Abstract
We explored changes in the cyclical two-finger force performance task caused by turning visual feedback off performed either by the index and middle fingers of the dominant hand or by two index fingers of two persons. Based on an earlier study, we expected drifts in finger force amplitude and midpoint without a drift in relative phase. The subjects performed two rhythmical tasks at 1 Hz while paced by an auditory metronome. One of the tasks required cyclical changes in total force magnitude without changes in the sharing of the force between the two fingers. The other task required cyclical changes in the force sharing without changing total force magnitude. Subjects were provided with visual feedback, which showed total force magnitude and force sharing via cursor motion along the vertical and horizontal axes, respectively. Further, visual feedback was turned off, first on the variable that was not required to change and then on both variables. Turning visual feedback off led to a mean force drift toward lower magnitudes while force amplitude increased. There was a consistent drift in the relative phase in the one-hand task with the index finger leading the middle finger. No consistent relative phase drift was seen in the two-person tasks. The shape of the force cycle changed without visual feedback reflected in the lower similarity to a perfect cosine shape and in the higher time spent at lower force magnitudes. The data confirm findings of earlier studies regarding force amplitude and midpoint changes, but falsify predictions of an earlier proposed model with respect to the relative phase changes. We discuss factors that could contribute to the observed relative phase drift in the one-hand tasks including the leader–follower pattern generalized for two-effector tasks performed by one person.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several recent studies have reported unintentional drifts during performance of accurate cyclical force production tasks after the visual feedback was turned off (Levy-Tzedek et al. 2011; Ambike et al. 2016a, b; Reschechtko et al. 2017). In particular, when subjects use a fingertip to produce a cyclical force profile, there is a consistent increase of the amplitude of this force profile while the force midpoint decreases. These effects have been observed when an auditory metronome was used to pace subjects throughout performance. To interpret the mentioned drifts in performance, some of these authors invoked the concept of neural control with time changes in referent coordinates (RC) for salient variables (Latash 2010; Feldman 2015) and proposed a model in which RC(t) time changes are caused by non-linear oscillators with parameters that drift toward their preferred magnitudes. In particular, RC drifts toward actual coordinate of the effector, a process addressed as RC-back-coupling (Zhou et al. 2014; Reschechtko et al. 2014), is proposed to cause the drift in the force midpoint level. A drift in another parameter causes an increase in the amplitude of the cyclical RC(t) process that translates into an increase in force amplitude.
In a two-finger task requiring production of cyclical patterns of total force without changes in the force sharing between the fingers (defined as fraction of total force produced by one finger), turning visual feedback off caused the described drifts in the forces generated by each of the fingers (Reschechtko et al. 2017). In addition, it was found that the relative phase of the two-finger forces did not maintain the in-phase relation required by the task: A phase drift was observed, on average about 0.5 rad over 20 s. A similar drift was observed when the task started with an out-of-phase finger force pattern (constant total force and cyclical changes in the sharing). This drift in relative phase is particularly surprising given the literature on two-finger actions, which reports that in-phase and out-of-phase movements by two fingers are the only stable regimes (with in-phase being the most stable across frequencies, Schöner and Kelso 1988; Kelso 1995).
The relative phase drifts were hypothesized to originate from different drift speeds in the dominant and non-dominant hands. This hypothesis is compatible with the dynamic dominance hypothesis (Sainburg 2005) on hemispheric specialization for the control of steady states (non-dominant hemisphere) and quick actions (dominant hemisphere). The hypothesis on different parameter drifts has been also corroborated by the significantly larger and faster force drift reported in the non-dominant hand following removal of the visual feedback in steady force production tasks (Parsa et al. 2016).
In the present study, we tested this hypothesis by observing the performance of (1) two fingers of the same hand; and (2) two fingers from different people as they performed tasks that required each finger to produce sinusoidal force profiles. Since both fingers of a hand are controlled by the same hemisphere, we did not expect to see consistent relative phase drifts, which were assumed to reflect hemisphere-specific differences (Hypothesis 1). On the other hand, both of an individual’s fingers are controlled by a single central nervous system helped by sensory feedback, when available. When two persons perform the task, each using only one finger, their brains are coupled only by visual and auditory sensory information. Turning visual feedback off in the presence of auditory feedback (the metronome) was expected to decouple the actions of two participants with respect to force magnitude while their overall timing structure remained intact. This was not expected to lead to a consistent phase drift (Hypothesis 2). In contrast to effects presumed to originate from inter-hemispheric differences, we predicted that drifts in amplitude and midpoint of individual finger force time profiles would be consistent with earlier observations such as an increase in the force amplitude and a drop in the force midpoint (Hypothesis 3).
To explore the specific hypotheses, we used two tasks similar to those used in the previous study (Reschechtko et al. 2017). The tasks were formulated with respect to total force magnitude produced by both fingers and force sharing between the two fingers; total force changes translated into vertical cursor motion on the feedback monitor, while sharing changes caused horizontal motion of the cursor on the monitor. To remove visual feedback on one of the variables (total force or sharing), motion of the cursor along the corresponding axis was frozen.
Additional analyses were motivated by the findings that falsified Hypothesis 1 (see “Results”): We observed phase drifts in one-hand tasks similar to those in the first study when the two index fingers were used to produce forces (Reschechtko et al. 2017). We then explored whether turning visual feedback off was associated with changes in the internal structure of the individual force cycles that could lead to the observed phase drifts.
Methods
Subjects
Thirteen healthy young adult subjects (7 females and 6 males; 23–30 years of age) participated in the present study. Thirteen pairs of subjects were formed (7 pairs of females and 6 pairs of males) from the same pool of subjects. To form a pair, two randomly chosen subjects of the same gender were selected; as such, a unique pair was a unique combination of individuals, but some subjects participated in more than one pair. Based on the earlier study (Reschechtko et al. 2017), which demonstrated medium to large effect sizes, this number of subjects was expected to provide at least 90% power for detecting significant differences across the main analyses at α = 0.05. All subjects self-identified as right-handed and had no hand injuries or neurological disorders, which would interfere with their ability to perform the experiment. All subjects provided informed consent in accordance with procedures approved by the Office for Research Protections at The Pennsylvania State University.
Apparatus
Data collection for this study used two multi-axis force/torque transducers (Nano 17, ATI Industrial Automation, Apex, NC). Each sensor operated in a networked force/torque system (Net F/T, ATI Industrial Automation), which transmitted force and torque values to the data collection computer at a rate of 1000 samples per second. The surface of each sensor was covered with 320-grit sandpaper to provide high friction. A customized application, developed in the LabVIEW programming environment (National Instruments, Austin, TX, USA), displayed feedback and logged data for subsequent offline analysis.
When a single subject used two fingers of the dominant hand to produce force, the force sensors were placed close to each other with a 3-cm distance between their centers. Subjects placed the index and middle fingers of their right hand on top of the sensors. When each of the two subjects used the right index finger to produce force, the sensors were spaced by a larger distance to allow comfortable placement of the fingers by two participants. Across all series, each subject sat on a chair (in two-subject series, the subjects sat side-by-side) in front of a 19″ monitor that was positioned about 1 m away from the subjects. The monitor was used to provide visual feedback on the total force measured by the two sensors (FTOT = F1 + F2) and on force sharing defined as the fraction of total force produced by one finger (S = F1/FTOT). Changes in FTOT moved the cursor on the screen up and down, while changes in S moved the cursor left and right (in the direction of a finger with higher S).
In all trials, subjects were instructed to not lift their fingers off the sensors at any time. An auditory metronome was used to pace the subjects at twice the task frequency (see below).
Experimental procedure
There were two main tasks: Force task and Share task. These terms indicate whether subjects were instructed to cyclically vary FTOT produced by both fingers (while keeping S steady), or to cyclically vary S (and keep FTOT constant). In the one-hand condition, FTOT was defined as FI + FM (where FI and FM are the forces produced by the index and middle fingers, respectively) and Share (S) was defined as FM/FTOT. The Force task involves two conditions, with equal shares of FTOT produced by the two fingers (1:1) and with the middle finger producing twice as much force as the index finger (1:2). The Share task also involved two conditions with changes in S about the mean of 1:1 and about the mean of 1:2, while FTOT was to be kept at 12 N (males) or 10 N (females). Subjects performed three practice trials and then three test trials per each initial sharing condition and task.
In the two-person condition, the two subjects (two males or two females, randomly assigned) pressed on the sensors using their right-hand index fingers. Similar to the one-hand trials, the subjects performed the Force and Share tasks. FTOT was defined as FS1 + FS2 (where the subscripts refer to the first and second subject, respectively) and S was defined as FS2/FTOT. Both tasks involved only one condition with equal shares of FTOT produced by the two subjects (1:1). Subjects performed three practice trials and then three test trials for each task.
Feedback in both conditions is illustrated in Fig. 1. In both conditions, the feedback consisted of a target with an inner line showing perfect performance (horizontal in Share task; vertical in Force task), and a rectangle around this line indicating “acceptable” error margins. In the Share task, the error margins were perfect performance ± 0.05 (S is unitless) in the S direction, and perfect performance ± 2 N in the FTOT direction for men or ± 1.5 N for women. Similarly, for the Force task the rectangle was perfect performance ± 0.05 in the S direction and perfect performance ± 2 N in the FTOT direction for men or ± 1.5 N for women.
An illustration of the two main experimental tasks. The feedback monitor showed a cursor that moved in the vertical direction with changes in total force and in the horizontal direction with changes in force sharing. The Force task required vertical cursor displacement, while the Share task required horizontal cursor displacement. Only one set of targets was shown in a given trial
During the Force task (Fig. 2), subjects were instructed to produce a cyclical profile of FTOT ranging from 8 N to 16 N for male subjects and 7 N to 13 N for female subjects (as in Reschechtko et al. 2017). These magnitudes were selected based on the cited previous study and pilot experiments to cause measurable force drifts while avoiding fatigue over the duration of the experiment. The subjects were told to press naturally with the fingers in such a way that the cursor moved in the vertical direction only and touched each of the targets on consecutive metronome beats. For the first 8 s of each trial, subjects were given feedback on both FTOT and S; for the next 15 s (8–23 s after trial onset), subjects received feedback on FTOT only (cursor motion along the horizontal direction was suppressed). Finally, for the last 15 s of the trial (23–38 s), subjects did not receive any visual feedback (the cursor disappeared). The instruction to the subjects was to “continue doing what you have been doing” after the first 8 s despite the feedback change.
Performance by a representative male subject in the one-hand task (a) and two-person task (same male subject with another male subject) (b). The Force task (left column) and the Share task (right column) are shown. Note that there was only one initial sharing condition for the two-person task (1:1) and two conditions for the one-hand task (from left to right: 1:1 and 1:2). Total force (FTOT) is shown by the solid gray line (values on the left y-axis), while Share (S) is shown by the dashed black line (values on the right y-axis). Vertical dashed lines show when visual feedback was turned off
During the Share task (Fig. 2), subjects were asked to maintain a constant level of FTOT (12 N for males and 10 N for females), while changing the sharing of FTOT between the index and middle fingers in the one-hand conditions and between the two subjects in the two-person conditions. The subjects were told to press naturally with the fingers in such a way that the cursor moved in the horizontal direction only and touched each of the targets on consecutive metronome beats. For the first 8 s, subjects were given feedback on both FTOT and S; for the next 15 s (8–23 s after trial onset), subjects received feedback on S only (cursor motion along the vertical direction was suppressed). For the last 15 s of the trial (23–38 s), the cursor disappeared and subjects did not receive any visual feedback. The instruction to the subjects was to “continue doing what you have been doing” after the first 8 s despite the feedback change.
In all trials, subjects were paced by a metronome at 2 Hz, such that subjects would produce maximal FTOT (or S) on one beat and minimal FTOT (or S) on the next beat, for a cycle frequency of 1 Hz. Subjects practiced each condition over three consecutive trials (38 s each) under full visual feedback and then performed three experimental trials in a row at the same condition. The rest time between conditions and tasks was 30 and 60 s, respectively. The order of conditions and tasks was randomized.
Note that the design of the experiment and the protocol were selected to match closely the earlier experiment with the cyclical force production by the two index fingers (Reschechtko et al. 2017). In contrast to the aforementioned study, we reduced the number of tasks (in particular, we used only two initial sharing conditions for the one-hand task and one sharing condition for the two-person task) to accommodate the one-hand and two-person tasks and avoid fatigue.
Data analysis
Data processing was performed offline using MATLAB (The MathWorks Inc., Natick MA). First, all data were low-pass filtered using a zero-lag 4th -order Butterworth filter at 10 Hz. Further quantitative analysis was performed on cycles in each of the three time windows: 3–8 s (under full visual feedback), 8–23 s (when only task-variable feedback was available), and 23–35 s (when no feedback was available) after trials began. These time windows will subsequently be referred to as Phase-1, Phase-2, and Phase-3, respectively. Phase-3 ended slightly before the end of the trial because subjects occasionally stopped force production in anticipation of the end of the trial. Cycles were defined by identifying every other time that the demeaned value of FTOT crossed 0. For each subject and each Task (force and share) × Initial-Sharing (1:1, 1:2) combination, the following quantities were calculated in each phase. In analysis, we focused primarily on the differences between Phase-1 and Phase-3.
Mean FTOT and S was computed for both Force and Share tasks, yielding the outcome variables FM and SM, respectively. The peak-to-peak amplitude of both FTOT and S were also computed and are subsequently referred to as FA and SA. Similar analysis was performed for the individual finger forces, FI and FM in the one-hand trials and for FS1 and FS2 in the two-person trials. For both tasks, average mean and peak-to-peak force values were computed over each of the three phases.
Relative phase analysis
We analyzed relative phase (RP) between FI and FM in the one-hand condition and between FS1 and FS2 in the two-person condition. For this purpose, continuous relative phase was computed (Lamb and Stöckl 2014) so that in each trial, each finger’s phase portrait (from 3 to 35 s after trial initiation) was centered and RP was subsequently computed for each trial by subtracting the phase angle—obtained by finding the Hilbert transform of the centered data—of the Middle finger (or S2) from that of the Index finger (or S1). For each trial, RP values were averaged in 1-s bins; the data for the bins were then averaged across all trials of a given task/initial sharing condition.
Harmonicity analysis
This analysis explored possible changes in the intrinsic structure of force cycles. Two methods were used. The first index quantified similarity of individual force cycles to a perfect cosine function. For this purpose, pairs of complete cycles at the end of Phase-1 and at the end of Phase-3 were selected. Two complete perfect cosine cycles (min-to-min) were fitted to each of those time intervals and then linear regression of the actual data against a cosine function was computed: f(t) = a + b cos·(ωt + φ0). The coefficient of determination (R2) between the data and the fitted cosine function was computed and z-transformed for statistical analysis. We will refer to this index as H1.
The second index (H2) quantified the width of the F(t) curve at the level of average over the cycle force values within individual cycles. The midpoint width was computed as a fraction of total width of the cycle. The time interval between the moments when F(t) crossed the average value within each cycle was computed and expressed as a fraction of the total cycle time. This analysis was performed for all cycles in all phases. This index reflected changes in the relative time spent at lower forces compared to the relative time spent at higher forces within a cycle.
Statistical analysis
To test the first hypothesis, relative phase (RP) in the one-hand task was subjected to the three-way ANOVA with repeated measures: Phase (1 and 3) × Initial-Sharing (1:1 and 1:2) × Task (Force and Share). To test the second hypothesis, a two-way ANOVA was run on RP for the data in the two-person task, Phase × Task, because there was only one initial sharing condition. To test the third hypothesis, ANOVA was run on the main characteristics of force and sharing (FA, FM, SA, SM). The one-hand data were analyzed using a three-way ANOVA with factors Phase, Initial-Sharing and Task. For the two-person data, the two-way ANOVA Phase × Task was used. Similarly, we explored changes in force profile characteristics (indices H1 and H2) over the phases in both tasks using the three-way ANOVA Phase × Initial-Sharing × Task for the one-hand data and Phase × Task for the two-person data.
Circular statistics was used to analyze RP. After running ANOVAs, significant main effects and interactions were further explored using pairwise contrasts with Bonferroni corrections. In some cases, one-way ANOVA was used for further analysis of significant effects in multi-factor ANOVAs. All statistical tests were run in SPSS 24 (IBM Corp, New York, USA). We assume statistical significance when p < 0.05.
Results
General patterns of performance
Manipulations of visual feedback led to consistent changes in performance across subjects. In particular, turning off feedback on the non-task variable only (FTOT in the Share task and S in the Force task) resulted in a consistent drift in the variable deprived of the feedback. These drifts typically led to an increase in the peak-to-peak cyclical changes of that variable and a drift of the mean FTOT value to lower magnitudes. Mean S drifted toward 0.5 (even sharing between fingers or subjects) in the 1:2 initial sharing condition only with no consistent drift in the 1:1 condition. After visual feedback was completely removed, the drift of the non-task variable continued, and the task variable began to show a drift pattern similar to those described for non-task variables.
Figure 2 illustrates typical performance by a representative subject (panel A) and a typical couple (panel B) using time series FTOT(t) and S(t) shown by the solid gray and dashed black lines, respectively. The times when visual feedback was manipulated are shown with dashed vertical lines. Note the visible drifts in both FTOT(t) and S(t) compatible with the described pattern.
Figure 3 illustrates the same data in the force–force space. Perfect task performance is shown as a black bar with a circle in the middle corresponding to the required midpoint values of FTOT and S. The solid blue trajectory shows the performance during the initial time interval (Phase-1) when full visual feedback was available. The dashed green line shows the performance after turning the non-task variable feedback off, and the thick red line shows the performance after feedback on both variables (FTOT and S) was turned off. Note that the described trends in the two performance variables were associated with the emergence of elliptical trajectories on the force–force plane in the one-hand conditions (panels A) suggesting a consistent phase lag between the individual finger force time profiles. In the two-person conditions (panels B), chaotic trajectories could be seen suggesting no consistent pattern across subject pairs.
Typical performance of the same subjects as in Fig. 2 in the Force task (left column) and Share task (right column). A The y-axis shows Middle finger force (FM), while the x-axis shows Index finger force (FI). The top and bottom rows show the 1:1 and 1:2 sharing conditions, respectively. B The y-axis shows finger force by subject S2 (FS2), while the x-axis shows finger force by subject S1 (FS1). Performance during the first 8 s is shown with solid blue lines; 8–23 s—with dotted green lines; and 23–35 s—with red lines. The black line represents the ideal task performance
Characteristics of the performance variable drifts
To explore the drifts in FTOT and S, we quantified the mean value over a cycle (FM and SM) and the peak-to-peak amplitude over a cycle (FA and SA). Figures 4 and 5 show averaged across subjects magnitudes of these four parameters at the end of Phase-1 (full visual feedback), at the end of Phase-2 (feedback on S only in the Share task and on FTOT only in the Force task), and at the end of Phase-3 (no visual feedback) for the one-hand (Fig. 4) and two-person tasks (Fig. 5), respectively. While we analyzed the data over all three phases, in this paper the main focus is on changes from Phase-1 to Phase-3.
Mean total force level (FM) and peak-to-peak amplitude (FA) and mean share level (SM) and peak-to-peak amplitude (SA) in the one-hand task with two initial sharing conditions (1:1 and 1:2). Averaged across subjects data are shown with standard error bars. Different phases are represented by different colors (white: Phase 1; gray: Phase 2; black: Phase 3)
Mean total force (FM) and peak-to-peak amplitude (FA) and mean share (SM) and peak-to-peak amplitude (SA) in the two-person task. Averaged across subject pairs data are shown with standard error bars. Different phases are represented by different colors (white: Phase 1; gray: Phase 2; black: Phase 3)
When the Share task was performed by two fingers of a hand, there was an increase in FA by an average of 2.89 ± 0.73 N (effect of Phase, F[1,30] = 165.6; p < 0.001 in the three-way ANOVA, Phase × Initial Sharing × Task) and a drop in FM, by an average of 1.15 ± 1.04 N (effect of Phase, F[1,30] = 12.5; p < 0.01) from Phase-1 to Phase-3. Turning the S feedback off led to an increase in SA, by an average of 0.16 ± 0.07 (effect of Phase, F[1,30] = 57.6; p < 0.001) while SM showed no significant overall change. There was, however, a significant interaction Phase × Initial Sharing on SM (F[1,30] = 4.49; p < 0.05). The interaction reflected a drift of SM toward lower values in the 1:2 condition, but not in the 1:1 condition. One-way post-hoc ANOVAs confirmed significant differences between values of SM in the 1:1 and 1:2 conditions by an average of 0.16 ± 0.004 in Phase-1 (F[1,30] = 664.8; p < 0.001) and by a smaller value, 0.11 ± 0.04, in Phase-3 (F[1,30] = 24.5; p < 0.001).
In the Force task, removing feedback led to an increase in FA, by an average of 2.69 ± 1.04 N (effect of Phase, F[1,30] = 36.4; p < 0.001) and in SA, by an average of 0.18 ± 0.16 (effect of Phase, F[1,30] = 19.3; p < 0.001) from Phase-1 to Phase-3. FM decreased by an average of 1.41 ± 0.42 N (effect of Phase, F[1,30] = 28.1; p < 0.01), while SM showed no significant change.
In the two-person condition, removing feedback led to an increase in FA, by an average of 4.20 ± 0.2 N (effect of Phase; F[1,22] = 32.4; p < 0.01 in a two-way ANOVA, Phase × Task) from Phase-1 to Phase-3; the pairwise comparisons showed that this effect was larger for the Share task, 4.97 ± 0.73 N, compared to the Force task, 2.70 ± 1.12 N (F[1,22] = 53.9; p < 0.001). FM dropped from Phase-1 to Phase-3 in both the Force task (on average by 0.69 ± 0.73 N) and Share task (on average by 0.94 ± 1.84 N), but this effect was under the level of significance. While SM did not show any significant effects, SA increased from Phase-1 to Phase-3, by an average of 0.29 ± 0.08 and 0.16 ± 0.01 in the Force and Share tasks, respectively (effect of Phase, F[1,22] = 55.04; p < 0.001 and interaction Phase × Task, F[1,22] = 4.52; p < 0.05).
To compare the effects between the one-hand and two-person tasks, a mixed-effects ANOVA was run for the 1:1 initial sharing condition only with the factor Effectors (Index–Middle fingers vs. Index–Index fingers) and Phase. In the Force task, the effect of Effectors on FA was significant (F[1,26] = 5.04; p < 0.05) reflecting higher values for the one-hand condition (2.8 ± 1.4 N) compared to two-person condition (2.66 ± 1.1 N).. SA also showed a significant effect of Effectors (F[1,26] = 21.6; p < 0.001) reflecting larger values for the two-person condition (0.29 ± 0.08) compared to the one-hand condition (0.19 ± 0.09). Note that this analysis described changes in S, a variable that was expected not to change in the Force task. There were no significant effects on SM.
In the Share task, FA showed a significant Phase × Effectors interaction (F[1,26] = 7.15; p < 0.05). Further analysis confirmed that the increase of FA from Phase-1 toward Phase-3 was larger in the two-person condition compared to the one-hand condition (5.06 ± 0.8 vs. 2.5 ± 0.8 N). Also SA showed a significant Phase × Effectors interaction (F[1,26] = 14.1; p < 0.01) reflecting the fact that the increase in SA was larger for the two-person condition compared to the one-hand condition (0.43 ± 0.07 vs. 0.34 ± 0.05).
Drifts in individual finger force patterns
The described drifts in the characteristics of FTOT and S were accompanied by consistent drifts in the individual finger forces. Figure 6 illustrates the typical individual finger force profiles. Panel A shows the time series of FM(t) and FI(t) for a representative subject in the one-hand condition, while Panel B shows FS1(t) and FS2 (t) for a representative couple in the two-person condition.
The individual finger force profiles for the one-hand tasks (A; FI—solid line; FM—dashed line) and the two-person tasks (B; FS1—solid line; FS2—dashed line) for each initial sharing pattern (rows) and task (columns) are shown for the same subjects as in Figs. 2 and 3. Dashed vertical lines correspond to the times at which visual feedback was turned off
For the sake of brevity, we do not present statistical analysis of the individual finger force characteristics. In both one-hand and two-person condition, and in both Force and Share tasks, there were no significant differences between the characteristics of the two finger forces and their changes from Phase-1 to Phase-3.
Drifts in finger force relative phase
We observed consistent drifts in the relative phase (RP) between the two finger force profiles for both Force task and Share task in the one-hand condition only. These drifts are illustrated in Fig. 7a. Over the trial duration, on average, the drift from Phase-1 to Phase-3 was, on average, 0.22 ± 0.03 rad (F[1,30] = 158.8; p < 0.01) in the Force task and about 0.2 ± 0.02 rad (F[1,30] = 22.6; p < 0.01) in the Share task. The index finger force consistently led the middle finger force.
A Relative phase of the middle finger force (FM) with respect to the index finger force (FI). Data for the two initial sharing conditions are shown with the solid and dashed lines. Note that data are jittered slightly along the time axis to avoid excessive overlap. B Relative phase of subject-2 finger force (FS2) with respect to subject-1 finger force (FS1). Averaged across subjects data are shown with standard error bars. Note the consistent phase drift in A but not in B and the very large error bars at later phases in B
In contrast, in the two-person condition, no consistent phase drift was observed in either task (Fig. 7b). There was much variability both within a trial (e.g., Fig. 3) and across the subject pairs. The latter was reflected in an increase in the standard deviation by about a factor of two over the trial duration.
Changes in the finger force cycle structure
Figure 8 shows a close-up illustration of FTOT in the two last cycles of Phase-1 and Phase-3 for a representative subject performing the Force task. Note the nearly sinusoidal shape of the cycles in Phase-1 (solid line), and the distorted cycle shapes with prolongation of the part at low forces in Phase-3 (dashed line). We explored these changes using two indices described in Methods: H1 quantified similarity of the force cycles to a perfect cosine function, and H2 reflected the relative width of the force pulse at the level of average over the cycle force values within individual cycles (illustrated with arrows in Fig. 8).
Overall, for both one-hand and two-person conditions, H1 dropped from Phase-1 to Phase-3 for FTOT in the Force task and increased for the non-task variables (FTOT for the Share task and S for the Force task). The latter effect reflects the fact that under visual feedback the non-task variables (which were supposed to be kept constant) showed relatively small-amplitude, irregular changes. Without visual feedback, these variables started to show rhythmical changes.
The second index, H2, is illustrated in Fig. 9. In the Force task H2 for FTOT decreased from Phase-1 to Phase-3 for both one-hand and two-person conditions. In the Share task, the changes in H2 differed between the two-person (H2 decreased) and one-hand (H2 increased) conditions. This was reflected in a significant Effectors × Phase interaction (F[1,26] = 7.43; p < 0.01).
Magnitudes of H2 for the Force task (left columns) and Share task (right columns). The index was computed for total force (FTOT), share (S), and forces of the individual fingers (index—FI, middle—FM, first subject—FS1, and second subject—FS2). Averaged across subjects data are shown with standard error bars
There was also a significant Effectors × Phase interaction (F[1,26] = 5.72; p < 0.05) on H2 computed for S in the Force task. The difference between the effectors was seen only in Phase-3 (p < 0.05 in the post-hoc comparison), on average by 0.04 ± 0.01. In the Share task, H2 for S did not show any significant effects.
Discussion
In both one-hand and two-person conditions we observed relative phase drifts caused by turning visual feedback off. However, only in the one-hand condition these drifts were consistent across subjects showing results similar to those of the previous study of two-hand actions by a single person (Reschechtko et al. 2017), resulting in a new phase pattern with the index finger leading the middle finger by over 0.2 rad. In two-person conditions, the drifts were inconsistent across subjects as reflected by no significant overall change in the relative phase accompanied by the large across-subject variability. Considering within-subject behaviors, the data speak against Hypothesis 1 because individual subjects did show consistent phase drifts. Hypothesis 2, however, was mostly confirmed: While phase drifts in two-person conditions took place, they were not reproducible and did not lead to a new stable pattern of finger forces (see Fig. 4). More detailed examination of the force cycles revealed a consistent change in the intrinsic structure of the force change that deviated from the perfect sine wave over the trial duration (Figs. 8, 9). We discuss possible causes for the changes in force cycle structure further in the Discussion.
Our third hypothesis received support in the study: Across conditions, we observed an increase in the amplitude and a drop in the midpoint of individual finger force time profiles (as well as in total force) consistent with earlier observations (Ambike et al. 2016a, b; Reschechtko et al. 2017). These data provide additional support for the idea that performance drifts caused by removing the visual feedback are caused by drifts in parameters of intrinsic (neural) oscillators.
Performance drifts caused by RC drifts
Unintentional drifts in force produced without visual feedback have been known for about 20 years (Slifkin et al. 2000; Vaillancourt and Russell 2002). Such drifts are observed over relatively short times (typical trials lasted about 20 s) and at relatively modest initial force magnitudes (within the range between 15 and 25% of MVC), which make effects of fatigue unlikely. Early papers suggested a hypothesis that these phenomena reflected limitations of working memory (Vaillancourt and Russell 2002). This hypothesis received support in later studies reporting significantly faster and larger force drift in patients with Parkinson’s disease (Vaillancourt et al. 2001; Jo et al. 2016), as well as by EEG and MRI studies (Vaillancourt et al. 2003; Poon et al. 2012). On the other hand, a recent study documented no downward force drift in experiments where subjects were asked to memorize a force level and reproduce it after comparable time intervals (Solnik et al. 2017). This study also showed that brief episodes of relaxation effectively removed the downward force drift, suggesting that continuous force production for an extended period was an important task feature leading to the force drift. Another study showed no drift in the force-matching task performed by the contralateral hand while the task hand showed a typical force drift (Reschechtko et al. 2018). These results suggest that, at some hierarchically high level of task specification, the task was not forgotten.
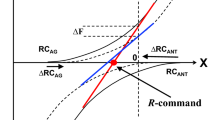
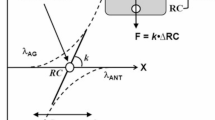
A conceptually different explanation, which does not negate possible involvement of memory-related mechanisms, has been suggested within the theoretical framework assuming that the central nervous system manipulates parameters of physical laws to perform intentional actions (reviewed in Latash 2016, 2017; Latash and Zatsiorsky 2016). In particular, force production may be viewed as a consequence of defining a referent coordinate (RC) for the effector, which is different from its actual coordinate (AC). The difference between RC and AC leads to active force production toward RC. If the effector is free to move, motion toward RC is observed until AC = RC where the effector comes to rest. If the effector cannot move, a complementary process has been postulated: An unintentional RC drift toward AC resulting in a force drop (this may be interpreted as the CNS “forgetting” the required RC). This drift, addressed as “RC-back-coupling” (Zhou et al. 2014), reflects the natural tendency of all physical systems to move toward states with lower potential energy. The assumed RC drift happens at all times, but subjects correct its effects on force if visual feedback (or another reliable sensory modality informing on actual force) is available. Recent studies have provided support for the described scheme by reconstructing RC during the force drift (Ambike et al. 2016b; Reschechtko and Latash 2017).
In this description, we do not differentiate between drifts in RC and in the c-command—a command that defines the spatial range where both agonist and antagonist muscles are active (Feldman 1980, 2015) and leads to effective modulation of the effector’s apparent stiffness (Latash and Zatsiorsky 1993). Drifts in both RC and c-command have been reported as possible causes for the force drift during steady force production tasks (Ambike et al. 2016b; Reschechtko et al. 2018).
Time profiles RC(t) may be viewed as the outputs of neuronal networks representing a time-varying, dynamical, system. Earlier studies of force drifts during cyclical force production (Levy-Tzedek et al. 2011; Ambike et al. 2016a) have suggested at least two processes with different characteristic times. The force cycle amplitude typically increases with the time exponent of about 1–2 s, while the force cycle midpoint drifts downward with typical time exponents of about 10–20 s. These observations suggest drifts in at least two parameters of the hypothetical dynamical system that happen at considerably different rates. The midpoint drift may be viewed as a reflection of the tendency to move toward lower potential energy (as in the aforementioned explanation of force drifts during constant force production). The amplitude drift has been interpreted as a reflection of a tendency to drift toward some preferred action amplitude (cf. Kay et al. 1987). These assumptions were formalized in a dynamical model (Reschechtko et al. 2017) able to account for such salient features of performance as a drop in force midpoint, an increase in the peak-to-peak force amplitude, and a phase drift between the right and left hands. Our current experiments have confirmed some of the model predictions such as the midpoint and amplitude drifts across one-hand and two-person tasks in addition to the cited earlier study demonstrating these effects in two-hand tasks performed by a single actor. The experiments, however, failed to confirm predictions related to the phase drift in two-effector tasks.
Possible causes of phase drifts
Early studies of two-effector tasks within the dynamical systems approach to motor control reported only two stable phase relations between the trajectories of two effectors, in-phase and out-of-phase (Schöner and Kelso 1988; reviewed in Kelso 1995). The out-of-phase regime was less stable and typically showed a transition to the in-phase movement with an increase in the action frequency. Those early findings were later summarized in a dynamical model, the so-called Haken-Kelso-Bunz model (Haken et al. 1985), which remains one of the classical models (for a review see Kelso 1995). During isometric cyclical force production tasks, anti-phase action was less stable than the in-phase pattern, but no switch to the in-phase pattern was observed with an increase in the action frequency (Carson 1995).
Other phase relations could also be observed under specific manipulations such as, for example, asymmetrical loading of the two effectors (Sternad et al. 1995, 1999). However, during natural two-finger action only stable in-phase and out-of-phase have been reported so far. By itself, manipulation of visual feedback is known to modify the stability of different rhythms. In particular, it could make the out-of-phase pattern more stable (Mechsner et al. 2001), lead to stable polyrhythm patterns, as well as to 1:1 patterns with a relative phase shift (Kovacs et al. 2009, 2010). The importance of visual feedback for stability of in-phase and out-of-phase coordination has been shown recently in isometric force production tasks (Lafe et al. 2016a, b). However, to our knowledge, our experiments are the first showing spontaneous phase desynchronization from both in-phase (Force task) and out-of-phase (Share task) regimes in symmetrical conditions and without changes in the action frequency. The phase drift caused by turning visual feedback off was on the order of 0.2–0.3 rad with the middle finger consistently trailing the index finger.
In addition, the formulation of the task and practice schedule could possibly condition our subjects to depend on the visual feedback for accurate performance. In such conditions, turning salient visual feedback off could cause major deterioration of performance (as in Kovacs et al. 2010) including an inability to correct errors in relative phase when they occur (Wilson et al. 2003, 2010; Wilson and Bingham 2008).
Compared to many earlier studies, the task used in our experiment was relatively complex because the subjects were required to produce not only properly phased finger force changes, encoded via required changes in FTOT and S, but also to produce coordinated peak-to-peak magnitudes of those changes. In addition, the formulation of the task in terms of FTOT and S time profiles could contribute to the task complexity. Indeed, even under full visual feedback, the subjects required substantial practice. This was particularly obvious in the two-person condition, when, after a comparable amount of practice, the subjects showed rather poor performance with large deviations of the non-task variable (S in the Force task and FTOT in the Share task) when visual feedback of both S and FTOT was available (e.g., Figs. 3, 4).
The different phase regimes observed in our experiment with and without visual feedback could reflect the different neurophysiological structures involved in the production of externally and intrinsically triggered actions. The different involvement of brain structures in the two types of actions has been documented in both animal studies (Mushiake et al. 1991; Schieber 1999) and brain imaging studies in humans (Debaere et al. 2001, 2003).
In a previous study (Reschechtko et al. 2017), we suggested a hypothesis that phase drifts were caused by different RC drifts in the dominant and non-dominant upper limbs. This hypothesis was motivated by both the dynamic dominance hypothesis (Sainburg 2005) and earlier observations showing faster force drifts in the dominant hand in steady force production tasks (Parsa et al. 2016a,b). It is also consistent with recent observations of changes in ongoing cyclical actions performed by a limb in the 1:2 bimanual force coordination task, which could be attributed to the production of force by the contralateral limb (Kennedy et al. 2016).
Our current observations disprove this hypothesis by showing consistent phase drifts in the one-hand condition, when hemispheric asymmetry cannot be invoked. What could be the reason for consistent phase drifts in the one-hand tasks? In the next section, we offer a tentative interpretation of these phenomena based on the “leader–follower” concept suggested for two-person tasks (Reed and Peshkin 2008; Ganesh et al. 2014; Mojtahedi et al. 2017; Bosga et al. 2010; Candidi et al. 2015; Davis et al. 2017), which we generalize here for one-person, two-effector tasks.
Possible causes of changes in the force cycle structure
Several factors could contribute to the observed changes in the force cycle structure from the beginning of the trial (Phase-1), when the force cycle resembled a sine wave, to the end of the trial (Phase-3), when the cycles typically showed sharper force peaks and prolonged dwell periods at low forces. First, there is pronounced asymmetry in the force generating capabilities of finger flexors and extensors; in particular, maximal voluntary contraction force into flexion is much larger than the MVC into extension (Shim et al. 2007; Oliveira et al. 2008). Another possible contributor to the asymmetry of actions into flexion and extension is the markedly larger enslaving (an index of unintentional finger force production when another finger of the hand produces force, Zatsiorsky et al. 2000) during extensor actions (Oliveira et al. 2008). Since extensor co-contraction could be expected in our tasks (cf. Li et al. 1999, 2001), the mentioned factors could lead to distortion of the force time profiles as compared to perfect sine patterns. These distortions can be corrected under visual feedback and become obvious without the feedback. Note that experiments with finger motion synchronization with the metronome showed a loss of anti-phase stability when the finger extended instead of flexed with a metronome beat (Kelso et al. 1990).
Another factor is the fact that our tasks were unidirectional, i.e., they required only flexion force production, and the subjects were explicitly required not to lift their fingers off the sensors at any time. This feature could condition the subjects to be more cautious at lower forces, particularly given the fact that the drifts in the force amplitude and midpoint could bring finger forces close to zero (see Fig. 3). This “floor effect” could slow down the force changes as a result of the speed-accuracy trade-off (Fitts 1954; Gorniak et al. 2008). Possible role of this factor may be explored in future studies by comparing force drifts in unidirectional and bidirectional force production tasks.
The observed changes in the force cycle structure could make the computed relative phase sensitive to the location of the peak force within the cycle. Here we would like to invoke the aforementioned phenomenon of “leader–follower” in two-person actions, which can be observed in force production tasks (Solnik et al. 2016; Mojtahedi et al. 2017). The idea can be generalized to single-person actions involving pairs of effectors that are markedly different in their ability to produce accurate contributions to the task. In both two-hand and one-hand two-finger tasks, the involved effectors were unequal. The use of only right-handed subjects in the previous study (Reschechtko et al. 2017) meant that the right index finger was likely more accurate than the left one, particularly in tasks involving relatively quick force changes (cf. Li et al. 2000; Zhang et al. 2006). The index finger of the right hand was likely more independently controlled (Li et al. 1998; Zatsiorsky et al. 2000) and more accurate (Gorniak et al. 2008) as compared to the middle finger. So, both effector pairs involved a “better controlled” effector and a “worse controlled” one. We hypothesize that in such tasks the “better controlled” effector becomes a leader in the pair leading to an earlier force pulse within each cycle. Indeed, the right index finger consistently led the left one in the previous study, and the index finger consistently led the middle finger in this study. This hypothesis is speculative and requires a follow-up study exploring different finger pairs (for example, the ring finger of the right hand working together with the index finger of the left hand).
Concluding comments
Our study produced both expected and unexpected results. The former include, in particular, the drifts in the force amplitude and midpoint, while the latter include consistent relative phase drifts in one-hand two-finger tasks. We view these results as incompatible with the model suggested in the previous study (Reschechtko et al. 2017). The findings suggest that there is a reason for the relative phase drift unrelated to the issue of hand dominance. Based on the documented changes in the force cycle structure, we have suggested that the phase drift could be caused by leader–follower roles played by the two fingers. In two-person tasks, the drifts became random because, after the visual feedback had been turned off, the two subjects performed independently and could not form a consistent leader–follower pair. At this time, this interpretation remains speculative and follow-up studies are needed to confirm or falsify it.
We believe that the main lessons of our study for the field of motor control are related to the issue of action stability, in particular to stability of natural actions involving multiple effectors. Participants showed loss of stability of performance very quickly after removal of the visual feedback. This loss was reflected in the drifts of the performance characteristics, such as midpoint and amplitude of cyclical force production, as well as in the relative phase drift. Note that several earlier studies quantified stability of action within the framework of the UCM hypothesis (Scholz and Schöner 1999) and documented a drop in the indices of stability during unintentional drifts in performance caused by turning visual feedback off. Such conclusions have been supported by the analysis of the structure of inter-trial variance (Parsa et al. 2016), of motor equivalence (Parsa et al. 2017), and of synergies within the space of hypothetical control variables (Ambike et al. 2016b, 2018; Reschechtko and Latash 2017). Taken together, all these studies have formed a strong body of evidence that links the observed changes in action stability to the theory of control with a hierarchy of RC time profiles (Latash 2016) defined by neural networks with parameters that show natural drifts; such drifts can be corrected with salient feedback. While this scheme can account for drifts in force amplitude and midpoint, explaining phase drifts remains a challenge, in particular given that the drifts happen even when the initial relative phase is expected to be very stable (in-phase or out-of-phase). We offered an explanation in an earlier section but admit that it remains speculative.
A few basic issues remain unclear. For example, why is visual feedback so crucial even for seemingly simple tasks that could potentially benefit from other sensory modalities? Can vision be successfully substituted by another modality to prevent loss of stability? These are some of the topics for future studies.
References
Ambike S, Mattos D, Zatsiorsky VM, Latash ML (2016a) The nature of constant and cyclic force production: Unintentional force-drift characteristics. Exp Brain Res 234:197–208
Ambike S, Mattos D, Zatsiorsky VM, Latash ML (2016b) Unsteady steady-states: central causes of unintentional force drift. Exp Brain Res 234:3597–3611
Ambike S, Mattos D, Zatsiorsky VM, Latash ML (2018) Systematic, unintended drifts in the cyclic force produced with the fingertips. Mot Control 22:82–99
Bosga J, Meulenbroek RG, Cuijpers RH (2010) Intra- and interpersonal movement coordination in jointly moving a rocking board. Mot Control 14:440–459
Candidi M, Curioni A, Donnarumma F, Sacheli LM, Pezzulo G (2015) Interactional leader-follower sensorimotor communication strategies during repetitive joint actions. J R Soc Interface 12(110):0644
Carson RG (1995) The dynamics of isometric bimanual coordination. Exp Brain Res 105:465–476
Davis TJ, Pinto GB, Kiefer AW (2017) The stance leads the dance: the emergence of role in a joint supra-postural task. Front Psychol 8:718
Debaere F, Van Assche D, Kiekens C, Verschueren SM, Swinnen SP (2001) Coordination of upper and lower limb segments: deficits on the ipsilesional side after unilateral stroke. Exp Brain Res 141:519–529
Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP (2003) Internal vs. external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage 19:764–776
Feldman AG (1980) Superposition of motor programs. I. Rhythmic forearm movements in man. Neurosci 5:81–90
Fitts PM (1954) The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47:381–391
Feldman AG (2015) Referent control of action and perception: Challenging conventional theories in behavioral science. Springer, NY
Ganesh G, Takagi A, Osu R, Yoshioka T, Kawato M, Burdet E (2014) Two is better than one: physical interactions improve motor performance in humans. Sci Rep 4:3824
Gorniak SL, Duarte M, Latash ML (2008) Do synergies improve accuracy? A study of speed-accuracy trade-offs during finger force production. Mot Control 12:151–172
Haken H, Kelso JAS, Bunz H (1985) A theoretical model of phase transitions in human hand movements. Biol Cybern 51:347–356
Jo HJ, Ambike S, Lewis MM, Huang X, Latash ML (2016) Finger force changes in the absence of visual feedback in patients with Parkinson’s disease. Clin Neurophysiol 127:684–692
Kay BA, Kelso JAS, Saltzman EL, Schoner G (1987) Space-time behavior of single and bimanual rhythmical movements: data and limit cycle model. J Exp Psychol Hum Percept Perform 13:178–192
Kelso JAS (1995) Dynamic patterns: The self-organization of brain and behavior. MIT Press, Cambridge
Kelso JAS, DelColle JD, Schöner G (1990) Action-perception as a pattern formation process. Attention performance XIII 5:139–169
Kennedy D, Rhee J, Shea CH (2016) Symmetrical and asymmetrical influences on force production in 1:2 and 2:1 bimanual force coordination tasks. Exp Brain Res 234:287–300
Kovacs AJ, Buchanan JJ, Shea CH (2009) Bimanual 1:1 with 90 degrees continuous relative phase: difficult or easy. Exp Brain Res 193:129–136
Kovacs AJ, Buchanan JJ, Shea CH (2010) Impossible is nothing: 5:3 and 4:3 multi-frequency bimanual coordination. Exp Brain Res 201:249–259
Lafe CW, Pacheco MM, Newell KM (2016a) Adapting relative phase of bimanual isometric force coordination through scaling visual information intermittency. Hum Mov Sci 47:186–196
Lafe CW, Pacheco MM, Newell KM (2016b) Bimanual coordination and the intermittency of visual information in isometric force tracking. Exp Brain Res 234:2025–2034
Lamb PF, Stöckl M (2014) On the use of continuous relative phase: Review of current approaches and outline for a new standard. Clin Biomech 29:484–493
Latash ML (2010) Motor synergies and the equilibrium-point hypothesis. Mot Control 14:294–322
Latash ML (2016) Towards physics of neural processes and behavior. Neurosci Biobehav Rev 69:136–146
Latash ML (2017) Biological movement and laws of physics. Mot Control 21:327–344
Latash ML, Zatsiorsky VM (1993) Joint stiffness: Myth or reality? Hum Move Sci 12: 653-692
Latash ML, Zatsiorsky VM (2016) Biomechanics and motor control: defining central concepts. Academic Press, New York
Levy-Tzedek S, Ben Tov M, Karniel A (2011) Rhythmic movements are larger and faster but with the same frequency on removal of visual feedback. J Neurophysiol 106:2120–2126
Li ZM, Latash ML, Zatsiorsky VM (1998) Force sharing among fingers as a model of the redundancy problem. Exp Brain Res 119:276–286
Li ZM, Zatsiorsky VM, Latash ML (1999) Contributions of the extrinsic and intrinsic hand muscles to the moments in finger joints. J Clin Biomechs 15:203–211
Li S, Danion F, Latash ML, Li Z-M, Zatsiorsky VM (2000) Characteristics of finger force production during one- and two-hand tasks. Hum Move Sci 19:897–924
Li ZM, Zatsiorsky VM, Latash ML (2001) The effect of finger extensor mechanism on the flexor force during isometric tasks. J Biomechs 34:1097–1102
Mechsner F, Kerzel D, Knoblich G, Prinz W (2001) Perceptual basis of bimanual coordination. Nature 414:69–73
Mojtahedi K, Whitsell B, Artemiadis P, Santello M (2017) Communication and inference of intended movement direction during human-human physical interaction. Front Neurorobot 11:21
Mushiake H, Inase M, Tanji J (1991) Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol 66:705–718
Oliveira MA, Hsu J, Park J, Clark JE, Shim JK (2008) Age-related changes in multi-finger interactions in adults during maximum voluntary finger force production tasks. Hum Mov Sci 27:714–727
Parsa B, O’Shea DJ, Zatsiorsky VM, Latash ML (2016) On the nature of unintentional action: A study of force/moment drifts during multi-finger tasks. J Neurophysiol 116:698–708
Parsa B, Terekhov A, Zatsiorsky VM, Latash ML (2017) Optimality and stability of intentional and unintentional actions: I. Origins of drifts in performance. Exp Brain Res 235:481–496
Poon C, Chin-Cottongim LG, Coombes SA, Corcos DM, Vaillancourt DE (2012) Spatiotemporal dynamics of brain activity during the transition from visually guided to memory-guided force control. J Neurophysiol 108:1335–1348
Reed KB, Peshkin MA (2008) Physical collaboration of human-human and human-robot teams. IEEE Trans Haptics 1:108–120
Reschechtko S, Latash ML (2017) Stability of hand force production: I. Hand level control variables and multi-finger synergies. J Neurophysiol 118:3152–3164
Reschechtko S, Zatsiorsky VM, Latash ML (2014) Stability of multifinger action in different state spaces. J Neurophysiol 112:3209–3218
Reschechtko S, Hasanbarani F, Akulin VM, Latash ML (2017) Unintentional force changes in cyclical tasks performed by an abundant system: Empirical observations and a dynamical model. Neurosci 350:94–109
Reschechtko S, Cuadra C, Latash ML (2018) Force illusions and drifts observed during muscle vibration. J Neurophysiol. https://doi.org/10.1152/jn.00563.2017. (in press)
Sainburg RL (2005) Handedness: differential specializations for control of trajectory and position. Exerc Sport Sci Rev 33:206–213
Schieber MH (1999) Voluntary descending control. In: Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR (eds) Fundamental neuroscience. Academic Press, San Diego etc, pp 931–949
Schoner G, Kelso JAS (1988) Dynamic pattern generation in behavioral and neural systems. Science 239:1513–1520
Shim JK, Oliveira MA, Hsu J, Huang J, Park J, Clark JE (2007) Hand digit control in children: age-related changes in hand digit force interactions during maximum flexion and extension force production tasks. Exp Brain Res 176:374–386
Slifkin AB, Vaillancourt DE, Newell KM (2000) Intermittency in the control of continuous force production. J Neurophysiol 84:1708–1718
Solnik S, Reschechtko S, Wu Y-H, Zatsiorsky VM, Latash ML (2016) Interpersonal synergies: Static prehension tasks performed by two actors. Exp Brain Res 234:2267–2282
Solnik S, Qiao M, Latash ML (2017) Effects of visual feedback and memory on unintentional drifts in performance during finger pressing tasks. Exp Brain Res 235:1149–1162
Sternad D, Collins D, Turvey MT(1995) The detuning factor in the dynamics of interlimb rhythmic coordination.Biol Cybern73:27–35
Sternad D, Turvey MT, Saltzman EL (1999) Dynamics of 1:2 coordination: Sources of symmetry breaking. J Mot Behav 31:224–235
Vaillancourt DE, Russell DM (2002) Temporal capacity of short-term visuomotor memory in continuous force production. Exp Brain Res 145:275–285
Vaillancourt DE, Slifkin AB, Newell KM (2001) Visual control of isometric force in Parkinson’s disease. Neurophysiologia 39:1410–1418
Vaillancourt DE, Thulborn KR, Corcos DM (2003) Neural basis for the processes that underlie visually guided and internally guided force control in humans. J Neurophysiol 90:3330–3340
Wilson AD, Bingham GP (2008) Identifying the information for the visual perception of relative phase. Percept Psychophys 70:465–476
Wilson AD, Bingham GP, Craig JC (2003) Proprioceptive perception of phase variability. J Exp Psychol Hum Percept Perform 29:1179–1190
Wilson AD, Snapp-Childs W, Coats R, Bingham GP (2010) Learning a coordinated rhythmic movement with task-appropriate coordination feedback. Exp Brain Res 205:513–520
Zatsiorsky VM, Li ZM, Latash ML (2000) Enslaving effects in multi-finger force production. Exp Brain Res 131:187–195
Zhang W, Zatsiorsky VM, Latash ML (2006) Accurate production of time-varying patterns of the moment of force in multi-finger tasks. Exp Brain Res 175:68–82
Zhou T, Solnik S, Wu Y-H, Latash ML (2014) Equifinality and its violations in a redundant system: Control with referent configurations in a multi-joint positional task. Mot Control 18:405–424
Acknowledgements
We are very much grateful to Dr. Mehdi Shahbazi for facilitating the visit of Ms. Fariba Hasanbarani to The Pennsylvania State University. The study was in part supported by NIH Grant NS095873.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hasanbarani, F., Reschechtko, S. & Latash, M.L. Performance drifts in two-finger cyclical force production tasks performed by one and two actors. Exp Brain Res 236, 779–794 (2018). https://doi.org/10.1007/s00221-018-5179-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-5179-5