One 9:e101496. https://doi.org/10.1371/journal.pone.0101496
Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W (1996) Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol 40:367–378. https://doi.org/10.1002/ana.410400306
Zoghi M, Pearce SL, Nordstrom MA (2003) Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle. J Physiol 550:933–946. https://doi.org/10.1113/jphysiol.2003.042606
Acknowledgements
We thank April Ren for the assistance with data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mooney, R.A., Cirillo, J. & Byblow, W.D. Adaptive threshold hunting for the effects of transcranial direct current stimulation on primary motor cortex inhibition. Exp Brain Res 236, 1651–1663 (2018). https://doi.org/10.1007/s00221-018-5250-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-5250-2