Abstract
The use of arm weight support (WS) to optimize movement quality may be an avenue for improved upper limb stroke rehabilitation; however, the underlying neurophysiological effects of WS are not well understood. Rehabilitation exercises may be performed when sitting or standing, but the interaction of posture with WS has not been examined until now. We explored the effect of posture with WS on corticomotor excitability (CME) in healthy adults. Thirteen participants performed static shoulder abduction in two postures (sitting and standing) at three levels of WS (0, 45, and 90 % of full support). Transcranial magnetic stimulation of primary motor cortex was used to elicit motor-evoked potentials (MEPs) in eight upper limb muscles. Stimulus–response (SR) curves were fitted to the MEP data using nonlinear regression. Whole-body posture interacted with WS to influence tonic activity and CME in all muscles examined. SR curve parameters revealed greater CME when standing compared to sitting for upper arm muscles, but lower CME to the shoulder, forearm, and hand. Distal to the shoulder, tonic activity and CME were modulated independent of any explicit differences in task requirements. Overall, these results support a model of integrated upper limb control influenced by whole-body posture and WS. These findings have implications for the application of WS in settings such as upper limb rehabilitation after stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a leading cause of adult disability with two-thirds of stroke survivors experiencing lingering upper limb impairment (Feigin et al. 2010; Mendis 2013). Weight support (WS) can be used to augment arm movements made during stroke rehabilitation therapy (Prange et al. 2006; Brewer et al. 2007; Kwakkel et al. 2008; Mehrholz et al. 2015) and may be applied manually, or through devices ranging from passive supports to sophisticated robotic systems (Loureiro et al. 2011). The benefits of WS for upper limb rehabilitation have been ascribed to increasing the intensity or volume of therapeutic exercises (Kwakkel and Meskers 2014). Beyond its role in facilitating increased training dosage, WS can also improve movement quality. For example, in reaching tasks the application of WS results in a reduction of antagonist muscle activity in both healthy older adults and chronic stroke patients (Prange et al. 2009a, b). WS can also lessen abnormal coupling of joint torques between the shoulder and elbow through a reduction in antigravity torques required for shoulder abduction (Dewald and Beer 2001; Beer et al. 2004). As a functional consequence, individuals who express the stereotyped flexor synergy can achieve greater elbow extension under gravity-compensated conditions, thereby increasing access to the reaching workspace (Sukal et al. 2007). To date, the neural mechanisms underlying transient changes in motor behavior with WS have received less attention and are not well understood.
Change in whole-body posture can also affect motor control of the upper limb, but its interaction with WS has not been investigated. Rehabilitation exercises may be performed when sitting or standing; thus, an interaction of posture with WS on upper limb control may inform its clinical application. Standing postures introduce balance requirements that alter the way arm movements are coordinated and increase the complexity of reaching and pointing tasks (Pozzo et al. 2001, 2002; Berrigan et al. 2006). Centrally mediated changes in the accessibility of muscles for activation may be assessed using transcranial magnetic stimulation (TMS). Compared to sitting, standing results in greater corticomotor excitability (CME) to the anterior deltoid, but no change in CME to the first dorsal interosseous (Kantak et al. 2013). Posture-related modulation of shoulder, but not hand, CME likely reflects a greater mechanical role played by proximal muscles in shifting the center of mass, e.g., to maintain stability in response to a perturbation. Neuromuscular activity is modulated across the limb with WS and may involve both excitatory and inhibitory mechanisms (Devanne et al. 2002; Runnalls et al. 2014, 2015). Whether the neural mechanisms underpinning posture-related changes in upper limb control interact with the neural linkages modulated by WS is unknown.
In the present study, we sought to examine the interaction of whole-body posture and WS on CME to upper limb muscles. TMS was used to elicit motor-evoked potentials (MEPs) from muscles in the shoulder, arm, forearm, and hand of healthy adults. We expected that tonic muscle activity would modulate with both WS and posture manipulations. It was hypothesized that tonic activity would be reduced with greater WS, and greater during standing compared to sitting. CME was examined by analyzing MEP area and comparing stimulus–response (SR) curves fitted to group means. It was hypothesized that SR curves would reflect greater CME with a standing posture, evident by steeper slope and associated parameters. Furthermore, we expected the magnitude of posture-related differences would be greater with WS.
Methods
Participants
Thirteen neurologically healthy right-handed adults without history of upper limb impairment participated in this study (mean age 28 years, range 20–50 years, 3 females). All participants gave written informed consent and were screened for contraindications to TMS by a neurologist. Study procedures were approved by the University of Auckland Human Participants Research Ethics Committee in accordance with the Declaration of Helsinki.
Design
All procedures were completed in a single-session using a repeated-measures design. Single-pulse TMS was used to elicit MEPs from muscles of the arm during 2 postures (sitting and standing) at 3 levels of WS (low, medium, and high). The order of the 6 experimental conditions was randomized between participants. Within each experimental condition, a range of TMS intensities was randomized on a trial-by-trial basis. Each session lasted approximately 3 h.
Posture and arm support
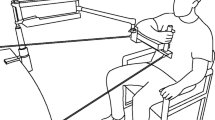
Figure 1 illustrates the sitting and standing experimental conditions. The right arm was supported by a SaeboMAS arm support system (Saebo Inc., Charlotte, NC, USA). Force was provided and adjusted via spring tension. A custom brace provided a rigid and cushioned surface for the forearm and hand. Elasticized fabric wrap was used to secure the forearm to the brace in a palm-down position. For both sitting and standing conditions, TMS was performed in a standardized arm position with the shoulder flexed forward approximately 80° and abducted 45° in the horizontal plane, and the elbow flexed at 90°. Joint angles were initially set using a goniometer and subsequently maintained by aligning a laser pointer to a reference point on the wall. The brace prevented rotation in the vertical plane ensuring the forearm was parallel to the floor. In the sitting condition, participants sat in a chair with their feet on the floor and left arm resting on their lap. In the standing condition, participants stood with their feet shoulder width apart and left arm resting at their side.
Three discrete levels of WS were defined relative to the force required to fully compensate for the weight of the arm. At low support (0 %), the device carried its own weight, but provided no additional support to the arm. The force required for full support (100 %) was determined using a force titration procedure. While maintaining the standardized arm position, supportive force was incrementally decreased from a superfluous setting requiring shoulder adduction. Full support (100 %) was defined as the last point before root-mean-square electromyogram amplitude (rmsEMG) in the anterior deltoid was observed to deflect away from the baseline activity that persists even with excessive support (Runnalls et al. 2014, 2015). Medium and high support levels were then defined as 45 and 90 % of full support.
Electromyography
Surface electromyography (EMG) was used to record activity from eight muscles of the right arm and hand: anterior deltoid (AD), biceps brachii (BB), triceps brachii (TB), brachioradialis (BRD), extensor carpi radialis (ECR), flexor carpi radialis (FCR), first dorsal interosseous (FDI), and abductor pollicis brevis (APB). Following standard skin preparation, self-adhesive Ag–AgCl electrodes (Blue Sensor N; Ambu, Denmark) were placed approximately 2 cm apart in a bipolar montage over the belly of each muscle. The common ground electrode was placed over the acromion process (Red Dot; 3 M Health Care, Canada). Signals were amplified (AMT-8; Bortec Biomedical, Calgary, Canada) with 1000× gain, band-pass filtered (10–1000 Hz), sampled at 2 kHz (CED Power 1401 mkII; Cambridge Electronic Design, Cambridge, UK), and saved for subsequent offline analysis using CED Signal software (v6.03c).
Transcranial magnetic stimulation
Single-pulse TMS was applied over the left motor cortex using a MagPro X100 magnetic stimulator and MC-B70 butterfly coil (MagVenture, Denmark). The coil was held tangentially to the scalp and angled approximately 45° away from midline. A monophasic pulse was used to induce a posterior-to-anterior current flow in M1. The coil was positioned at the optimal site for eliciting MEPs in the right ECR muscle. A single experimenter conducted all the tests. Task motor threshold (MT) for the right ECR was defined as the minimum stimulus intensity that elicited a 50-µV MEP in four out of eight trials while seated with the arm in the standardized position at the high support level.
For each of the six experimental conditions, stimulus–response (SR) curves were collected using a single stimulation site to concurrently elicit MEPs in all muscles. Eleven stimulus intensities were set relative to task motor threshold of ECR: −10, −5, 0, +5, +10, +15, +20, +25, +30, +35, +40 % of maximum stimulator output (% MSO). Stimulation was based on the site and threshold for ECR because it was somatotopically central and most consistently captured the stimulus–response range for the set of examined muscles. For each curve, 88 stimuli were delivered in a randomized order (8 stimuli for each of the 11 intensities). To mitigate fatigue, participants rested their arm on a table for approximately 15 s after every six stimuli.
Data analysis
Individual EMG traces were inspected for the presence of an appropriate stimulus artifact and absence of phasic muscle activity. Trials that did not meet these criteria were discarded from further analysis. Measures were taken from individual raw EMG traces. The main dependent measure, MEP area, was calculated over a 20-ms window determined manually for each muscle for each participant. To account for systematic differences in MEP size between participants, raw MEP area values were normalized between 0 and 1 across conditions within each muscle. As a covariate, background muscle activity was measured as the rmsEMG amplitude over a 50-ms window preceding the stimulus.
Statistical analysis
Analyses of background muscle activity and MEP area were performed using R 3.1.2 (R Core Team 2014) with the nlme: linear and nonlinear mixed effects models (Pinheiro et al. 2015) and predictmeans: Calculate predicted means for linear models packages (Luo et al. 2014). Outlying data points were identified by analyzing background muscle activity on a within-subject basis. Observations of rmsEMG more than 1.5× the interquartile range either above the third quartile or below the first quartile, along with their associated MEP values, were excluded from further analysis. Data were log-transformed to better satisfy the assumption of normally distributed residuals.
To assess the interaction of weight support and posture on background muscle activity across the upper limb, separate linear mixed effects analyses were performed for each muscle. In each case, background muscle activity was modeled as a function of support level and posture as factors, with random intercepts for subject. The sequential sum of squares was used for Wald tests of model terms (Pinheiro and Bates 2000). As a measure of effect size, log response ratios were calculated for differences between marginal means (Hedges et al. 1999). For support level, the response ratio was expressed as the natural logarithm of high support relative to low with negative values indicating less muscle activity with high support. For posture, the response ratio was expressed as the natural logarithm of standing relative to sitting; negative values indicate less muscle activity when standing.
For MEP area, separate linear mixed effects models were constructed for each muscle. In each case, mep area was modeled as a function of stimulus intensity, support level, and posture as factors. background muscle activity was included as a continuous covariate term. The error term included random slopes for background muscle activity and random intercepts for subject. Each model was subsequently used to infer predicted means and standard errors for mep area at the median value of the background muscle activity distribution (Welham et al. 2004). This procedure permitted comparisons of MEP area between experimental conditions by accounting for underlying differences in background muscle activity.
Stimulus–response curves were fitted to group-level data for each muscle using nonlinear regression in Prism 7 (GraphPad, San Diego, CA, USA). For each experimental condition, a three-parameter Boltzmann function was fitted to both observed and predicted mean MEP areas (Devanne et al. 1997). To improve the rate at which nonlinear regression converged on a fit, the upper plateau was constrained to its theoretical range of normalized MEP area between 0 and 1. Similarly, the half-maximal stimulus intensity (S50) was constrained to be between 0 and 40 % MSO above task motor threshold. The slope was unconstrained. Omnibus extra sum-of-squares F tests were used to assess whether individual regression curves for each condition fit the data significantly better than a single curve for the muscle across conditions. To examine whether the posture manipulation shifted the SR curve within each support level, log response ratios were calculated as the natural logarithm of the standing value divided by the sitting value for the S50 and slope parameters that defined each curve. For each muscle, the best-fit parameters were analyzed separately using one-way ANOVA. Planned tests were then conducted on the difference between postures within each support level. Multiple comparisons were corrected by controlling the false discovery rate (Q = 0.05) with a two-stage step-up method (Benjamini et al. 2006).
Results
Data from all 13 participants were included in the analysis. Of the 88 stimuli delivered to each participant per condition, an average of 79 traces (range 64–86) were retained in the final analysis. Trials containing outlying values of background muscle activity were discarded. Example EMG traces are presented in Fig. 2.
a Single EMG traces recorded from a representative participant. Traces from all muscles were recorded simultaneously during the seated high support condition. Intensity of the stimulus was 35 %MSO above task motor threshold. b Average EMG traces for TB and ECR as representative muscles from the same participant and stimulus intensity. Color shade corresponds to WS level (color figure online)
Effects of weight support and posture on background muscle activity
Group means for background muscle activity are presented in Fig. 3. There were significant main effects of both the support level and posture factors, as well as a concomitant interaction between support level and posture in all muscles (Table 1). As expected, the magnitude of the support-level effect was greatest for proximal muscles AD, BB, and TB. The direction of the effect was uniform across muscles, with less background muscle activity at high support. For the effect of posture on background muscle activity, the magnitude and direction of the response were not consistent across all muscles.
Background muscle activity responds to changes in support level and posture. Group averages for background muscle activity are plotted at low (0 %), medium (45 %), and high (90 %) levels of weight support. Solid lines represent data from the seated condition, while dashed lines are used for the standing condition. Error bars represent ±1 SEM
Effects of weight support and posture on stimulus–response curves
The left column of Fig. 4 presents SR curves fitted to group means of observed MEP area. Significant effects of the experimental manipulations on background muscle activity warranted further analysis of MEP data using values derived from the statistical models. Mean MEP area and standard error were predicted for each combination of support level, posture, and stimulus intensity. The procedure accounted for covarying background muscle activity. SR curves fitted to the predicted means are presented in the right column of Fig. 4. For all muscles, extra sum-of-squares F tests indicated that SR curves for each condition fit the data significantly better than a single regression curve (AD: F (15,46) = 30.72, p < 0.0001; BB: F (15,46) = 207.6, p < 0.0001; TB: F (15,43) = 108.0, p < 0.0001; BRD: F (15,48) = 18.04, p < 0.0001; ECR: F (15,48) = 14.46, p < 0.0001; FCR: F (15,45) = 4.64, p < 0.0001; FDI: F (15,47) = 9.02, p < 0.0001; APB: F (15,42) = 2.95, p = 0.0029).
SR curves shift in response to changes in support level and posture. On the left, SR curves are fitted to group means of observed MEP area. On the right, SR curves are fitted to means predicted using the linear mixed effects model for the median level of background muscle activity (color figure online)
Shifts in SR curves were examined by testing for differences in the in the S50 and slope parameters that defined each curve. Omnibus results of the one-way ANOVAs for curve parameters are presented in Table 2 and represented by asterisks next to muscle labels in Figs. 5 and 6. Response ratios for posture-related change in the S50 and slope parameters are presented as bars in Figs. 5 and 6, respectively. For S50, the average magnitude of change across muscles was greatest at high support (5.8 % MSO) followed by low (3.9 % MSO) and medium support (2.0 % MSO). Similarly for slope, the average magnitude of change was also greatest at high support (0.022 % MSO−1) followed by low (0.014 % MSO−1) and medium support (0.006 % MSO−1). Specific tests for posture-related differences in the S50 and slope parameters within support levels are represented by asterisks next to individual bars in Figs. 5 and 6.
Shift in S50 parameter plotted as log response ratios between postures. Positive response ratios reflect larger S50 values for standing relative to sitting. Bar shading represents support level. Vertical lines indicate the mean across support levels for each muscle. Statistical significance is indicated next to muscle labels for omnibus tests and next to the respective bar for planned comparisons (*p < 0.05, **p < 0.01, ***p < 0.001) (color figure online)
Shift in slope parameter plotted as log response ratios between postures. Negative response ratios reflect smaller (less steep) slopes for standing relative to sitting. Bar shading represents support level. Vertical lines indicate the mean across support levels for each muscle. Statistical significance is indicated next to muscle labels for omnibus tests and next to the respective bar for planned comparisons (*p < 0.05, **p < 0.01) (color figure online)
Discussion
In this study, we examined the interaction of change in whole-body posture and systematic variation of arm weight support (WS) on corticomotor excitability (CME) to upper limb muscles. In support of our hypothesis, there was an interaction of whole-body posture and WS on CME for all muscles examined. In line with previous findings, tonic activity of muscles across the upper limb was less when WS was high, compared to when WS was medium or absent (low). Tonic muscle activity was also affected by posture. However, the hypothesis that activity would be greater when standing was found for only a subset of muscles (AD, BB, APB). As expected, CME modulated with WS and posture manipulation. Consistent with our hypothesis, analyses of CME measures indicated a trend for smaller half-maximal stimulus intensity (S50) and larger slope parameters to accompany standing for BB and TB. In contrast, muscles in the shoulder, forearm, and hand exhibited the opposite pattern reflecting lower CME when standing. We also expected that the magnitude of posture-related differences would be largest with greatest levels of WS, but support for this hypothesis was equivocal. While the S50 and slope parameters both exhibited the largest average difference at high support, the smallest magnitude difference occurred with medium rather than low support. Apart from the direct effect of WS on AD activity, the observed modulation of tonic activity and CME across upper limb muscles occurred independent of any differences in explicit task requirements.
Interactions of weight support and posture on tonic muscle activity and CME
The change in tonic muscle activity in response to changes in WS provides evidence for a common neural drive to muscles of the upper limb. Tonic muscle activity diminished with greater WS as indicated by values of background EMG (Table 1; Fig. 3). The largest magnitude of EMG activity and the greatest difference between high and low WS were exhibited by AD. This finding reflects the role of AD as the principal muscle generating antigravity torque and confirms the efficacy of the WS manipulation. The finding is consistent with the results reported by previous studies employing multiple levels of WS (Coscia et al. 2014; Runnalls et al. 2014, 2015). Changes in WS did not alter the task requirements for forearm and hand muscles because the forearm was fully supported and secured to the brace. Task requirements did not vary for BB and TB because they were not oriented to act against gravity. Observed differences in tonic activity were involuntary and remote to the primary action of WS at the shoulder. This tendency for WS to influence tonic muscle activity is indicative of a common neural drive across the upper limb.
Dissociation between muscles for the response to sitting versus standing suggests the influence of whole-body posture on tonic activity of upper limb muscles is mediated by distinct mechanisms in addition to common neural drive. As evidenced by response ratios (Table 1), AD was the most sensitive muscle to WS, but the least sensitive to posture. The relatively small response of tonic muscle activity to change in posture may reflect a strong independent voluntary descending drive to maintain shoulder abduction. In more distal muscles that receive mostly involuntary input, larger relative responses may indicate that the signals conveying postural information interact with neural linking mechanisms responsible for distributing common drive. The factors determining whether a muscle will express greater tonic activity in sitting or standing are not clear from the present results. A reciprocal relation between agonist and antagonist is supported for BB and TB. It is possible that mechanical restriction from the WS brace contributed to the absence of similar reciprocity in the forearm. Future studies may be warranted to investigate the impact of WS on peripheral nerve conduction. A non-global influence on tonic activity across the upper limb refutes the hypothesis that standing would result in a general increase of muscle activity and suggests postural information can modulate neural drive on a muscle-specific basis.
Posture exerted an influence on upper limb CME over and above those changes evident in tonic muscle activity alone. This was borne out in statistical analyses of MEP area which accounted for differences in background EMG. A similar postural manipulation without WS was previously reported to elicit increased CME to the proximal AD, but not the distal FDI (Kantak et al. 2013). The present findings indicate that whole-body posture can affect CME to the arm, forearm, and hand, as evidenced by SR curve parameters (Table 2; Figs. 5, 6). The discrepancy between the earlier reports and present findings could be attributed to the arm postures examined. Kantak et al. tested the arm in a resting state hanging at the side, whereas the present study examined a task-relevant arm posture that elicited involuntary tonic activity. The additional neural elements engaged by the reaching-related arm posture could provide a substrate for interaction with whole-body postural information. Consistent with previous findings, an up-regulation of CME with less WS (Fig. 4) appears to subserve both voluntary activity in AD and involuntary activity in more distal upper limb muscles. Whole-body posture also influences CME; however, the factors determining whether changing posture has a facilitatory or inhibitory effect for a specific muscle are not clear.
Postural demands and mechanisms for integrated upper limb control
Integrated control of neuromuscular activity may facilitate the coordination of voluntary actions like forward reaching and involuntary actions for postural stabilization. In the present study, changes in tonic activity and CME provide evidence for integrated control along the proximal–distal axis. Unlike previous reports of distal responses to shoulder activation (Devanne et al. 2002; Runnalls et al. 2014, 2015) and shoulder position (Dominici et al. 2005; Ginanneschi et al. 2005, 2006), the present findings do not exhibit a clear anatomical or task-related pattern. Differential modulation of CME to upper limb muscles could reflect non-universal membership within specific neural linkages or synergies, or it may be an expression of multiple synergies with complex or competing interactions. Further studies are warranted to distinguish between these possibilities.
Modulation of CME with whole-body posture could reflect the priming of a response that satisfies potential mechanical demands imposed by the specific task. Standing postures have greater stability requirements than sitting and require larger displacements of the arm for compensatory reactions to perturbations (Allum et al. 2002; Roos et al. 2008). Standing also increases the complexity of arm dynamics for goal-directed movements like reaching (Berrigan et al. 2006). One or more posture-sensitive upper limb synergies may act to prepare the arm for its altered biomechanical role. For example, standing may necessitate a general increase of CME to muscles that have a significant influence on the center of mass. Putative posture-sensitive neural linkages may interact with those that respond to descending drive to the shoulder and are thus sensitive to WS.
It is worth considering the neural mechanisms that may mediate the proximal–distal neural linkages and shape motor output. In primary motor cortex, anatomical colocation of muscle representations may facilitate functional interaction. Multiple non-contiguous representations overlap with those of other muscles in animals (Donoghue et al. 1992; Schneider et al. 2001; Rathelot and Strick 2006) and humans (Sanes et al. 1995; Devanne et al. 2006). Furthermore, representations of distal forelimb muscles are systematically surrounded by those of proximal muscles (Park et al. 2001). Functionally, intracortical disinhibition has been implicated as a mechanism contributing to modulation of CME with shoulder activation and whole-body posture (Devanne et al. 2002; Kantak et al. 2013). Passive shoulder position influences distal CME through intracortical facilitation (Ginanneschi et al. 2005, 2006). Subcortical and spinal mechanisms may also play a role. Anatomically, divergence of descending corticomotor pathways can provide correlated input to multiple motor neuron pools (McKiernan et al. 1998). Propriospinal neurons link multiple spinal segments and can modulate descending drive to the forearm (Pauvert et al. 1998; Pierrot-Deseilligny 2002). Additionally, spinal interneuron circuits are a substrate for stable muscle synergies (Bizzi and Cheung 2013). Functionally, differences in limb position can impact motor neuron excitability through multiple proprioceptive inputs (Mogk et al. 2014; Nuzzo et al. 2016). Intrinsic electrical properties of spinal motor neurons vary with Ia afferent input (Hyngstrom et al. 2007) and shape motor output through nonlinear integration of descending synaptic and neuromodulatory inputs (Binder et al. 1993; Heckman et al. 2008). For example, the intrinsic excitability of human triceps brachii motor neurons is greater than that of biceps brachii, potentially indicating an enhanced role of persistent inward currents for postural or anatomical antigravity muscles (Wilson et al. 2015). In summary, there are many neural elements and mechanisms that may act to link neuromuscular activity to control movement and posture of the upper limb. It is likely that multiple mechanisms are sensitive to posture and WS, thus contributing to the complex pattern of CME modulation observed in this study.
Potential limitations
A limitation of the present study is the absence of a dynamic movement task. Although participants were required to accurately maintain their static posture, there was no dynamic component to challenge stability or introduce a goal-directed movement intention. It is unclear whether additional dynamic constraints would have biased CME in a more consistent pattern. The present study was conducted with healthy adults who may easily adapt reaching behavior across levels of WS (Coscia et al. 2014). Future studies may be warranted to investigate the interaction of posture and WS in the elderly and in those with motor impairment such as after stroke. It is possible a sensorimotor system with reduced capacity would be less adaptable at a neural level to posture and WS manipulation.
Conclusions
A novel combination of WS and posture manipulations led to changes in tonic muscle activity across the upper limb and some modulation of CME to muscles in the arm, forearm, and hand. Tonic activity and CME are not uniformly greater in standing compared to sitting. Whole-body posture may increase or decrease CME depending on the muscle and level of WS. The results support a model of integrated upper limb control and suggest posture-sensitive neural linkages may be distinct from those responsible for modulation with WS. These findings may have relevance for upper limb rehabilitation, e.g., after stroke. With further characterization, the combination of WS and posture manipulation may create avenues to uniquely balance CME for optimal engagement in rehabilitation exercises.
References
Allum JHJ, Carpenter MG, Honegger F et al (2002) Age-dependent variations in the directional sensitivity of balance corrections and compensatory arm movements in man. J Physiol (Lond) 542:643–663. doi:10.1113/jphysiol.2001.015644
Beer RF, Dewald JPA, Dawson ML, Rymer WZ (2004) Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res 156:458–470. doi:10.1007/s00221-003-1807-8
Benjamini Y, Krieger AM, Yekutieli D (2006) Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93:491–507. doi:10.1093/biomet/93.3.491
Berrigan F, Simoneau M, Martin O, Teasdale N (2006) Coordination between posture and movement: interaction between postural and accuracy constraints. Exp Brain Res 170:255–264. doi:10.1007/s00221-005-0210-z
Binder MD, Heckman CJ, Powers RK (1993) How different afferent inputs control motoneuron discharge and the output of the motoneuron pool. Curr Opin Neurobiol 3:1028–1034
Bizzi E, Cheung VCK (2013) The neural origin of muscle synergies. Front Comput Neurosci 7:1–6. doi:10.3389/fncom.2013.00051
Brewer BR, McDowell SK, Worthen-Chaudhari LC (2007) Poststroke upper extremity rehabilitation: a review of robotic systems and clinical results. Top Stroke Rehabil 14:22–44. doi:10.1310/tsr1406-22
Coscia M, Cheung VCK, Tropea P et al (2014) The effect of arm weight support on upper limb muscle synergies during reaching movements. J Neuroeng Rehabil 11:1–15. doi:10.1186/1743-0003-11-22
Devanne H, Lavoie BA, Capaday C (1997) Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114:329–338. doi:10.1007/PL00005641
Devanne H, Cohen LG, Kouchtir-Devanne N, Capaday C (2002) Integrated motor cortical control of task-related muscles during pointing in humans. J Neurophysiol 87:3006–3017
Devanne H, Cassim F, Ethier C et al (2006) The comparable size and overlapping nature of upper limb distal and proximal muscle representations in the human motor cortex. Eur J Neurosci 23:2467–2476. doi:10.1111/j.1460-9568.2006.04760.x
Dewald JPA, Beer RF (2001) Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve 24:273–283
Dominici F, Popa T, Ginanneschi F et al (2005) Cortico-motoneuronal output to intrinsic hand muscles is differentially influenced by static changes in shoulder positions. Exp Brain Res 164:500–504. doi:10.1007/s00221-005-2270-5
Donoghue JP, Leibovic S, Sanes JN (1992) Organization of the forelimb area in squirrel monkey motor cortex: representation of digit, wrist, and elbow muscles. Exp Brain Res 89:1–19
Feigin VL, Barker-Collo S, Parag V et al (2010) Auckland stroke outcomes study. Part 1: gender, stroke types, ethnicity, and functional outcomes 5 years poststroke. Neurology 75:1597–1607. doi:10.1212/WNL.0b013e3181fb44b3
Ginanneschi F, Santo F, Dominici F et al (2005) Changes in corticomotor excitability of hand muscles in relation to static shoulder positions. Exp Brain Res 161:374–382. doi:10.1007/s00221-004-2084-x
Ginanneschi F, Dominici F, Biasella A et al (2006) Changes in corticomotor excitability of forearm muscles in relation to static shoulder positions. Brain Res 1073–1074:332–338. doi:10.1016/j.brainres.2005.12.021
Heckman CJ, Johnson M, Mottram C, Schuster J (2008) Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 14:264–275. doi:10.1177/1073858408314986
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156
Hyngstrom AS, Johnson MD, Miller JF, Heckman CJ (2007) Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nat Neurosci 10:363–369. doi:10.1038/nn1852
Kantak SS, Wittenberg GF, Liao W-W et al (2013) Posture-related modulations in motor cortical excitability of the proximal and distal arm muscles. Neurosci Lett 533:65–70. doi:10.1016/j.neulet.2012.10.048
Kwakkel G, Meskers CGM (2014) Effects of robotic therapy of the arm after stroke. Lancet Neurol 13:132–133. doi:10.1016/S1474-4422(13)70285-0
Kwakkel G, Kollen BJ, Krebs HI (2008) Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. NNR 22:111–121. doi:10.1177/1545968307305457
Loureiro RCV, Harwin WS, Nagai K, Johnson M (2011) Advances in upper limb stroke rehabilitation: a technology push. Med Biol Eng Comput 49:1103–1118. doi:10.1007/s11517-011-0797-0
Luo D, Ganesh S, Koolaard J (2014) Predictmeans: calculate predicted means for linear models. R package version 0.99. http://CRAN.R-project.org/package=predictmeans. Accessed 1 Mar 2016
McKiernan BJ, Marcario JK, Karrer JH, Cheney PD (1998) Corticomotoneuronal postspike effects in shoulder, elbow, wrist, digit, and intrinsic hand muscles during a reach and prehension task. J Neurophysiol 80:1961–1980
Mehrholz J, Pohl M, Platz T et al (2015) Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev. doi:10.1002/14651858.CD006876.pub4
Mendis S (2013) Stroke disability and rehabilitation of stroke: World Health Organization perspective. Int J Stroke 8:3–4. doi:10.1111/j.1747-4949.2012.00969.x
Mogk JPM, Rogers LM, Murray WM et al (2014) Corticomotor excitability of arm muscles modulates according to static position and orientation of the upper limb. Clin Neurophysiol 125:2046–2054. doi:10.1016/j.clinph.2014.02.007
Nuzzo JL, Trajano GS, Barry BK et al (2016) Arm posture-dependent changes in corticospinal excitability are largely spinal in origin. J Neurophysiol 115:2076–2082. doi:10.1152/jn.00885.2015
Park MC, Belhaj-Saif A, Gordon M, Cheney PD (2001) Consistent features in the forelimb representation of primary motor cortex in rhesus macaques. J Neurosci 21:2784–2792
Pauvert V, Pierrot-Deseilligny E, Rothwell JC (1998) Role of spinal premotoneurones in mediating corticospinal input to forearm motoneurones in man. J Physiol (Lond) 508(Pt 1):301–312
Pierrot-Deseilligny E (2002) Propriospinal transmission of part of the corticospinal excitation in humans. Muscle Nerve 26:155–172. doi:10.1002/mus.1240
Pinheiro J, Bates D (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Pinheiro J, Bates D, R Core Team (2015) Nlme: linear and nonlinear mixed effects models. R package version 3.1-122. http://CRAN.R-project.org/package=nlme. Accessed 1 Mar 2016
Pozzo T, Ouamer M, Gentil C (2001) Simulating mechanical consequences of voluntary movement upon whole-body equilibrium: the arm-raising paradigm revisited. Biol Cybern 85:39–49. doi:10.1007/PL00007995
Pozzo T, Stapley PJ, Papaxanthis C (2002) Coordination between equilibrium and hand trajectories during whole body pointing movements. Exp Brain Res 144:343–350. doi:10.1007/s00221-002-1052-6
Prange GB, Jannink MJA, Groothuis-Oudshoorn CGM et al (2006) Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J Rehabil Res Dev 43:171–184
Prange GB, Jannink MJA, Stienen AHA et al (2009a) Influence of gravity compensation on muscle activation patterns during different temporal phases of arm movements of stroke patients. NNR 23:478–485. doi:10.1177/1545968308328720
Prange GB, Kallenberg LAC, Jannink MJA et al (2009b) Influence of gravity compensation on muscle activity during reach and retrieval in healthy elderly. J Electromyogr Kinesiol 19:e40–e49. doi:10.1016/j.jelekin.2007.08.001
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org. Accessed 1 Mar 2016
Rathelot J-A, Strick PL (2006) Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci U S A 103:8257–8262. doi:10.1073/pnas.0602933103
Roos PE, McGuigan MP, Kerwin DG, Trewartha G (2008) The role of arm movement in early trip recovery in younger and older adults. Gait Posture 27:352–356. doi:10.1016/j.gaitpost.2007.05.001
Runnalls KD, Anson G, Wolf SL, Byblow WD (2014) Partial weight support differentially affects corticomotor excitability across muscles of the upper limb. Physiol Rep 2:e12183. doi:10.14814/phy2.12183
Runnalls KD, Anson G, Byblow WD (2015) Partial weight support of the arm affects corticomotor selectivity of biceps brachii. J Neuroeng Rehabil 12:1–10. doi:10.1186/s12984-015-0085-6
Sanes JN, Donoghue JP, Thangaraj V et al (1995) Shared neural substrates controlling hand movements in human motor cortex. Science 268:1775–1777
Schneider C, Zytnicki D, Capaday C (2001) Quantitative evidence for multiple widespread representations of individual muscles in the cat motor cortex. Neurosci Lett 310:183–187. doi:10.1016/S0304-3940(01)02105-X
Sukal TM, Ellis MD, Dewald JPA (2007) Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp Brain Res 183:215–223. doi:10.1007/s00221-007-1029-6
Welham S, Cullis B, Gogel B et al (2004) Prediction in linear mixed models. Aust NZ J Stat 46:325–347. doi:10.1111/j.1467-842X.2004.00334.x
Wilson JM, Thompson CK, Miller LC, Heckman CJ (2015) Intrinsic excitability of human motoneurons in biceps brachii versus triceps brachii. J Neurophysiol 113:3692–3699. doi:10.1152/jn.00960.2014
Acknowledgments
The SaeboMAS device was generously provided by Henry Hoffman at Saebo Incorporated. KR was supported by a University of Auckland Doctoral Scholarship. The research was funded by a University of Auckland FRDF grant to WB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Runnalls, K.D., Anson, G. & Byblow, W.D. Posture interacts with arm weight support to modulate corticomotor excitability to the upper limb. Exp Brain Res 235, 97–107 (2017). https://doi.org/10.1007/s00221-016-4775-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4775-5