Abstract
The purpose of this study was to investigate the effect of 2 weeks of ankle joint immobilization on triceps surae neural activation, with particular emphasis on the potential differences between the monoarticular soleus and the biarticular gastrocnemius muscles. Seventeen male volunteers were divided into the immobilized group (IG, n=8) and the control group (CG, n=9). Elastic adhesive bandages and an ankle stabilization orthosis were used to immobilize the ankle joint only. The plantar flexor torque obtained during maximal voluntary contractions (MVC) and after single, paired and tetanic stimuli applied at rest was measured. The associated EMG activity from the soleus and gastrocnemius muscles was also recorded, and their activation levels were estimated by means of the twitch interpolation technique. After immobilization, triceps surae maximal voluntary torque significantly decreased by 17% (P<0.001). Strength losses were accompanied by a decrement in activation level (−6%, average of the three techniques used) and in maximal 100 Hz tetanic force (−11%). A significant decrease in the soleus (−22%, P<0.05) but not in the gastrocnemius EMG activity, normalized to respective M-waves, was also found. It was concluded that the reduced voluntary torque output after immobilization could be attributed to both muscular and neural alterations. These latter selectively involved the monoarticular soleus muscle, while neural drive to the biarticular gastrocnemii, which had not been immobilized in their function as knee flexors, was preserved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well established that reduced physical activity induced by immobilization can lead to a decrease in maximal voluntary strength (Davies et al. 1987; Hortobagyi et al. 2000; Thom et al. 2001; White et al. 1984). Many studies have shown that such impairment is typically associated with significant loss of muscle mass (Hespel et al. 2001; Miles et al. 1994; Thom et al. 2001; Veldhuizen et al. 1993). For example, Veldhuizen et al. (1993) found that the strength of the quadriceps femoris muscle decreased by 53% after 4 weeks of knee immobilization, whereas the decrease in the anatomical cross-sectional area (CSA) assessed by computed tomography was 21%. The same discrepancies between strength and anatomical CSA reductions, measured by ultrasonography or nuclear magnetic resonance imaging, have been observed following 10 and 14 days of knee immobilization respectively, by Thom et al. (2001) and Hespel et al. (2001). Whatever the methodology used to measure the anatomical CSA, the magnitude of the voluntary strength loss appears to be 3- to 4-fold greater than the magnitude of muscle atrophy (Hespel et al. 2001; Thom et al. 2001), therefore suggesting that immobilization may also affect neural activation. This point of view is reinforced by the greater reduction in maximal voluntary contraction (MVC) compared with maximal tetanic force observed after short- or long-term immobilization (Davies et al. 1987; Duchateau and Hainaut 1987; White et al. 1984), which indicates an inability of the central nervous system to activate the immobilized muscle adequately. Consequently, it may be inferred that strength losses induced by immobilization are be attributable to muscular as well as neural alterations.
Many investigations have examined surface electromyographic (EMG) activity following unloading interventions in humans and have reported either significant reductions (Deschenes et al. 2002; Dudley et al. 1992; Hortobagyi et al. 2000; Schulze et al. 2002; Seki et al. 2001b) or no changes (Berg and Tesch 1996). For example, lower vastus medialis and vastus lateralis EMG activity has been observed following 3 weeks of knee immobilization (Hortobagyi et al. 2000) and after 2 weeks of limb suspension (Deschenes et al. 2002), whereas no changes in the average EMG activity of three muscles of the quadriceps has been reported following 10 days of lower limb unloading (Berg and Tesch 1996). Nevertheless, in this latter study the selective adaptation of monoarticular muscles would have been masked by the fact that the EMGs of the vastus medialis, the vastus lateralis and the biarticular rectus femoris muscle were averaged together. Indeed, Deschenes et al. (2002) recently suggested that in its hip flexor function, the rectus femoris muscle would continue to be regularly recruited during the forward swing phase of crutch-assisted ambulation. The same is true for the triceps surae (TS) muscle, since the monoarticular soleus is maintained at a near constant length during ankle (but not knee) immobilization, while the biarticular gastrocnemii are immobilized in their function of plantar flexors but continue to be regularly activated in their function of knee flexors. Therefore, it can be hypothesized that short-term ankle joint immobilization would mainly impair neural activation of the monoarticular soleus and have little or no effect on the biarticular gastrocnemii.
The main aim of the present study was to investigate the effect of 2 weeks of ankle joint immobilization on TS neural activation, with particular emphasis on the potential differences between the soleus and gastrocnemius EMG activity. The EMG and mechanical response obtained under maximal voluntary and electrically evoked (single, paired and tetanic stimuli) conditions were used to distinguish neural adaptations from contractile changes. For the first time in an immobilization study, neural activation was carefully investigated by using the twitch interpolation technique and three different formulas for the estimation of the voluntary activation level and by normalizing the EMG activity from the soleus and the gastrocnemius muscles to the amplitude of their respective M-waves.
Methods
Subjects
After receiving ethical approval from the University of Burgundy Committee on Human Research, 17 male subjects gave written consent to participate in this study. Subjects who had had ankle surgery or any other orthopedic and neurological abnormalities of the lower limb were excluded from the study. Eight subjects [age 25.8 (1.6) years, height 176.4 (2.0) cm, body mass 70.0 (2.6) kg] were assigned randomly to the immobilized group (IG), with the remaining subjects assigned to the control group [CG; n=9, age 24.7 (1.1) years, height 181.3 (2.1) cm, body mass 76.8 (3.1) kg]. For all subjects, the neuromuscular properties of the right TS were tested before and after a 2-week period, with the IG subjects immobilized as detailed below. All procedures used in this study conformed with the Declaration of Helsinki.
Experimental design
On the basis of a previous power analysis where repeated measures of isometric torque were performed, a sample size of ten subjects was required. Because of technical and financial limitations, eight volunteers participated in the IG. We expected that the use of eight subjects would lead to insufficient statistical power. However, in previous immobilization studies using fewer or the same number of subjects, a significant decline of muscle strength was demonstrated (Duchateau and Hainaut 1987, Duchateau and Hainaut 1990; Kitahara et al. 2003; White et al. 1984; Thom et al. 2001; Veldhuizen et al. 1993). Consequently, we concluded that eight subjects would be sufficient for this study.
Muscle immobilization
Before immobilization, the skin of the leg was cleaned with household soap and shaved. The right ankle joint was then held flexed at 90° (i.e., the sole of the foot perpendicular to the tibia) as detailed below. Two tapes, the anchors, were applied in a circular way and without tension. The proximal anchor was located distal to the muscle belly of the gastrocnemii and the distal anchor was at mid-foot level. A first elastic adhesive bandage, the stirrup, (6 cm, Elastoplaste HB, Smith & Nephew, Le Mans, France) was applied from the medial to the lateral side of the leg, beginning and ending at the upper anchor, with tension on the lateral side. Next, a second bandage, the horseshoe, was applied medially from the lower anchor on the mid-foot around the Achilles tendon, crossing the first bandage in the area of the anterior talofibular ligament on the lateral side and finishing on the lower anchor laterally. This procedure was repeated three times making sure to slightly overlap each successive stirrup and horseshoe strap. The strapping was then maintained by an ankle stabilization orthosis (Aircast, Europa, Stephanskirchen, Germany). In this way, only the ankle joint was immobilized (Fig. 1a, b). The subjects were then fitted with crutches and instructed not to use their immobilized ankle during the experimentation, while knee extension and flexion were still possible. Each subject reported to the laboratory every 3–4 days to have the strapping replaced.
Stimulation and mechanical recordings
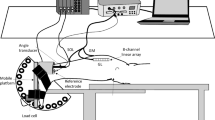
During testing, subjects were seated with the trunk inclined at 30° with respect to the vertical and with a 120° angle at the knee and a 90° angle at the ankle joint. The foot was secured to a pedal equipped with strain gauges, which had been developed by the local engineering school. The posterior tibial nerve was stimulated using a cathode ball electrode (0.5 diameter) pressed and maintained by the experimenter in the popliteal fossa. The cathode was hand-held by the same experimenter throughout the testing session. The anode was a large rectangular electrode (5 cm×10 cm, Medicompex, Ecublens, Switzerland), placed on the anterior surface of the knee. The percutaneous electrical stimulus was a rectangular pulse (1 ms duration) delivered by a Digitimer stimulator (Model DS7, Hertfordshire, England).
EMG recordings
The EMG activity was recorded from the soleus, and the medial and lateral gastrocnemius muscles, by means of two silver-chloride surface electrodes of 10 mm diameter, with an inter-electrode (center to center) distance of 2.5 cm. For the soleus, the recording electrodes were placed along the mid-dorsal line of the leg, ~5 cm below the insertion of the gastrocnemii on the Achilles tendon. The medial and lateral gastrocnemius electrodes were fixed lengthwise over the middle of the muscle belly. The reference electrode was placed in a central position on the same leg. The placement of the electrodes was marked on the skin with indelible ink so that it could be exactly repositioned for the post-tests. Low impedance (<5 kΩ) between the electrodes was obtained by abrading the skin with emery paper and cleaning with alcohol. EMG signals were amplified with a bandwidth frequency ranging from 15 Hz to 2 kHz (common mode rejection ratio =90 dB; Z input =100 MΩ; gain =1,000).
Experimental procedure
Each subject was initially familiarized with several submaximal (1–20 mA) single electrical stimuli over a period of 10 min. The current intensity was then progressively increased until the maximal intensity, i.e., when plantar flexor twitch torque and soleus M-wave amplitude reached a maximal value. At this intensity, four single pulses with a 5-s interval between stimuli, three doublets (i.e., paired stimuli with a 10-ms interval) each separated by 5 s and two 500-ms tetani (20 Hz and 100 Hz) were delivered under resting conditions. This tetanus duration was long enough to induce a plateau in the mechanical response and less painful for the subjects than longer-duration tetani. Two-minute rest periods were allowed between different stimuli and between tetani. The subject was then asked to perform three 3-s isometric MVC of the plantar flexor muscles. Paired stimuli were delivered 4 s before the MVC (resting doublet), over the isometric plateau (superimposed doublet) and 4 s after the contraction (potentiated doublet) to assess the level of voluntary activation. Each muscular action was separated by 3 min to minimize the effects of fatigue. Immediately after this testing session, IG subjects were immobilized for 2 weeks as previously described. The subjects were then asked to return to the laboratory at the end of immobilization period to repeat the same testing session.
Data analysis
Mechanical and EMG traces were digitized on-line (sampling frequency 2 kHz) and stored with commercially available software (Tida, Heka Elektronik, Lambrecht/Pfalz, Germany) for data analysis. Torque and EMG from the highest MVC were analyzed over a 1-s period once the torque had reached a plateau and before the superimposed stimuli. For the soleus muscle, the EMG root mean square (RMS) values were calculated and then normalized to the peak-to-peak amplitude of the maximal M-wave (i.e., RMS/M ratio). For the gastrocnemius muscles, the sum of RMS values (i.e., medial + lateral gastrocnemius) was normalized to the sum of the two M-waves to obtain the gastrocnemius RMS/M ratio. Concerning the electrically evoked contractions, EMG and mechanical signals were averaged and peak-to-peak amplitude (A) and duration (D) of the soleus, medial and lateral gastrocnemius maximal M-wave were calculated. The gastrocnemius amplitude and duration were then averaged together. The following twitch contractile properties were calculated: (1) peak twitch (Pt), the highest value of twitch torque production; (2) time-to-peak twitch (TPT), the time to obtain twitch maximal torque, calculated from the origin of the mechanical signal; (3) half-relaxation time (HRT), the time to obtain half of the decline in twitch maximal torque. For the paired stimuli, the peak torque (PtPS) and the time-to-peak twitch (TPTPS) were measured. Three different techniques were used to estimate the individual level of voluntary activation (VA).
Firstly, the ratio of the amplitude of superimposed doublet over the size of the doublet evoked at rest was calculated (i.e., VA-RD), as proposed by Klass et al. (2004): VA−RD (%) = (1− superimposed doublet/resting doublet) ×100.
Secondly, the ratio of the amplitude of superimposed doublet over the size of the potentiated doublet was calculated (i.e., VA-PD), as suggested by Behm et al. (1996): VA−PD (%) = (1− superimposed doublet/potentiated doublet) ×100.
Thirdly, the central activation ratio (CAR) was calculated according to the following equation (Kent-Braun and Le Blanc 1996): CAR = [MVC/total force (i.e., superimposed doublet + MVC)] ×100. For the 20 Hz and 100 Hz tetani, the respective peak torque (P100 and P20) and the P20/P100 and the Pt/P100 ratios were calculated.
Statistical analysis
Ordinary statistical methods including means and their standard errors (SE) were calculated for each parameter. Two-factor (group/time) ANOVA with repeated measures on time were used to compare the dependent variables. When significant treatment effects occurred, Student-Newman-Keuls post hoc tests were used to test differences among the means. The level of significance was fixed at P≤0.05 for all the procedures. The statistical analyses were undertaken by using Statistica software for Microsoft Windows (StatSoft, version 6.1, Tulsa, USA).
Results
At baseline, no significant differences were found between the two groups for the ensemble of variables considered.
MVC, EMG activity and activation level
There was a significant group × time interaction for MVC (P<0.01). The MVC torque significantly decreased by 17 (3)% after immobilization [124 (6) N.m vs 103 (7) N.m, P<0.001] while no significant changes occurred in the CG [139 (13) N.m vs 138 (12) N.m].
The soleus RMS/M ratio showed a significant group × time interaction (P<0.05). The ratio was significantly decreased by 22 (8)% (P<0.05; Fig. 2a) as a result of the immobilization, whereas no changes were observed in the CG. There was no group × time interaction for the gastrocnemii RMS/M ratio (P>0.05) (Fig 2b).
Normalized EMG activity (RMS/M ratio) for respective muscles obtained during maximal plantar flexion before (black bars) and after (white bars) the 2-week period in IG (n=8) and CG (n=9). a Soleus, b gastrocnemius. All values are means (SE). §P<0.05 Significantly lower than the value before immobilization
A significant group × time interaction was observed for the three methods used to estimate the central activation (P<0.05). After immobilization, VA-RD, VA-PD and CAR were significantly decreased by 8 (4)%, 8 (4)% and 4 (1)% (P<0.05), respectively (Table 1) while no changes were observed in the CG (Table 1).
Contractile properties and M-waves
No interactions for Pt, PtPS and TPTPS were noted between the groups and the sessions (Table 2). However, TPT and HRT showed significant group × time interactions (P<0.01 and P<0.05, respectively). TPT increased significantly after immobilization [+17 (4)%, P<0.001] while HRT displayed a trend towards a significant increase [+12 (4)%, P=0.06]. Furthermore, post-immobilization TPT was found to be higher (P<0.01) in the IG compared to the CG (Table 2). These two parameters remained unchanged in the CG (Table 2).
A significant group × time interaction was noted for P100 (P<0.05). The torque evoked by the 100-Hz tetanic stimulation decreased significantly [−11 (3)%, P<0.05] after immobilization whereas no change was observed in the CG (Table 2). No interaction between group and time was observed for the 20-Hz tetanic torque. However, there was a significant group × time interaction for the P20/P100 ratio (P<0.05). This ratio significantly increased [+24 (10)%, P<0.05] following immobilization but remained unchanged in the CG. Finally, no significant group × time interaction was observed for the Pt/P100 ratio.
A significant interaction between the group and the sessions was noted for the soleus M-wave duration. This duration increased significantly [+32 (12)%, P<0.05; Table 3] after immobilization but remained unchanged in the CG (Table 3). No interactions were observed for the other characteristics of the M-waves (Table 3).
Discussion
This study demonstrated that 2 weeks of ankle joint immobilization significantly reduced the MVC of the triceps surae muscle in able-bodied humans, and this was accompanied by a significant decrease in the triceps surae voluntary activation, the normalized EMG activity for the soleus but not for the gastrocnemius muscles, and the maximal 100 Hz tetanic force.
The relative decrement in MVC torque obtained in the present study (17%) is similar to the results of Davies et al. (1987) and White et al. (1984), who recorded a 19% and 24% reduction respectively in plantar flexion torque following 2 weeks of immobilization. The 17% decrease in MVC induced by the present immobilization period was higher than the reduction in 100 Hz tetanic force (−11%), in line with previous research (Davies et al. 1987; Duchateau and Hainaut 1987). This allows us to infer that peripheral changes cannot fully account for the voluntary strength reductions observed here. Several authors reported a decrease in maximal tetanic force after immobilization in both human (Davies et al. 1987; Duchateau and Hainaut 1987; Seki et al. 2001b) and animal muscles (Booth 1982) that was attributed to a reduction in anatomical CSA. However, many other studies in rats (Gardetto et al. 1989) and in humans (Larsson et al. 1996; Narici et al. 2003; Widrick et al. 1999) showed that specific tension (i.e., force per CSA) of single fibers (Gardetto et al. 1989; Larsson et al. 1996; Widrick et al. 1999) or whole muscle (Narici et al. 2003) can also be reduced following hindlimb suspension (Gardetto et al. 1989), bed-rest (Larsson et al. 1996) or spaceflight (Narici et al. 2003; Widrick et al. 1999) experiments. Muscle damage (Narici et al. 2003) and/or a decreased number of force-generating cross-bridges (Gardetto et al. 1989; Larsson et al. 1996; Widrick et al. 1999) are classically proposed to explain this latter alteration. It can therefore be conjectured that the decrement in strength induced by the present immobilization treatment could be partially ascribed to muscular changes (i.e., muscular atrophy or specific tension decrement), although this was not directly quantified. Nevertheless, the ~35% relative difference between the MVC and the maximal tetanic force loss indicated an inability of the central nervous system to activate the muscle adequately after immobilization.
Contrary to the 100 Hz tetanus findings, we observed no changes in the peak torque for 20 Hz tetanus after immobilization, and, as a consequence, the P20/P100 ratio increased significantly. Our results are consistent with several reports (Davies et al. 1987; Seki et al. 2001b; White et al. 1984) which showed no changes in 20 Hz tetanus properties following immobilization. Another common finding is that the TPT increased significantly while the associated peak torque was unchanged following immobilization (Davies et al. 1987; White et al. 1984). It is likely that the longer TPT observed here after the intervention resulted in a reduction of tetanic fusion frequency (Seki et al. 2001b). Thus, at the lower frequency range (i.e. 20 Hz), where the twitches are largely unfused, the longer TPT might induce a better twitch summation caused by alteration in excitation-contraction coupling. In contrast, in the higher frequency range, where the twitches are largely fused, the evoked contraction force might be more influenced by the atrophy induced by immobilization (Seki et al. 2001b).
Changes in twitch contractile properties and in the associated M-wave characteristics reflect alterations of the excitation-contraction coupling (Desmedt and Hainaut 1968; Seki et al. 2001a). In the current study, the longer TPT could be related to Ca2+ kinetic modifications, i.e., slower Ca2+ release. However, Thom et al. (2001) reported no alteration in the maximal rate of sarcoplasmic reticulum Ca2+ release following 10 days of knee immobilization. Nevertheless, a slowing of the conduction velocity along the membranes of the muscle fibers would have occurred, as indicated by the increase in soleus M-wave duration. This longer M-wave could be ascribed to an alteration of the neuromuscular transmission due to immobilization as previously reported by Grana et al. (1996) for the soleus muscle. Such an increase in M-wave duration may also contribute to the longer TPT observed in the current study. Similarly, Duchateau and Hainaut (1987) found a longer TPT and an increased adductor pollicis M-wave duration following 6 weeks of human thumb immobilization. TPT and M-wave results taken as a whole indicate that the present immobilization program resulted in a slowing of the excitation-contraction coupling. In the current study, a trend towards a significant increase was observed for the HRT. Two authors (Davies et al. 1987; White et al. 1984) have reported an increased HRT following short-term immobilization of the triceps surae muscle and this enhancement could be explained by a reduction in the uptake of Ca2+ (Thom et al. 2001). To summarize, even though changes in ionic membrane and intracellular processes took place following the present immobilization program, the exact origin of these alterations remains to be determined.
Although very few studies have directly examined neural activation following disuse, many investigators have suggested that neural adaptations are partly responsible for the strength losses observed (Antonutto et al. 1999; Davies et al. 1987; Duchateau and Hainaut 1987; Kawakami et al. 2001; Seki et al. 2001a; White et al. 1984). For example, Duchateau and Hainaut (1987) reported a greater reduction in MVC than in maximal tetanic force, suggesting an inability of the central nervous system to activate the muscle adequately after immobilization. An impairment of the maximal voluntary activation estimated by using the twitch interpolation technique has been previously reported for the knee extensor muscles after 20 days of bed-rest (Kawakami et al. 2001) and after 8 weeks of immobilization in a cast (Vandenborne et al. 1998). The present study demonstrated a significant reduction of voluntary activation following shorter-term immobilization (2 weeks) in healthy humans. Possible changes in voluntary activation induced by immobilization were studied with three different techniques in our investigation. The triceps surae maximal voluntary activation was assessed by using both potentiated and resting doublets, and the central activation ratio was estimated according to the formula proposed by Kent-Braun and Le Blanc (1996). After 2 weeks of immobilization, the VA-RD, VA-PD and CAR were significantly decreased by 8%, 8% and 4%, respectively. These results demonstrated that immobilization-induced strength losses could also be ascribed to an impaired ability to activate motor units. These methods allow the study of the level of activation of all the muscles innervated by the tibial nerve. Nevertheless, the twitch interpolation technique does not permit the investigation of the neural activation of individual muscles and thus the potential selective effect of immobilization on the monoarticular soleus muscle as opposed to the biarticular gastrocnemii.
The soleus and gastrocnemius RMS EMG values obtained during voluntary contractions were normalized to the respective M-wave in order to better characterize the neural activation of each muscle composing the triceps surae. Soleus activity significantly decreased while no changes were observed for the gastrocnemius muscles. Similar to the present findings obtained on the monoarticular soleus, the EMG activity from the monoarticular vastii muscles has been found to be reduced following 3 weeks of knee immobilization (Hortobagyi et al. 2000). On the other hand, it is not surprising to observe that gastrocnemius EMG activity was not significantly lower after our single-joint immobilization program. Indeed, even if the gastrocnemii were immobilized in their main function (i.e., plantar flexion), their knee flexion activity during crutch-aided ambulation would have counteracted the deleterious effects of immobilization, also in line with the observations of Deschenes et al. (2002). To the best of our knowledge, the present study is the first that has demonstrated a selective impairment of the neural drive to the monoarticular soleus following short-term immobilization of the ankle joint.
In conclusion, the present study has demonstrated that not only muscular but also neural alterations are responsible for the plantar flexor MVC torque reduction induced by 2 weeks of immobilization of the ankle joint. Single ankle joint immobilization significantly reduced the neural drive of the monoarticular soleus muscle but not of the biarticular gastrocnemii. Future research is warranted to ascertain whether short-term immobilization of two contiguous joints (e.g., knee and ankle or hip and knee) would alter the neural activation of monoarticular and biarticular muscles to the same extent.
References
Antonutto G, Capelli C, Girardis M, Zamparo P, di Prampero PE (1999) Effects of microgravity on maximal power of lower limbs during very short efforts in humans. J Appl Physiol 86:85–92
Behm DG, St-Pierre DM, Perez D (1996) Muscle inactivation: assessment of interpolated twitch technique. J Appl Physiol 81:2267–2273
Berg HE, Tesch PA (1996) Changes in muscle function in response to 10 days of lower limb unloading in humans. Acta Physiol Scand 157:63–70
Booth FW (1982) Effect of limb immobilization on skeletal muscle. J Appl Physiol 52:1113–1118
Davies CT, Rutherford IC, Thomas DO (1987) Electrically evoked contractions of the triceps surae during and following 21 days of voluntary leg immobilization. Eur J Appl Physiol Occup Physiol 56:306–312
Deschenes MR, Giles JA, McCoy RW, Volek JS, Gomez AL, Kraemer WJ (2002) Neural factors account for strength decrements observed after short-term muscle unloading. Am J Physiol Regul Integr Comp Physiol 282:R578–R583
Desmedt JE, Hainaut K (1968) Kinetics of myofilament activation in potentiated contraction: staircase phenomenon in human skeletal muscle. Nature 217:529–532
Duchateau J, Hainaut K (1987) Electrical and mechanical changes in immobilized human muscle. J Appl Physiol 62:2168–2173
Duchateau J, Hainaut K (1990) Effects of immobilization on contractile properties, recruitment and firing rates of human motor units. J Physiol (Lond) 422:55–65
Dudley GA, Duvoisin MR, Adams GR, Meyer RA, Belew AH, Buchanan P (1992) Adaptations to unilateral lower limb suspension in humans. Aviat Space Environ Med 63:678–683
Gardetto PR, Schluter JM, Fitts RH (1989) Contractile function of single muscle fibers after hindlimb suspension. J Appl Physiol 66:2739–2749
Grana EA, Chiou-Tan F, Jaweed MM (1996) Endplate dysfunction in healthy muscle following a period of disuse. Muscle Nerve 19:989–993
Hespel P, Op’t Eijnde B, Van Leemputte M, Urso B, Greenhaff PL, Labarque V, Dymarkowski S, Van Hecke P, Richter EA (2001) Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J Physiol (Lond) 536:625–633
Hortobagyi T, Dempsey L, Fraser D, Zheng D, Hamilton G, Lambert J, Dohm L (2000) Changes in muscle strength, muscle fibre size and myofibrillar gene expression after immobilization and retraining in humans. J Physiol (Lond) 524:293–304
Kawakami Y, Akima H, Kubo K, Muraoka Y, Hasegawa H, Kouzaki M, Imai M, Suzuki Y, Gunji A, Kanehisa H, Fukunaga T (2001) Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. Eur J Appl Physiol 84:7–12
Kent-Braun JA, Le Blanc R (1996) Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve 19:861–869
Kitahara A, Hamaoka T, Murase N, Homma T, Kurosawa Y, Ueda C, Nagasawa T, Ichimura S, Motobe M, Yashiro K, Nakano S, Katsumura T (2003) Deterioration of muscle function after 21-day forearm immobilization. Med Sci Sports Exerc 35:1697–1702
Klass M, Guissard N, Duchateau J (2004) Limiting mechanisms of force production after repetitive dynamic contractions in human triceps surae. J Appl Physiol 96:1516–1521
Larsson L, Li X, Berg HE, Frontera WR (1996) Effects of removal of weight-bearing function on contractility and myosin isoform composition in single human skeletal muscle cells. Pflugers Arch 432:320–328
Miles MP, Clarkson PM, Bean M, Ambach K, Mulroy J, Vincent K (1994) Muscle function at the wrist following 9 days of immobilization and suspension. Med Sci Sports Exerc 26:615–623
Narici M, Kayser B, Barattini P, Cerretelli P (2003) Effects of 17-day spaceflight on electrically evoked torque and cross-sectional area of the human triceps surae. Eur J Appl Physiol 90:275–282
Schulze K, Gallagher P, Trappe S (2002) Resistance training preserves skeletal muscle function during unloading in humans. Med Sci Sports Exerc 34:303–313
Seki K, Taniguchi Y, Narusawa M (2001a) Effects of joint immobilization on firing rate modulation of human motor units. J Physiol (Lond) 530:507–519
Seki K, Taniguchi Y, Narusawa M (2001b) Alterations in contractile properties of human skeletal muscle induced by joint immobilization. J Physiol (Lond) 530:521–532
Thom JM, Thompson MW, Ruell PA, Bryant GJ, Fonda JS, Harmer AR, De Jonge XA, Hunter SK (2001) Effect of 10-day cast immobilization on sarcoplasmic reticulum calcium regulation in humans. Acta Physiol Scand 172:141–147
Vandenborne K, Elliott MA, Walter GA, Abdus S, Okereke E, Shaffer M, Tahernia D, Esterhai JL (1998) Longitudinal study of skeletal muscle adaptations during immobilization and rehabilitation. Muscle Nerve 21:1006–1012
Veldhuizen JW, Verstappen FT, Vroemen JP, Kuipers H, Greep JM (1993) Functional and morphological adaptations following four weeks of knee immobilization. Int J Sports Med 14:283–287
White MJ, Davies CT, Brooksby P (1984) The effects of short-term voluntary immobilization on the contractile properties of the human triceps surae. Q J Exp Physiol 69:685–691
Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JL, Riley DA, Karhanek M, Trappe SW, Trappe TA, Costill DL, Fitts RH (1999) Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol (Lond) 516:915–930
Acknowledgements
The authors are indebted to the eight subjects who endured 2 weeks of ankle joint immobilization and to Mr Yves Ballay for his excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gondin, J., Guette, M., Maffiuletti, N.A. et al. Neural activation of the triceps surae is impaired following 2 weeks of immobilization. Eur J Appl Physiol 93, 359–365 (2004). https://doi.org/10.1007/s00421-004-1225-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-004-1225-z