Abstract
The supplementary motor area (SMA) is believed to be highly involved in the planning and execution of both simple and complex motor tasks. This study aimed to examine the role of the SMA in planning the movements required to complete reaction time, balance, and pegboard tasks using anodal transcranial direct current stimulation (tDCS), which passes a weak electrical current between two electrodes, in order to modulate neuronal activity. Twenty healthy adults were counterbalanced to receive either tDCS (experimental condition) or no tDCS (control condition) for 3 days. During administration of tDCS, participants performed a balance task significantly faster than controls. After tDCS, subjects significantly improved their simple and choice reaction time. These results demonstrate that the SMA is highly involved in planning and executing fine and gross motor skill tasks and that tDCS is an effective modality for increasing SMA-related performance on these tasks. The findings may be generalizable and therefore indicate implications for future interventions using tDCS as a therapeutic tool.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The supplementary motor area (SMA) is highly involved in planning both simple and complex motor behaviors, including involvement in action sequencing (e.g., a person deciding to turn their hand before vs. after picking up a small object), learning (e.g., becoming more accurate at a novel balance task), the executive control of movement (e.g., monitoring for errors and “tuning out” distraction from the environment), and acquiring grammar (Nachev et al. 2008; Nguyen et al. 2014; Ullman 2006; Vollman et al. 2013).
The SMA, as part of the larger supplementary motor cortex (SMC), is comprised of well-defined connections to various cortical and corticostriatal pathways. The SMA is somatotopically organized and possesses direct reciprocal connections with the primary motor cortex (M1)—most likely because the SMA is very closely related to motor output (Nachev et al. 2008). Independent of the primary motor cortex (M1), the SMA seems to play a pivotal role in planning motor actions before movement begins (Stephan et al. 1995; Taube et al. 2015). According to surface electroencephalogram (EEG), both self-paced and externally cued movements are preceded by a slow-rising movement-related potential over the SMA that occurs as early as 3 s prior to moving—implying that the SMA plays a key role in planning voluntary actions (Cui and MacKinnon 2009). However, the specific role of the SMA with regard to preparing a movement, organizing the spatial/temporal parameters of motor output, and initiating actions remains to be elucidated (Carlsen et al. 2015; Nachev et al. 2008).
Reaction time (RT) tasks represent one method to assess the planning capabilities of the SMA. RT involves three main components: stimulus identification, response selection, and movement execution. Faster RTs indicate more efficient planning for a movement (e.g., the more prepared the motor system is to move, the faster a person will be able to react to a stimulus and reach for a target) (Niemi and Näätänen 1981; Rinehart et al. 2001). While simple reaction time (SRT) tasks only manipulate stimulus identification (e.g., by varying the time between each subsequent stimulus), choice reaction time (CRT) tasks involve more unpredictability, which slows the response selection process and results in slower RTs (e.g., if one is unsure about which of two targets they will need to reach for, they will not be able to fully plan the movement until they identify the stimulus, causing them to react slower). For this study, we used SRT and CRT, which both involve planning calculations via the SMA, as a measure of the preparatory state of the motor system.
During demanding balance tasks, the SMA, in conjunction with the basal ganglia (BG) and cerebellum via frontal/BG cortex circuits and frontal/cerebellar circuits, contributes to the planning of whole body movements (Della Sala et al. 2002; Malouin et al. 2003; Taube et al. 2015; Taubert et al. 2010; Walenski et al. 2006). In balance, the BG are particularly important in postural flexibility, sensorimotor integration, and learning movement sequences (Goble et al. 2011; Visser and Bloem 2005). The cerebellum is largely involved in coordinating complex multi-joint movements, maintaining upright posture, using feedforward and feedback to maintain balance, and correcting errors based on sensory feedback (Morton and Bastian 2004). Thus, during the balance task, faster speed and greater accuracy indicated an increased preparatory state of the motor system and therefore more effective motor planning calculations via the SMA and its functional pathways.
As the SMA has been implicated in planning for grasping motions (Grèzes and Decety 2002; Nachev et al. 2005), upper extremity fine motor skill tasks. We used a Grooved Pegboard test (Lafayette Instruments) as a third assessment of SMA functioning; faster completion of this challenging fine motor skill task indicated more effective planning.
tDCS
A form of noninvasive brain stimulation, transcranial direct current stimulation (tDCS), passes a weak electrical current between two electrode sponges, the anode and cathode, placed on the subject’s scalp (Filmer et al. 2014). Research has shown that anodal tDCS transiently facilitates (i.e., depolarizes) and cathodal stimulation defacilitates (i.e., hyperpolarizes) neuronal resting membrane potential without actually inducing action potentials (Filmer et al. 2014; Nitsche et al. 2003a). Anodal tDCS may be applied to a cortical region to improve the functional connectivity of a given pathway and elicit behavioral changes, such as improvements in motor (Boggio et al. 2006; Madhavan and Shah 2012), cognitive (Miniussi et al. 2008), and speech (Schneider and Hopp 2011) abilities.
Anodal tDCS applied to M1 has been shown to produce positive motor learning (Boggio et al. 2006; Galea and Celnik 2009; Hunter et al. 2009; Nitsche et al. 2003b; Reis et al. 2009). For instance, tDCS to M1 in healthy adults has been shown to increase neuronal excitability by up to 40% during stimulation and even after stimulation has been discontinued (Nitsche and Paulus 2000). Moreover, anodal stimulation of left M1 has been found to increase motor performance of the contralateral hand, while stimulation of the premotor cortex and prefrontal cortex does not influence motor performance (Nitsche et al. 2003a). Moreover, imaging studies using functional magnetic resonance imaging (fMRI) have indicated that tDCS is also capable of reaching subcortical areas via corticostriatal pathways (Polanía et al. 2012), and clinical studies have supported these findings. Anodal tDCS applied to M1 improves gait and bradykinesia in patients with Parkinson’s disease, suggesting that tDCS is able to influence deep brain structures such as the BG to elicit measurable behavioral changes (Benninger et al. 2010).
Only a few studies have examined whether stimulating the SMA results in similar motor behavior improvements (Vollman et al. 2013; Carlsen et al. 2015; Hayduk-Costa et al. 2013; Carter et al. 2015; Bolzoni et al. 2015). Vollman et al. (2013) found short-term improvements in learning in a visuomotor pinch force task during anodal tDCS to the SMA. Carlsen et al. (2015) noted faster premotor RT after only 10 min of anodal tDCS to the SMA; improvements persisted for 40 min after stimulation. Anodal tDCS to the SMA led to earlier movement initiation and decreased inhibition abilities during a wrist flexion RT task (Hayduk-Costa et al. 2013). Anodal tDCS of the SMA also resulted in increased accuracy and speed during a bimanual dynamic phase coordination task (Carter et al. 2015).
In addition to a lack of literature regarding the effects of tDCS to the SMA, the vast majority of tDCS studies have tested only very simple motor tasks. While a few studies have examined effects of tDCS on complex motor behaviors including balance (Kaski et al. 2013), lower extremity movements (Kaski et al. 2013; Jeffery et al. 2007; Madhavan et al. 2011; Tanaka et al. 2009), and language acquisition (Schneider and Hopp 2011), more research is needed to observe if tDCS is capable of influencing complex, multi-step motor behaviors.
In this study, we investigated the effects of applying anodal tDCS to the SMA on performance during four tasks: simple reaction time (SRT), choice reaction time (CRT), dynamic balance, and the Grooved Pegboard test. Each task included a quantifiable motor planning component (and thus SMA involvement). By testing multiple motor tasks of varying complexity and stimulating the SMA with anodal tDCS, we were able to assess the degree of involvement of the SMA in each task and observe whether tDCS was effective in augmenting motor planning. It was hypothesized that anodal tDCS would result in an increased state of preparation of the motor system and elicit in improvements in response time and overall speed/accuracy on each task.
Methods
Participants
Twenty right-handed young adults, aged 20–22 (7 M, 13 F), volunteered as subjects. No subjects disclosed neurologic, sensory, or motor impairments. All participants provided written informed consent, and this study was approved by the Institutional Review Board and performed in accordance with the Declaration of Helsinki.
tDCS
Stimulation was delivered to participants using a Dupel iontophoresis device (Empi Inc.). Two electrode sponges (each 25 cm2, Amrex) saturated with sterile saline (0.9% NaCl) were placed on the scalp. This system has been used previously (Carlsen et al. 2015) The anode was placed over FC1 and FC3, which corresponds to the left SMA based on the International 10–20 extended system for EEG electrode placement by first measuring and marking the skull similar to previous studies (Cui et al. 1999; Oostenveld and Praamstra 2001; Stock et al. 2013).
The cathode was used as a reference lead and placed over the right supraorbital area. Over the course of 3 days, participants received a total dosage of 120 mA min (40 mA min × 3 days − 0.4 mA × ~90 min). This dosage is well within safe limits (Liebetanz et al. 2009) and previously used for therapeutic treatment improvements (Ardolino et al. 2005; Brunoni et al. 2012; Nitsche and Paulus 2000). As sponges in this experiment possessed a large surface area, stimulation likely affected the entire SMC (Miranda et al. 2006; Wagner et al. 2007).
Only a transient minimal superficial external integumentary physical risk to participants existed, and participants were fully informed of these risks before beginning the study. Systematic investigations of the behavioral effects of tDCS have been conducted for at least the past 40 years (Priori 2003). Protocols for administering tDCS have been comprehensively investigated, and side effects from stimulation are limited to a mild tingling sensation, itching, and fatigue (Poreisz et al. 2007; Madhavan and Shah 2012).
Experimental design
Subjects were counterbalanced to complete either the tDCS or control condition first. Subjects completed a total of six sessions: three tDCS sessions and three control sessions. Sessions one, two, and three took place on consecutive days, and sessions for the other condition took place on consecutive days one week later. Subjects completed four tasks each day: SRT, CRT, dynamic balance, and the Grooved Pegboard test.
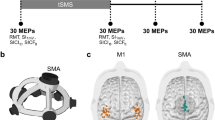
The testing protocol for the tDCS condition (Fig. 1) was structured as follows: (1) Performing the motor tasks before receiving the first tDCS dose; (2) Completing the motor tasks halfway through the second tDCS session while receiving stimulation; (3) Completing the motor tasks after receiving the final tDCS dose. On days 1 and 3, participants were instructed to sit quietly (i.e., read a book, work on their laptop, or watch television) while receiving tDCS.
During the control condition, participants completed the motor tasks for three consecutive days but did not receive tDCS in order to measure the motor learning expected to occur with practice (Fig. 1). No sham tDCS condition was implemented because this study employed motor tasks that were not likely susceptible to placebo effects. The motor tasks were not cognitively demanding, and participants could not easily detect their own performance improvements (e.g., RT scores were never relayed to subjects, so they remained largely unaware of their competence on these tasks) (Rinehart et al. 2001).
Reaction time tasks
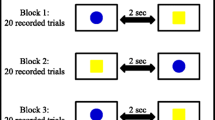
RT measures included an SRT and a CRT task using a MOART reaction time and movement time panel (Lafayette Instruments) (Fig. 2). Both RT and movement time (MT) were recorded for each task. During the SRT task, an auditory and visual stimulus occurred simultaneously and participants were instructed to remove their finger from the home key (SRT) and touch a target directly in front of them (MTSRT) as quickly as possible.
During the CRT task, participants also had a simultaneous visual and auditory stimulus which they responded to by lifting (CRT) and then touching whichever of two targets lit up red as quickly as possible (MTCRT). One target was situated on the ipsilateral side to the individual’s dominant hand, and the other target was on situated on the contralateral side. Both tasks included delays of between 1 and 4 s between each subsequent stimulus to make the stimulus timing unpredictable. Participants performed five practice trials in order to familiarize them with the task (Pascual-Leone et al. 1992), followed by 25 recorded trials for both SRT and CRT.
Balance tasks
As another measure of motor planning, we used a dynamic balance aiming task; the Limits of Stability (LOS) dynamic balance task using a Biodex Balance System (Biodex). This task required participants, while standing on a firm/stationary surface, to correctly shift their center of pressure (CoP) to reach the indicated target on a screen in front of them and pause for 0.25 s before moving to next target (Fig. 3). Similar to one’s finger during the SRT and CRT tasks, participants’ CoP functioned as an aiming tool during the balance task. However, this task represented a more complex version of SRT and CRT. After seeing the indication to proceed (i.e., stimulus identification), participants had to determine which of the eight targets was correct and plan how to manipulate their body to reach the target (i.e., response selection). Participants then had to effectively coordinate their visual, vestibular, and somatosensory systems, as well as coordinate their muscles to shift their CoP, to complete the desired movement (i.e., movement execution). Thus, compared to SRT and CRT, the balance task required more complex planning.
Subjects were familiarized with the Biodex machine through performing a static balance task before each assessment. Participants then completed one LOS trial for practice followed by two recorded trials during each testing period. While the SRT and CRT tasks were able to differentiate RT from MT, this task did not distinguish between the participant’s RT to each new stimulus and the MT taken to reach to the next target. Multiple targets were linked together, and RT and MT were both imbedded within the task.
Fine motor skill task
Participants completed the Grooved Pegboard test (Lafayette Instruments) (Fig. 4). Subjects were instructed to use only their dominant hand, pick up one peg at a time, and turn it to fit into the slots on the board, progressing from left to right to fill each row. Participants were allotted a familiarization period prior to completing the task each day. Two trials were recorded during each session, and video footage was collected to calculate time taken from when subjects touched the first peg to when they released the final peg.
Data analysis
Data were analyzed using SPSS (version 22). A 2 (time) × 2 (condition) MANOVA was conducted. A post hoc paired sample t tests were conducted for pre-post for the tDCS and control conditions for SRT, CRT, dynamic balance speed and accuracy. We then conducted a 2 (online vs. offline) MANOVA on day 2 for these same variables. We separated these from the original analysis because of the tDCS being applied in this session we wanted to compare performance when receiving online versus offline on these tasks. The alpha level was set at p < 0.05, trends were reported at 0.1, and with a Bonferroni correction alpha would be set at p < 0.005. Because of the exploratory nature of this study, we have reported it all and discussed results based on the alpha level of 0.05. In addition, effect sizes are reported with using partial η 2 with over 0.26 a large effect size, 0.13 medium effect size.
Results
All participants tolerated tDCS treatment without any adverse effects. The overall MANOVA (SRT, CRT, MTSRT, MTCRT; balance accuracy, balance time, and pegboard time), showed a significant time effect (F (14,66) = 2.82, p = 0.002, partial η 2 = 0.374). The condition effect was not significant (F (14,66) = 0.76, p = 0.63, partial η 2 = 0.29) nor was condition × time (F (14,66) = 1.26, p = 0.26, partial η 2 = 0.21).
A MANOVA was conducted for CRT and SRT tasks, and there was not a significant condition effect (F (2,18) = 0.396, p = 0.69, partial η 2 = 0.042) or condition × time (F (2,18) = 1.715, p = 0.208, partial η 2 = 0.16). There was a significant time effect (F (2,18) = 8.85, p = 0.002, partial η 2 = 0.5). Univariate analysis showed significant time effect for CRT (F (1,19) = 17.29, p = 0.001, partial η 2 = 0.48) and a marginal trend toward a significant time × condition effect for SRT (F (1,19) = 2.44, p = 0.13, partial η 2 = 0.11). SRT time effect (partial η 2 = 0.04), SRT condition effect (partial η 2 = 0.002), CRT condition effect (partial η 2 = 0.03), and CRT time × condition (partial η 2 = 0.08) were not significant (p > 0.05). Post hoc t tests were conducted for time for the tDCS condition. As indicated in Figs. 5 and 6, after 3 days of tDCS stimulation, the tDCS condition resulted in a significant time (pre vs. post) effect for SRT (t = 2.13, p < 0.05) and CRT (t = 3.89, p < 0.001). For the control condition, there were no time effects for SRT (p > 0.05) or CRT (p > 0.05). The MANOVA for day 2 analysis (online vs. not online) was not significant (p < 0.05) and post hoc analysis also showed no significant effects or trends for SRT, CRT, or MT measures (p < 0.05) and effect sizes (partial η 2) were small.
The MANOVA for balance measures showed a significant time effect (F (2,18) = 22.5, p = 0.001, partial η 2 = 0.71). There were not significant condition (partial η 2 = 0.009) or condition × time effects (partial η 2 = 0.02). Univariate analysis showed significant time effects for balance accuracy (F (1,19) = 44.12, p = 0.001, partial η 2 = 0.70) and balance time (F (1,19) = 4.02, p = 0.05, partial η 2 = 0.18). As depicted in Fig. 7, while receiving stimulation (on Day 2), there was a trend on the MANOVA for condition (online vs. offline) (F (2,18) = 3.13, p = 0.068, partial η 2 = 0.26). Univariate analysis showed significant differences for time (F (1,19) = 6.61, p = 0.02, partial η 2 = 0.26) but not for accuracy (F (1,19) = 2.24, p = 0.15, partial η 2 = 0.11). Post hoc analysis showed that for the tDCS condition showed significantly faster times compared to the control condition (t = −2.57, p < 0.05). No significant condition effect occurred for dynamic balance accuracy (p > 0.05), but the means were in the direction that would be expected if tDCS was improving accuracy (µ tDCS = 65.6 ± 16%; µ con = 59.5 ± 20%).
For the Grooved Pegboard test, there was a significant time effect (F (1,19) = 11.56, p = 0.003, partial η 2 = 0.38). There were not significant condition (partial η 2 = 0.001) or condition × time effects (partial η 2 = 0.03), p < 0.05. There was not a significant condition effect between tDCS stimulation on Day 2 and no stimulation (p > 0.05, partial η 2 = 0.03).
Discussion
This study examined whether anodal tDCS to the SMA influences performance on RT, balance, and pegboard tasks. These tasks varied in complexity, and each required some degree of planning (and thus involvement of the SMA) to execute. Improvements occurred with tDCS in RT and balance: SRT and CRT values were significantly faster after 3 days of tDCS stimulation and dynamic balance speed significantly improved (with a trend toward improved accuracy) while receiving tDCS on Day 2. Conversely, RT from pre-post and time to complete balance task pre-post did not change in the control condition. There were significant improvements in time to complete the Grooved Pegboard test for both groups, but tDCS did not improve performance with receiving stimulation or show greater benefits in performance.
tDCS likely facilitated a greater state of preparation to perform each task through modulating the SMA (Filmer et al. 2014; Nitsche et al. 2003a) and may have also helped to facilitate movement initiation, contributing to the faster response times measured during the tDCS condition (Carlsen et al. 2015). Improvements were evident in tasks of varying complexity, from simple RT to a complex balance aiming task. This indicates that the SMA is involved in the planning of a wide range of motor behaviors and that tDCS is able to enhance SMA planning processes for a variety of behaviors.
tDCS may have also improved the functional connectivity of the SMA’s pathways, cortico–cortico connectivity between the SMA and M1, corticostriatal connections, and SMA–cerebellum connectivity (Bonelli and Cummings 2007; Filmer et al. 2014; Hamada et al. 2009; Nachev et al. 2008; Polanía et al. 2012). As corticostriatal circuits are heavily implicated in planning of actions, it would logically follow that tDCS improved the efficiency of these circuits (Schneider et al. 2013).
Specifically during the balance task, possibly increasing the functional connectivity of the BG-thalamocortical motor pathway may have led to more efficient updating and refining of the subjects’ motor plans as subjects responded to the visual feedback evaluating their performance on the Biodex screen.
An interesting finding included that improvements in balance speed occurred only during tDCS (Day 2), while RT improvements persisted even after tDCS was discontinued (Day 3). RT improvements likely emerged faster because an upper extremity reaching RT task is simple and quickly mastered by participants; thus, tDCS facilitation of planning had a larger impact on behavioral output than was seen in the balance task.
Dynamic balance aiming represents a more complex task, and increasing the amount of information participants was required to coordinate (e.g., choosing between eight possible targets and controlling aiming with center of pressure) and involving a greater number of muscles and systems (e.g., visual, vestibular, and somatosensory). This task also required significantly more time to learn compared to the RT tasks, and participants did not reach a plateau in performance even after 3 days of testing. Thus, after only a short timeframe of practice (3 days), the balance task may have been too complex to result in performance improvements that lasted after tDCS was discontinued. As tDCS was able to influence SRT and CRT after it was turned “off,” a future investigation should assess whether allowing more time to practice the balance task during tDCS is able to facilitate more efficient motor planning to result in balance improvements that remain after tDCS is discontinued.
Other motor studies support the idea of training balance during tDCS. Anodal tDCS to M1 (which via cortico–cortico connections is associated with the SMA) results in improvements in gait and balance (Kaski et al. 2013), ankle control (Madhavan et al. 2011), and leg pinch force (Tanaka et al. 2009). However, no previous studies have yet observed the effects of tDCS to the SMA on planning for any lower extremity behaviors. As definite neurophysiological benefits exist for training lower extremity tasks during tDCS to M1, similarly beneficial, potentially long-lasting effects would likely result from training balance during tDCS to the SMA—which would be a meaningful future study to conduct.
Therapeutic applications of tDCS
Over fifteen million American adults report difficulties performing basic activities of daily living (e.g., cooking meals and dressing themselves) independently (Brault 2012). Thus, developing more effective motor therapies is of the utmost importance. Low cost, portable, and user-friendly tDCS is a promising tool for future translation into clinical settings, as only 3 days of stimulation resulted in some improvements to RT and balance in healthy adults. Future studies should investigate whether tDCS is effective in populations with motor planning deficits.
For this study, we applied tDCS for a longer duration of time (up to 85 min). Longer stimulation duration increases the likelihood of achieving lasting motor improvements (Ardolino et al. 2005; Brunoni et al. 2012; Nitsche and Paulus 2000) and allows more time for training while receiving tDCS, which may also contribute to lasting motor benefits (Kaski et al. 2013; Madhavan et al. 2011). Most importantly, as external sensations (e.g., itching and tingling) are often detectable at currents greater than 0.4 mA (Nitsche and Paulus 2000), using a lower current density increases the likelihood of tolerability by those who might be hypersensitive to external stimuli.
Conclusion
In twenty neurotypical young adults, anodal tDCS applied to the SMA across 3 days was effective in eliciting significant improvements in SRT and CRT pre- versus post-tDCS, and increased proficiency on the dynamic balance task during stimulation, while no significant changes were evident in the control condition. Although the specific neurophysiological mechanisms by which tDCS operates to transiently modulate excitability in cortical regions and the optimal parameters for stimulation remain largely unknown, targeting the SMA with low-intensity current was effective in improving various motor behaviors in healthy adults. While this study examined a sample of healthy college students and found small, short-term differences in performance, future studies will include investigating how tDCS modulation of the SMA might augment performance in populations with motor planning deficits.
References
Ardolino G, Bossi B, Barbieri S, Priori A (2005) Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol 568:653–663. doi:10.1113/jphysiol.2005.088310
Benninger DH, Lomarev M, Lopez G, Wassermann EM, Li X, Considine E, Hallett M (2010) Transcranial direct current stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry 81:1105–1111. doi:10.1136/jnnp.2009.202556
Boggio PS, Castro LO, Savagim EA, Braite R, Cruz VC, Rocha RR, Rigonatti SP, Silva MT, Fregni F (2006) Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett 404:232–236. doi:10.1016/j.neulet.2006.05.051
Bolzoni F, Bruttini C, Esposti R, Castellani C, Cavallari P (2015) Transcranial direct current stimulation of the SMA modulates anticipatory postural adjustments without affecting the primary movement. Behav Brain Res 291:407–413. doi:10.1016/j.bbr.2015.05.044
Bonelli RM, Cummings JL (2007) Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci 9:141–151
Brault MW (2012) Americans with disabilities: 2010. Current Population Reports, US Census Bureau
Brunoni AR, Boggio PS, Bikson M, Bolognini N, Nitsche MA, Fregni F et al (2012) Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul 5:175–195. doi:10.1016/j.brs.2011.03.002
Carlsen AN, Eagles JS, MacKinnon CD (2015) Transcranial direct current stimulation over the supplementary motor area modulates the preparatory activation level in the human motor system. Behav Brain Res 279:68–75. doi:10.1016/j.bbr.2014.11.009
Carter MJ, Maslovat D, Carlsen AN (2015) Anodal direct current stimulation applied over the supplementary motor area delays spontaneous antiphase-to-in-phase transitions. J Neurophysiol 113:780–785. doi:10.1152/jn.00662.2014
Cui RQ, MacKinnon CD (2009) The effect of temporal accuracy constraints on movement-related potentials. Exp Brain Res 194:477–488. doi:10.1007/s00221-009-1725-5
Cui RQ, Hunter D, Lang W, Deeke L (1999) Neuroimage of voluntary movement: topography of the Bereitschaftspotential, a 64-channel DC current source density study. NeuroImage 9:124–134
Della Sala S, Francescani A, Spinnler H (2002) Gait apraxia after bilateral supplementary motor area lesion. J Neurol Neurosurg Psychiatry 72:77–85. doi:10.1136/jnnp.72.1.77
Filmer HL, Dux PE, Mattingley JB (2014) Applications of transcranial direct current for understanding brain function. Trends Neurosci 37:742–753. doi:10.1016/j.tins.2014.08.003
Galea JM, Celnik P (2009) Brain polarization enhances the formation and retention of motor memories. J Neurophysiol 102:294–301. doi:10.1152/jn.00184.2009
Goble DJ, Coxon JP, Van Impe A, Geurts M, Doumas M, Wenderoth N, Swinnen SP (2011) Brain activity during ankle proprioceptive stimulation predicts balance performance in young and older adults. J Neurosci 31:16344–16352. doi:10.1523/JNEUROSCI.4159-11.2011
Grèzes J, Decety J (2002) Does visual perception of object afford action? Evidence from a neuroimaging study. Neuropsychologia 40:212–220. doi:10.1016/S0028-3932(01)00089-6
Hamada M, Hanajma R, Terao Y, Okabe S, Nakatani-Enomoto S, Furubayashi T, Matsumoto H, Shirota O, Ugawa Y (2009) Primary motor cortical metaplasticity induced by priming over the supplementary motor area. J Physiol 587:4845–4862. doi:10.1113/jphysiol.2009.179101
Hayduk-Costa G, Drummond NM, Carlsen AM (2013) Anodal tDCS over SMA decreases the probability of withholding an anticipated action. Behav Brain Res 257:208–214. doi:10.1016/j.bbr.2013.09.030
Hunter T, Sacco P, Nitsche MA, Turner DL (2009) Modulation of internal model formation during force field-induced motor learning by anodal transcranial direct current stimulation of primary motor cortex. J Physiol 587:2949–2961. doi:10.1113/jphysiol.2009.169284
Jeffery DT, Norton JA, Roy FD, Gorassini MA (2007) Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Exp Brain Res 182:281–287
Kaski D, Dominguez RO, Allum JH, Bronstein AM (2013) Improving gait and balance in patients with leukoaraiosis using transcranial direct current stimulation and physical training: an exploratory study. Neurorehabil Neural Repair 27(9):864–871. doi:10.1177/1545968313496328
Liebetanz D, Koch R, Mayenfels S, Konig F, Paulus W, Nitsche MA (2009) Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol 120:1161–1167. doi:10.1016/j.clinph.2009.01.022
Madhavan S, Shah B (2012) Enhancing motor skill learning with transcranial direct current stimulation—a concise review with applications to stroke. Front Psychiatry 3:1–9. doi:10.3389/fpsyt.2012.00066
Madhavan S, Weber KA, Stinear JW (2011) Non-invasive brain stimulation enhances motor control of the hemiparetic ankle: implications for rehabilitation. Exp Brain Res 209:9–17. doi:10.1007/s00221-010-2511-0
Malouin F, Richard CL, Jackson PL, Dumas F, Doyon J (2003) Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp 19:47–62
Miniussi C, Cappa SF, Cohen LG, Floel A, Fregni F, Nitsche MA, Oliveri M, Pascual-Leone A, Paulus W, Priori A, Walsh V (2008) Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul 1:326–336. doi:10.1016/j.brs.2008.07.002
Miranda PC, Lomarev M, Hallet M (2006) Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol 117:1623–1629. doi:10.1016/j.clinph.2006.04.009
Morton SM, Bastian AJ (2004) Cerebellar control of balance and locomotion. Neuroscientist 10:247–259. doi:10.1177/1073858404263517
Nachev P, Rees G, Partion A, Kennard C, Husain M (2005) Volition and conflict in human medial frontal cortex. Curr Biol 15:122–128. doi:10.1016/j.cub.2005.01.006
Nachev P, Kennard C, Husain M (2008) Functional role of the supplementary and pre-supplementary motor area. Nat Rev Neurosci 9:856–869. doi:10.1038/nrn2478
Nguyen VT, Breakspear M, Cunnington R (2014) Reciprocal interactions of the SMA and cingulate cortex sustain premovement activity for voluntary actions. J Neurosci 34:16397–16407. doi:10.1523/JNEUROSCI.2571-14.2014
Niemi P, Näätänen R (1981) Foreperiod and simple reaction time. Psychol Bull 89:133–162
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527:633–639. doi:10.1111/j.1469-7793.2000.t01-1-00633.x
Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W (2003a) Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 553:293–301. doi:10.1113/jphysiol.2003.049916
Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W et al (2003b) Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 15:619–626. doi:10.1162/089892903321662994
Oostenveld R, Praamstra P (2001) The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol 112:713–719. doi:10.1016/S1388-2457(00)00527-7
Pascual-Leone A, Brasil-Neto J, Valls-Sole J, Cohen LG, Hallett M (1992) Simple reaction time to focal transcranial magnetic stimulation. Comparison with reaction time to acoustic, visual and somatosensory stimuli. Brain 115:109–122. doi:10.1093/brain/115.1.109
Polanía R, Paulus W, Nitsche MA (2012) Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp 33:2499–2508. doi:10.1002/hbm.21380
Poreisz C, Boros K, Antal A, Paulus W (2007) Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull 72:208–214. doi:10.1016/j.brainresbull.2007.01.004
Priori A (2003) Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol 114:589–595. doi:10.1016/S1388-2457(02)00437-6
Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E et al (2009) Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci 106:1590–1595. doi:10.1073/pnas.0805413106
Rinehart NJ, Bradshaw JL, Brereton AV, Tonge BJ (2001) Movement preparation in high-functioning Asperger disorder: a serial choice reaction time task involving motor reprogramming. J Autism Dev Disord 31:79–88
Schneider HD, Hopp JP (2011) The use of the Bilingual Aphasia Test for assessment and transcranial direct current stimulation to modulate language acquisition in minimally verbal children with autism. Clin Linguist Phon 25:640–654. doi:10.3109/02699206.2011.570852
Schneider HD, Livitz IE, Schneider D (2013) Sustainable learning for sustainability. JOTSC 10:124–147. doi:10.1179/1477963313Z.0000000009
Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS (1995) Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol 73:373–386
Stock A, Wascher E, Beste C (2013) Differential effects of motor efference copies and proprioceptive information on response evaluation processes. PLoS ONE 8:e62335. doi:10.1371/journal.pone.0062335
Tanaka S, Hanakawa T, Honda M, Wanatabe K (2009) Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Exp Brain Res 196:459–465. doi:10.1007/s00221-009-1863-9
Taube W, Mouthon M, Leukel C, Hoogewoud HM, Annoni JM, Keller M (2015) Brain activity during observation and motor imagery of different balance tasks: an fMRI study. Cortex 64:102–114. doi:10.1016/j.cortex.2014.09.022
Taubert M, Draganski B, Anwander A, Muller K, Horstmann A, Villringer A, Ragert P (2010) Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci 30:11670–11677. doi:10.1523/JNEUROSCI.2567-10.2010
Ullman MT (2006) Is Broca’s area part of a basal ganglia thalamocortical circuit? Cortex 42:480–485. doi:10.1016/S0010-9452(08)70382-4
Visser JE, Bloem BR (2005) Role of the basal ganglia in balance control. Neural Plast 12:161–174. doi:10.1155/NP.2005.161
Vollman H, Conde V, Sewerin S, Taubert M, Sehm B, Witte OW, Villringer A, Ragert P (2013) Anodal transcranial direct current stimulation (tDCS) over supplementary motor area (SMA) but not pre-SMA promotes short-term visuomotor learning. Brain Stimul 6:101–107. doi:10.1016/j.brs.2012.03.018
Wagner T, Valero-Cabre A, Pascual-Leone A (2007) Noninvasive human brain stimulation. Annu Rev Biomed Eng 9:527–565. doi:10.1146/annurev.bioeng.9.061206.133100
Walenski M, Tager-Flusberg H, Ullman MT (2006) Language in autism. In: Moldin SO, Rubenstein JLR (eds) Understanding Autism: From basic neuroscience to treatment. CRC Press, Boca Raton, pp 175–203
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hupfeld, K.E., Ketcham, C.J. & Schneider, H.D. Transcranial direct current stimulation (tDCS) to the supplementary motor area (SMA) influences performance on motor tasks. Exp Brain Res 235, 851–859 (2017). https://doi.org/10.1007/s00221-016-4848-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4848-5