Abstract
Daily Achilles tendon vibration has been shown to increase muscle force, likely via corticospinal neural adaptations. The aim of the present study was to determine the extent by which corticospinal excitability is influenced during direct Achilles tendon vibration. Motor-evoked potentials (MEPs) were elicited in the soleus (SOL), gastrocnemius medialis (GM) and tibialis anterior (TA) by transcranial magnetic stimulation of the motor cortical area of the leg with and without Achilles tendon vibration at various frequencies (50, 80 and 110 Hz). Contralateral homologues were also investigated. SOL and GM MEP amplitude significantly increased by 226 ± 188 and 66 ± 39 %, respectively, during Achilles tendon vibration, without any difference between the tested frequencies. No MEP changes were reported for TA or contralateral homologues. Increased SOL and GM MEP amplitude suggests increased vibration-induced corticospinal excitability independent of vibration frequency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic vibration of the Achilles tendon has the potential to increase maximal plantar flexion strength in both the vibrated (Lapole and Pérot 2010) and the contralateral non-vibrated side (Lapole et al. 2013), likely resulting from demonstrated neural adaptations (Lapole and Pérot 2010). While enhanced activation capacities were partly explained by an increase in soleus (SOL) spinal excitability (Lapole and Pérot 2012; Lapole et al. 2013), neural adaptations were also suggested to have supraspinal origins (Lapole et al. 2013). Tendon and muscle vibration are known to be powerful stimuli for muscle spindle primary endings and, to a lesser degree, secondary spindle and Golgi afferents (Eklund and Hagbarth 1966; Roll et al. 1989). While proprioceptive inputs are essential at the spinal level (Gandevia 2001), they also play a major role in motor control at the cortical level (Wiesendanger and Miles 1982). It has previously been reported that the motoneuron firing rate may be reduced by up to 30 % in the absence of afferent feedback (Macefield et al. 1993). Moreover, modulation of afferent inputs can induce persistent neuroplastic changes in motor cortical areas, suggesting that projections from the somesthetic cortex modulate motor cortical excitability. For instance, 2 h of peripheral electrical stimulation of the hand muscles was reported to increase motor cortical excitability (Ridding et al. 2000; Kaelin-Lang et al. 2002). The hypothesis that vibratory stimuli may also influence the cortical level is supported by studies showing that vibration can generate evoked cortical potentials in sensory and motor cortical areas of the upper limbs (Munte et al. 1996; Naito et al. 1999; Naito and Ehrsson 2001).
Human cortical excitability can be assessed by transcranial magnetic stimulation (TMS). Using this technique, numerous upper-limb studies have demonstrated an increase in corticospinal excitability indicated by increased motor-evoked potential (MEP) amplitude or area within seconds of the initiation of vibration of various hand and wrist muscles (Claus et al. 1988; Kossev et al. 1999; Siggelkow et al. 1999; Kossev et al. 2001; Rosenkranz et al. 2003; Rosenkranz and Rothwell 2003; Steyvers et al. 2003; Rosenkranz and Rothwell 2006) with a simultaneous decrease in the excitability of antagonist muscles (Siggelkow et al. 1999; Kossev et al. 2001; Rosenkranz et al. 2003; Rosenkranz and Rothwell 2003, 2006). Effects to corticospinal excitability were also demonstrated in the contralateral non-vibrated side. Kossev et al. (2001) reported a significant decrease in antagonist MEP area during vibration and a tendency towards an increase in agonist MEP area. The vibration-induced increased MEP amplitude was also reported to be frequency dependent (Siggelkow et al. 1999; Steyvers et al. 2003). A significant increase in extensor carpi radialis MEP amplitude was reported during 80- and 120-Hz but not 160-Hz vibration (Siggelkow et al. 1999). Similarly, it was shown that 75-Hz vibration resulted in greater flexor carpi radialis MEP amplitude increase than vibration at 120 Hz, while a 20-Hz frequency had no effect on MEP amplitude (Steyvers et al. 2003). Dependence of MEP amplitude increase in both wrist flexor and extensor muscles on vibration frequency has mainly been discussed regarding the excitation of muscle spindle primary endings. Indeed, Ia afferents can fire synchronously with vibration frequencies up to 80–100 Hz (Roll et al. 1989). Since 20-Hz vibration is known to entrain a suboptimal primary ending firing rate (Roll et al. 1989), insufficient central temporal summation of Ia afferent discharge was suggested to account for the lack of MEP amplitude change (Steyvers et al. 2003). Similarly, a high vibration frequency (i.e. 160 Hz) was ineffective in facilitating MEPs due to overstimulation of Ia afferents (Siggelkow et al. 1999), leading to a possible decrease in their firing rate (Roll et al. 1989). Hence, vibration-induced MEP amplitude increase is believed to be directly mediated by the induced discharge of spindle primary endings.
Despite lower-limb muscles being of fundamental importance because of their functional role in locomotion, corticospinal excitability of leg muscles has rarely been investigated. In early stages of immobilization, reduced neural drive accounts for a large portion of the decrease in plantar flexor strength loss (Duchateau 1995; Gondin et al. 2004); therefore, it is important to develop effective countermeasures to neuromuscular deconditioning. With both potential ipsilateral and contralateral effects, a tendon vibration program might be beneficial for individuals who are hypoactive or immobilized, and not candidates for whole-body vibration. To our knowledge, only one study has investigated leg MEP amplitudes during vibration (Mileva et al. 2009). This study reported increased tibialis anterior (TA) MEP amplitude during 30-Hz whole-body vibration with no change in SOL MEP size. In this study, vibration was applied simultaneously to both limbs while maintaining a squat position, entraining different neural mechanisms than with localized vibration exposure (Rittweger 2010). More recently, the acute after-effects of a single 1-h bout of 50-Hz Achilles tendon vibration were investigated and increased SOL and TA corticospinal excitability was reported (Lapole et al. 2012). It is not known whether SOL corticospinal excitability is modulated during Achilles tendon vibration in the same way as in upper-limb muscles. Furthermore, it is not known whether MEP amplitude changes also occur in synergist, antagonist and contralateral muscles during Achilles tendon vibration.
Therefore, the main aim of the present study was to elucidate how corticospinal excitability is influenced during direct Achilles tendon vibration. We investigated the effect of different vibration frequencies within the optimal range of Ia afferent discharge on MEP response amplitudes of the vibrated muscles [SOL and gastrocnemius medialis (GM)], their antagonist (TA) and their contralateral homologues.
Methods
Participants
Twelve healthy (free of lower-limb injury during the previous 3 months) right-leg dominant participants (two females and ten males; age: 28 ± 8 years; height: 176 ± 9 cm; body mass: 72 ± 7 kg) provided written informed consent prior to participating in the study. This study conformed to the standards from latest revision of the Declaration of Helsinki, and all procedures were approved by Comité de Protection des Personnes Sud-Est 1, France. All participants were free of contraindications to TMS, acute and chronic neurological disorders and trauma. They were instructed to abstain from caffeine for a minimum of 12 h before each session. Participants were seated in a custom-built chair with hips, knees and ankles at 90° of extension for all measures.
Electromyographic activity
EMG of SOL, GM and TA was recorded with pairs of self-adhesive surface electrodes (Meditrace 100, Covidien, Mansfield, OH, USA) in bipolar configuration with a 30-mm interelectrode distance and the reference on the medial malleolus. SOL electrodes were placed 2 cm below the insertion of the gastrocnemii on the Achilles tendon, and GM and TA electrodes were placed on the muscle belly according to SENIAM recommendations (Hermens et al. 2000). A low impedance (<5 kΩ) between electrodes was verified after shaving, gently abrading the skin and then cleaning it with isopropyl alcohol. Signals were analogue-to-digitally converted at a sampling rate of 2,000 Hz by PowerLab system (16/30-ML880/P, ADInstruments, Bella Vista, Australia) and octal bio-amplifier (ML138, ADInstruments; common mode rejection ratio = 85 dB, gain = 5,000 for SOL and 2,000 for GM and TA) with bandpass filter (10–500 Hz) and analysed offline using Labchart 7 software (ADInstruments).
Transcranial magnetic stimulation
Transcranial magnetic stimulation was performed using a Magstim 2002 stimulator (The Magstim Company Ltd, Whitland, UK) and a 110-mm concave double-cone coil (maximum output of 1.4 T). The coil was positioned over the leg area of the motor cortex along the nasal-inion axis to induce a postero-anterior current. Optimal coil position was selected so as to elicit the largest right SOL MEP amplitude. TMS intensity was based upon SOL MEP amplitude since TA and GM MEPs are more easily elicited than SOL MEPs. This ensured the presence of MEPs in all tested muscles when a SOL MEP was identified. Moreover, the selection of the hot spot along the nasal-inion axis permitted both motor cortices to be stimulated to the same degree; thus, permitting simultaneous bilateral recording of MEPs. The coil position was drawn directly on the silicone swim cap worn by the participants. Before the delivery of each stimulus, the coil position was verified with regard to the marks on the swim cap. Resting motor threshold (rMT) was determined as the intensity to elicit SOL MEP amplitudes higher than 50 μV in at least three of five consecutive trials with the muscle in the relaxed state (Perez et al. 2004). During the protocol, TMS was delivered at 120 % SOL rMT, i.e. 64 ± 20 % maximal stimulator output.
Achilles tendon vibration
Low-amplitude (1 mm) Achilles tendon vibration of the dominant leg (personal preference) was performed by tendon vibrator (VB 115, Techno Concept, Mane, France) strapped immediately proximal to the malleoli. The chosen vibration frequencies were 50, 80 and 110 Hz. All chosen frequencies are known to be in the range where Ia afferents can fire synchronously with the vibration frequency (Roll et al. 1989). During control conditions (i.e. without vibration), the vibrator remained strapped to the Achilles tendon. Participants were instructed to fully relax their muscles and to keep their eyes closed in order to prevent the occurrence of tonic vibratory reflexes induced by vision (Roll et al. 1980). Experiments were conducted in a quiet room to prevent auditory disruptions. EMG data were verified to ensure that tonic vibratory reflexes were not evoked, and that participants were not performing very weak voluntary contractions. This occurred by analysing background activity as the mean value of the rectified EMG over a 100-ms period before delivery of TMS.
Experimental protocol
Figure 1 illustrates the experimental set-up. TMS was delivered at 120 % SOL rMT in a control condition and during Achilles tendon vibration at 50, 80 and 110 Hz. For each condition, two series (A and B) were performed in order to take into account both possible variability in vibration-induced effects and a time effect. Each series consisted of 10 stimuli delivered at randomly determined interstimulus intervals of 3–10 s. Vibration was continuously applied for a maximal total time of 60 s per series. The first TMS pulse was delivered ~3 s after the beginning of vibration. The eight series were performed in a randomized order with 60 s of rest between series. EMG activity was recorded from SOL, GM and TA in both right (vibrated) and left (contralateral) legs. The size of the MEPs was measured as the peak-to-peak amplitude of the non-rectified response. For each series of 10 TMS pulses, the three maximal responses of each muscle were averaged. Mean MEP amplitudes at each vibrating frequency were normalized and expressed as a percentage of the mean control MEP for each muscle.
Statistics
Statistical analyses were performed using SPSS software (SPSS 21, SPSS, Chicago, IL). The distribution of each variable was examined with the Kolmogorov–Smirnov normality test, and homogeneity of variance was verified by Levene’s test. For each muscle, a linear mixed model for repeated measures was first performed on MEP amplitude and background EMG activity. Condition (vibration vs. control), series (A and B) and frequency (50, 80 and 110 Hz) were considered as fixed main effects, as were their interactions. Participants were taken as a random factor. To compare for vibration-induced changes, the mean MEP amplitudes at each vibrating frequency were then normalized and expressed as a percentage of the mean control MEP for each muscle. Since there was no series main effect or interaction effect, series A and B were averaged and a two-way repeated measures ANOVA was performed to compare for mean vibration-induced changes with the factors frequency (50, 80 and 110 Hz vibration) and muscle (SOL, GM and TA in both vibrated and contralateral legs). When the ANOVA identified significant effects, post hoc Student–Newman–Keuls testing was performed. Data are presented as mean ± SD. Statistical significance was set at P < 0.05.
Results
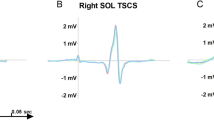
There was no main effect or interaction effect on background EMG activity, confirming the absence of voluntary activation and/or tonic vibratory reflex during vibration conditions. Table 1 presents the raw background EMG activities for all tested muscles. No series effect was found for MEP amplitude for the tested muscles. Raw traces are illustrated in Fig. 2, and raw MEP amplitudes (with series A and B averaged) are presented in Table 2. The linear mixed model identified a significant condition effect for the SOL and GM muscles, with increased MEP amplitude during vibration (P = 0.006, and 0.007, respectively). There was no effect of condition for the antagonist TA (P = 0.734) nor for the contralateral SOL, GM and TA (P = 0.637, 0.188 and 0.554, respectively). No effect of frequency was found, nor was there an interaction effect.
When expressing MEP amplitude as a percentage of the control MEP (Fig. 3), the two-way repeated measures ANOVA confirmed that there was no vibration frequency effect (P = 0.498). There was a significant muscle effect (P < 0.001) with the normalized vibrated SOL MEP amplitude being significantly greater than all other tested muscles (Fig. 2; P < 0.001).
Effect of frequency on vibration-induced MEP amplitude changes. Mean (±SD) MEP amplitudes as a function of vibration frequency (50 Hz in white; 80 Hz in grey; and 110 Hz in black) for each tested muscle. MEP amplitudes are expressed as a percentage of the mean control MEP elicited at rest without vibration. Mean control MEP (i.e. 100 % on the figure) is represented by the black dotted line. Results are presented for the SOL, GM and TA of the right vibrated leg (a) and for the contralateral non-vibrated leg (b). *Significant differences in absolute MEP amplitude value when compared to control condition. At all three frequencies, the vibrated SOL increased significantly more than all other tested muscles (# P < 0.001)
Discussion
A fundamental result of the present study is the significant increases in SOL and GM MEP amplitudes by 226 ± 188 and 66 ± 39 %, respectively. Despite weaker corticomotoneuronal projections to lower-limb muscles, especially SOL (Brouwer and Ashby 1990), this finding is in accordance with studies reporting similar findings in various hand and wrist muscles when subjected to tendon or muscle vibration (Claus et al. 1988; Kossev et al. 1999; Siggelkow et al. 1999; Kossev et al. 2001; Rosenkranz et al. 2003; Rosenkranz and Rothwell 2003, 2006; Steyvers et al. 2003). In the only study investigating the effects of whole-body vibration on leg muscle MEPs, SOL MEP amplitude was unaffected, while TA corticospinal excitability increased (Mileva et al. 2009). In that study, TMS intensity was adjusted to be suprathreshold for TA (120 % of rMT for TA). Since corticomotoneuronal projections are stronger to the TA than to the SOL (Bawa et al. 2002), the TMS intensity employed in Mileva et al. (2009) was likely too low to sufficiently depolarize SOL pyramidal cells, possibly explaining the lack of changes in SOL MEP amplitude. Moreover, differences in vibration paradigm may explain the discrepancy. While tendon vibration is known to mainly activate Ia afferents (Roll et al. 1989), whole-body vibration activates proprioceptive receptors of all antagonist/synergist muscles and sensory afferents of the foot sole, acting both bilaterally and simultaneously on motor and sensory afferents of all lower-limb muscles (Mileva et al. 2009).
In the present study, since both SOL and GM were solicited by Achilles tendon vibration, an increase in their MEP amplitudes was expected. Interestingly, the magnitude of vibration-induced MEP amplitude increase was much greater for the SOL than GM. This discrepancy could be due to the fact that the density of muscle spindles is higher in SOL than in GM (Proske 1997), making the SOL more sensitive to vibration than the GM.
MEP amplitude depends on the synaptic relays of the corticospinal projections at both the cortical and spinal levels (Devanne et al. 1997). Thus, contribution of spinal or peripheral mechanisms to the increased MEP amplitudes cannot be ruled out. However, it was reported that M- and F-waves (two means of assessing sarcolemmal and motoneuronal excitability, respectively) remain unchanged during vibration (Rollnik et al. 2001). In preliminary experimentation, we also investigated SOL and GM M-waves during vibration and found no effect of vibration (unpublished data). Thus, MEPs were not normalized with regard to the M-wave in the present study. Moreover, it has been well-established that mechanisms causing increased MEP amplitude during vibration in the upper limbs are cortical in origin (Kossev et al. 1999). The preclusion of spinal contributions in the present study is further supported by the unaffected postsynaptic excitability of SOL motoneurons during 25-Hz SOL vibration (Abbruzzese et al. 1997) and the absence of background EMG activity during Achilles tendon vibration. However, changes in spinal excitability cannot be ruled out since vibration-induced Ia afferent activity may have partially depolarized motoneuronal cells without causing them to discharge. Thus, motoneurons would have been more responsive to subsequent TMS. In the future, investigation of cervicomedullary-evoked potentials (McNeil et al. 2013) will enable verification that vibration has no impact on motoneuronal excitability that may have been undetectable with the present protocol.
Previous studies demonstrated that vibration-induced MEP amplitude increases may be directly mediated by the induced discharge of spindle primary endings and is frequency dependent (Siggelkow et al. 1999; Steyvers et al. 2003). Low (20 Hz) and high (160 Hz) frequencies were suggested to be ineffective in facilitating MEPs due to suboptimal and over-stimulation of Ia afferents, respectively (Siggelkow et al. 1999; Steyvers et al. 2003). In the present study, the tested frequencies were within the range of optimal Ia afferent firing rate in response to vibration (Roll et al. 1989). Since vibration-induced SOL and GM MEP amplitudes increased by similar magnitudes at vibration frequencies of 50, 80 and 110 Hz, it can be suggested that all three frequencies were similarly effective in modulating MEP amplitude through their respective capacities to induce firing of Ia afferents.
The present study also investigated vibration-induced MEP changes in the antagonist TA. No changes in MEP amplitude were reported during vibration. This result is in opposition with previous upper-limb studies reporting decreased MEP size of antagonist muscles during agonist vibration (Siggelkow et al. 1999; Kossev et al. 2001; Rosenkranz et al. 2003; Rosenkranz and Rothwell 2003, 2006). Despite the recognized antagonist inhibition in upper-limb muscles during vibration, the present study did not observe antagonist inhibition during Achilles tendon vibration. Further studies are needed to explain this discrepancy.
In the present study, no effects were reported in contralateral muscles. Conversely, a non-significant increase in contralateral agonist MEP area was reported in a study investigating crossed-effects of extensor carpi radialis muscle vibration (Kossev et al. 2001). The authors also reported a significant decrease in contralateral antagonist MEP area. It was suggested that the facilitatory action of muscle vibration may be mediated via transcallosal pathways that in turn cause inhibition of cortical outputs to the contralateral antagonist (Kossev et al. 2001). It was more recently reported that vibration-induced proprioceptive input in the first dorsal interosseous may reduce the MEP amplitude of the contralateral homologous muscle with effects occurring in the cortex ipsilateral to the stimulus and mediated via transcallosal fibres (Swayne et al. 2006). Discrepancies with the present study in leg muscles remain to be elucidated.
In conclusion, Achilles tendon vibration increased MEP amplitude of plantar flexor muscles, suggesting the ability of vibration to modulate plantar flexor corticospinal excitability. While other vibration paradigms have demonstrated efficacy such as repeated flexor carpi radialis and biceps brachii vibration in neurorehabilitation and motor recovery in stroke patients (Marconi et al. 2011), further investigations must now focus on the effects on corticospinal excitability with chronic Achilles tendon vibration. For instance, long-term potentiation of corticospinal excitability would be beneficial in rehabilitation as a countermeasure to the reduced neural drive observed in early stages of immobilization (Duchateau 1995; Gondin et al. 2004).
Abbreviations
- GM:
-
Gastrocnemius medialis
- MEP:
-
Motor-evoked potential
- rMT:
-
Resting motor threshold
- SOL:
-
Soleus
- TA:
-
Tibialis anterior
- TMS:
-
Transcranial magnetic stimulation
References
Abbruzzese M, Minatel C, Faga D, Favale E (1997) Testing for pre-synaptic and post-synaptic changes in the soleus H reflex pathway following selective muscle vibration in humans. Neurosci Lett 231:99–102
Bawa P, Chalmers GR, Stewart H, Eisen AA (2002) Responses of ankle extensor and flexor motoneurons to transcranial magnetic stimulation. J Neurophysiol 88:124–132
Brouwer B, Ashby P (1990) Corticospinal projections to upper and lower limb spinal motoneurons in man. Electroencephalogr Clin Neurophysiol 76:509–519
Claus D, Mills KR, Murray NM (1988) Facilitation of muscle responses to magnetic brain stimulation by mechanical stimuli in man. Exp Brain Res 71:273–278
Devanne H, Lavoie BA, Capaday C (1997) Input–output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114:329–338
Duchateau J (1995) Bed rest induces neural and contractile adaptations in triceps surae. Med Sci Sports Exerc 27:1581–1589
Eklund G, Hagbarth KE (1966) Normal variability of tonic vibration reflexes in man. Exp Neurol 16:80–92
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81:1725–1789
Gondin J, Guette M, Maffiuletti NA, Martin A (2004) Neural activation of the triceps surae is impaired following 2 weeks of immobilization. Eur J Appl Physiol 93:359–365. doi:10.1007/s00421-004-1225-z
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10:361–374
Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG (2002) Modulation of human corticomotor excitability by somatosensory input. J Physiol 540:623–633
Kossev A, Siggelkow S, Schubert M, Wohlfarth K, Dengler R (1999) Muscle vibration: different effects on transcranial magnetic and electrical stimulation. Muscle Nerve 22:946–948
Kossev A, Siggelkow S, Kapels H, Dengler R, Rollnik JD (2001) Crossed effects of muscle vibration on motor-evoked potentials. Clin Neurophysiol 112:453–456
Lapole T, Pérot C (2010) Effects of repeated Achilles tendon vibration on triceps surae force production. J Electromyogr Kinesiol 20:648–654. doi:10.1016/j.jelekin.2010.02.001
Lapole T, Pérot C (2012) Hoffmann reflex is increased after 14 days of daily repeated Achilles tendon vibration for the soleus but not for the gastrocnemii muscles. Appl Physiol Nutr Metab 37:14–20. doi:10.1139/h11-129
Lapole T, Deroussen F, Perot C, Petitjean M (2012) Acute effects of Achilles tendon vibration on soleus and tibialis anterior spinal and cortical excitability. Appl Physiol Nutr Metab 37:657–663. doi:10.1139/h2012-032
Lapole T, Canon F, Perot C (2013) Ipsi- and contralateral H-reflexes and V-waves after unilateral chronic Achilles tendon vibration. Eur J Appl Physiol 113:2223–2231. doi:10.1007/s00421-013-2651-6
Macefield VG, Gandevia SC, Bigland-Ritchie B, Gorman RB, Burke D (1993) The firing rates of human motoneurons voluntarily activated in the absence of muscle afferent feedback. J Physiol 471:429–443
Marconi B, Filippi GM, Koch G et al (2011) Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabil Neural Repair 25:48–60. doi:10.1177/1545968310376757
McNeil CJ, Butler JE, Taylor JL, Gandevia SC (2013) Testing the excitability of human motoneurons. Front Hum Neurosci 7:152. doi:10.3389/fnhum.2013.00152
Mileva KN, Bowtell JL, Kossev AR (2009) Effects of low-frequency whole-body vibration on motor-evoked potentials in healthy men. Exp Physiol 94:103–116. doi:10.1113/expphysiol.2008.042689
Munte TF, Jobges EM, Wieringa BM, Klein S, Schubert M, Johannes S, Dengler R (1996) Human evoked potentials to long duration vibratory stimuli: role of muscle afferents. Neurosci Lett 216:163–166
Naito E, Ehrsson HH (2001) Kinesthetic illusion of wrist movement activates motor-related areas. NeuroReport 12:3805–3809
Naito E, Ehrsson HH, Geyer S, Zilles K, Roland PE (1999) Illusory arm movements activate cortical motor areas: a positron emission tomography study. J Neurosci 19:6134–6144
Perez MA, Lungholt BK, Nyborg K, Nielsen JB (2004) Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res 159:197–205. doi:10.1007/s00221-004-1947-5
Proske U (1997) The mammalian muscle spindle. News Physiol Sci 12:37–42
Ridding MC, Brouwer B, Miles TS, Pitcher JB, Thompson PD (2000) Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res 131:135–143
Rittweger J (2010) Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol 108:877–904. doi:10.1007/s00421-009-1303-3
Roll JP, Gilhodes JC, Tardy-Gervet MF (1980) Effects of vision on tonic vibration response of a muscle or its antagonists in normal man. Experientia 36:70–72
Roll JP, Vedel JP, Ribot E (1989) Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res 76:213–222
Rollnik JD, Siggelkow S, Schubert M, Schneider U, Dengler R (2001) Muscle vibration and prefrontal repetitive transcranial magnetic stimulation. Muscle Nerve 24:112–115
Rosenkranz K, Rothwell JC (2003) Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol 551:649–660. doi:10.1113/jphysiol.2003.043752
Rosenkranz K, Rothwell JC (2006) Differences between the effects of three plasticity inducing protocols on the organization of the human motor cortex. Eur J Neurosci 23:822–829. doi:10.1111/j.1460-9568.2006.04605.x
Rosenkranz K, Pesenti A, Paulus W, Tergau F (2003) Focal reduction of intracortical inhibition in the motor cortex by selective proprioceptive stimulation. Exp Brain Res 149:9–16. doi:10.1007/s00221-002-1330-3
Siggelkow S, Kossev A, Schubert M, Kappels HH, Wolf W, Dengler R (1999) Modulation of motor evoked potentials by muscle vibration: the role of vibration frequency. Muscle Nerve 22:1544–1548
Steyvers M, Levin O, Verschueren SM, Swinnen SP (2003) Frequency-dependent effects of muscle tendon vibration on corticospinal excitability: a TMS study. Exp Brain Res 151:9–14. doi:10.1007/s00221-003-1427-3
Swayne O, Rothwell J, Rosenkranz K (2006) Transcallosal sensorimotor integration: effects of sensory input on cortical projections to the contralateral hand. Clin Neurophysiol 117:855–863. doi:10.1016/j.clinph.2005.12.012
Wiesendanger M, Miles TS (1982) Ascending pathway of low-threshold muscle afferents to the cerebral cortex and its possible role in motor control. Physiol Rev 62:1234–1270
Acknowledgments
We sincerely acknowledge Dr. Léonard Féasson for conducting medical inclusions and Régis Bonnefoy for technical assistance. We also thank Rodolphe Testa and Thierry Busso for helping with statistical analysis. J.T. was supported by a doctoral research grant from the Rhône-Alpes Region. P.A. was supported by a doctoral research grant from the General Directorate for Armament, Ministry of Defence (France).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lapole, T., Temesi, J., Gimenez, P. et al. Achilles tendon vibration-induced changes in plantar flexor corticospinal excitability. Exp Brain Res 233, 441–448 (2015). https://doi.org/10.1007/s00221-014-4125-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-014-4125-4