Abstract
The present work aimed to evaluate three varieties of peanut (Arachis hypogaea L.) regarding the content of anthocyanins and total phenolics, antioxidant activity, phenolic profile and the alkaloids trigonelline and theobromine. The total phenolic content was analyzed by the Fast-Blue and Folin–Ciocalteau methods, while the antioxidant activity was analyzed by the β-carotene/linoleic acid, ABTS+ and FRAP methods. The phenolic profile and alkaloids were determined using high performance liquid chromatography (HPLC–DAD-UV–Vis). Peanuts with beige shells stood out with higher antioxidant activity and total phenolic content, while peanuts with purple shells stood out with higher anthocyanin content. Eight phenolic compounds and the alkaloids trigonelline and theobromine have been identified in peanut varieties. However, only four phenolics (gallic, syringic, ferulic and transcinnamic acids), in addition to the two alkaloids, were identified simultaneously in the three varieties studied. Chlorogenic acid was absent in red and purple-seeded varieties, catechin and resveratrol in varieties with red and beige coats, and p-coumaric acid in those with beige coats. For the first time, trigonelline and theobromine were identified in peanuts. Therefore, the bioactive composition and antioxidant activity of peanuts depend on its genotype.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arachis Hypogaea L. is a herbaceous plant belonging to the Fabaceae family, commonly known as Leguminoseae. Its fruits develop, atypically, underground, and have a leathery coat that protects the seeds, called peanuts. Its origin is believed to date back to Central America, from where it spread to other parts of the world [1], being cultivated and consumed in the tropics and subtropics, in more than 100 countries [2]. China is the largest producer of peanuts with an estimated production of 18.3 million tons in 2021, followed by India which produced just over 10 million tons, also in 2021 [3]. The peanut is the second most important legume cultivated globally, and contributes greatly to the diet of many people [4]
In Brazil, according to the National Supply Company (Conab), peanut production in 2023 was approximately 893 thousand tons, having great importance from an economic point of view for the country. The production of legumes is largely intended for export of the grain in natura or manufactured mainly as oil, which has contributed to the development of agriculture and Brazilian agroindustry [5].
Peanuts are an important vehicle for oils, proteins, vitamins B (thiamine, riboflavin, niacin, folate, and vitamin B6), vitamin E, and fiber [6]. In addition to its nutritional value, it is considered an excellent source of functional compounds [7], with emphasis on phytosterols, phenolic compounds, stilbenes, lignans, and isoflavonoids. It presents high antioxidant activity, which is related to the combat of various diseases [8]. Its intake is related to improving memory function and the response to stress in young people [6], in addition to reducing the risk of diabetes, cardiovascular diseases, cancer, Alzheimer's, and gallstones [9].
Raw peanut kernel contains polyphenols, p-hydroxybenzoic acid, chlorogenic acid, p-coumaric acid, ferulic acid, catechin, epicatechin, resveratrol, quercertin, kaempferol, and procyanidins [6, 10,11,12,13]. The composition and amount of bioactive compounds are different in each of the parts of the peanut [6]. The tegument, a thin layer that superficially covers the grain, is the fraction of the grain that most concentrates bioactive compounds. It can have different colors, such as reddish pink, black, white, and even multicolored, depending on the variety [14]. Therefore, the seed coat influences the content of phenolic compounds and the antioxidant activity of peanuts [15]. In addition to the seed coat, other factors can influence the composition of phenolic compounds in peanuts, such as genotype, growing conditions, disease resistance, post-harvest storage, and heat treatment [12, 16].
After an extensive review of the literature, no reports were found on the presence of theobromine and trigonelline in peanuts. The beneficial health effects of these alkaloids have been reported in recent years [17,18,19,20,21]. Furthermore, there was a lack of studies on the phenolic profile and antioxidant capacity of fresh peanuts, and no studies were found that investigated the correlation between the color of the seed coat and the presence of bioactive compounds. Therefore, the objective of the present work was to evaluate the content of bioactive compounds and the antioxidant activity of three peanut varieties, seeking to correlate these variables with each other and with the color of the seed coat.
Material and methods
Experimental design
The experiment was conducted in a simple completely randomized design (DIC) consisting of 3 varieties of peanuts (Arachis hypogaea L.), easily distinguishable by the color of the seed coat, namely: “Virgínia” (beige), “Valência” (red), and “ Roxo creole” (purple), in 6 replicas.
Plant material
Peanuts (Arachis hypogaea L.) of three varieties, classified according to the color of the seed coat as beige, red, and purple, of the varieties “Virgínia”, “Valência”, and “Roxo creole”, respectively (Fig. 1), were harvested in the city of Santana do Garambéu in Campos das Vertentes, Minas Gerais, Brazil (Latitude: 21° 34′ 30″ South, Longitude: 44° 4′ 49″ West, 1044.52 m altitude). The region’s climate is classified According to Köppen's [22], as type Cwb, mild temperate (mesothermal) or high-altitude tropical, with dry winters and rainy summers. The average annual precipitation is 1.387 mm and the average annual temperature is 18.4 °C [23]. Most of the soil in the region is described as dystrophic Tb haplic cambisols (66%), followed by red-yellow latosols (9.4%), red latosols (7%), litholic neosols (5.95%), humic cambisols (4.77%), fluvic neosols (2.51%), and hydromorphic soils (1.09%) [24].
The peanuts were harvested at the ideal point of maturation, which the ideal harvest point being considered when 70–80% of the pods had a dark internal color, and when the grains were filled according to the characteristic color of each variety. Approximately 3 kg of peanuts from each variety were separated and packaged in six low-density polyethylene packages, each containing about 500 g of peanuts, considered replicates.
The peanuts were transferred to the Fruit and Vegetable Post-harvest Laboratory at the Federal University of Lavras, where they were prepared and analyzed, discarding those with the presence or signs of pathogens, pests, and defects. Immediately after peeling, the grains with the seed coat intact were analyzed for color, texture, size, and mass and then homogenized in an industrial blender, sprayed with liquid nitrogen, and stored in an ultrafreezer (Coldlab-CL374-86 V) at −80 °C, until the moment of carrying out the other analyses.
Analysis
Anthocyanins
The total anthocyanin content was estimated spectrophotometrically [25]. The anthocyanic compounds were extracted by exposing 1 g of sample to 25 mL of ethanol acidified with hydrochloric acid, until pH = 1), for 1 h, at room temperature. The extract was filtered through filter paper (qualitative filter paper, 15 cm in diameter, Unifil®), completing the volume to 50 mL with acidified ethanol. After this procedure, a spectrophotometer was read at a wavelength of 535 nm, considering the acidified ethanol as white. The results were expressed in milligrams of cyanidin-3-glycoside per 100 g of sample, according to the following formula.
Total anthocyanins (mg 100 g−1) = (A*MM*FD/€*1)*100, where: A = sample absorbance; MM = molecular mass of cyanidin 3-glucoside = 449.2; FD = dilution factor; € = molar absorptivity = 26,900.
Total phenolics and antioxidant activity
The extracts for determining total phenolics and antioxidant activity were obtained as described by Whaterhouse [26], with some adaptations. 2.5 g of sample were placed in a centrifuge tube together with 20 mL of 50% methanol and homogenized on a shaking table for 30 min, protected from light. They were centrifuged at 5,300 g for 5 min at 4 °C, with the supernatant transferred to a 50 mL volumetric flask and reserved. 20 mL of acetone were added to the residue from the first extraction and the extract was taken to an ultrasound bath for 30 min, and filtered through filter paper (qualitative filter paper, 15 cm in diameter, Unifil®). The filtrate was transferred to a flask to which was added 50 mL of distilled water. The extracts were used for spectrophotometric determination of total phenolics, using the Folin–Ciocalteau [27] and Fast Blue [28] methods, and antioxidant activity, using the β-Carotene/linoleic acid methods [29], ABTS+ [30], and FRAP [31]. All measurements were performed in triplicate using a 96-well microplate reader (Biochrom, EZ Read 2000). 30 µL of the extract was mixed with 150 µL of 10% (v/v) Folin–Ciocalteau reagent. After four minutes, another 120 µL of a 4% (w/v) sodium carbonate solution was added. This reaction mixture was incubated for 2 h, away from light, and the total phenolic content was determined at 720 nm. Two hundred microliters of the extract were mixed with 20 µL of Fast Blue reagent (0.1%, v/v) and 20 µL of sodium hydroxide (5%, w/v), and the absorbance measured at 420 nm, after 1.5 h of incubation in the dark. Total phenolic results were reported as gallic acid equivalents in milligrams per 100 g of sample fresh weight (mg GAE 100 g−1 FM).
The determination of antioxidant activity through the β-carotene/linoleic acid method was based on the oxidation of β-carotene (discoloration) induced by the oxidative degradation products of linoleic acid. Solutions were prepared by mixing 270 μL of β–carotene/linoleic acid system solution and 20 μL of extract. The mixture was kept in a water bath at 40 °C for two hours, and its absorbance was determined at 470 nm. The results were expressed as percentage of oxidation inhibition.
The determination of antioxidant activity by the ABTS+ method was based on the capture of the ABTS+ radical by an antioxidant. Briefly, the ABTS+ solution was prepared by reacting the diammonium salt 2,2′-azi-nobis (3-ethylbenzothiazoline-6-sulfonic acid) at a concentration of 7 mmol L−1 with 2.45 mmol L−1 potassium persulfate at room temperature for 16 h. The obtained solution was then diluted with ethanol to an absorbance of 0.70 ± 0.05 at 734 nm. Aliquots of 3 μL of sample extracts were pipetted together with 297 μL of ABTS+ solution. After 6 min of reaction, protected from light, the absorbance was measured at 734 nm. Antioxidant activity results were expressed as µMol equivalents of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (TROLOX) per gram of fresh sample, based on a calibration curve with known concentrations of TROLOX [30].
Antioxidant activity was also measured based on iron reducing power (FRAP) in the presence of antioxidants. FRAP reagent was prepared by mixing 40 mM TPTZ (diluted in 40 mM HCl), 300 mM acetate buffer (pH 3.6), and 20 mM FeCl3 in a ratio of 10:1:1 (v/v/v). A 9 µL aliquot of the extract was mixed with 269 µL of the FRAP reagent and 27 µL of distilled water, incubated at 37 °C for 30 min, and the absorbance was read at 595 nm. For calibration, a five-point standard curve (0–2000 uM) was prepared using ferrous sulfate (FeSO4 • 7H2O) as reference, and the results were reported as µM ferrous sulfate per gram of fresh mass (µM FeSO4 g−1 FM).
Profile of phenolics, trigonelline and theobromine
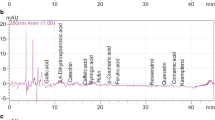
Phenolic compounds and alkaloids were extracted from 2.5 g of sample, homogenized in 20 mL of 70% (v/v) HPLC grade methanol, and kept in an ultrasonic bath, at room temperature, for 1 h. The extract obtained was centrifuged at 8,832 g for 15 min at 4 °C, and was subsequently filtered through quantitative filter paper with 25 µm porosity. Before injection, the extracts were filtered again using a membrane filter with a diameter of 13 mm and a pore size of 0.22 µm. The determination and quantification of phenolics were carried out at the Chemical Analysis and Prospecting Center of the Federal University of Lavras, using a high-efficiency liquid chromatograph (HPLC–DAD-UV–Vis), model Shimadzu, consisting of a quaternary pump LC-20AT, degasser DGU-20A5 (serial no.: L20244808404), injector SIL-20A (serial no.: L20164503197), controller CBM-20A (serial no.: L20234505269), oven CTO-20AC (serial no.: L20214503287), detector SPDM-20A (serial no.: L20154503047), RID-10A detector (series no.: C20934806770), and FRC-10A fraction collector (serial no.: C20374504580). The analysis followed the following parameters: oven temperature 35 °C; Shim-pack VP-ODS column 250 mm × 4.6 mm × 5 µm; and pre-column Shim-pack GVP-ODS 10 mm × 4.6 mm × 5 µm. The mobile phase consisted of 2% (v/v) glacial acetic acid in water type 1.5.10.2 (mobile phase A) and 70:28:2% (v/v/v) methanol/water/acetic acid (mobile phase B), at a flow rate of 1.0 mL min−1 with a gradient elution program running for 65 min. The injection volume was 20 µL and phenolic compounds were detected at 280 nm. Standard solutions were prepared by diluting trigonelline, theobromine, catechin, vanillin, resveratrol, gallic, chlorogenic, caffeic, syringic, p-, o- and m-coumaric, ferulic, rosmarinic, and trans-cinnamic acids in methanol. Discovery curves were found from injections of ten different concentrations of each standard, in duplicate. Phenolic compounds and alkaloids were identified by comparing the retention times of sample peaks with the times of available standards. The results were expressed in mg 100 g−1 of the fresh sample.
Statistical analysis
The results obtained were subjected to analysis of variance and the Tukey test, at 5% significance, using the SISVAR program [32]. The results of the analyzes were autoscaled and then analyzed through principal component analysis (PCA) and hierarchical cluster analysis (HCA), using the Chemoface software (version 1.61, Brazil) [33]. The figures were processed using the Inkscape software (version 0.92.4, USA). Pearson correlations (P < 0.05) between parameters were estimated using Jamovi software (version 2.3.17).
Results and discussion
The total phenolic content and antioxidant activity of the analyzed peanuts are presented in Table 1.
The anthocyanin content differed statistically depending on the peanut varieties (p < 0.05) (Table 1). The purple-coated variety (Fig. 1C) stood out with the highest concentration of anthocyanins, four times higher than that found in beige-coated peanuts (Fig. 1A) and twice as high as that found in red-coated peanuts (Fig. 1B). Thus, the color of the peanut coat can be associated with the anthocyanins present in it. In fact, anthocyanins are pigments that give red, bluish, purple, blackish colors to different vegetables. The beige color is more associated with anthoxanthins than anthocyanins, although both groups of pigments fall into the phenolic class of flavonoids. This fact may explain the lower concentration of anthocyanins found in beige peanuts, compared to the red and purple ones. Positive correlation between seed coat color and anthocyanin content present in peanuts has been reported [34, 35].
The total phenolic contents, determined by the Folin–Ciocalteau and Fast-Blue methods, are presented in Table 1. Peanuts with beige coats have a higher content of total phenolic compounds, differing statistically (p < 0.05) from the others, in every determination method (Table 1). However, the lowest total phenolic content was observed in peanuts with purple skins when the Folin–Ciocalteau method was adopted, and in peanuts with red skins when the Fast blue method was adopted (p < 0.05; Table 1). According to Attree et al. [34], the peanut seed coat contributes most of the phenolic compounds present in the raw grain. The average total phenolics found in beige and red varieties, using the Folin–Ciocalteau method, were higher than the range of 185.4 to 300.9 mg GAE 100 g−1, reported by Yang et al. [36], when analyzing several peanut cultivars using the same method. On the other hand, the average content of total phenolics found in purple peanuts fits the range reported by Yang et al. [36]. Differences in total phenolic concentrations, using the same determination method, can be justified by cultivation conditions, genotypes, and post-harvest treatments.
Although the Folin–Ciocalteau method is traditional and the most used in determining total phenolics, its results are usually underestimated due to its low specificity. The Fast Blue method is based on the coupling of phenolic compounds with diazonium salt, which results in the formation of azo compounds [28]. Fast Blue BB salt contains a diazonium group (N = N) that reacts with the OH group of phenolic compounds. Therefore, the Fast Blue method is much more specific than the Folin–Ciocalteau, generating results that are closer to reality, and is currently the most recommended. The greater specificity of the Fast Blue method justifies the higher levels of total phenolics observed, when compared to the Folin–Ciocalteau.
As observed for total phenolics, peanuts with beige coats presented the highest antioxidant activities measured by the β-Carotene/linoleic acid, ABTS+, and FRAP methods (p < 0.05; Table 1). On the other hand, red-coated peanuts did not differ from purple-coated peanuts in terms of antioxidant activity measured by the ABTS+ and FRAP methods (p < 0.05; Table 1). However, greater antioxidant activity was observed in red peanuts, compared to the purple ones, when using the β-Carotene/linoleic acid method (p < 0.05; Table 1).
The highest antioxidant activity, observed in light-seeded peanuts, may be associated with their higher concentration of total phenolics (Table 1), since phenolics generally have a high antioxidant capacity. However, the antioxidant action of other compounds cannot be ruled out. Vitamin E, for example, is effective in inhibiting lipid peroxidation. In fact, vitamin E is present in appreciable quantities in peanuts [6].
Attree et al. [34] report that the seed coat contributes to 88.2–95.8% of the total antioxidant activity of peanuts. The antioxidant activity determined by the ABTS+ method in purple and red peanuts, from 50.77 to 51.04 µM of TROLOX g−1 (Table 1), respectively, is similar to that described by de Camargo et al. [37] when analyzing whole peanut flour, with an average of 49.38 µM TROLOX g−1. The FRAP values found for peanuts were higher than those reported by [36], from 8.8 to 13.61 µM of ferrous sulfate g−1.
Eight phenolic compounds and the alkaloids trigonelline and theobromine were identified and quantified in the peanut varieties studied (Table 2). The following compounds were identified, concomitantly, in the three peanut varieties, in decreasing order of concentration: trans-cinnamic acid > trigonelline > ferulic acid > syringic acid > theobromine > gallic acid. However, chlorogenic acid was found only in peanuts with beige skins, catechin and resveratrol were only found in peanuts with purple skins, and p-coumaric acid only in peanuts with red and purple skins.
Phenolic compounds are secondary bioactive metabolites that have in common an aromatic ring linked to a hydroxyl. Found in high amounts in plants, they are synthesized through the phenylpropanoid route, which begins with the condensation of phosphoenolpyruvate with erythrose 3 phosphate, originating from the glycolytic and alternative pentose pathways, respectively, giving rise to dehydroquinic acid and, subsequently, dehydroshikimic acid and shikimic acid. The phenylpropanoid route, also known as the shikimic acid route, is so called because the basic structure of its compounds is phenyl propane (C6–C3). However, some important phenolics are synthesized even before the formation of shikimic acid, such as quinic acid, which has dehydroquinic acid as its immediate precursor, and gallic acid, which can be formed from quinic acid or dehydroquinic acid. Phenylalanine ammonia lyase is a key enzyme in the phenylpropanoid pathway, which catalyzes the transformation of phenylalanine into cinnamic acid. As derivatives of cinnamic acid, there are, in sequence, coumaric, caffeic, ferulic, sinapic, and syringic acids. Chlorogenic acid is a product of the condensation of caffeic acid with quinic acid. Coumaric acid can form coumaroyl CoA, which, when linked to three malonyl CoA molecules, can give rise to resveratrol and catechins.
Gallic acid was the phenolic compound found in the lowest concentration in peanuts, not differing (p < 0.05) between the peanut varieties analyzed (0.52–0.57 mg 100 g−1) (Table 2). Despite being the phenolic found in lower concentration in peanuts, the levels found are higher than those reported for the peanut varieties Silihong (4.58 ± 0.38 µg g−1), Silihei (5.47 ± 1.60 µg g−1), and Xiaobaisha (0.66 ± 0.08 µg g−1) [38]. Gallic acid or 3,4,5-trihydroxybenzoic acid, found in abundance in the plant kingdom, is a colorless or slightly yellowish crystalline compound, having a range of applications in the pharmaceutical and food industries [39]. There are many beneficial effects of gallic acid on health, such as antimicrobial and anticancer activity, and protection against gastrointestinal, cardiovascular, metabolic, and neuropsychological diseases. These effects are attributed to the antioxidant and anti-inflammatory potential of this compound [40].
Chlorogenic acid was identified only in peanuts with beige skins (38.03 ± 4.50 mg 100 g−1) (Table 2), at a concentration higher than those reported by Zhou et al. [38] when analyzing the peanut varieties Silihong (19.46 ± 1.69 µg g−1), Silihei (10.52 ± 1.89 µg g−1), and Xiaobaisha (19.63 ± 4.97 µg g−1). Chlorogenic acid is a phenolic compound that is poorly soluble in nonpolar solvents and soluble in polar solvents [41]. The functions of this compound in the body include antioxidant activity, liver and kidney protection, antibacterial, antitumor, sugar regulation, lipid metabolism, and anti-inflammatory and nervous system protection [41].
As for syringic acid, the highest concentration was observed in peanuts with beige skins (7.24 ± 0.31 mg 100 g−1), which was statistically different (p < 0.05, Table 2) from peanuts with purple skins. The observed means are higher than those reported by Zhou et al. [38], when analyzing the peanut varieties Silihong (5.87 ± 0.01 µg g−1), Silihei (2.26 ± 0.45 µg g−1), and Xiaobaisha (5.31 ± 0.87 µg g−1). Syringic acid has a series of therapeutic applications, such as antioxidant, anti-inflammatory, anticancer, antidiabetic, antiendotoxic, neuroprotective, cardioprotective, and hepatoprotective effects [42].
The highest concentration of ferulic acid was found in red-seeded peanuts (17.76 ± 1.29 mg 100 g−1), which differed statistically from the others (p < 0.05, Table 2). However, the average levels observed in the peanut varieties studied are higher than those reported for the peanut varieties Silihong (1.36 ± 0.21 µg g−1), Silihei (1.67 ± 0.18 µg g−1), and Xiaobaisha (1.16 ± 0.17 µg g−1) [38]. Ferulic acid is a phenolic with antioxidant properties, often found cross-linked with lignin and polysaccharides to form part of the cell wall of plants [43]. Among the proven pharmacological properties of ferulic acid are: broad action on oxidative stress, inflammation, vascular endothelial injury, fibrosis, apoptosis, platelet aggregation, and cancer, in addition to neuroprotective and antibacterial effects [43].
Trans-cinnamic acid, an isomer of cinnamic acid, was the phenolic compound found in the highest concentration in peanuts, with the red seed coat standing out with the highest average (102.53 ± 0.91 mg 100 g−1) (p < 0.05, Table 2). The cinnamic acid content found in the present study is higher than that reported by Zhang et al. [44], 90.26 ± 5.57 µg g−1, in peanut sprouts. Its implementation in the diet can help preventing obesity [45], diabetes [46], and aging [47].
Catechin was identified only in peanuts with purple coats (1.33 ± 0.3 mg 100 g−1) (Table 2), its content being higher than those reported by Putra et al. [48], when analyzing peanut skin, and by Zhang et al. [44], when analyzing peanut sprouts, (188.66 µg g−1 and 70.97 ± 2.60 µg g−1), respectively. There are several beneficial effects from ingesting foods rich in catechin, such as: neuroprotective, cardioprotective, antiatherosclerotic, antitumor, anti-aging, and anti-inflammatory [49].
P-coumaric acid was identified only in peanuts with red (2.5 ± 0.3 mg 100 g−1) and purple (1.49 ± 0.13 mg 100 g−1) coats, with a significant difference between both (p < 0.05, Table 2). These values are higher than those observed by Phan-Thien et al. [50], when analyzing five peanut genotypes, with averages ranging from 53.7 ± 5.5 µg g−1 to 169 ± 9.9 µg g−1, and by Zhang et al. [44], when analyzing peanut sprouts, which presented an average of 144.90 ± 8.33 µg g−1. Among the beneficial properties of p-coumaric acid are hepatoprotective [51], antioxidant [52], anti-inflammatory, and antiapoptotic activities [53].
Resveratrol was identified only in purple-seeded peanuts (5.38 ± 0.83 mg 100 g−1) (Table 2), in a concentration higher than those reported by Rudolf and Resurreccion [54], in the order of 0.48 ± 0.08 µg g−1, when studying peanuts not subjected to abiotic stresses, Phan-Thien et al. [50], when evaluating five peanut genotypes, with averages ranging from 2.75 ± 0.2 µg g−1 to 5.59 ± 0.31 µg g−1, and Zhang et al. [44], when analyzing peanut sprouts, which presented an average of 25.83 ± 1.89 µg g−1. Resveratrol acts in plant defensive responses, having a signaling function [55], with fungal invasion and mechanical damage being stimuli for its accumulation [56]. Among the biological functions of resveratrol in the body are: anti-obesity, anti-diabetic, neuroprotective, cardioprotective, and chemo-protective [12]. Variations in the concentrations of phenolic compounds, when comparing data from the present work with those from other authors, can be justified by the effect of genotype, soil, and climate factors and post-harvest treatments.
Thousands of alkaloids with extremely divergent structures have already been identified in vegetables, although they normally have a nitrogenous heterocyclic structure in common. Trigonelline (1-Methylpyridinium-3-carboxylate or simply N-methyl nicotinic acid) and theobromine (3,7 dehydro-3,7 dimethyl-1H-purine-2,6-dione, or simply 3,7-dimethylxanthine) are among the most studied alkaloids, found, for example, in coffee (in concentrations rangig from 0.2–6.3 g 100 g−1) and cocoa (ranging between 5.73–7.606 g 100 g−1) for the compounds respectively [57, 58]. However, reports on the presence of these two alkaloids in peanuts were not found in the literature, and this is the first work to identify them in varieties of this legume. The trigonelline content found in peanuts was 7, 8, 10.5, and 17.8 times higher than that of theobromine, in peanuts with beige, red, and purple skins, respectively. Furthermore, when compared to phenolics, trigonelline was the third most abundant compound in the peanuts under study, behind only trans-cinnamic and chlorogenic acid. The concentration of trigonelline differed statistically among peanuts with different coat colors (p < 0.05, Table 2), with the purple coat having the highest concentration of the alkaloid, with an average of 23.63 ± 1.31 mg 100 g−1. As for theobromine, the purple-coated variety had the lowest content of this compound (1.33 ± 0.23 mg 100 g−1), statistically different (p < 0.05, Table 2) from the red-coated peanut. Studies have suggested several beneficial effects of these alkaloids on human health, such as reducing allergic symptoms and preventing kidney and cardiovascular diseases, promoted by trigonelline [11, 17, 20] and prevention of colon cancer and therapeutic potential to prevent obesity and its associated diseases, promoted by theobromine [18, 19, 21].
Principal component analysis (PCA) and cluster grouping analysis (HCA)
The concentrations of total and individual phenolics, as well as the alkaloids identified in each type of peanut, alongside their respective antioxidant activities, were subjected to multivariate analysis. Using PCA, it was found that PC1 and PC2 cumulatively explained 100% of the total variation in the data, 63.77% and 36.22%, respectively (Fig. 2a). Considering a loading of 0.68 (Table 3), the variables responsible for the separation in PC1 (Fig. 2a) were total phenolics, chlorogenic acid, syringic acid, and antioxidant activity, which correlated positively with each other and negatively with trigonelline and catechin, which makes it possible to distinguish peanuts with beige coats from others. In fact, from the visual analysis in Fig. 2a, the antioxidant activity, the content of total phenolics and chlorogenic and syringic acids are close in the PC1 space, next to the beige peanut, in a position diametrically opposite to trigonelline and catechin. On the other hand, it appears from PC2 (Fig. 2a, Table 3), that the lower the total phenolic content determined by the Fast Blue method, catechin and resveratrol, the higher the concentrations of gallic, ferulic, trans-cinnamic acids, p-coumaric, and theobromine, correlations associated with purple and red peanuts.
HCA corroborates the results extracted from PCA. In the dendrogram presented in Fig. 2b, the greater the Euclidean distance, the lower the similarity between the samples. Therefore, peanuts with red and purple coats form a group, according to their similarities, which is distinguished from peanuts with beige coats.
Pearson correlation
The Pearson correlation matrix (Table 4) confirms the results and interpretations extracted from the PCA. Positive and negative correlations between the studied variables were observed, with correlation coefficients as low and negligible as −0.012, between anthocyanins and ferulic acid, and as high and significant as -1.0 between beta-carotene and trigonelline and 1.0 between chlorogenic acid and ABTS+ and chlorogenic acid and FRAP (Table 4).
A high positive correlation was noted between the antioxidant activity measured by the β-carotene/linoleic acid, ABTS+, and FRAP methods in peanuts. These results are similar to those obtained by Wootton-Beard et al. [59] and Rumpf et al. [60], who found a strong positive correlation between FRAP and ABTS+ in vegetables. It is noteworthy that the extraction solvent of plant materials significantly influences their antioxidant activity [61], as well as the origin and structure of the sample [60].
The antioxidant activity of peanuts was positively correlated to the total phenolic content in all the methods used (Table 4). The results obtained by the β-carotene/linoleic acid method had a higher correlation with those obtained by the Follin Ciocalteau method, while those obtained by the FRAP method showed a higher correlation with Fast Blue. In fact, phenolic compounds, due to the hydroxyls present in their structure, play an important antioxidant role, acting to scavenge free radicals from cells, which justifies the results found.
In general, antioxidant activity and total phenolic content showed a positive correlation with the phenolic compounds, gallic, chlorogenic, and syringic acids and with the theobromine alkaloid, and a negative correlation with the other identified phenolics and with the trigonelline alkaloid. Also, in general, the identified phenolic acids (gallic, chlorogenic, syringic, ferulic, trans-cinnamic, and p-coumaric) and the theobromine were positively correlated to each other but negatively to catechin, resveratrol, anthocyanins, and trigonelline. Exceptions to these correlations were noted, but with low and negligible correlation coefficients.
It is worth highlighting the strong positive correlation (R2 = 1.00) between chlorogenic acid and antioxidant activity measured by the ABTS+ and FRAP methods and between resveratrol and catechin. According to Marinova et al. [62], the presence of chlorogenic acid in food is an important determinant of its antioxidant activity. The higher its concentration, the greater the antioxidant activity. In fact, chlorogenic acid has four hydroxyls, the structure of phenolic compounds responsible for their antioxidant properties. It is noteworthy, however, that chlorogenic acid was found only in peanuts with beige coats (Table 2). On the other hand, catechin and resveratrol, which showed a strong positive correlation, were identified only in purple-coated peanuts (Table 2). The strong correlation found between catechin and resveratrol can be justified by the common precursors of both compounds. They are synthesized from the condensation of a Cumaroyl-CoA molecule with three malonyl CoA molecules, with stilbene synthase responsible for the synthesis of resveratrol and chalcone synthase for the synthesis of catechin.
Catechins are predominantly found in mesophyll cells in close proximity to epidermal cells [63], coexisting with protoanthocyanidins [64], which are considered a form of defense against biotic and abiotic stresses [65]. Protoanthocyanidins come from catechins and epicatechins [66], which may justify the positive correlation between resveratrol and catechins, which share the same substrate, and anthocyanins, derived from catechins, as well as the higher anthocyanin content found in purple-coated peanuts.
To date, no researcher has associated the color of the peanut seed coat with its chemical composition. However, in wines the amount of resveratrol can significantly influence pigmentation reactions and color intensity [67]. Since chlorogenic acid was found only in peanuts with beige coats, and catechins and resveratrol in peanuts with purple coats, it is suggested that the color of the coat is an indicator of specific phenolic compounds in peanuts, consequently, of their antioxidant activity. It should be noted that chlorogenic acid was found only in peanuts with beige coats, which presented the highest antioxidant activity among the peanuts evaluated (Table 1) and that there was a strong positive correlation between both variables, which further reinforces the association between color of the tegument and the concentration of phenolics and their antioxidant activity, as already demonstrated in PCA HCA.
Conclusions
The bioactive composition and antioxidative activity of peanuts depend on its genotype. For the first time trigonelline and theobromine were identified in peanuts. An unprecedented correlation has been demonstrated between the color of the peanut seed coat and its bioactive composition and antioxidant activity.
Data availability
Data will be available on request.
References
Settaluri VSK, Puppala CVK, Sundaram NJ (2012) Peanuts and their nutritional aspects—a review. Food Nutr Sci 3:1644–1650. https://doi.org/10.4236/fns.2012.312215
Guo C, Xie YJ, Zhu MT, Xiong Q, Chen Y, Yu Q, Xie JH (2020) Influence of different cooking methods on the nutritional and potentially harmful components of peanuts. Food Chem 316:1–9. https://doi.org/10.1016/j.foodchem.2020.126269
Statista (2023). Production share of peanuts worldwide in 2021, by leading country. https://www.statista.com/statistics/1030846/major-producers-of-peanut-worldwide/. Accessed 18 Feb 2024
Bodoira R, Rossi Y, Velez A, Montenegro M, Martínez M, Ribotta P, Maestri D (2022) Impact of storage conditions on the composition and antioxidant activity of peanut skin phenolic-based extract. Molecules 57(10):6471–6479. https://doi.org/10.1111/ijfs.15964
Companhia Nacional de Abastecimento (Conab) (2024). Amendoim. https://www.conab.gov.br/info-agro/safras/serie-historica-das-safras/itemlist/category/899-amendoim. Accessed 20 April 2024.
Çiftçi S, Suna G (2022) Functional components of peanuts (Arachis Hypogaea L.) and health benefits: a review. Future Foods 5:100140. https://doi.org/10.1016/j.fufo.2022.100140
Francisco MLDL, Resurreccion AVA (2008) Functional components in peanuts. Crit Rev Food Sci Nutr 48:715–746. https://doi.org/10.1080/10408390701640718
Jung M, Kim J, Ahn SM (2020) Factors associated with frequency of peanut consumption in Korea: a national population-based study. Nutrients 12(1207):1–15. https://doi.org/10.3390/nu12051207
Arya SS, Salve AR, Chauhan S (2016) Peanuts as functional food: a review. J Food Sci Technol 53(1):31–41. https://doi.org/10.1007/s13197-015-2007-9
Win M, Abdul Hamid A, Baharin B, Anwar F, Mc S, Dek M (2011) Phenolic compounds and antioxidant activity of peanut’s skin, hull, raw kernel and roasted kernel flour. Pak J Bot 43:1635–1642
Chen GH, Yang CY, Lee SJ, Wu CC, Tzen JTC (2014) Catechin content and the degree of its galloylation in oolong tea are inversely correlated with cultivation altitude. J Food Drug Anal 22:303–309. https://doi.org/10.1016/j.jfda.2013.12.001
Toomer OT (2018) Nutritional chemistry of the peanut (Arachis hypogaea). Crit Rev Food Sci Nutr 58(17):3042–3053. https://doi.org/10.1080/10408398.2017.1339015
Kyei SK, Akaranta O, Darko G (2020) Synthesis, characterization and antimicrobial activity of peanut skin extract-azo-compounds. Scientific African 8:1–14. https://doi.org/10.1016/j.sciaf.2020.e00406
Zhao X, Chen J, Du F (2012) Potential use of peanut by-products in food processing: a review. J Food Sci Technol 49(5):521–529. https://doi.org/10.1007/s13197-011-0449-2
Chuenchom PSP, Senawong T, Jogloy S (2016) Antioxidant capacity and phenolic content evaluation on peanut skins from 3 peanut types. Chiang Mai J Sci 43(1):123–137
Guo J, Chen H, Liu N, Chen W, Zhou X, Luo H, Huang L, Li W, Wu B, Huai D, Lei Y, Liao B, Jiang H (2022) Identification and validation of a major locus with linked marker for resveratrol content in culitivated peanut. Euphytica 218(15):1–10. https://doi.org/10.1007/s10681-022-02969-2
Nugrahini AD, Ishida M, Nakagawa T, Nishi K, Sugahara T (2020) Trigonelline: Na alkaloid with anti-degranulation properties. Mol Immunol 118:201–209. https://doi.org/10.1016/j.molimm.2019.12.020
Shojaei-Zarghani S, Yari Khosroushahi A, Rafraf M (2021) Oncopreventive effects of theanine and theobromine on dimethylhydrazine-induced colon cancer model. Biomed Pharmacother 134:1–9. https://doi.org/10.1016/j.biopha.2020.111140
Cadoná FC, Dantas RF, de Mello GH, Silva FP Jr (2022) Natural products targeting into cancer hallmarks: an update on caffeine, theobromine, and (+)–catechin. Food Sci Nutr 62(26):7222–7241. https://doi.org/10.1080/10408398.2021.1913091
Peerapen P, Boonmark W, Thongboonkerd V (2022) Trigonelline prevents kidney stone formation processes by inhibiting calcium oxalate crystallization, growth and crystal-cell adhesion, and downregulating crystal receptors. Biomed Pharmacother 149:1–14. https://doi.org/10.1016/j.biopha.2022.112876
Tanaka E, Mitani T, Nakashima M, Yonemoto E, Fujii H, Ashida H (2022) Theobromine enhances the conversion of white adipocytes into beige adipocytes in a PPARγ activation-dependent manner. J Nutr Biochem 100:1–11. https://doi.org/10.1016/j.jnutbio.2021.108898
Köppen W (1936) Das geographische system der klimatologie. Borntrager, Berlim, p 44
Climate-Data.Org. (2024) Clima Santana de Garambéu (Brasil). Disponível: https://pt.climate-data.org/america-do-sul/brasil/minas-gerais/santana-do-garambeu-176063/>. Accessed 22 Apr 2024.
Araújo AR, Oliveira JM, Pereira P, Curi N, Marques AFSM, Marques JJGSM (2019) Geomorfologia, solos e aptidão agrícola das terras da bacia do alto Rio Grande, Minas gerais. editora UFLA, Lavras, p 239
Barcia M, Pertuzatti P, Jacques A, Godoy H, Zambiazi R (2012) Bioactive compounds, antioxidant activity and percent composition of jambolão fruits (Syzygium cumini). Nat Prod J Shariah 2(2):129–138. https://doi.org/10.2174/2210315511202020129
Whaterhouse AL (2002) Polyphenlics: determination of total phenolic. In: Wrolstad RE (ed) Current protocols in food analytical chemistry. John Wiley, New York, pp 1–8
Paradiso VM, Castellino M, Renna M, Gattullo CE, Calasso M, Terzano R, Allegretta I, Leoni B, Caponio F, Santamaria P (2018) Nutritional characterization and shelf-life of packaged microgreens. Food Funct 9(11):5629–5640. https://doi.org/10.1039/C8FO01182F
Medina MB (2011) Determination of the total phenolics in juices and superfruits by a novel chemical method. J Funct Foods 3(2):79–87. https://doi.org/10.1016/j.jff.2011.02.007
Rufino MDSM, Alves RE, de Brito ES, Pérez-Jiménez J, Saura-Calixto F, Mancini-Filho J (2010) Bioactive compounds and antioxidante capacities of 18 no-traditional tropical fruits from Brazil. Food Chem 121(4):996–1002. https://doi.org/10.1016/j.foodchem.2010.01.037
Auzanneau N, Weber P, Kosińska-Cagnazzo A, Andlauer W (2018) Bioactive compounds and antioxidant capacity of Lonicera caerulea berries: comparison of seven cultivars over three harvesting years. J Food Compos Anal 66:81–89. https://doi.org/10.1016/j.jfca.2017.12.006
Pulido R, Bravo L, Saura-Calixto F (2000) Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem 48(8):3396–3402. https://doi.org/10.1021/jf9913458
Ferreira DF (2010) SISVAR–Sistema de análise de variância. In (Version 5.8) https://des.ufla.br/~danielff/programas/sisvar.html. Accessed 1 Dec 2023
Nunes CA, Freitas MP, Pinheiro ACM, Bastos SC (2012) Chemoface: a novel free user-friendly interface for chemometrics. J Braz Chem Soc 23(11):2003–2010. https://doi.org/10.1590/S0103-50532012005000073
Attree R, Du B, Xu B (2015) Distribution of phenolic compounds in seed coat and cotyledon, and their contribution to antioxidant capacities of red and black seed coat peanuts (Arachis hypogaea L.). Ind Crops Prod 67:448–456. https://doi.org/10.1016/j.indcrop.2015.01.080
Kuang Q, Yu Y, Attree R, Xu B (2017) A comparative study on anthocyanin, saponin, and oil profiles of black and red seed coat peanut (Arachis hypogacea) grown in China. Int J Food Prop 20(1):131–140. https://doi.org/10.1080/10942912.2017.1291676
Yang QQ, Kim GAKF, Luo Q, Corke H (2020) Phenolic profile, antioxidant and antiproliferative activities of diverse peanut cultivars. J Food Measure Characteriz 14:2361–2369. https://doi.org/10.1007/s11694-020-00483-4
Camargo AC, Regitano-d’Arce MAB, Shahidi F (2017) Phenolic profile of peanut by-products: antioxidant potential and inhibition of alfa-glucosidase and lipases activities. J Am Oil Chem Soc 94:959–971. https://doi.org/10.1007/s11746-017-2996-9
Zhou Z, Fan Z, Meenu M, Xu B (2021) Impact of germination time on resveratrol, phenolic acids, and antioxidant capacities of different varieties of peanut (Arachis hypogaea Linn.) from China. Antioxidants 10:1714
Fernandes FHA, Salgado HRN (2016) Gallic acid: review of the methods of determination and quantification. Anal Chem 46:257–267. https://doi.org/10.1080/10408347.2015.1095064
Kahkeshani N, Farzaei F, Fotouhi M, Alavi SS, Bahramsoltani R, Naseri R, Momtaz S, Abbasabadi Z, Rahimi R, Farzaei MH, Bishayee A (2019) Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci 22(3):225–237. https://doi.org/10.22038/ijbms.2019.32806.7897
Wang L, Pan X, Jiang L, Chu Y, Gao S, Jiang X, Zhang Y, Chen Y, Luo S, Peng C (2022) The biological activity mechanism of chlorogenic acid and its applications in food industry: a review. Front Nutr 9(943911):1–22. https://doi.org/10.3389/fnut.2022.943911
Srinivasulu C, Ramgopal M, Ramanjaneyulu G, Anuradha CM, Suresh Kumar C (2018) Syringic acid (SA)–a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed Pharmacother 108:547–557. https://doi.org/10.1016/j.biopha.2018.09.069
Li D, Rui YX, Guo SD, Luan F, Liu R, Zeng N (2021) Ferulic acid: a review of its pharmacology, pharmacokinetics and derivatives. Life Sci 284:1–13. https://doi.org/10.1016/j.lfs.2021.119921
Zhang L, Qu H, Xie M, Shi T, Shi P, Yu M (2023) Effects of different cooking methods on phenol content and antioxidant activity in sprouted peanut. Molecules 28(12):4684
Wang Z, Ge S, Li S, Lin H, Lin S (2020) Anti-obesity effect of trans-cinnamic acid on HepG2 cells and HFD-fed mice. Food Chem Toxicol 137:111148. https://doi.org/10.1016/j.fct.2020.111148
Adisakwattana S (2017) Cinnamic acid and its derivatives: mechanisms for prevention and management of diabetes and its complications. Nutrients 9(163):1–27
Hseu YC, Korivi M, Lin FY, Li ML, Lin RW, Wu JJ, Yang HL (2018) Trans-cinnamic acid attenuates UVA-induced photoaging through inhibition of AP-1 activation and induction of Nrf2-mediated antioxidant genes in human skin fibroblastos. J Dermatol Sci 90:123–134. https://doi.org/10.1016/j.jdermsci.2018.01.004
Putra NR, Rizkiyah DN, Qomariyah L, Aziz AHA, Veza I, Yunus MAC (2023) Experimental and modeling for catechin and epicatechinrecovery from peanut skin using subcritical etanol. J Food Process Eng 46(e14275):1–11. https://doi.org/10.1111/jfpe.14275
Pedro A, Maciel GM, Ribeiro V, Haminiuk C (2019) Fundamental and applied aspects of catechins from differentsources: a reviews. Int J Food Sci Technol 55(2):429–442. https://doi.org/10.1111/ijfs.14371
Phan-Thien KY, Wright GC, Lee NA (2014) Peanut antioxidants: Part 2. Quantitation of free and matrix-bound phytochemicals in five selected genotypes with diverse antioxidante capacity by high performance liquid chromatog-raphy (HPLC). LWT Food Sci Technol 57:312–319. https://doi.org/10.1016/j.lwt.2013.12.020
Parvizi F, Yaghmaei P, Haeri Rohani SA, Mard AS (2020) Hepatoprotective properties of p-coumaric acid in a rat model of ischemia-reperfusio. Avicenna J Phytomed 10(6):633–640
Boo YC (2019) p-coumaric acid as an active ingredient in cosmetics: a review focusing on its antimelanogenic effects. Antioxidants 8(275):1–16
Rafiee Z, Moaiedi MZ, Gorji AV, Mansouri E (2020) Mitigates doxorubicin-induced nephrotoxicity through suppression of oxidative stress, inflammation and apoptosis. Arch Med Res 51:32–40. https://doi.org/10.1016/j.arcmed.2019.12.004
Rudolf JR, Resurreccion AVA (2005) Elicitation of resveratrol in peanut kernels by application of abiotic stresses. J Agric Food Chem 53:10186–10192. https://doi.org/10.1021/jf0506737
Yin Y, Hu J, Yang Z, Fang W, Yang J (2023) Effects of methyl jasmonate and NaCl treatments on the resveratrol accumulation and defensive responses in germinated peanut (Arachis hypogaea L.). Plant Physiol Biochem 194:664–673. https://doi.org/10.1016/j.plaphy.2022.12.012
Sanders TH, McMichael RW, Hendrix KW (2000) Occurrence of resveratrol in edible peanuts. J Agric Food Chem 48:1243–1246. https://doi.org/10.1021/jf990737b
Peláez PP, Bardón I, Camasca P (2016) Methylxanthine and catechin content of fresh and fermented cocoa beans, dried cocoa beans, and cocoa liquor. Sci Agropec 7(4):355–365. https://doi.org/10.17268/sci.agropecu.2016.04.01
Konstantinidis N, Franke H, Schwarz S, Lachenmeier DW (2023) Risk assessment of trigonelline in coffee and coffee by-products. Molecules 28(3460):1–22. https://doi.org/10.3390/molecules28083460
Wootton-Beard PC, Moran A, Ryan L (2011) Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res Int 44(1):217–224. https://doi.org/10.1016/j.foodres.2010.10.033
Rumpf J, Burger R, Schulze M (2023) Statistical evaluation of DPPH, ABTS, FRAP, and folin-ciocalteu assays to assess the antioxidant capacity of lignins. Int J Biol Macromol 233:1–9. https://doi.org/10.1016/j.ijbiomac.2023.123470
Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins Byrne D (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal 19(6):669–675. https://doi.org/10.1016/j.jfca.2006.01.003
Marinova EM, Toneva A, Yanishlieva N (2009) Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem 114:1498–1502. https://doi.org/10.1016/j.foodchem.2008.11.045
Tounekti T, Joubert E, Hernández I, Munné-Bosch S (2013) Improving the polyphenol content of tea. Crit Rev Plant Sci 32(3):192–215. https://doi.org/10.1080/07352689.2012.747384
Tanaka T, Matsuo Y, Kouno I (2020) Chemistry of secondary polyphenols produced during processing of tea and selected foods. Int J Mol Sci 11(1):14–40
Rauf A, Imran M, Abu-Izneid T, Haq IU, Patel S, Pan X, Naz S, Silva AS, Saeed F, Suleria HAR (2019) Proanthocyanidins: a comprehensive review. Biomed Pharmacother 116:1–6. https://doi.org/10.1016/j.biopha.2019.108999
Liu C, Wang X, Shulaev V, Dixon RA (2016) A role for leucoanthocyanidin reductase in the extension of proanthocyanidins. Nature plants 2:1–7. https://doi.org/10.1038/nplants.2016.182
Uysal RS, Issa-Issa H, Sendra E, Carbonell-Barrachina ÁA (2023) Changes in anthocyanin pigments, trans-resveratrol, and colorimetric characteristics of Fondillón wine and other “Monastrell” wines during the aging period. Eur Food Res Technol 249(7):1821–1831. https://doi.org/10.1007/s00217-023-04256-3
Acknowledgements
The authors would like to thank the Central of Analysis and Chemical Prospecting of the Federal University of Lavras, and Finep, To The National Council of Technological and Scientific Development (CNPq:304413/2016-0; 302699/2019-8), the Minas Gerais Research Support Foundation (FAPEMIG: PPM-00458-15; PPM-00355-17), and the Higher Education Personnel Improvement Coordination (CAPES: 88881.068456/2014-01) for financial support.
Author information
Authors and Affiliations
Contributions
Gilson Gustavo Lucinda Machado: Conceptualization, Investigation, Methodology, Data curation, Formal analysis, Writing – original draft. Ana Beatriz Silva Araújo: Conceptualization, Formal Analysis. Ana Cristina Freitas de Oliveira Meira: Conceptualization, Formal analysis. Carlos Henrique Milagres Ribeiro: Conceptualization, Formal analysis. Ingrid Alves Santos: Conceptualization, Formal analysis. Lorrane Ribeiro de Souza: Conceptualization, Formal analysis. Elano Pinheiro Pereira: Conceptualization, Formal analysis. Eduardo Valério de Barros Vilas Boas: Conceptualization, Fundraising, Project Administration, Supervision, Validation, Visualization, Writing—Original Draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical requirements
This article does not contain any studies with human or animal subject.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Machado, G.G.L., Araújo, A.B.S., de Oliveira Meira, A.C.F. et al. Bioactive capacity of peanuts with different coat colors. Eur Food Res Technol (2024). https://doi.org/10.1007/s00217-024-04572-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00217-024-04572-2