Abstract
Both self-paced movements (internally generated) and movements paced by a fixed interval cue (externally cued) are preceded by a slow-rising movement-related potential (MRP) of similar timing, magnitude and topography. When the timing of the external cue is variable (temporally unpredictable), this MRP is absent. These findings have been interpreted to suggest that MRPs reflect neural activity mediating the preparation of temporally predictive movements, irrespective of whether the movement is internally generated or externally cued. However, the apparent similarity between MRPs preceding self-paced and predictably cued movements might be explained by the absence of control for the timing of movement onset, that is, MRPs preceding regularly-paced cues may simply reflect activity associated with self-paced movements initiated at times that more or less coincide with the timing of the imperative cue. The objective of this study was to reexamine the comparison of MRPs preceding temporally predictive (self-paced and predictably cued) versus reactive movements. To circumvent the potential confound of movement onset timing, constraints were placed on the temporal accuracy of movements cued by a regularly-paced tone. This design permitted post-hoc classification of trials into predictive or reactive movements to the tone. Three movement initiation conditions were tested: (1) self-paced (SP), (2) in response to an irregularly-paced cue (IC), and (3) in response to a regularly-paced cue (RC). In the latter condition, subjects were trained to initiate movement to within less than one simple reaction time in at least 50% of trials, and MRPs were compared between movements that were initiated “too early” (predictive), “too late” (reactive), or were temporally accurate (predictive). Cerebral potentials were recorded from 87 scalp surface electrodes. Consistent with previous studies, an early slow-rising MRP with maximum negativity over the midline frontal cortex was present only when the timing of movement onset could be predicted in advance (SP and RC conditions). Moreover, MRPs for movements that were temporally accurate or were initiated “too early” were significantly larger than the MRPs that preceded SP movements (P < 0.018). In contrast, movements initiated in reaction to the cue (IC condition), even when the timing of the cue could be predicted in advance (movement initiated “too late” in the RC condition), were associated with a significant attenuation of premovement activity (P < 0.005). Differences between conditions (RC > SP > IC) were significantly greater over the midline frontal cortex than the contralateral or ipsilateral sensorimotor cortex (P < 0.038). These findings show that the imposition of accuracy constraints on the timing of movement onset markedly enhances preparatory activity in the cortical or subcortical networks that mediate the predictive initiation of movement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Self-paced (internally generated) movements and movements triggered in response to external cues in the surrounding environment (externally generated) are often considered to be mediated by largely separate neural pathways (Goldberg 1985; Passingham 1987). Yet, under certain conditions, both modes of initiation appear to share an overlapping cortical and subcortical network (Jahanshahi et al. 1995; Jenkins et al. 2000). Intermittent self-paced movements are preceded by a slow-rising movement-related potential (MRP) that can begin more than 1.5 s prior to movement onset (Kornhuber and Deecke 1965). An inherent property of self-paced movements is that the task and onset timing of movement are known and therefore predictive strategies can be used to prepare the movement well in advance. Similarly, predictive strategies can be implemented prior to externally cued movements when the task is known and the onset timing of the imperative cue to initiate movement can be predicted in advance. Under these conditions, externally cued movements are preceded by a MRP that has timing and topography similar to the MRP that precedes self-paced movements (Thickbroom and Mastaglia 1985; Cunnington et al. 1995; Jahanshahi et al. 1995; Jankelowitz and Colebatch 2002). In contrast, an early slow-rising MRP is absent when the timing of the imperative cue to initiate movement cannot be predicted in advance, even when the task is known. Thus, the dichotomy between self-paced and externally cued movements holds when the timing of the imperative cue is unpredictable, but the extent to which self-paced and predictably cued movements are mediated by similar neuroanatomical substrates remains unclear.
A variety of studies have directly compared movement-related activity associated with self-paced movements and movement triggered by a regularly-paced (fixed interval) cue. EEG studies have shown that the early components of the MRP for both modes were similar in timing and magnitude, suggesting equivalent activation of the mesial frontal cortex, whereas the magnitude of the later stages of preparation (NS’ or NS2 component) tended to be larger for self-paced movements (Thickbroom and Mastaglia 1985; Cunnington et al. 1995; Jahanshahi et al. 1995; Jankelowitz and Colebatch 2002). Neuroimaging studies have also shown that self-paced and regularly-cued movements are associated with activation of a common set of cortical regions including the contralateral sensorimotor cortex, rostral and caudal supplementary motor area (SMA), and bilateral dorsal premotor cortex (Jahanshahi et al. 1995; Jenkins et al. 2000). Differences in activity between initiation modes were confined to regions of the right dorsolateral prefrontal cortex (Jahanshahi et al. 1995; Jenkins et al. 2000), pre-SMA and the cingulate motor areas (Dieber et al. 1999). In contrast, movements triggered by temporally unpredictable (random) cues were characterized by the absence of early components of MRPs (onset near 100 ms prior to movement onset) (Papa et al. 1991; Cunnington et al. 1995; Jahanshahi et al. 1995; Jankelowitz and Colebatch 2002) and markedly reduced regional cerebral blood flow in regions of the SMA (Jenkins et al. 2000). Based on these findings, it has been proposed that self-paced movements and movements triggered by temporally predictable cues are mediated by a largely common network of cortical and subcortical regions, but preparatory activity tends to be higher prior to self-paced movements (Nachev et al. 2008).
However, interpretation of the findings of the studies cited above is confounded by the fact that the timing of movement onset relative to the external cue was not controlled. This leaves the possibility that the similarities in cerebral activity between self-paced and externally triggered movements were due to subjects performing cued movements in a self-paced manner. In other words, the subjects performed self-paced movements at regular intervals that, more or less, coincided with the rate of the cue. It is also possible that the MRPs were generated from the averaging of trials with movement onsets that were timed to coincide with the cue (predictive) and movements initiated in reaction to the cue. As noted above, MRPs preceding temporally predictable and unpredictable cues are markedly different.
The purpose of this study was to reexamine the comparison between MRPs that proceed temporally predictive (both self-paced and regularly-cued movements) and reactive movements.
For this purpose, a paradigm was developed that allowed the separation and analysis of trials associated with temporally predicted initiation from trials initiated in reaction to the imperative “go” cue. This separation was accomplished by imposing tight constraints on the accuracy of movement onset timing when the movements were paced by a regular (fixed interval) cue. We hypothesized that MRPs preceding accurately-timed, and thus temporally predictive, movements would be enhanced relative to self-paced movements, and that the early components of the MRP would be significantly attenuated prior to reactive movements relative to both temporally predictive and self-paced movements.
Methods
Subjects
Nine right-handed neurologically healthy subjects (2 females, 7 males), ranging in age from 27 to 49 years (average age = 33 ± 10) participated in these experiments. Hand dominance was assessed using a modified Oldfield’s handedness questionnaire (Oldfield 1971; Salmaso and Longoni 1985). The experiments were approved by the Institutional Review Board at Northwestern University and written informed consent was obtained prior to inclusion into the study.
Experimental design
The experiment was conducted in two sessions on separate days. During the first session, subjects were trained to perform the movement task to within specified temporal accuracy constraints. Only those subjects who could perform the task within these constraints participated in the second session.
Training session
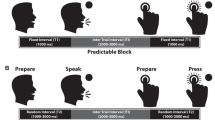
Subjects sat in a comfortable chair with their right forearm restrained in a brace in a pronated position with the palm of the hand facing downward on the surface of a table. The movement performed was a ballistic wrist extension movement from neutral to an angle of approximately 30°–45° (see Fig. 1). Electromyographic (EMG) activity was recorded from bipolar surface electrodes placed over the motor point of the extensor carpi radialis longus muscle (ECRL). EMG signals were amplified (2,000×), filtered (30–1,000 Hz) and rectified on-line.
Examples of wrist extensor EMG activity for the three tasks. The regularly-paced cue task (RC) involved the presentation of an acoustic cue (vertical black arrow) once every 3.5 s. For the irregularly-paced cue task (IC), the acoustic tone was presented randomly between 3.5 and 10 s. The self-paced task (SP) required subjects to initiate movement without the acoustic cue every 4–10 s
Two sets of experiments were conducted during training. The first experiment was used to establish the minimum voluntary reaction (1RTmin) for each subject (see Fig. 4). Subjects were instructed to initiate the movement as soon as possible following the onset of an auditory tone (50 ms, 1,000 Hz). The tone was presented randomly every 3.5–10 s (irregular cue, IC). EMG onset was estimated using an analog threshold detector that generated a trigger during the initial rising phase of the rectified and low-pass filtered EMG signal. The threshold was adjusted to signal EMG onset with an accuracy of between 10 and 20 ms. The trigger signal was collected at a rate of 1,000 Hz using a data acquisition board (PCI-MIO-16XE, National Instruments, Austin, TX, USA) and control software (LabView 7, National Instruments). The timing of the trigger was then analyzed relative to the onset of the “go” tone and plotted on a histogram. A total of 50 trials were performed consecutively. The minimum reaction time was chosen as the time bin (10 ms increments) with the lowest reaction time that contained two or more trials. This minimum reaction time value served as our estimate of the minimum time required for a subject to voluntarily initiate movement in reaction to the auditory tone. The average minimum reaction time across subjects was 142 ± 25 ms (range = 110–190 ms).
For the second experiment, subjects performed the same task with the tone presented at regular intervals of once every 3.5 s (regularly-paced cue, RC). Subjects were trained to initiate EMG onset to within ± one minimum reaction time (Figs. 1, 4). Feedback was provided after each movement specifying whether the movement was too early (RC1), too late (RC4) or within the reaction time criteria (RC3, RC4). Trials with EMG onset within this tight temporal constraint were defined as being temporally accurate. Only those subjects that could perform the task after training with over 50% of trials within the timing accuracy constraints participated in the second session.
Data collection session
Subjects performed the same ballistic wrist extension movements described above under three different cueing conditions (Fig. 1): (1) self-paced movements performed once every 4–10 s (SP task); (2) movements cued by an acoustic tone presented randomly every 3.5–10 s (irregularly-paced cue, IC); and (3) movements cued by the same tone presented every 3.5 s (regularly-paced cue, RC) (same as the training paradigm). During the RC task, subjects were instructed to initiate movement within the accuracy constraints determined during the training session (±1 minimum reaction time). Feedback of movement timing accuracy was not provided on a trial-by-trial basis, but instead was provided after 50 trials were completed. During the data collection, subjects were instructed to fix their gaze on a 2 cm2 green circle presented on a computer monitor placed in the center of their field of view and to try to prevent eye blinks and eye movements during data collection.
Data acquisition
EEG was recorded from a montage of 87 scalp surface electrodes placed at sites based on the 10/20 system with additional interpolated locations. EEG signals were differentially amplified (gain = 2,500) with respect to a reference electrode placed over the right mastoid process, filtered from DC-200 Hz, and sampled at 1,000 Hz (Neuroscan Synamps, USA). Electrode impedances were below 15 K. Electroculography (EOG) was recorded using electrodes placed over the upper and lower canthi of one eye (band-pass = 1–200 Hz). EMG recordings were obtained from the ECRL muscle as described above.
At the end the MRP data collection, somatosensory evoked potentials (SEPs) were recorded. SEPs were produced by electrically stimulating the median nerve using surface electrodes placed over the nerve at the wrist crease. Square wave pulses of 0.2 ms duration were delivered at random time intervals between 1 and 2 s. The intensity of the stimulus was sufficient to elicit activation of the thenar musculature and a small twitch of the thumb. Epochs were recorded from 40 ms prior, to 60 ms after, the stimulus. Data was collected at 5,000 Hz (bandpass filtered from 5 to 1,000 Hz, 24 dB/octave) and a total of 1,000–1,200 artifact-free epochs were collected, baseline corrected (−40 to −1 ms) and averaged.
MRP data analysis
All signals were baseline corrected for DC bias by subtracting the average voltage from −2,000 to −1,800 ms prior to EMG onset. EMG signals were then rectified and onset of muscle activity was marked based on the threshold trigger pulse and adjusted manually if needed. The data was then epoched into single movement segments from 2,000 ms prior, to 500 ms after, EMG onset. Trials containing eye movements, blinks, or other artifacts were rejected before averaging across trials.
The magnitude of the premovement potentials were quantified by calculating the average integrated signal at four regions of interest (ROI): ROI1 = midline anterior frontal (electrodes FP1, FP2, AF3, AFz, AF4, AFF1, and AFF2), ROI2 = midline central (electrodes FCz, FCC1, FCC2, and Cz); ROI3 = contralateral sensorimotor (electrodes C5, C3, C1, FCC5, FCC1, CCP5, and CCP3), and ROI4 = ipsilateral sensorimotor cortex (electrodes C2, C4, C6, FCC4, FCC6, CCP4, CCP6). The average integrated EEG at each ROI was calculated over six time bins (−1,700 to −1,600 ms, −1,300 to −1,200 ms, −900 to −800 ms, −500 to −400 ms, −300 to −200 ms and −100 to 0 ms). Wrist extensor EMG activity was quantified by measuring the area under the rectified EMG signal from onset to 100 ms.
A three-way ANOVA was conducted using the factors task condition (SP, IC, RC), region of interest (ROIs 1–4) and time bin as the repeated measures (SPSS Version 11.5, Chicago, USA). In the presence of a significant main effect of time, the temporal evolution of differences between task conditions, ROIs and task condition × ROI interaction effects was examined using a series of two-way ANOVAs with planned contrasts at each time bin. Post-hoc analysis of interaction effects were conducted using two-tailed Students t tests with Bonferroni correction for multiple comparisons. Differences were considered significant with a P value of less than 0.05.
Results
The topography of the voltage distribution on the scalp surface for the grand average MRPs is shown in Fig. 2a. MRP waveforms at selected electrodes are presented in Fig. 2b. The waveforms for the RC task were derived only from trials that were temporally accurate (RC2 and RC3). For both the SP and RC tasks, the MRPs were characterized by an early, slow-rising scalp surface negativity over the region of the vertex (Cz), positivity over the anterior midline frontal cortex (AFz), and a reversal (isopotential) of the potential over the mid-frontal cortex (Fz). In contrast, premovement activity was absent for the IC task until approximately 130 ms prior to EMG onset. Premovement activity was greater over the contralateral than the ipsilateral sensorimotor cortex for the SP and RC tasks. The average wrist extensor EMG activity was reduced for the RC task, but there were no significant differences across tasks (F 2 = 1.537, P = 0.245). For comparison, Fig. 2a also shows the topography of the grand average SEP at 19.8 s produced by stimulation of the right median nerve. Note the reversal of the potential immediately below the hand region of the contralateral sensorimotor cortex (C3).
a Topography of the voltage distribution on the scalp surface across tasks and time bins. The waveforms were derived from the grand average MRPs for each condition across subjects. The waveforms for the RC task were derived only from trials that were temporally accurate (RC2 and RC3). Note the reversal potential over the midline frontal cortex (near Fz). The head map on the bottom left shows the voltage distribution for the grand average right median nerve SEP at 19.8 ms. Note the reversal potential immediately below the C3 electrode (contralateral sensorimotor cortex). Electrode locations were estimated based upon standard 10–20 coordinates. Scalp voltage maps were interpolated with a 2nd order spherical spline. The black circles on the scalp surface map indicate the locations of electrodes Fz, Cz and C3. b The grand average potentials across subjects at selected electrode locations over the midline anterior frontal (AFz), midline frontal (Fz), midline central (Cz), contralateral (C3) and ipsilateral sensorimotor (C4) cortex. The plot on the lower right shows the average rectified EMG signal in the wrist extensors (ECRL) across tasks
The average integrated magnitude of the potentials for the SP, IC and RC task conditions are shown in Fig. 3. Statistical analysis of the data showed significant main effects of task condition (F 2 = 30.886, P < 0.001), ROI (F 3 = 23.111, P < 0.001), and time (F 5 = 73.063, P < 0.001) and significant interaction effects for task condition × ROI (F 6 = 4.707, P < 0.001), task condition × time (F 10 = 19.276, P < 0.001), ROI × time (F 15 = 16.272, P < 0.001) and task condition × ROI × time (F 30 = 5.464, P < 0.001). Significant main effects of task condition were observed at time bins from −900 to −100 ms (F 2 > 10.67, P < 0.001). Planned contrasts between conditions showed that the premovement potentials for the RC task were larger than the potentials for the SP and IC tasks (P < 0.019) from −900 to −100 ms. The SP potentials were larger than the IC potentials (P < 0.003) from −500 to −100 ms. Significant main effects of ROI were observed at time bins from −1,300 to −100 ms (F 3 > 6.531, P < 0.003). Activity over the midline frontal region (ROI2) and midline anterior frontal (ROI1) was significantly greater than activity over the contralateral (ROI3) and ipsilateral (ROI4) sensorimotor cortex (P < 0.008) from −900 to −100 ms. Activity over ROI1 was significantly greater than ROI2 from −500 to −100 ms (p < 0.05) and activity over ROI3 was significantly greater than ROI4 only at the −100 to 0 ms time bin. The effects of task condition (RC > SP > IC) were significantly greater over the midline frontal cortex (ROI2) than over the contralateral and ipsilateral sensorimotor cortex (ROIs 3 and 4) from −900 to −100 ms (P < 0.038).
Average magnitude of the scalp surface potentials across tasks at four regions of interest (ROI): midline anterior frontal (ROI1), midline central (ROI2), contralateral sensorimotor (ROI3) and ipsilateral sensorimotor (ROI4). The magnitude reflects the average integrated potential across selected electrodes in the region of interest. Six time intervals were analyzed: −1,700 to −1,600 ms, −1,300 to −1,200 ms, −900 to −800 ms, −500 to −400 ms, −300 to −200 ms and −100 to 0 ms. The mean ± 1 standard error at each time interval are shown
Subsets of trials for the RC task were examined to assess if the premovement potentials were affected by the accuracy of movement onset (Fig. 4a). Individual trials were separated into four reaction bins depending upon the timing of EMG onset relative to the tone: RC1 = onset early and temporally inaccurate (between 1 s and −1RTmin); RC 2 = onset early but temporally accurate (less than −1RTmin); RC 3 = onset late but temporally accurate (0 ms to +1RTmin); RC4 = onset late and temporally inaccurate (between +1RTmin and 1 s). Movements that were initiated too late (RC4) were considered to be “reactions” to the stimulus in the same manner as the IC condition, whereas movements that were initiated too early or within the accuracy constraints were considered to reflect temporally predicted movement onsets. An example of the distribution of trials within each category for a single subject is shown in Fig. 4b.
a Classification of trials for the regularly-paced cue (RC) task according to the individual’s reaction time (RT) relative to the onset of the auditory tone at 0 s. Reaction times less than the individual’s fastest voluntary reaction when the timing of the cue was irregular (1RT) were classified as being temporally accurate (RC2 and RC3), whereas reactions that were too early (RC1) or too late (RC4) were classified as being temporally inaccurate. b Example of the distribution of RC task trials according to RT in a single subject. Trial counts have been summed within 25 ms bins
The grand average premovement potentials for each category of EMG onset timing are summarized in Fig. 5. The timing and magnitude of the potentials preceding movements with early onset (RC1) and those that were temporally accurate (RC2 and RC3) were markedly larger than the potentials that preceded movements that were too late (RC4). A three-way ANOVA with planned contrasts, similar to the methods used to examine differences between the SP, IC and RC tasks, was implemented. This analysis revealed main effects of reaction time bin (F 3 = 15.140, P < 0.001), ROI (F 3 = 19.897, P < 0.001) and time (F 5 = 74.134, P < 0.001) and significant interaction effects for reaction time bin × ROI (F 9 = 4.076, P < 0.001), reaction time bin × time (F 15 = 16.04, P < 0.001), ROI × time (F 15 = 17.297, P < 0.001) and reaction time bin × ROI × time (F 45 = 4.426, P < 0.001). The main effects of reaction time bin were significant at time bins from −900 to −100 ms (F 3 > 3.188, P < 0.043). Planned contrasts between conditions showed that the premovement potentials for the RC1, RC2 and RC3 time bins were larger than the potentials for the RC4 bin (P < 0.05) from −500 to −100 ms. Significant main effects of ROI were observed at time bins from −1,300 to −100 ms (F 3 > 9.977, P < 0.001). Activity over the midline frontal (ROI2) and midline anterior frontal (ROI1) regions was significantly greater than activity over the contralateral (ROI3) and ipsilateral (ROI4) sensorimotor cortex (P < 0.011) from −900 to −100 ms. The effects of reaction time bin (RC1, RC2 and RC3 > RC4) were significantly greater over the midline frontal cortex (ROI2) than over the contralateral and ipsilateral sensorimotor cortex (ROIs 3 and 4) from −900 to −300 ms (P < 0.046).
a The grand average potentials for the regularly-paced cue task (RC) after the trials were sorted according to reaction time and categorized as being too early (RC1), too late (RC4) or temporally accurate (RC2 and RC3). The potentials are shown at selected electrode locations over the midline anterior frontal (AFz), midline frontal (Fz), midline central (Cz), contralateral (C3) and ipsilateral sensorimotor (C4) cortex. The plot on the lower left shows the average rectified EMG signal in ECRL across reaction time bins. b Average magnitude of the scalp surface potentials for trials categorized as RC1, RC2, RC3 or RC4. The four regions of interest (ROI) and six time intervals were the same as those described in Fig. 3. The mean ± 1 standard error at each time interval are shown
Since movements that were initiated “too late” (RC4) were associated with an MRP, this potential was compared separately to both the IC and SP conditions using a repeated measures ANOVA. The magnitude of the MRP for RC4 movements was significantly greater than for the IC task (F 1 = 25.822 9.977, P < 0.001). In contrast, there was no main effect of task condition between the RC4 movements and the SP task (F 1 = 0.625, P = 0.877) and no task condition × ROI × time interaction effect.
Discussion
There are two novel findings from this study. First, the imposition of tight constraints on the timing of movement onset resulted in a marked increase in the magnitude of the MRP. Specifically, there was a significant increase in the magnitude of the MRP preceding movements accurately-timed to an external cue (RC) in comparison to the same movements that were self-paced (SP). Second, MRPs were significantly reduced in magnitude when movements were initiated in reaction to the imperative cue, even when the stimulus timing was known (RC4 trials), and were absent when stimulus timing was unpredictable (IC condition). The discussion below relates these results to the literature examining the effects of temporal predictability of external cues on MRPs and reactive versus predictive control of movement initiation.
Effects of the temporal predictability of movement onset
A variety of studies have examined the effects of temporal predictability and duration estimation on cortical potentials associated with movement preparation (e.g. Walter et al. 1964; McAdam 1966; Elbert et al. 1991; Macar et al. 1999). For the most part, questions have been addressed using a contingent negative variation (CNV) paradigm. The CNV is a movement-related potential that is generated in the delay interval between an instruction or warning stimulus and a subsequent imperative stimulus to initiate movement (Walter et al. 1964). For long inter-stimulus intervals (in the seconds range), components of the CNV that are related to the second stimulus are characterized by a slow-rising potential that is similar to the MRP that precedes SP movements (Rohrbaugh et al. 1976; Hamano et al. 1997; Cui et al. 2000a). Walter et al. (1964) were the first to show that the CNV developed when subjects were asked to initiate movement at the expected time of the second stimulus, even in the absence of the imperative cue. This finding provided evidence that the CNV was related not only to motor preparation but also to the subject’s estimate of the duration of the inter-stimulus interval. Subsequent studies showed that the magnitude of the CNV correlates with the estimated duration of the inter-trial interval (McAdam 1966; Ruchkin et al. 1977; Macar et al. 1999, 2002). Yet, few studies have examined the effects of temporal uncertainly on the CNV by manipulating the probability of timing of the second stimulus across conditions. Trillenberg et al. (2000) showed that reaction times decreased and CNVs increased with increasing a posteriori probability of occurrence of the imperative stimulus. However, the CNVs and reactions times recorded by Trillenberg et al. (2000) were obtained using a choice reaction time task, thus the results reflect anticipatory changes in cortical activity prior to reactive movements.
The RC and IC task conditions tested in the present study allowed comparison of MRPs under temporally predictable and unpredictable conditions respectively. Similar to the findings of previous EEG studies, movements triggered by a regularly-paced cue were preceded by a slow-rising MRP and this potential was absent when the timing of the cue was unpredictable (Kutas and Donchin 1980; Papa et al. 1991; Cunnington et al. 1995; Jankelowitz and Colebatch 2002). These differences have been interpreted to reflect a preferential role of the mesial frontal cortex, in particular the supplementary motor area (SMA), in the preparation and initiation of temporally predictable cues. This interpretation was corroborated by a positron emission tomography study showing that regularly-paced movements were associated with a marked increase in the magnitude and extent of regional cerebral blood flow in the region of SMA (Jenkins et al. 2000). Yet, in contrast to these findings, several neuroimaging studies showed that mesial motor cortical activation was greater when movements were paced by an irregularly-paced cue compared to a regularly-paced cue (Lutz et al. 2000; Thickbroom et al. 2000; Toma et al. 2003), suggesting preferential involvement of the SMA in temporally unpredictable movements. The apparent discrepancy between studies is best explained by differences in the inter-stimulus interval. It is well recognized that the duration and variability of the foreperiod preceding an imperative stimulus is a major determination of reaction times (Niemi and Näätänen 1981) and that this effect is related to the temporal uncertainty of the imperative stimulus. In general, RTs increase with increasing duration and variability of the foreperiod (Klemmer 1956). Studies that used an inter-stimulus interval of greater than 3 s for the regular cue and a wide range of variability for the irregular cue (e.g. greater than 5 s), such as in the present study, showed greater activation in the mesial frontal cortex with regularly-paced movements (Jahanshahi et al. 1995; Jenkins et al. 2000). Studies that showed increased mesial frontal activation for irregularly-paced cues had mean inter-stimulus intervals of less than 2 s and timing variability of less than 0.5 s (Lutz et al. 2000; Thickbroom et al. 2000; Toma et al. 2003). Thickbroom et al. (2000) explained these findings using a model whereby, in the face of temporal uncertainty, mesial motor cortical activity is increased and maintained in an elevated state until the appearance of the imperative stimulus. In contrast, the build-up of preparatory activity is timed to coincide with the estimated timing of the imperative cue during a regularly-paced task, and thus a prolonged period of elevated anticipatory activity is not required. This model is likely to hold for short inter-stimulus intervals and a narrow range of onset times, yet the physiological cost of maintaining long-lead activity may be too high for longer intervals and high temporal uncertainty. Under those conditions, individuals may be more likely to react to the imperative stimulus rather than generate an early MRP.
MRPs preceding self-paced and regular-cued movements
In keeping with the findings of previous studies, the MRP associated with the RC task was remarkably similar in timing and topography to the MRP that precedes self-paced movements (Kutas and Donchin 1980; Thickbroom and Mastaglia 1985; Cunnington et al. 1995; Jankelowitz and Colebatch 2002). In both the SP and RC conditions, the MRP was characterized by an early, slow-rising scalp surface negativity over the region of the vertex (Cz), positivity over the anterior midline frontal cortex (AFz), and a reversal (isopotential) of the potential over the mid-frontal cortex (Fz). Note that this topography was markedly different from the SEP evoked by electrical stimulation of the median nerve (Fig. 2a). The SEP at this time point represents activity associated with synaptic input to area 3b on the posterior bank of the central sulcus, immediately posterior to the hand region of the primary motor cortex (Allison et al. 1991). Thus, the topography of the MRP for both the SP and RC movements was consistent with evidence that motor regions of the mesial frontal cortex including the SMA, dorsal premotor cortex and cingulate motor areas contribute to the early premovement potential (Ikeda et al. 1992; Jahanshahi et al. 1995; MacKinnon et al. 1996; Ball et al. 1999; Cui and Deecke 1999; Cui et al. 1999; Erdler et al. 2000; Jenkins et al. 2000). However, other cortical and subcortical regions also likely contribute to the MRP, such as the primary motor cortex, pre-SMA, dorsolateral prefrontal cortex, and basal ganglia (Jahanshahi et al. 2001; Jahanshahi and Hallett 2003; Rektor 2002). In keeping with this interpretation, positron emission tomography studies have shown that SP and RC movements are associated with the activation of a common network of regions including the SMA, dorsal premotor cortex, contralateral sensorimotor cortex and cingulate motor areas (Jahanshahi et al. 1995; Jenkins et al. 2000). Taken together, these findings have been interpreted to suggest that movements initiated under temporally predictable conditions, irrespective of whether the movements are self-paced or externally triggered, are mediated by a largely common network of motor cortical and subcortical regions (Nachev et al. 2008). Our results are consistent with this view in terms of the similarly of topography of the SP and RC potentials, yet there were distinct differences in the magnitude of the MRP depending upon the initiation mode (SP vs. RC) or whether movements were initiated in prediction of, or reaction to, the imperative “go” cue (RC1–3 vs. RC4).
Predictive versus reactive initiation of movement
Previous studies have shown that the early components of the MRP for both SP and RC movements were similar in timing and magnitude, suggesting equivalent activation of the mesial frontal cortex, whereas the magnitude of the later stages of preparation (NS’ or NS2 component) tended to be larger for SP movements (Jankelowitz and Colebatch 2002; Papa et al. 1991; Cunnington et al. 1995; Jahanshahi et al. 1995). However, in those studies, MRPs were generated from the averaging of all trials for a given task condition, irrespective of the timing of movement onset relative to the cue. Thus, the MRP may have been composed of a mixture of movements initiated in a self-paced manner that, more or less, corresponded to the timing of the cue, movements that were initiated in reaction to the cue, or movements that were timed to coincide with the cue.
The present study was designed to dissociate predictive from reactive movements on a trial-by-trial basis. This was done was imposing tight constraints on the temporal accuracy of movement onset during the RC task. This constraint resulted in a significant increase in early premovement activity relative to the SP task. The effects of task condition were greater over the midline frontal cortex, suggesting that the modulation of preparatory activity was most prevalent within mesial premotor regions such as the SMA. A comparable change in the MRP was also observed for movement initiated “too early” (RC1 trials). Thus, temporal accuracy was not required for the enhanced MRP. These findings are consistent with the idea that the imposition of constraints on the timing of movement onset resulted in a significant facilitation of cerebral processes mediating the preparation of temporally predictive movements.
Previous studies using the CNV paradigm have shown that an important element of the preparatory potential is the related to the subject’s estimate of the timing of the imperative cue (Walter et al. 1964; McAdam 1966; Macar et al. 1999; Pfeuty et al. 2005). As subjects learn the inter-stimulus interval, based on performance feedback, there is an augmentation of the CNV (McAdam 1966). When repetitive movements are paced by a fixed interval cue, the slope of the CNV is adjusted to ensure that the peak magnitude of the potential coincides with the predicted (implicit) timing of the imperative cue (Pfeuty et al. 2005; Praamstra et al. 2006). These findings are in accord with microelectrode recordings in monkeys that have shown that the timing of preparatory neural activity in primary and premotor regions of the frontal cortex is adjusted to the temporal regularity of cue presentation (Riehle et al. 1997; Heinen and Liu 1997; Lucchetti and Bon 2001). It has been proposed that these adjustments in the timing of neural activity, and corresponding changes in MRPs, reflect increasing (climbing) neural activity that encodes the passage of time between two behaviorally relevant, and temporally predictable, events (Durstewitz 2003; Reutimann et al. 2004; Pfeuty et al. 2005; Praamstra et al. 2006). Climbing activity, as well as synchronization of the firing patterns of a large ensemble of neurons might explain the enhancement of the MRP associated with the RC task.
When the RC task trials were sorted according to reaction time, MRPs associated with temporally accurate movements (RC2 and RC3) and movements initiated “too early (RC1) were significantly larger than the MRPs for movements initiated “too late” (RC4 trials). The RC1-3 trials were considered to reflect temporally predictive movements whereas the RC4 trials were classified as temporally reactive movements. However, it is unlikely that the MRP associated with the RC4 trials reflects a purely reactive mode of movement initiation since preparatory activity was comparable in magnitude and time course to the SP task and significantly increased relative to the IC task. Thus, the most parsimonious explanation for the reduction of the MRP for the RC4 trials is that the data set included subsets of both temporally predictive, but nonetheless “late” trials, and purely reactive trials. Designation of trials as predictive or reactive was based on the specification of a stationary minimum reaction time latency (1RT). The 1RT estimate was based on a sample of 50 movements for the IC task and the selection of the shortest reaction time bin with two or more trials. Due to the low number of trials used to define 1RT, it is possible that we underestimated the minimum reaction time (i.e. the true 1RT was longer). In that case, many trials may have been classified as reactive (RC4) when it fact the subject accurately predicted the timing of the tone (RC3). It has been shown that temporal estimates of movement onset that are “too long” are associated with an increased CNV relative to estimates that are “too short” or accurate (Macar et al. 1999). Thus, temporary predictive movements that were initiated “too late” would be expected to have MRP magnitudes comparable to, or greater than, the R1-3 trials and their incorporation into the RC4 average would have markedly increased the magnitude of the MRP. The absence of accuracy constraints on the timing of movement onset may also explain the findings of studies that showed no difference in the magnitude of MRPs preceding self-paced and regularly-cued movements (Cunnington et al. 1995; Jankelowitz and Colebatch 2002). Without accuracy constraints there is likely to be a greater proportion of reactive trials relative to predict trials. This would tend to decrease the magnitude of the ensemble averaged trials for the cued condition and create the impression that MRPs preceding regularly-cued movements were comparable to SP movements.
Role of attention
It is also possible that differences in attention contributed to differences in the MRPs between tasks and trial types. Subjects commented that the imposition of a tight temporal accuracy criterion made the task demanding and that successful completion of more than 50% of trials within the reaction time criteria required vigilant attention to the task. Accordingly, trials in which subjects reacted too late (RC4) to the tone may have simply reflected a momentary lapse of attention to the task. Increased attentional demands could be reflected in a generalized facilitation of preparatory movement-related neurons, particularly in the mesial frontal cortex and dorsolateral prefrontal cortex. This latter region has been implicated in “attention to action” (Passingham 1996) and cognitive timing (Lewis and Miall 2006) and contributes to MRPs (Jahanshahi et al. 2001). Increasing the attentional aspects of a movement task has been shown to increase in the magnitude of the MRP (Dirnberger et al. 2000), but this increase was small in comparison to the two-fold increase observed in the present study. Thus, increases in both preparatory motor and attentional aspects of the task may have contributed to the enhanced MRPs.
What is the function of early preparatory movement-related cortical activity?
The function of MRPs has been the subject of considerable investigation over the past four decades. A variety of studies have shown that the magnitude of MRPs that precede self-paced movements can be modulated by changes in force output, task complexity and for unilateral versus bilateral movements (Becker and Kristeva 1980; Lang et al. 1988, 1990; Kristeva et al. 1990; Simonetta et al. 1991; Kitamura et al. 1993; Cui et al. 2000b; Slobounov et al. 2002). Yet even the most rudimentary movements (e.g. index finger abduction) are preceded by MRPs. Thus, the above factors contribute to the scaling of the MRP, but are not prerequisites for the generation of the premovement potential. It has also been hypothesized that MRPs reflect activity associated with planning “when” to initiate a movement (Deecke et al. 1985). Our data would seem to be compatible with this idea. Yet, the fact that both simple and complex movements can be evoked by electrical stimulation of premotor regions, such as the SMA (Penfield and Welch 1951), suggests that its role is not simply as a timing keeper, and that it must play an important role in motor output. More likely, the slow-rising slope of the MRP reflects synaptic activity associated with the progressive preparation of the motor plan in anticipation of the intended timing of movement initiation. When the timing of voluntary or imposed movement can be predicted in advance, anticipatory adjustments in reflex excitability, postural adjustments and accompanying synergist and antagonist muscle activity can be timed to coincide with the movement. In this manner, stable postures and unexpected deviations from intended movement trajectories can be minimized. When the timing of an imposed movement is unpredictable, there is an unexpected deviation of the limb away from the desired posture or trajectory (Hugon et al. 1982). Since the premotor cortex and SMA send extensive descending projections to subcortical and segmental regions that mediate reflex and postural control (Keizer and Kuypers 1989; He et al. 1995), as well as projections to the primary motor cortex (Muakkassa and Strick 1979; Luppino et al. 1993), these regions are well suited to mediate the interaction between the intended movement and its postural requirements. This may explain why increased force output, movement complexity, or bimanual movements, each of which imposes demands on muscle activation and postural accompaniment, are associated with increased MRPs.
References
Allison T, McCarthy G, Wood CC, Jones SJ (1991) Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. Brain 114:2465–2503
Ball T, Schreiber A, Feige B, Wagner M, Lucking CH, Kristeva-Feige R (1999) The role of higher-order motor areas in voluntary movement as revealed by high-resolution EEG and fMRI. Neuroimage 10:682–694
Becker W, Kristeva R (1980) Cerebral potentials prior to various force deployments. Prog Brain Res 54:189–194
Cui RQ, Deecke L (1999) High resolution DC-EEG analysis of the Bereitschaftspotential and post movement onset potentials accompanying uni- or bilateral voluntary finger movements. Brain Topogr 11:233–249
Cui RQ, Huter D, Lang W, Deecke L (1999) Neuroimage of voluntary movement: topography of the Bereitschaftspotential, a 64-channel DC current source density study. Neuroimage 9:124–134
Cui RQ, Egkher A, Huter D, Lang W, Lindinger G, Deecke L (2000a) High resolution spatiotemporal analysis of the contingent negative variation in simple or complex motor tasks and a non-motor task. Clin Neurophysiol 111:1847–1859
Cui RQ, Huter D, Egkher A, Lang W, Lindinger G, Deecke L (2000b) High resolution DC-EEG mapping of the Bereitschaftspotential preceding simple or complex bimanual sequential finger movement. Exp Brain Res 134:49–57
Cunnington R, Iansek R, Bradshaw JL, Phillips JG (1995) Movement-related potentials in Parkinson’s disease. Brain 118:935–950
Deecke L, Kornhuber HH, Lang W, Lang M, Schreiber H (1985) Timing function of the frontal cortex in sequential motor and learning tasks. Human Neurobiol 4:143–154
Dieber M-P, Honda M, Ibanez V, Sadato N, Hallett M (1999) Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol 81:3065–3077
Dirnberger G, Reumann M, Endl W, Lindinger G, Lang W, Rothwell JC (2000) Dissociation of motor preparation from memory and attentional processes using movement-related cortical potentials. Exp Brain Res 135:231–240
Durstewitz D (2003) Self-organizing neural integrator predicts interval times through climbing activity. J Neurosci 23:5342–5353
Elbert T, Ulrich R, Rockstroh B, Lutzenberger W (1991) The processing of temporal intervals reflected in CNV-like brain potentials. Psychophysiology 28:648–655
Erdler M, Beisteiner R, Mayer D, Kaindl T, Edward V, Windischberger C, Lindinger G, Deecke L (2000) Supplementary motor area activation preceding voluntary movement is detectable with a whole-scalp magnetoencephalography system. Neuroimage 11:697–707
Goldberg G (1985) Supplementary motor area structure and function: review and hypotheses. Behav Brain Sci 8:567–615
Hamano T, Luders HO, Ikeda A, Collura TF, Comair YG, Shibasaki H (1997) The cortical generators of the contingent negative variation in humans: a study with subdural electrodes. Electroenceph clin Neurophysiol 104:257–268
He SQ, Dum RP, Strick PL (1995) Topographic organization of corticospinal projections from the frontal lobe: motor areas on the medial surface of the hemisphere. J Neurosci 15:306–3284
Heinen SJ, Liu M (1997) Single-neuron activity in the dorsomedial frontal cortex during smooth-pursuit eye movements to predictable target motion. Visual Neurosci 14:853–865
Hugon M, Massion J, Wiesendanger M (1982) Anticipatory postural changes induced by active unloading and comparison with passive unloading in man. Pflugers Archiv—Europe J Physiol 393:292–296
Ikeda A, Luders HO, Burgess RC, Shibasaki H (1992) Movement-related potentials recorded from supplementary motor area and primary motor area Role of supplementary motor area in voluntary movements. Brain 115:1017–1043
Jahanshahi M, Hallett H (2003) The Bereitschaftspotential, movement-related cortical potentials, In: Jahanshahi M and Hallett M (eds), Kluwer, New York
Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ (1995) Self-initiated versus externally triggered movements I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118:913–933
Jahanshahi M, Dirnberger G, Liasis A, Towell A, Boyd S (2001) Does the pre-frontal cortex contribute to movement-related potentials? Recordings from subdural electrodes. Neurocase 7:495–501
Jankelowitz SK, Colebatch JG (2002) Movement-related potentials associated with self-paced, cued and imagined arm movements. Exp Brain Res 147:98–107
Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ (2000) Self-initiated versus externally triggered movements II. The effect of movement predictability on regional cerebral blood flow. Brain 123:1216–1228
Keizer K, Kuypers HG (1989) Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis). Exp Brain Res 74:311–318
Kitamura J, Shibasaki H, Takagi A, Nabeshima H, Yamaguchi A (1993) Enhanced negative slope of cortical potentials before sequential as compared with simultaneous extensions of two fingers. Electroenceph Clin Neurophysiol 86:176–182
Klemmer ET (1956) Time uncertainty in simple reaction time. J Exp Psychol 51:179–184
Kornhuber HH, Deecke L (1965) Hirnpotentialänderungen bei Willkürbewegungen und passiven bewegungen des menschen: bereitschaftspotential und reafferente potential. Pflugers Arch 284:1–17
Kristeva R, Cheyne D, Lang W, Lindinger G, Deecke L (1990) Movement-related potentials accompanying unilateral and bilateral finger movements with different inertial loads. Electroenceph Clin Neurophysiol 75:410–418
Kutas M, Donchin E (1980) Preparation to respond as manifested by movement-related brain potentials. Brain Res 202:95–115
Lang W, Lang M, Uhl F, Koska C, Kornhuber A, Deecke L (1988) Negative cortical DC shifts preceding and accompanying simultaneous and sequential finger movements. Exp Brain Res 71:579–587
Lang W, Obrig H, Lindinger G, Cheyne D, Deecke L (1990) Supplementary motor area activation while tapping bimanually different rhythms in musicians. Exp Brain Res 79:504–514
Lewis PA, Miall RC (2006) A right hemispheric prefrontal system for cognitive time measurement. Behav Processes 71:226–234
Lucchetti C, Bon L (2001) Time-modulated neuronal activity in the premotor cortex of macaque monkeys. Exp Brain Res 141:254–260
Luppino G, Matelli M, Camarda R, Rizzolatti G (1993) Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol 338:114–140
Lutz K, Specht K, Shah NJ, Jancke L (2000) Tapping movements according to regular and irregular visual timing signals investigated with fMRI. Neuroreport 11:1301–1306
Macar F, Vidal F, Casini L (1999) The supplementary motor area in motor and sensory timing: evidence from slow brain potential changes. Exp Brain Res 125:271–280
Macar F, Lejeune H, Bonnet M, Ferrara A, Pouthas V, Vidal F, Maquet P (2002) Activation of the supplementary motor area and of attentional networks during temporal processing. Exp Brain Res 142:475–485
MacKinnon CD, Kapur S, Hussey D, Verrier MC, Houle S, Tatton WG (1996) Contributions of the mesial frontal cortex to the premovement potentials associated with intermittent hand movements in humans. Hum Brain Mapp 4:1–22
McAdam DW (1966) Slow potential changes recorded from humanbrain during learning of a temporal interval. Psychonom Sci 6:435–436
Muakkassa KF, Strick PL (1979) Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized ‘premotor’ areas. Brain Res 177:176–182
Nachev P, Kennard C, Husain M (2008) Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9:856–869
Niemi P, Näätänen R (1981) Foreperiod and simple reaction time. Psychol Bull 89:133–162
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Papa SM, Artieda J, Obeso JA (1991) Cortical activity preceding self-initiated and externally triggered voluntary movement. Mov Disord 6:217–224
Passingham RE (1987) Two cortical systems for directing movement. Ciba Found Symp 132:151–164
Passingham RE (1996) Attention to action. Philos Trans R Soc Lond Biol 351:1473–1479
Penfield W, Welch K (1951) The supplementary motor area of the cerebral cortex: a clinical and experimental study. AMA Arch Neurol Psychiat 66:289–317
Pfeuty M, Ragot R, Pouthas V (2005) Relationship between CNV and timing of an upcoming event. Neurosci Lett 382:106–111
Praamstra P, Kourtis D, Kwok HF, Oostenveld R (2006) Neurophysiology of implicit timing in serial choice reaction-time performance. J Neurosci 26:5448–5455
Rektor I (2002) Scalp-recorded Bereitschaftspotential is the result of the activity of cortical and subcortical generators—a hypothesis. Clin Neurophysiol 113:1998–2005
Reutimann J, Yakovlev V, Fusi S, Senn W (2004) Climbing neuronal activity as an event-based cortical representation of time. J Neurosci 24:3295–3303
Riehle A, Grun S, Diesmann M, Aertsen A (1997) Spike synchronization and rate modulation differentially involved in motor cortical function. Science 278:1950–1953
Rohrbaugh JW, Syndulko K, Lindsley DB (1976) Brain wave components of the contingent negative variation in humans. Science 191:1055–1057
Ruchkin DS, McCalley MG, Glaser EM (1977) Event related potentials and time estimation. Psychophysiology 14:451–455
Salmaso D, Longoni AM (1985) Problems in the assessment of hand preference. Cortex 21:49–533
Simonetta M, Clanet M, Rascol O (1991) Bereitschaftspotential in a simple movement or in a motor sequence starting with the same simple movement. Electroenceph clin Neurophysiol 81:129–134
Slobounov S, Johnston J, Chiang H, Ray W (2002) Movement-related EEG potentials are force or end-effector dependent: evidence from a multi-finger experiment. Clin Neurophysiol 113:1125–1135
Thickbroom GW, Mastaglia FL (1985) Cerebral potentials preceding self-paced and visually triggered saccades—a study of presaccadic potentials. Electroenceph clin Neurophysiol 62:277–289
Thickbroom GW, Byrnes ML, Sacco P, Ghosh S, Morris IT, Mastaglia FL (2000) The role of the supplementary motor area in externally timed movement: the influence of predictability of movement timing. Brain Res 874:233–241
Toma K, Ozawa M, Matsuo K, Nakai T, Fukuyama H, Sato S (2003) The role of the human supplementary motor area in reactive motor operation. Neurosci Lett 344:177–180
Trillenberg P, Verleger R, Wascher E, Wauschkuhn B, Wessel K (2000) CNV and temporal uncertainty with ‘ageing’ and ‘non-ageing’ S1–S2 intervals. Clin Neurophysiol 111:1216–1226
Walter W, Cooper R, Aldridge V, McCallum W, Winter A (1964) Contingent negative variation: an electric sign of sensorimotor association and expectancy in the human brain. Nature 203:380–384
Acknowledgments
We thank Dr. Jun Yao for assistance with the data analysis and review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, R., MacKinnon, C.D. The effect of temporal accuracy constraints on movement-related potentials. Exp Brain Res 194, 477–488 (2009). https://doi.org/10.1007/s00221-009-1725-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-009-1725-5