Abstract
Recent empirical research has revealed differences in functional capacity between the upper and lower visual fields (VFs), with the lower VF exhibiting superiority in visual perception skills. Similarly, functional differences between the left and right hemispheres elicit a predominance for visuospatial processing in the left visual field (left VF). Both anatomical as well as evolutionary arguments have been adopted in accounting for these variations in function. Preceding upper and lower VF research has typically investigated either static stimulus perception or the visual processing of upper limb action. The aim of the current research was to investigate whether the lower VF benefits associated with limb control transcend to visual anticipation (the perception of motion). Methods were based on Khan and Lawrence (Exp Brain Res 164:395–398, 2005), who investigated upper/lower VF differences in visuomotor control, but utilising a representational momentum paradigm to isolate perceptual processes. Thirty-two participants were randomised into either a left or right VF group and completed a perceptual computer-based task in the upper and lower VF, where they were required to judge the final position of a moving object before it disappeared. Two aspects of the distributions of same responses were then analysed; the central tendency (weighted means) and the variability. Results revealed that in the left VF, weighted means for the lower VF were significantly greater than for the upper VF [t(14) = 2.242, p = 0.042]. In both left and right VFs, variability was greater in the upper compared to lower VF. This provides new findings regarding visual processes in the different visual fields. While visual search and large scene perception has been found to be superior in the upper VF, here we find that visual anticipation, like target-directed visuomotor skill, is superior in the lower VF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent empirical research has revealed advantages in the lower VF for both visual perception and visual-based control of actions (Carlsen et al. 2007; Danckert and Goodale 2001; Khan and Lawrence 2005; Krigolson and Heath 2006), whereas an upper VF advantage is reported for tasks involving visual search (Danckert and Goodale 2003; Efron et al. 1990; Fecteau et al. 2000; Lee et al. 2009; Previc 1990; Previc and Blume 1993). From an anatomical standpoint, explanations for these differences may reside from the fact that there is a greater density of ganglion cells (responsible for transmitting image forming and non-image forming information to brain regions) appearing in the superior hemiretina of the eye compared to the inferior hemiretina (Curcio et al. 1987; Curcio and Allen 1990). Additionally, in the striate cortex of each VF, the lower VF is represented above the calcarine fissure and the upper VF below. Thus, in the extrastriate cortex, the visual fields are represented dorsally and ventrally, respectively (Felleman and Van Essen 1991; Galati et al. 2000; Rubin et al. 1996).
In agreement with the functional segregation of the dorsal and ventral visual cortical pathways (Goodale and Milner 1992), Previc (1990; also see Danckert and Goodale 2003) proposed an evolutionary explanation to account for the upper and lower VF differences with the former primarily responsible for processing vision in extrapersonal space (beyond reaching distance) and the latter in peripersonal space (close to the body). The evolution of everyday human daily needs and action has resulted in a specialisation of the upper VF for large scene perception and visual search (ventrally controlled visual processing), whereas a specialisation for target-directed actions such as reaching and pointing/grasping (dorsally controlled visual processing) has occurred within the lower VF.
These upper and lower VF differences have recently been reported to be dependent on the location of stimuli within the left and right visual fields with only the left VF revealing different response properties between the upper and lower VF (Lee et al. 2009). These findings may be due to the functional differences between the left and right hemispheres (Heilman and Ven Den Abell 1980) with the right hemisphere (left VF) having a predominance for visuospatial processing and the left hemisphere (right VF) for verbal processing (Lee et al. 2009).
Because the majority of the upper and lower VF research has investigated either static stimulus perception (Carlsen et al. 2007) or the visual processing of upper limb action (Danckert and Goodale 2001; Khan and Lawrence 2005), the aim of the current research was to investigate the implications of the previously reported lower VF benefits on visual anticipation. In order to achieve this, the methods of the current study were to some extent a replication of Khan and Lawrence’s (2005) investigation into differences in visuomotor control between the upper and lower VFs. Here, researchers adopted a visuomotor task in which participants were required to move a cursor from a home position to a target under concurrent full vision conditions. Results revealed that movement endpoints were more accurate and less variable in the lower VF. Additionally, both online and offline movement adjustments were greater in the lower VF, indicating that movement control within this field is more adjustable compared to the movements in the upper VF. To extend the understanding of the upper and lower VF differences, the current study endeavoured to isolate perceptual processes to determine whether visual anticipation might contribute to the improved motor performance observed in the research of Khan and Lawrence. In addition, because recent research has suggested the upper and lower VF differences are removed in the right VF, we also investigated the interaction effects between the upper/lower and the left/right VFs during visual anticipation.
To achieve this, we utilised a representation momentum (RM) paradigm that has been adopted by researchers to investigate the visual perception of moving objects. Specifically, RM refers to the phenomenon that when observers attempt to remember the final observed position of an object undergoing implied or actual motion, they tend to misremember the final location as further along in the direction of continued motion (Freyd and Finke 1984). In Freyd and Finke’s (1984) seminal article, participants were required to view a sequence of three images of a rectangle, each indicating an implicit direction of the rectangle’s rotation. The task was to remember the orientation of the third rectangle prior to the presentation of a test rectangle either in the same position as the third rectangle or rotated in the implicit or reverse direction. Participants experienced difficulty in detecting a difference between the third and fourth rectangle when the fourth rectangle was rotated in the implicit direction, suggesting that the participant remembered the third rectangle as being rotated further forward from its original position. This effect is now well established in the literature and has been demonstrated using a diverse selection of tasks and stimuli, for example continuous vertical and horizontal motion of simple objects (Hubbard and Bharucha 1988), static stimuli where motion is implied (Freyd and Pantzer 1995; Freyd et al. 1988; Reed and Vinson 1996) and more recently, in the complex and dynamic environment of a driving simulator (Blättler et al. 2010; Thornton and Hayes 2004).
Research has investigated the effects of various factors on RM, such as stimulus speed (Freyd and Finke 1985) and acceleration (Finke et al. 1986), conceptual knowledge regarding the stimulus (Reed and Vinson 1996), expertise (Blättler et al. 2010), and age (Piotrowski and Jakobson 2011). In addition, RM has been investigated within special populations such as children born preterm and at term (Taylor and Jakobson 2010), and those individuals with autism spectrum disorder (Hudson et al. 2012). The typical RM effects observed within past research have been hypothesised to reflect an anticipatory component of perception which supports effective action (for a review of RM findings and theory see Hubbard 2005). Thus, if processes associated with RM support action, it might be expected that the RM effect should be stronger in the lower VF than in the upper VF, because utilisation of visual feedback for the control of action is superior in the lower VF (Khan and Lawrence 2005).
In the current investigation, participants were asked to perform either a left VF or a right VF RM task in both the upper and lower VF. It was hypothesised that the RM effect would be greater in lower VF compared to that in upper VF. That is, participants would extrapolate the predicted motion of objects and recall the last observed position of objects further forward in the lower VF compared to the upper VF. Furthermore, because the upper and lower VF differences are removed in the right VF (Lee et al. 2009), it was expected that our hypothesised differences in object anticipation between the upper and lower VF would be stronger in the left VF.
Methods
Participants
Thirty-two undergraduate students (29 males, three females; mean age 21.23, SD = 2.16) from the School of Sport, Health and Exercise Sciences, Bangor University, participated as partial fulfilment of a course requirement. All were naïve to the research hypotheses and inexperienced at the experimental task. They gave their informed written consent prior to participation and all reported normal or corrected-to-normal vision. The experiment was conducted in accordance with the ethical guidelines laid down by the Ethics Committee of the School of Sport, Health and Exercise Sciences, Bangor University, for research involving human participants.
Apparatus and stimuli
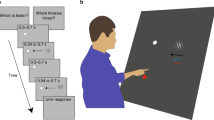
Stimuli were presented on a 22″ View Sonic FuHzion monitor (120 Hz) that was positioned on a table top in front of the participants. The monitor was surrounded on all sides by 50 cm of black card to prevent any logos, scratches, or scuffs on the monitor casing being used as visual reference points during the experimental trials. The participant’s head was placed in a chin/head rest such that their face was parallel to the monitor and their eyes were at a distance of 40 cm and directly in line with the monitor’s horizontal centre line. A fixation point, consisting of a 4-mm black circle, was positioned at the centre point of the monitor and viewing position was centred so that each eye was equidistant from the fixation point.Footnote 1 The stimulus cursor consisted of an 8-mm black circle that appeared in the upper or lower VF and moved horizontally from the periphery to the centre line. The start position of the upper VF stimuli in the right VF was located 24 cm to the right and 8 cm above the fixation point, and direction of movement was right to left. These distances were the same for the left VF, but the orientation of the start position was located to the left of the participant and direction of movement was left to right. In both the right and left, upper VF conditions, the stop position was located 8 cm directly above the fixation point, and in the lower VF conditions, the stop position was located 8 cm below the fixation point. The distance of 8 cm from the fixation point to the stop position created a visual angle of 16°. Both direction of motion (i.e. towards the centre) and the 16° visual angle were direct replications of Khan and Lawrence’s (2005) methods, in an effort to determine whether visual anticipation is at least partly responsible for the reported functional benefits of visuomotor control in the lower VF.
To ensure participants maintained fixation during the experimental trials, a Sony CVX-VIP colour video camera (lens diameter = 8 mm) was positioned to capture video of the participants’ eyes. The experimenter monitored the output from the camera using a digital Sony Video Walkman GV/D 900E placed on the experimenter’s desk.
Task and procedure
Participants were randomly assigned to either a right or left VF group. Due to the demanding nature of the task in maintaining fixation while making perceptual judgements away from the fovea, researchers made the decision to use the direction component as a between-subject factor. This also enabled them to manage the large number of experimental trials participants were required to complete. They were told that their eye movements were being monitored, and at the beginning of each trial, participants were required to maintain their gaze on the fixation point. A warning tone, informing the participant that the trial was about to begin, was then presented and the stimulus cursor appeared at the start location. Following a variable foreperiod (1500–2500 ms), the cursor travelled across the monitor screen (at one of five movement time speeds; 400, 425, 450, 475, 500 ms) and terminated directly above (upper VF condition) or below (lower VF condition) the fixation point. Motion of the cursor was at a constant velocity (i.e. motion did not contain acceleration and deceleration) and movement time speeds equated to 0.60, 0.56, 0.53, 0.51, and 0.48 m/s, respectively. Upon reaching the termination point, the cursor disappeared for a period of 250 ms before reappearing at one of three possible test positions; either forward (+5 mm); directly in line with (0 mm); or backwards (−5 mm) of the termination point. Participants were then asked to verbally report whether the test cursor appeared in the same location (same) as the termination point, or whether it appeared too far forwards (forward) or too far backwards (back) along the direction of motion. The experimenter recorded the response on a Compusys computer for later analysis. Trials for which eye movements occurred were marked for exclusion and subsequently omitted from later analyses. Percentages of these trials were extremely low, with an average of 1 % eye movement trials in the lower VF and 2 % in the upper VF.
Participants completed two blocks of 90 trials, one where the cursor moved in the upper VF and one where the cursor moved in the lower VF. The order of visual fields was counterbalanced between participants. Within each block of trials, the cursor travelled at each of the five different movement time speeds and reappeared at each of the three test positions. The numbers of trials within the components of these two factors were equal, and the order of the trials was randomised within each block. Thus for both the upper and lower VFs, this resulted in a total of 30 trials at each test position with six of those occurring at each speed. At the beginning of each block, participants were given seven familiarisation trials.
Dependent measures and analyses
Two aspects of the distributions of same responses were analysed; the central tendency and the variability.
Central tendency (RM)
The RM effect can be quantified by calculating the central tendency of the distribution of same responses across test positions; the central tendency represents the remembered position of the target. Under conditions of RM, the distribution of same responses is biased towards the forward compared to the backward test positions. Central tendency was estimated by calculating the mean probe position endorsed as same for each condition (upper/lower VF × speed) for each participant. For this calculation, each same response was weighted by the probe position value at which it occurred (i.e. a same response at the backward test position would be assigned a value of −1, a same response at the true same position would be assigned a value of 0, and a same response at the forward test position would be assigned a value of +1), and the average of these weighted same responses was calculated. This measure of central tendency has been termed the weighted mean (Freyd and Jones 1994).
Variability
The variability of the distribution represents the precision of responses about the central tendency.Footnote 2 The variability associated with the distribution of same responses was estimated by calculating the average absolute deviation score for each participant. For each condition, the absolute difference between the test position value (−1, 0, or +1) associated with each same response and that participant’s weighted mean was calculated. These absolute deviations were summed and divided by the total number of same responses.Footnote 3
Analyses
To test whether the weighted means in the upper and lower fields represented significant forward RM shifts, one-sample t tests were conducted, separately for the left and right VF groups. To test for differences among conditions, the weighted mean and average absolute deviation scores were subjected to 2 × 2 × 5 (upper/lower VF × stimulus side × speed) mixed model ANOVAs with upper/lower VF and speed as within-subject factors and stimulus side as a between-subject factor. Significant between-subject effects were broken down using Tukey’s HSD post hoc tests, while significant within-subject effects were broken down into their simple main effects. In line with the assumptions of ANOVA, homogeneity of variance and sphericity were tested; for all computational statistics conducted, there were no violations of homogeneity or sphericity.
Results and discussion
Data screening
Data from one participant who misunderstood the “forward” and “backward” response mappings in the lower VF condition were excluded from the data set. Data from a second participant were excluded because there were no same responses in some conditions and therefore weighted means could not be calculated for those conditions. Data from the remaining 30 participants (15 participants for each stimulus side) were analysed.
Descriptive statistics
Figure 1 shows the mean distributions of same responses as a function of probe position for upper and lower VF, shown separately for the two stimulus sides. Data are collapsed across target speed and participant.
Percent “same” responses as a function of test position for stimuli appearing in the upper and lower VFs shown separately for the left and right VF groups. Test position values are relative to the final seen position, with positive values indicating positions further in the direction of continued motion. Error bars indicate standard error of the mean
Inferential statistics
Central tendency (RM)
Weighted means for the left VF group were significantly greater than zero in both upper VF [0.11; t(14) = 2.359, p = 0.033] and lower VF [0.29; t(14) = 4.217, p = 0.001; see Fig. 2]. The weighted means were not significantly different from zero for the right VF group in either the upper VF (0.01; p = 0.900) or the lower VF (−0.06; p = 0.496).
The ANOVA on the weighted means revealed a main effect of stimulus side [F (1, 28) = 7.387, p = 0.011, η 2 = 0.209] with greater weighted means for left VF (0.20) than for right VF (−0.03). There was a significant upper/lower VF × stimulus side interaction [F (1, 28) = 4.258, p = 0.048, η 2 = 0.132]. Post hoc analyses (Tukey HSD) revealed that for left VF, weighted means for lower VF (M = 0.29, SD = 0.26) were significantly greater than for upper VF (M = 0.11, SD = 0.19) (p < 0.05), whereas for the right VF group, there was no significant difference in weighted means for the two visual fields. Furthermore, in the lower VF, left VF target motion produced significantly greater weighted means (M = 0.29, SD = 0.26) than right VF target motion (M = −0.06, SD = 0.33) (p < 0.05), whereas in the upper VF, there was no effect of stimulus side.
These central tendency results establish that the anticipatory RM bias occurs in both the upper and lower VF, but only for the stimuli presented in the left VF. Moreover, for these left VF stimuli, the RM effect is stronger in the lower VF compared to the upper VF. This upper/lower VF difference was predicted based on findings that visual perception for the control of action is superior in the lower VF (Danckert and Goodale 2001; Khan and Lawrence 2005); this connection between RM and control of action will be addressed further in the “General discussion”.
The difference in RM between the upper and lower VF only occurs in the left VF, which supports the findings of Lee et al. (2009) that upper/lower VF differences in visual processing occur in the left VF only.
Variability
The analysis revealed a significant main effect for upper/lower VF [F (1, 28) = 13.961, p = 0.001, η 2 = 0.333], with greater average absolute deviation in upper VF (0.52) than in lower VF (0.43). No other main effects or interactions were significant. This result indicates that although performance in the lower VF was less accurate in the sense that the anticipatory RM bias was larger, responses were less variable, which may reflect superior deployment of visual attention (Prinzmetal and Wilson 1997).
General discussion
It was predicted that RM would be larger in the lower VF, based on the finding that visual control of upper limb target-directed movements has been found to be superior in the lower VF (Danckert and Goodale 2001; Khan and Lawrence 2005). It was hypothesised that if anticipatory visual processes support this visuomotor skill, then RM should also be stronger in the lower VF. The findings support this hypothesis in that performance for lower VF stimuli was less variable and showed a stronger RM bias than in the upper VF for left VF stimuli. As predicted, this was not the case for the right VF, which may be because upper/lower VF perceptual differences are not found in the right VF (Lee et al. 2009). However, in the present study, the null result in the right VF could also be due to the fact that RM biases did not occur in the right VF and therefore RM effects were insufficiently strong to demonstrate upper/lower VF differences. This result is consistent with previous findings that RM effects are stronger for stimuli in the left VF than for stimuli in the right VF (White et al. 1993). An additional consideration is that stimuli in the left VF condition moved from left to right, whereas stimuli in the right VF moved from right to left. Previous findings indicate that memory distortions are greater when viewing objects moving from left to right. Halpern and Kelly (1993) have presented multiple explanations for this finding. One proposition is left and right hemispheric functional differences, with the right hemisphere (left VF) predisposed to visuospatial processing and the left hemisphere (right VF) predisposed to verbal processing. An alternative or supplementary explanation used to account for this finding is reading habits, which for English speakers/readers is typically done in the left-to-right direction. Halpern and Kelly suggest that the process of tracking ahead when reading in this direction may extend to the tracking of visual objects and hence account for a larger memory bias when processing objects moving in the left-to-right direction.
However, a consequence and limitation of replicating Khan and Lawrence’s (2005) methods, albeit to investigate perceptual as opposed to motor effects, was that potential visual hemisphere and direction of motion confounders were not possible to disentangle. That is, it cannot be confirmed whether an RM effect in the left VF was a result of direction of motion specifically (left to right), or because motion occurred primarily in the left VF. Additionally, left and right VFs were based on the region that motion of the stimulus cursor occurred in, i.e. the right visual field group completed trials in which the stimulus began on the right-hand side of the monitor and travelled in a right-to-left direction towards the centre of the monitor. However, it should be noted that in some trials (+ 5 mm test position), the test cursor would have reappeared a small distance (5 mm; just over half the diameter of the cursor) into the opposite visual field; therefore, stimuli have not been completely isolated to the right or left VF. Disentangling the source of the differential RM effects in the left and right VF remains a topic for future investigation.
The current research provides support for the theory that the anticipatory visual processes that underlie RM are an adaptive response to action demands in the everyday environment (see Hubbard 2005). Thorpe et al. (1996) suggest that it takes up to 150 ms to visually process an object. Thus, viewing a moving object should theoretically result in a consequential delay between perceptual and actual location of that object. However, in reality, we are able to compensate for this latency by further extrapolating an object’s motion (Khurana and Nijhawan 1995; Berry et al. 1999). This strategy may be necessary in everyday life to elicit an effective motor response, such as reaching or grasping a falling object. Shepard’s (1981) work has previously advocated some perceptual processes as being a function of the enduring characteristics of our environment. This notion is also in line with Danckert and Goodale (2003) who suggest that functional benefits of visuomotor control in the lower VF are likely a consequence of evolution as a result of these tasks predominantly occurring in the lower VF. With this in mind, researchers have suggested that one possible function of the RM effect is in bridging the gap between perception and action (Hubbard, 2005). The current findings suggest that participants were better able to accomplish this functional displacement in the lower VF, which is consistent with visuomotor superiority (Khan and Lawrence 2005) and other perceptual processing advantages previously identified in this region; Carlsen et al. (2007) found that performance in a visual inspection task was superior when performed in the lower versus upper VF.
The physiological characteristics of humans, whether a consequence of evolution or not, facilitate our understanding of perceptual processes. Researchers (e.g. Lennie 1981; Berry et al. 1999) have long since indicated the pertinence of retinal ganglion cells in perceptual processing as well as confirming a disproportionate ganglion cell distribution favouring the lower VF (Curcio et al. 1987; Curcio and Allen 1990). This, combined with an over-representation of the lower VF in the dorsal stream, provides a physiological explanation for this greater RM shift in the lower VF.
In conclusion, these results provide new findings regarding visual processes in the upper and lower VFs. While visual search and large scene perception has been found to be superior in the upper VF, here we find that visual anticipation, like target-directed visuomotor skill, is superior in the lower VF. This is consistent with the theory that anticipatory processes associated with RM support successful action, and future research testing RM and visuomotor tasks concurrently between the upper and lower VFs can test this link directly. Moreover, many perceptual processes modulate the magnitude of the RM effect, including expectations based on the identity of the object (Reed and Vinson 1996; Vinson and Reed 2002) and expectations regarding the unfolding event (Verfaillie and d’Ydewalle 1991). Future research is needed to determine how these modulatory effects may differ between the upper and lower VFs.
Notes
To verify these vertical and horizontal eye positions, participants viewed the fixation point through the 8-mm-diameter circular bore of a cylinder (25 mm diameter; 32 mm length) that was placed perpendicular against the monitor screen; adjustments to the chin/head rest were made until the fixation point appeared to the participant to be centred in the cylindrical bore.
Hayes and Freyd (2002) have demonstrated in an RM paradigm that precision of responses about the central tendency decreases under dual task conditions. Other perceptual judgments, such as of colour, orientation, and static location, also become less precise under dual task conditions (e.g. Prinzmetal et al. 1998). It might be expected, then, that any differences in attentional deployment to the upper versus lower visual fields might be reflected in changes in the variability of the distributions of same responses.
References
Berry MJ Jr, Brivanlou IH, Jordan TA, Meister M (1999) Anticipation of moving stimuli by the retina. Nature 398:334–338
Blättler C, Ferrari V, Didierjean A, Elslande P, Marmèche E (2010) Can expertise modulate representational momentum? Vis Cognit 18(9):1253–1273
Carlsen AN, Maslovat D, Chua R, Franks IM (2007) Perceptual processing time differences owing to visual field asymmetries. Neuroreport Vis 18(10):1067–1070
Curcio CA, Allen KA (1990) Topography of ganglion cells in human retina. J Comparative Neurol 300:5–25
Curcio CA, Sloan KR, Packer O, Hendrickson AE, Kalina RE (1987) Distribution of cones in human and monkey retina: individual variability and radial asymmetry. Science 236:579–581
Danckert J, Goodale M (2001) Superior performance for visually guided pointing in the lower visual field. Exp Brain Res 137:303–308
Danckert J, Goodale M (2003) Ups and downs in the visual control of action. In: Johnson-Frey SH (ed) Taking action: cognitive neuroscience perspectives on intentional actions. MIT Press, Cambridge, Mass, pp 29–64
Efron R, Yund E, Nichols D (1990) Detectability as function of target location: effects of spatial configuration. Brain Cognit 12:102–116
Fecteau JH, Enns JT, Kingstone A (2000) Competition-induced visual field differences in search. Psychol Sci 11(5):386–393
Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1:1–47
Finke RA, Freyd JJ, Shyi GCW (1986) Implied velocity and acceleration induce transformations of visual memory. J Exp Psychol Gen 115:175–188
Freyd JJ, Finke RA (1984) Representational momentum. J Exp Psychol Learn Mem Cogn 10:126–132
Freyd JJ, Finke RA (1985) A velocity effect for representational momentum. Bull Psychon Soc 23(6):443–446
Freyd JJ, Jones KT (1994) Representational momentum for a spiral path. J Exp Psychol Learn Mem Cogn 20(4):968–976
Freyd JJ, Pantzer TM (1995) Static patterns moving in the mind. In: Smith SM, Ward TB, Finke RA (eds) The creative cognition approach. MIT Press, Cambridge, MA, pp 181–204
Freyd JJ, Pantzer TM, Chang JL (1988) Representing statics as forces in equilibrium. J Exp Psychol Gen 117:395–407
Galati G, Lobel E, Vallar G, Berthoz A, Pizzamiglio L, Le Bihan D (2000) The neural basis of egocentric and allocentric coding of space in humans: a functional magnetic resonance study. Exp Brain Res 133:156–164
Goodale MA, Milner AD (1992) Separate visual pathways for perception and action. Trends Neurosci 15(1):20–25
Halpern AR, Kelly MH (1993) Memory biases in left versus right implied motion. J Exp Psychol Learn Mem Cogn 19:471–484
Hayes AE, Freyd JJ (2002) Representational momentum when attention is divided. Vis Cognit 9:8–27
Heilman KM, Ven Den Abell T (1980) Right hemisphere dominance for attention. Neurology 30(3):327
Hubbard TL (2005) Representational momentum and related displacements in spatial memory: a review of the findings. Psychon Bull Rev 12(5):822–851
Hubbard TL, Bharucha JJ (1988) Judged displacement in apparent vertical and horizontal motion. Percept Psychophys 44:211–221
Hudson M, Burnett HG, Jellema T (2012) Anticipation of action intentions in autism spectrum disorder. J Autism Dev Disord 42:1684–1693
Keppel G (1991) Design and analysis: a researcher’s handbook, 3rd edn. Prentice Hall, Upper Saddle River, NJ
Khan MA, Lawrence GP (2005) Differences in visuomotor control between the upper and lower visual fields. Exp Brain Res 164:395–398
Khurana B, Nijhawan R (1995) Extrapolation or attention shift? Nature 378:565–566
Krigolson O, Heath M (2006) A lower visual field advantage for endpoint stability but no advantage for online movement precision. Exp Brain Res 170:127–135
Lee B, Kaneoke Y, Kakigi R, Sakai Y (2009) Human brain response to visual stimulus between lower/upper visual fields and cerebral hemispheres. Int J Psychophysiol 74:81–87
Lennie P (1981) The physiological basis of variations in visual latency. Vis Res 21:815–824
Piotrowski AS, Jakobson LS (2011) Representational momentum in older adults. Brain Cogn 77:106–112
Previc F (1990) Functional specialization in the lower and upper visual fields in humans: its ecological origins and neurophysiological implications. Behav Brain Sci 13:519–575
Previc F, Blume J (1993) Visual search asymmetries in three-dimensional space. Vis Res 33:2697–2704
Prinzmetal W, Wilson A (1997) The effect of attention on phenomenal length. Perception 26(2):193–205
Prinzmetal W, Amiri H, Allen K, Edwards T (1998) Phenomenology of attention: 1. color, location, orientation, and spatial frequency. J Exp Psychol Hum Percept Perform 24(1):261–282
Reed CL, Vinson NG (1996) Conceptual effects on representational momentum. J Exp Psychol Hum Percept Perform 22(4):839–850
Rubin N, Nakayama K, Shapley R (1996) Enhanced perception of illusory contours in the lower versus upper visual hemifields. Science 271(5249):651–653
Shepard RN (1981) Phsychophysical complementarity. In: Kubovy M, Pomerantz JR (eds) Perceptual organisation. Erlbaum, Hillsdale, NJ, pp 279–341
Taylor NM, Jakobson LS (2010) Representational momentum in children born preterm and at term. Brain Cogn 72:464–471
Thornton IM, Hayes AE (2004) Anticipating action in complex scenes. Vis Cognit 11:341–370
Thorpe S, Fize D, Marlot C (1996) Speed of processing in the human visual system. Nature 381:520–522
Verfaillie K, d’Ydewalle G (1991) Representational momentum and event course anticipation in the perception of implied periodical motions. J Exp Psychol Learn Mem Cogn 17(2):302–313
Vinson N, Reed CL (2002) Sources of object-specific effects in representational momentum. Visual Cognit 9(1/2):41–65
White H, Minor SW, Merrell J, Smith T (1993) Representational-momentum effects in the cerebral hemispheres. Brain Cogn 22(2):161–170
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gottwald, V.M., Lawrence, G.P., Hayes, A.E. et al. Representational momentum reveals visual anticipation differences in the upper and lower visual fields. Exp Brain Res 233, 2249–2256 (2015). https://doi.org/10.1007/s00221-015-4294-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4294-9