Abstract
Chickpea is the world’s second most widely grown pulse. This legume will become increasingly important due to its natural drought and heat tolerance ability, and its capacity to fix atmospheric N2 in symbiosis with rhizobia what makes this pulse a low-water and carbon fingerprint crop. The aim of this study was to assess the nutritional value, the mineral composition, and the phenolic compound profiles of ten Spanish chickpea genotypes. Seed morphological characteristics were also determined as useful traits for analyzing plant biodiversity. Most of these advanced lines and/or recombinant inbred lines (RILs) were derived from intraspecific crosses among kabuli-type chickpeas genotypes. The variety Kasin and two RILs, namely 5-RIL-33 and 5-RIL-92, shared the same parental lines, one of them from India (WR315) of desi type. Only one genotype (5-RIL-33) has colored grains and pink flowers (common desi-type traits). These three genotypes were resistant to both ascochyta blight [Ascochyta rabiei (Pass.) Labr] and Fusarium oxysporum f. sp. ciceris race 5. The protein content of all genotypes was higher than 20% with some outstanding lines having > 25%. Other functional components such as crude fat, fiber, and carbohydrates contents and minerals were broadly uniform across the studied material. The analysis of the phenolic compounds on methanolic seed extracts reveals common features as the presence of gentisic and 4-hydroybezoic acids, besides l-glutamic, citric, and succinic organic acids. In contrast, some compounds such as gallic acid, gallocatechin, and rutin are exclusively present in the colored 5-RIL-33 line, in addition to the reference Apulian black chickpea variety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chickpea (Cicer arietinum L.) is one of the earliest cultivated legumes and belongs to the family Fabaceae (subfamily Faboideae). Remains of this pulse from the Middle East have been found dating to around 7500–9000 years ago. Cultivated chickpeas are divided into two main types, namely “desi” and “kabuli” [1, 2]. The “desi” types have pigmented vegetative parts and pink flowers, and seeds are generally small and colored (mostly dark) with a thick seed coat. The “desi” chickpeas occupy about 80–85% of the chickpea cultivation areas in the world and are mainly grown in South Asia (India), East Africa, and Australia. The “kabuli” types have non-pigmented vegetative parts, white flowers, and are relatively larger, having a thin coat and whitish or cream colored testa, and are mostly cultivated in the Mediterranean Basin, the Near East, and East Asia [3]. A third chickpea type, “pea-like” has also been described usually in germplasm collections and breeding populations [4].
Today, chickpea is the world’s second most widely grown pulse after soybean and its cultivation is well adapted to the climate and agronomic features of the Mediterranean basin. It will become increasingly important facing the forecast climate change scenario due to its natural drought and heat tolerance ability, and its capacity to fix atmospheric N2 in symbiosis with rhizobia soil bacteria which also increase the soil fertility. All these facts make this pulse a low water and carbon fingerprint crop. At the same time, it is the most important food legume cultivated among cool season food legumes in the arid and semi-arid regions of the world under rainfed conditions. In recent years, India has been the leading producer of chickpeas with a total production of more than 11 million metric tons of chickpeas in 2020 and Turkey is the second with an estimated 630,000 metric tons. In Spain, the surface dedicated to grain legumes cultivation in 2021 exceeded 360,000 ha raising a total production of 431,796 tons where chickpeas represent a 12% of surface cultivation and 9% of the grain legumes production. It is noticeable that less than a 1% of the production is dedicated to human consumption (https://www.mapa.gob.es/es/estadistica).
Pulses have gained more attention since the United Nations General Assembly declared 2016 as the International Year of Pulses (IYP) recognizing their importance for nutrition, health, and agriculture. The IYP contributed to increase the awareness on the multiple benefits of pulses for humans and agriculture and, thus, increase pulse consumption and production. To encounter the challenge of providing affordable, nutritious foods at low environmental costs, pulses can play an important role. Legumes such as soybean, chickpeas, lentils, peas, beans, peanuts, and forage legumes such as alfalfa, clover, etc., are used worldwide for human food supply as well as for animal feed purposes. Along with their environmental, nutritional, and agronomic benefits, pulses foster sustainable agriculture, contribute to climate change mitigation and adaptation, and promote biodiversity [5, 6].
Most countries face some form of malnutrition, ranging from undernutrition and micronutrient deficiencies to obesity and diet-related diseases. In this context, pulses are important food crops and should be part of a healthy diet because they are recognized as being readily available sources of protein, complex carbohydrates, fibers, vitamins, minerals, and bioactive compounds while being low in fat [7,8,9]. Then, pulses have been used as plant-based solutions in the food system, which deserve the attention of many researchers, food technologists, and marketers. For nutritionists, pulses are considered healthy and high nutrient-like protein-rich diet, that mainly decrease the risk of stroke and heart diseases. Even though grain legumes were part of many traditional diets, pulse consumption has decreased globally; in Spain, the current human consumption rate is 2.5 kg/year/per capita far from the 4 kg/year/per capita of the beginning of the century being chickpeas the preferred food legume followed by lentils and common beans.
Materials and methods
Plant material
Chickpea genotypes used in this work and the parental lines are listed in Table 1. This material has been provided and informed (biological status and parental lines) by Dr. J. Rubio (Department of Plant Breeding and Biotechnology), IFAPA (Andalusian Institute of Agrarian, Fishing, Food Investigation and Ecological Production), Center Alameda del Obispo, Córdoba, Spain. The original material has been multiplied under field conditions, during 2022 at the Agriculture Experimental Station Tomejil, (IFAPA-Center Las Torres, Seville, Spain). Seeds have been stored at 8ºC till its use for chemical and nutritional analyses. In addition, a black-pigmented chickpea type Apulian black, local variety (Cece nero rugoso della Murgia), kindly provided by Dr. A.R. Piergiovanni (Institute of Biosciences and BioResources, Bari, Italy), has been used as reference genotype for some purposes.
Seed morphological and physical characteristics
Shape, ribbing, and color of seed were accomplished by visual assessment (VG) following the codes of UPOV (International Union for the Protection of new varieties of Plants) [10], by two independent observers. Seed coat incidence was calculated based on three independent samples of ten seeds following Avola et al. [11]. 100-seed weight (100SW) was gravimetrically determined on three independent samples. The seed shape analysis (length, width, and circularity) of the genotypes has been analyzed by traitor [12], a computer-aided image analysis system. Briefly, selected 80 seeds/genotype were set up in matrices of 8 files × 10 columns, with the ventral side of seed touching the surface of a scanner HP OfficeJet 8600. Images were taken with HP Easy Scan 2.0, in a blue background. A coin was used to normalize measurements to the nearest 0.1 mm. A matrix of 775 records of the ten genotypes has been used for graphical and statistical analyses. The interaction between length and circularity was performed in R (lineal model circularity ~ variety* length).
Proximate and mineral composition of seeds
Dry and raw seeds of each genotype were ground and sieved at 1 mm to obtain the corresponding flour. Sample flours were sent to authoritative specialized analyses unit Laboratorio Agroalimentario de Córdoba, (AGAPA) for proximate and mineral composition determination. The constituents referred as mandatory nutrition declaration (energy, fat, carbohydrates, sugar, salt, and protein) on EU Regulation No. 1169/2011 (art. 30) plus fiber content, ash, and humidity were determined. Mineral components: N, P, K, Ca, Mg, Na, Fe, Mn, and Cu were determined by ICP-OES (Inductively Coupled Plasma-Optical Emission Spectrometer).
Phenolic compounds determination
Methanolic extracts (methanol:water, 70:30) of chickpea flours were analyzed by UHPLC–HRMS (Ultra High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry) by the target screening method against more than 90 phenolic compounds at CITIUS (Centre of Research, Technology and Innovation University of Seville, Spain).
Results and discussion
The genetic background and denomination of the chickpea germplasm used in this study are shown in Table 1. Most of the chickpea genotypes derived from interspecific crosses of three kabuli-type parental lines from Russia, Syria, and Spain. However, two RILs (Recombinant Inbred Lines): 5-RIL-33 and 5-RIL-92 plus the variety Kasin, derived from reciprocal crosses that include the desi type from India (WR315) and the kabuli-type from Russia (ILC3279), respectively. 5-RIL-33 and 5-RIL-92, although derived from the same parental lines cross (female × male), have segregated distinctive morphological characteristics such as seed type and color, flower color, seed weight (100SW), and shape (Table 2).
All genotypes were resistant to ascochyta blight (Ascochyta rabiei) [13], and most of them showed sensitivity to Fusarium oxysporum f. sp. ciceris race 5 [14], exception made of 5-RIL-33, 5-RIL-92, and Kasin variety, which were resistant probably due to the desi genotype WR315 partner on their pedigree.
Morphological seed traits such as seed shape and size have been accepted as useful tools for studying plant biodiversity and to characterize intra- and inter-species variation as well as for genotypic discrimination and local varieties improvement [15, 16]. Thus, with the aim of distinguishing this set of Spanish chickpea genotypes, we have assessed six traits: seed size, 100SW (100-seed weight), shape, seed color, coat %, and ribbing. The morphological characteristics of the studied chickpeas are shown in Table 2 and Fig. 1. Most of the genotypes belong to kabuli type, exception made of 5-RIL-33 which belongs to desi type, thus exhibiting characteristics of this group such as pink flowers, angular shape, small seeds, reddish brown color, and thick coat denoted by the highest coat % and strong ribbing. Among the kabuli genotypes, 5-RIL-92, RR-33, RR-51, and RR-98 form a sub-group that shows intermediate characteristics such as medium size, 100SW ranging 16–30 g, round shape, grayed brown color, and absent to very weak ribbing. Half of the genotypes (BT-, meaning good size) could be gathered as a second sub-group of the kabuli seeds, as they present the highest seed size and 100SW (> 50 g), in agreement with the macrocarpa kabuli-type descriptors [1, 2]. These BT-chickpea seeds have an intermediate shape, whitish color, and medium to strong ribbing. The absence of ribs was well correlated with the round shape of seeds. In addition to the visual determination of shape and ribbing, digital seed morpho-metrics characterization has been used for cultivar discrimination in this work and those of others [12, 15, 17], which allowed a high number of replicates. Results of the relationship between length and width, and seed circularity and length are shown in Figs. 2 and 3, respectively. Those genotypes called BT-grouped together, as well as those assigned to round shape (5-RIL-92, RR-33, RR-51 and RR-98, Table 2), accordingly this image analysis is in agreement with the visual observations of shape. As stated by Cervantes et al. [15], modeling seed shape is an easy approximation that may help to understand and quantify differences between related genotypes among other purposes.
The Apulian black cultivar Cece nero (desi type), included as reference, presents pink flowers, small seeds, and angular shape; it showed the highest seed-coat value (14.2%). The analysis of seed-coat incidence of the Spanish genotypes ranged from 5% to 9.7%, corresponding the upper value to 5-RIL-33. In contrast to Gil and Cubero [18], who found that correlation between seed-coat thickness with seed size was always negative and low, in this study, we have found a high and positive correlation (r = 0.85) between the variables seed-coat thickness (seed coat %) and seed size. Most probably, the morphological trait of ribbing, which is well associated with the seed size, as all big-seeded genotypes (BT-) (Table 2) exhibited strong ribbing, may account for this correlation. The intermediate values of coat % in our study are in accordance with those from the Sicilian kabuli-type cultivars [11].
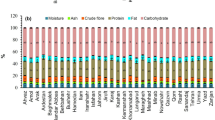
The proximate composition of seeds is presented in Table 3. We have determined the constituents referred as mandatory nutrition declaration on EU Regulation No. 1169/2011 (art. 30), plus fiber, ashes, and humidity content. The energy values slightly varied from 370 to 380 kcal/100 g, and it was positively correlated with the total fat content (saturated plus unsaturated) (r = 0.856). Energy values of this set of chickpeas are consistent with those reported for other grain legumes [19]. Fat values ranged from 5.8 to 7.2% among the Spanish genotypes. These values are higher than those previously reported for Sicilian cultivars (average 4.4%) [11], and for chickpea accessions (average 3.6%) reported by Costantini et al. [20]. Nonetheless, some Ecuadorian chickpea varieties had up to 7.4% of fat content [21]. Saturated fat content of the studied chickpeas did not exceed 1%. Carbohydrates available content represented more than 50% of seed composition in the studied seeds and agreed with other studies [20]. Protein content ranged from 21.8 to 26.3%, there are some outstanding breeding lines such as BT3-13, BT5-7, BT6-19 plus Kasin variety with ≥ 25%. This value exceeds the reported data of 18, 19.4, 21.5, and 24% for the Pakistani, other Spanish varieties, Ecuadorian, and Sicilian chickpeas, respectively. Costantini et al. [20] reported the proximate and mineral composition of twelve chickpea genotypes from different geographical origin, which had an average of 20% protein content. Thus, all genotypes of our study afford for a greater protein content. Salt content of seeds, calculated in base of sodium content × 2.5, is below 0.05% in all samples. Crude fiber averages 3.6%, like values previously reported for Sicilian seeds and others. However, 5-RIL-33 had a fiber value of 6.6%, most probably related with its highest seed-coat proportion (9.7%), and the strong ribbing morphology (Table 2). In the study of Costantini et al. [20], data of dietary fiber were higher, with an average value of 18%. Nonetheless, this value, lowered to less than 10%, when considering only the kabuli-type accessions, indicating that desi-type accessions had higher values of fiber. The mineral fraction of seeds (ashes) accounts for a 3% like values reported elsewhere in the literature. The nutritional constituents of our Spanish kabuli genotypes were also contrasted with the values reported in the FAO’s user guide [7]. We have taken into account the reported values from kabuli-type accessions (CIA001, CIA006, from Australia), (CIA004 from Canada) and, (CIA002 from India); all comparisons were made based on mature, whole, dried and raw seeds analyses. Protein, available carbohydrates, and fat contents showed to be higher in our samples, but fiber content (averaging 3.6%) was very low in comparison with FAO’s kabuli accessions data (ranging from 13 to 21%).
The humidity content of seeds was quite uniform, with values ranging from 7.9 to 8.8%, although higher contents of water has been described for Ecuadorian chickpea varieties, ranging 9–12% [21], and 8.6–10.3% for Pakistani kabuli-type genotypes [22]. Khattak et al. [22] found a strong positive correlation between seed size and protein content, and only positive correlation with seed moisture content. Our results did not reveal such interactions; on the contrary, in this study, there was a low and positive correlation (r = 0.29) among seed size with protein content, and negative correlation between seed size and humidity (r = − 0.38).
The seed concentrations of macro- and micronutrients elements are summarized in Table 4. Nitrogen % content analysis gathers the genotypes in two groups with mean ranging 357–324 and 347–317 mg/100 g, that did significantly differ one from another. P content was quite uniform across all genotypes in this study, with a general mean of 371 mg/100 g). BTs advanced lines plus Kasin variety did show the highest content of K (> 1100 mg/100 g). In general, the values of macronutrients (N, P, K, Ca, and Mg) obtained in this study agree with those reported by Costantini et al. [20] involving 12 chickpeas and those by Vandermark et al. [9] involving 22 chickpeas genotypes, but higher than those reported in FAO’s database [7]. In relation to micronutrients, both RILs had the lowest values of Fe and Mn content, while Cu concentration did not show significant differences across all ten genotypes. Contrasting our values of micronutrients composition, with those of the surveys mentioned above, Spanish genotypes have higher Fe and Mn concentrations, and an intermediate Cu content. Most probably, these differences may be due to soil chemical characteristics of the place where chickpeas were cultivated. The EU Regulation No. 1169/2011 on the provision of food information to consumers, established that when claiming a significant amount of a listed nutrients, the food should meet a 15% of the nutrient reference values (NRV) supplied by 100 g (Annex XIII). In our study, all chickpea genotypes can be claimed as containing significant amounts of P, K, Ca, Mg, Fe, Mn, Cu, and, Zn. It is noteworthy that the content in P, Fe, and Mn exceeds NVR values 3, 4, and 21 times, respectively (Table 4). However, grain legumes are mostly consumed after processing (hydration, boiling and cooking); it is well known that soaking and cooking may lead to losses in some nutrients [11, 23], nonetheless, nutrient retention factors (RFs) have been established in FAO’s user guide [7], and were defined as the coefficient expressing the preservation of nutrients in a food or dish after storage, preparation, warm holding, or re-heating. In the case of boiled pulses, the RFs are applied to minerals, vitamins and inositol. Applying these RFs (ranging 0.7–0.9) to minerals content, our chickpea samples would still contain, even after processing, significant amounts of minerals according with the EU Regulation No. 1169/2011.
Legumes have gaining additional interest because they are excellent sources of bioactive compounds and can be important sources of ingredients for uses in functional foods and other applications. A target analysis including more than 90 polyphenol compounds have been conducted in the methanolic extracts of the 10 chickpea genotypes and the Apulian black variety; results of seed phenolic composition are shown in Table 5. All genotypes present two polyphenolic compounds: gentisic acid and 4-hydroxybenzoic acid (except 5-RIL-92 that did not contain 4-hydroxybenzoic acid); in addition, organic acids such as glutamic, citric, and succinic acids are common in these chickpea seeds. Some genotypes present unique compounds. Thus, gallic acid, gallocatechin (flavanol), and rutin (quercitin flavonol) were only detected in 5-RIL-33 and Apulian black variety extracts; 2,4-dihydroxybenzoic acid has been found exclusively in 5-RIL-92 and Apulian black variety. On the other hand, p-coumaric acid was found in both inbred lines 5-RIL33 and 5-RIL92 plus BT6-19 and Apulian black seeds. Abscisic acid was present only in BT3-23 seeds and protocatechuic in BT6-19. In summary, colored seed genotypes share a high number and assortment of phenolics acids. It is well-known that the content of bioactive compounds of legumes is generally affected by planting environmental and genetic factors such as cultivar, cultivation year, cultivation location, and temperature [24, 25]. Hoek et al. [25] observed a significant genotype × environment interaction, although differences between the cultivars having the highest and lowest, total and individual isoflavone contents, were relatively consistent across the 16 environments tested. In our study, differences in polyphenol profiles may be mainly due to the genetic factor more than to environment component, as all chickpea genotypes were cropped in the same cultivation location and year. These data are in accordance with those obtained by Lin and Lai [24] on bioactive compound in legumes, they concluded that the dark-coat seeds, such as azuki beans and black soybeans, contained high amounts of phenolic compounds and contributed to high antioxidative ability. In agreement with Lin and Lai [24] work, results on 17 chickpea lines having colored seed coats [26] established that colored seed contained up to 13- and 11-fold more total polyphenol and total flavonoid content, respectively. This characteristic, high bioactive compounds content in colored seeds, seems a general rule in other legumes such as Phaseolus vulgaris and Vigna subterranea [27, 28], which reinforce the worldwide accepted importance of grain legumes consumption as source of bioactive compound in addition to their nutritional and mineral provisions. Further work should be done to valorize the role of the unique phenolic compounds found in this study in the human diet.
Conclusions
In summary, proximate, mineral composition, and polyphenols content have allowed us the genotypic discrimination of ten Spanish chickpea genotypes. The protein content of this set of chickpea genotypes (>25%), to our best knowledge overcome the described values of other genotypes. So, this study could be an useful tool to guide farmers and breeders in choosing chickpea genotypes, taking into account the nutritional composition and, the consumer preferences (morphological characteristics). Moreover, as three of these genotypes are resistant to both Ascochyta blight and Fusarium oxysporum, they could be recommended depending on the annual incidence of these diseases. These results also reinforce the idea of healthy habit of legumes consumption based on (1) alternative to the consumption of animal proteins, (2) their antioxidant capacity, and (3) their advantages in the forecast climate change scenario.
Data availability
The data generated and analyzed during the current study will be available from the corresponding author on reasonable request.
References
van der Maessen LJG (1972) Cicer L., a monograph of the genus, with special reference to the chickpea (Cicer arietinum L.), its ecology and cultivation. Landbouwhogeschool Wageningen. Veenman-341
Toker C (2009) A note on the evolution of kabuli chickpeas as shown by induced mutations in Cicer reticulatum Ladizinsky. Gen Resour Crop Evol 56:7–12
Eker T, Sari D, Sari H, Sule TH, Toker C (2022) A kabuli chickpea ideotype. Sci Rep. https://doi.org/10.1038/s41598-022-05559-3
Knights EJ, Wood JA, Harden S (2011) A gene influencing seed shape of desi type chickpea (Cicer arietinum L.). Plant Breed 130:278–280
Magrini MB, Anton M, Chardigny JM, Duc G, Duru M, Jeuffroy MH, Meynard JM, Micard V, Walrand S (2018) Pulses for sustainability: breaking agriculture and food sectors out of lock-in. Front Sustain Food Syst. https://doi.org/10.3389/fsufs.2018.00064
Oliveira B, de Moura AP, Cunha LM (2019) Increasing pulse consumption to improve human health and food security and to mitigate climate change. In: Castro P, Azul A, Leal Filho W, Azeiteiro U (eds) Climate change-resilient agriculture and agroforestry: ecosystem services and sustainability. Springer, London. https://doi.org/10.1007/978-3-319-75004-0_2
FAO/INFOODS (2017) Global food composition data base for pulses. User guide. https://www.fao.org/infoods/infoods/tables-and-databases/faoinfoods-databases. Accessed 15 Mar 2023
Margier M, Georgé S, Hafnaoui N, Remond D, Nowicki M, Du Chaffaut L, Amiot MJ, Reboul E (2018) Nutritional composition and bioactive content of legumes: characterization of pulses frequently consumed in France and effect of the cooking method. Nutrients. https://doi.org/10.3390/nu10111668
Vandemark GJ, Grusak MA, McGee RJ (2018) Mineral concentrations of chickpea and lentil cultivars and breeding lines grown in the US Pacific Northwest. Crop J. https://doi.org/10.1016/j.cj.2017.12.003
UPOV (International Union for the Protection of new varieties of Plants) (2020) Guidelines for the conduct of tests for distinctness, uniformity and stability. Code(s) CICER_ARI Cicer arietimum L.
Avola G, Patanè C, Barbagallo RN (2012) Effect of water cooking on proximate composition of grain in three Sicilian chickpeas (Cicer arietinum L.). Food Sci Technol 49:217–220
Dayrell RLC, Ott T, Horrocks T, Poschlod P (2023) Automated extraction of seed morphological traits from images. Methods Ecol Evol 14:1708–1718
Gil J, Castro P, Millán T, Madrid E, Rubio J (2017) Development of new kabuli large-seeded chickpea materials with resistance to Ascochyta blight. Crop Pasture Sci. https://doi.org/10.1071/CP17055
Caballo C, Madrid E, Gil J, Chen W, Rubio J, Millán T (2019) Saturation of genomic region implicated in resistance to Fusarium oxysporum f.sp. ciceris race 5 in chickpea. Mol Breed. https://doi.org/10.1007/s11032-019-0932-.4
Cervantes E, Martín JJ, Saadaou E (2016) Updated methods for seed shape analysis Hindawi Publishing Corporation Scientifica, pp 10. ID 5691825
Khamassi K, Babay E, Rouissi M, Dakhlaoui A, Rayda Ben Ayed R, Hanana M (2021) Genetic variability of tunisian faba beans (Vicia faba L.) based on seeds’ morphophysical properties as assessed by statistical analysis. Hindawi J Food Qual. https://doi.org/10.1155/2021/9493607
Daniel IO, Adeboye KA, Oduwaye OO, Porbeni J (2012) Digital seed morpho-metric characterization of tropical maize inbred lines for cultivar discrimination. Int J Plant Breed Genet 6:245–251
Gil J, Cubero JI (1993) Inheritance of seed coat thickness in chickpea (Cicer arietinum L.) and its evolutionary implications. Plant Breed 111:257–260
Moreiras O, Carbajal A, Cabrera L, Cuadrado C (2013) Tablas de composición de alimentos. Guía de Prácticas. Piramide, Madrid
Costantini M, Summo C, Centrone M, Rybicka I, Dagostino M, Annicchiarico P, Caponio F, Pavan S, Tamma G, Pasqualon A (2021) Macro- and micro-nutrient composition and antioxidant activity of chickpea and pea accessions. Polish J Food Nutr Sci. https://doi.org/10.31883/pjfns/135813
Polo Chávez IA (2012) Determinación proximal de los principales componentes nutricionales de seis variedades de leguminosas: arveja, garbanzo, haba, lenteja, maní y soya. Bachelor´s Degree. Pontificia Universidad Católica del Ecuador, Facultad de Ciencias Exactas y Naturales, Escuela de Ciencias Químicas
Khattak AB, Shah Khattak GS, Mahmood Z, Bibi N, Ihsanullah I (2006) Study of selected quality and agronomic characteristics and their interrelationship in kabuli-type chickpea genotypes (Cicer arietinum L.). Int J Food Sci Technol 41:1–5
Güzel D, Sayar S (2012) Effect of cooking methods on selected physicochemical and nutritional properties of barlotto bean, chickpea, faba bean, and white kidney bean. J Food Sci Technol 49:89–95
Lin P-Y, Lai H-M (2006) Bioactive compounds in legumes and their germinated products. J Agric Food Chem 54:3807–3814
Hoeck JA, Fehr WR, Murphy PA, Welk GA (2000) Influence of genotype and environment on isoflavone contents of soybean. Crop Sci 40:48–51
Segev A, Badani H, Kapulnik Y, Shomer I, Oren-Shamir M, Shmuel Galili S (2010) Determination of polyphenols, flavonoids, and antioxidant capacity in colored chickpea (Cicer arietinum L.). J Food Sci. https://doi.org/10.1111/j.1750-3841.2009.01477.x
Hungría M, Phillips DA (1993) Effects of a seed color mutation on Rhizobial nod-gene-inducing flavonoids and nodulation in common bean. MPMI 6:418–422
Puozaa DK, Jaiswal SK, Dakora FD (2021) Black seed-coat pigmentation is a marker for enhanced nodulation and N2 fixation in bambara groundnut (Vigna subterranea L. Verdc.) landraces. Front Agron 3:692238
Acknowledgements
The authors acknowledge Dr. Miguel Camacho for images-scanner acquisition and data management, and Laboratorio Agroalimentario de Córdoba (AGAPA-Andalucía) for proximate composition assessment. This work was supported by PRIMA PCI2020-112151 project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they do not have any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work. We have not used AI-assisted technologies in the writing process.
Compliance with ethics requirements
The article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brun, P., Camacho, M., Perea, F. et al. Characterization of Spanish chickpea genotypes (Cicer arietinum L.): proximate, mineral, and phenolic compounds composition. Eur Food Res Technol 250, 1007–1016 (2024). https://doi.org/10.1007/s00217-023-04437-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04437-0