Abstract
Khao-Mak is a traditional dessert from Thailand with a unique flavor profile from low alcohol and lactic acid. Khao-Mak is made from cooked glutinous rice fermented with the starter culture (Look-Pang), Look-Pang including yeast, molds, lactic acid bacteria, and herbs. Khao-Mak is made from white glutinous rice, pigmented rice (Kam-Doi, Leum-Pua), and germinated pigmented rice (Kam-Doi, Leum-Pua). This investigation focused on the physico-chemical, metabolites and flavor profiles of Khao-Mak in the fermentation period to optimize the best time for fermentation and biomarkers. The best time for fermentation was 2 days, because after day 2 of fermentation the physico-chemical properties slightly changed. Leum-Phua is the best rice variety for producing Khao-Mak in this research, because Leum-Phua was found to have the highest total anthocyanin content (TAC), total phenolic content (TPC) and %DPPH. In addition, metabolomics and flavoromics analysis results discovered 37 and 48 compounds, respectively, produced during fermentation processes. The metabolites and flavor compounds found were used to differentiate all samples using principal component analysis (PCA). Khao-Mak had strong alcohol, wine-like, whiskey-like, solvent-like, sweet and fruity flavors. Heat plot of metabolites and flavor compounds showed that lactic acid, acetic acid, 3-methyl-1-butanol, 2-methyl-1-propanol, and 1-propanol were in greater proportions than other compounds and used as biomarkers for fermentation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glutinous rice (Oryza sativa L. var. glutinosa) is used to make a traditional fermented dessert in Thailand called “Khao-Mak” [24]. Khao-Mak is produced from cooked white rice and a fermentation starter called “Look-Pang”, which consists of semicircle starch balls including molds (Aspergillus sp., Rhizopus sp., and Mucor sp.), yeast (Saccharomyces cerevisiae and Candida sp.), and lactic acid bacteria with pepper, garlic, and galangal as antibacterial agents in rice flour. During processing, enzymes from the mold (alpha-amylase, glucoamylase), hydrolyze starch in rice into sugar, which is then partially fermented to alcohol by yeast. Lactic acid bacteria also generate organic acids such as lactic acid [17]. This has a probiotic that consists of live microorganisms and can have health benefits on the host when administered in adequate amounts.
Pigmented rice and germinated rice are popular in Thailand. This rice contains more nutrition than white rice and has antioxidant activity, high vitamins B and E, dietary fiber, and GABA, and therefore used as an ingredient in snacks and desserts. Khao-Mak produced from pigmented rice has high antioxidant activity and GABA, and the flavor of volatile compounds in pigmented rice is higher than in white rice [26]. However, there is still a lack of research information on metabolomics changes in Khao-Mak fermentation.
At present, metabolomics technology has recently been used extensively to study metabolites in the fermentation process of fermented foods [4]. Gas chromatography (GC) and liquid chromatography (LC) are two of the most commonly used metabolomics techniques [18]. Metabolomics based on GC–MS has been successfully applied to the study of rice koji and Chinese rice wine, allowing researchers to uncover dynamic changes in metabolite profiles and optimize fermentation time. This research aims to investigate the combined metabolomics by UHPLC-Orbitrap Exploris MS and flavoromics by GC–TOF–MS to analyze the dynamic changes in the metabolite and flavor profiles in Khao-Mak to optimize the best time for fermentation and biomarkers of Khao-Mak.
Khao-Mak is produced from five different rice varieties. White glutinous rice (Khiaw Ngoo) is suitable for making Khao-Mak because of its fragrance. Kam Doi (germinated Kam Doi) is also a flavorful locally sourced black sticky rice that contains high flavonoids and anthocyanins. Furthermore, Leum Phua (germinated Leum Phua) is another unique variety of aromatic, glutinous rice, and research suggests that it contains high antioxidant properties.
Materials and methods
Materials and chemicals reagents
White glutinous rice was obtained from ‘Khao Niew Khiaw Ngoo’ in Chiang Saen district, Chiang Rai province in northern Thailand. Pigmented rice ‘Kam-Doi’ was obtained from Doi Saket district, Chiang Mai province in northern Thailand and ‘Leum-Pua’ in Phop Phra district, Tak province in northern Thailand. All rice was purchased from a local farmer and had been harvested in December 2020. Then it was vacuum-sealed-packed (1 kg/pack) and transported by truck to the laboratory at Kasetsart University, Bangkok, Thailand. Look-Pang (starter culture) was purchased from the local market in Lam Luk Ka district, Pathum Thani province in central Thailand. The chemicals and standards were either HPLC or analytical grade for physico-chemical analysis, GC and uHPLC, purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA).
Preparation of Khao-Mak
The traditional method of Wongsa et al. was used to make Khao-Mak. Briefly, rice samples were cooked in a rice cooker. Thereafter, the cooked rice was cooled down to room temperature and the water drained for 15 min. The obtained rice (300 g) was mixed with Look-Pang starter powder (0.6 g) in a plastic cup. The plastic containers were closed tightly and kept at 30 °C for 3 days.
Physico-chemical analysis of Khao-Mak
The pH values and alcohol content of Khao-Mak samples from all treatments were examined by a pH meter (Oakton, IL, USA) and a vinometer, respectively. Khao-Mak samples were ground, and the TSS of the resulting liquid was evaluated by a refractometer (Krüss, Hamburg, Germany).
Reducing sugar content
The rice sample and fermentation broth of Khao-Mak 10 g were mixed and diluted by ten times. The amount of reducing sugar was measured by the 3,5-dinitrosalicylic acid (DNS) assay [6] with 1 mL of the extracted sample, adding 1 mL of DNS solution, boiling for 5 min, and then cooling down in ice rapidly. The absorbance of each sample was measured at a wavelength of 540 nm using a spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). For quantification, a glucose standard curve with a concentration between 0 and 1 mg/mL was used.
Total anthocyanin content (TAC)
The rice sample and fermentation broth of Khao-Mak 10 g were mixed and diluted by ten times. TAC was measured by the pH difference method [2]. To 20 μL of the extracted sample, 3 mL of pH 1.0 KCl buffer was added, and to another 20 μL of the extracted sample, 3 mL of sodium acetate pH 4.5 buffer was added. The two test tubes were measured for absorbance at 510 and 700 nm using a spectrophotometer. The total anthocyanin content of the sample was calculated from the following equation:
where A = (A510 nm – A700 nm) pH 1.0 – (A510 nm – A700 nm) pH 4.5, MW = 449.2 g/mol/cm (molecular weight of cyanidin-3-glucoside), e = 26,900 L/mol/cm (molar absorptivity), L = 1 cm (length of cuvette), DF = dilution factor of sample solution.
Total phenolic content (TPC)
The rice sample and fermentation broth of Khao-Mak 10 g were extracted with 70% acetone 90 mL and centrifuged for precipitation. TPC was measured by the Folin–Ciocalteu assay [5]. The extracted sample of 50 µL was added to 125 µL of 7.5% sodium carbonate solution, and then 25 µL of 0.1 N Folin–Ciocalteu reagent was allowed to react for 30 min at ambient temperature. The absorbance was measured at a wavelength of 755 nm using a spectrophotometer. The results were expressed as mg gallic acid equivalent (GAE)/g of extract.
DPPH (2, 2-diphenyl-1-picrylhydrazyl) scavenging assay
The DPPH radical-scavenging effect of the sample was determined according to the method of Manosroi et al. [16] The spectrophotometer was used to measure the absorbance at wavelength 517 nm. The DPPH radical-scavenging activity was measured by calculating using the following formula:
Acontrol is the absorbance of the control reaction, Asample is the absorbance of the sample.
Metabolomics
The metabolite extraction technique was adapted from Jin et al. [9] with slight modifications for the sample. 50 mg of Khao-Mak was ground, then 1.5 mL of methanol/dichloromethane (7/3, v/v) was added into a centrifuge tube. After being vortexed for 60 s, the samples were extracted for 30 min into an ultrasonic bath at ambient temperature. The extracts were centrifuged at 4000 g for 10 min, and the supernatants were filtered through a 0.22 µm syringe filter and transferred to LC–MS vials.
The metabolomics analyzed in this study were untargeted metabolomics using a UPLC (Vanquish Flex UHPLC, Thermo Scientific, Germering, Germany) system connected to a binary pump system. The mass spectrometer was attached to Orbitrap Exploris 120 with electrospray ionization (H-ESI) and a hybrid quadrupole-orbitrap mass spectrometer (Thermo Scientific, Bremen, Germany). The columns used for the UPLC system were Hypersil Gold C-18 columns (2.1 × 150 mm, 1.9 µm) (Thermo Fisher Scientific Inc., Waltham, MA, USA). The protocol method used was followed as in Jin et al. [9]. A (0.1% formic acid in water) and B [0.1% formic acid in isopropanol/acetonitrile (4/6, v/v)] were used for the mobile phase. The mass spectrometry analysis was performed in each mode of electrospray ionization-positive mode as follows: range of mass-to-charge ratio (m/z): 70–700, full MS scan: 60,000 resolution, data-dependent MS2: 15,000 resolution, collision energy: 30, 50, 150 eV.
Flavoromics
The extraction was followed as in Wattanakul et al. [25]. The extraction was done using the autosampler headspace solid-phase microextraction (HS-SPME) method. The sample (3 g) was put into a 20 mL headspace vial and 10 μL of 0.1 mg/mL methyl nonanoate in methanol (internal standard) and 1 g of sodium chloride were added into the vial, and n-alkane (C6–C30) groups were used as standards to determine the retention index (RI). A 50/30 μm divinylbenzene/carboxen/polydimethylbenzene (DVB/CAR/PDMS) fiber was used to absorb the volatile compound.
Flavor compounds in Khao-Mak were measured and identified using a 6890N GC coupled with a time-of-flight mass spectrometer (Leco Corp., St. Joseph, MI, USA). To separate volatile compounds, a Stabilwax fused silica column (30 m 0.25 mm 0.25 m film thickness) with a cross-bond polyethylene glycol stationary phase was utilized. The method used followed that by Wattanakul et al. [25]. The identification and concentration of flavor compounds were performed using the ChromaTOF-GC Software v4.50.8.0.
Statistical data analysis
The physico-chemical data were analyzed at least twice, and the results were given as means of standard deviation (SD). The International Business Machines Statistical Package for the Social Sciences (SPSS) software version 22 was used to do a one-way analysis of variance (ANOVA) (SPSS Inc., Chicago, IL, USA). Duncan's multiple range test was used to assess significant differences between treatments at the 95% confidence level (P ≤ 0.05).
The data of metabolite by UHPLC were analyzed to identify the type of metabolites with Compound Discoverer software version 3.3 (Thermo Fisher Scientific, USA) by searching two databases, 1. mzCloud (https://www.mzcloud.org) and 2. ChemSpider compound (http://www.chemspider.com).
The HP-ChemStation A.06.03 tool was used to combine the peak regions of metabolites (Hewlett Packard). The reference standards comparison approach was used to identify metabolites. Each fraction's internal standard was used to calculate the concentration of each discovered metabolite in mg/mL.
The mass spectra and genuine standards were used to provide a preliminary identification of the targeted components connected to the Khao-Mak. NIST mass spectral database version 2.0 was used to compare mass spectra (National Institute of Standards and Technology, Gaithersburg, MD, USA). RI of the n-alkanes series (C6–C30) was used to calculate and compare to RI data obtained in the literature using the same GC column polarity. The authenticity of several taste components that had been tentatively identified was validated using authentic standards. The internal standard approach was used to determine the relative concentration of all flavor compounds. Each standard compound's and internal standards' calibration curves demonstrated acceptable linearity (R2 > 0.98).
Using the XLSTAT-base version 2018.3 software (Addinsoft, New York, NY, USA), the relative concentrations of metabolites and flavor compounds were subjected to PCA and AHC. Non-parametric correlation network analysis of the correlation/association tests option in the XLSTAT-base program was used to perform Spearman's rank correlation of all metabolites and flavor compounds at a significance level of (P ≤ 0.05).
Results and discussion
Physico-chemical properties of Khao-Mak during fermentation
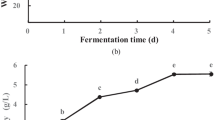
Khao-Mak was produced from five rice varieties, White glutinous rice (W), Kam-Doi (KD), germinated Kam-Doi (KDG), Leum-Pua (LP), and germinated Leum-Pua (LPG). The fermentation broth and rice were mixed to measure the physico-chemical properties consisting of TSS, reducing sugar, pH, and %alcohol during the fermentation period. The results are shown in Fig. 1
Physico-chemical attributes. A Total soluble solids, B reducing sugar, C alcohol content, and D pH of Khao-Mak produced from W (black triangle), KD (multipy), KDG (black diamond), LP (black circle) and LPG (black square) during the fermentation period (0–3 days). Values are means ± standard deviation (SD). Means in the same column indicated by different letters are significantly different at 95% confidence level (p ≤ 0.05)
The TSS of Khao-Mak from five rice varieties increased throughout the fermentation period. The TSS rapidly increased from day 0 and slightly increased after day 2 of fermentation. This was because mold saccharification due to total soluble solids indicates the content of various sugars such as sucrose, glucose, fructose, organic acids, and minerals dissolved in water [21, 26]. The amount of TSS found was consistent with the amount of reducing sugar. The maximum reducing sugar content was detected on day 1 of fermentation in pigmented rice and white rice which was the highest reducing sugar on day 2 of fermentation [19]. Then reducing sugar content rapidly decreased and the %alcohol increased. These results suggest that yeast consumed reducing sugar from saccharification and converted it to ethanol. During the fermenting process, the alcohol content increases. Khao-Mak produced from W showed higher content of alcohol than other rice varieties. These results are in line with Wongsa (2018). The pH value of Khao-Mak of the five rice varieties decreased over the fermentation period. It rapidly decreased on day 1 of the fermentation and slightly decreased until the end because lactic acid bacteria convert sugar to acid [23].
TAC, TPC, and DPPH of Khao-Mak during fermentation
Anthocyanin and phenolic compounds are secondary metabolites produced in plants. The results of TAC, TPC, and %DPPH radical scavenging are shown in Fig. 2 On day 0 of fermentation, the highest TAC and TPC were found in LP, followed by KDG, KD, LPG and W, respectively. TAC and TPC increased throughout the fermentation period [30]. The enzyme from microorganisms and alcohol would break the bonds between anthocyanin and phenolic compounds with other substances during the fermentation process, releasing anthocyanin and phenolic compound monomers or antioxidants. According to Kong et al. [11], acid or weak acid can cause a partial or complete hydrolysis of anthocyanin molecules. Therefore, the total anthocyanin content could be decreased. A higher amount of TPC and TAC was found to have higher antioxidant activity. The DPPH radical model was a wide and quick tool for estimating free radical-scavenging activity [5]. The ability of antioxidants to donate hydrogen was thought to respond to their effect on DPPH radical scavenging. This scavenging was observed as a change in color from purple to yellow. As a result, DPPH was commonly used as a substrate to assess antioxidant activity [14]. Khao-Mak produced from LP had the highest %DPPH radical-scavenging contents (74.43%) at the end of the fermentation period, followed by KDG, KD, LPG, and W, respectively.
The total anthocyanin content (A), total phenolic content (B), and %DPPH radical scavenging (C) of Khao-Mak produced from W (black triangle), KD (multipy), KDG (black diamond), LP (black circle), and LPG (black square) during the fermentation period (0–3 days). Values are means ± standard deviation (SD). Means in the same column indicated by different letters are significantly different at 95% confidence level (p ≤ 0.05)
The result of physico-chemical properties after 0–2 days of fermentation changed rapidly and then after 2 days slightly changed. Khao-Mak from W, KD, KDG, LP, and LPG fermentation for 2 days was within the standard Thai community product standards such as TSS around 40–50%, alcohol not exceeding 0.5%, pH 4–4.5. So, the recommended optimum time to ferment Khao-Mak was 2 days.
Metabolomics and flavoromics of Khao-Mak
In conclusion, there were around 100 chromatograms of both identified and unidentified metabolite profiles by metabolomics approach. There were 38 identifiable peaks, accounting for 40% of all metabolites in Khao-Mak produced from five rice varieties during fermentation for 0–3 days. All 38 metabolites could be divided into five groups, including 7 organic acids, 15 amino acids, 4 flavonoids, 9 fatty acids, and 2 sugar compounds. From the overall chromatograms, the flavoromics analysis produced more than 400 peaks. The substances were identifiable among these 48 peaks, making up around 12% of all the volatile compounds in Khao-Mak. All 48 flavor compounds may be classified into eight classes: 14 alcohol, 10 esters, 8 aldehydes, 7 volatile acids, 6 ketones, 1 acetol, 1 benzaldehyde, and 1 lactone. There were ~ 350 unidentified volatile compounds. PCA was applied to all metabolic and flavor chemicals.
All of the metabolites and flavor compounds during the Khao-Mak fermentation were separated along the PC1 and PC2 axes, accounting for almost 70% of the total variables, according to a PCA biplot (Fig. 3). The PCA biplot was used to group samples based on all compounds found in Khao-Mak. The PCs demonstrated eigenvalues of 57.79% and 12.39% of the overall variation for PC1 and PC2, respectively, at 0–3 days of Khao-Mak fermentation. Based on the variations in rice varieties, metabolites and flavoring compounds were divided into five clusters. On PC2, a general description of the variations between rice varieties could be found. The W was reliant on cluster 1, the KD on cluster 2, the KDG on cluster 3, the LD on cluster 4, and the LPG on cluster 5, demonstrating that throughout Khao-Mak fermentation, most of the sugars, amino acids, aldehydes, alcohols, and volatile acids increase, while most lipids decrease.
Coupled metabolomics–flavoromics analysis identified 5 compounds outstanding than other compounds. Lactic acid and acetic acid could be possible biomarkers for improper fermentation. Ethyl oleate, 3-methyl-1-butanol, 2-methyl-1-propanol, and 1-propanol could be used as biomarkers for monitoring fermentation.
Agglomerative hierarchical clustering analysis (AHC) based on the fermentation period (0–3 days) was performed to confirm PCA grouping (Fig. 4). The dendrogram verified that the rice varieties were similar, with differences in five groups: group 1 W; group 2 LPG; group 3 KDG; group 4 KD; and group 5 LP.
To look into compounds during Khao-Mak fermentation, a heat map analysis based on relative concentrations was used (Fig. 5). A heat map of all metabolites is shown in Fig. 5A. Carbohydrates in Khao-Mak were hydrolyzed by yeast and mold during their alcoholic metabolism. Sugar has the potential to serve as a substrate for the synthesis of aromatic amino acids and phenols.
The organic acid contents were increased during fermentation. Lactic, citric, and pyroglutamic acids were most abundant in Khao-Mak from W, KD, KDG, LP, and LPG metabolites. An increase in acid has also been reported in Korean fermented brown rice [](Jin, M. et al., 2020). Lactic acid is produced when pyruvate reacts with lactate dehydrogenase. Pyruvate is an important intermediate product in several metabolic pathways. It can be [20]
The branched-chain and aromatic amino acids such as valine, leucine, and tryptophan are assimilated into small amino acids. Consequently, these small molecules are involved in the Ehrlich pathway of yeast and converted to higher alcohols. So amino in Khao-Mak was increased on the 1st and 2nd days of fermentation and decreased on the final day of fermentation. Khao-Mak from W, KD, KDG, LP, and LPG exhibited a similar trend.
The flavonoid compounds in Khao-Mak increased during fermentation because of flavonoids extracted from rice [8]. Many factors influence the number of flavonoids extracted during fermentation, including the duration of skin maceration, ethanol concentration, pH, and pectolytic enzymes [13].
The last group in metabolomics was the fatty acids. These compounds were decreased in the fermentation period. Khao-Mak from W, KD, KDG, LP, and LPG exhibited a similar trend. A decrease in fatty acid has also been reported in Chinese rice wine [1, 10]. Yeast could convert fatty acid to create a membrane for ethanol tolerance [15].
One of the most important flavor groups in Khao-Mak was volatile alcohol as shown in Fig. 5B. The most abundant alcohols observed were higher alcohols such as 3-methyl-1-butanol (isoamyl alcohol), 1-propanol, and 2-methyl-1-propanol (isobutyl alcohol). These flavors are also apparent in Thai rice wine. Alcohols increased as a result of their formation during fermentation via the Ehrlich pathway with amino acids. In this pathway, the transformation of amino acids to ketones and aldehydes also occurred. Finally, amino acids may be reduced to higher alcohols via NADH-dependent chemical reactions [3, 7, 22]. Furthermore, 2-methyl-1-propanol from the valine metabolism shows a significant impact on the overall sensory complexity of rice wine [29].
Esters were the second abundant group of volatile compounds identified such as pentanoic acid, ethyl ester (ethyl pentanoate), ethyl acetate, and hexanoic acid, ethyl ester (ethyl hexanoate). Esters are formed by the esterification of fatty acids [27]. On the other hand, the interaction of alcohols with acetyl-CoA produces acetate esters [12]. Ethyl esters are produced via various mechanisms, such as from medium- and long-chain fatty acids and from the reaction between acyl-CoA and alcohols. In addition, the level of esters in the samples was impacted by starter cultures, fermentation conditions, and rice species [8]. Volatile losses may occur as a result of oxidation or other chemical reactions.
The precursor’s relation of all metabolites and flavor compounds during Khao-Mak fermentation is shown in Fig. 6. Flavor compounds were mostly associated with lipid composition. Lipids showed a strong positive correlation (r > 0.7) with acetol, alcohol, aldehyde, esters, ethers, ketones, lactones and volatile acids. However, lipids had a strong negative correlation (r < – 0.7) with some alcohols, some aldehydes, benzaldehyde, and some esters. This might occur since those compounds are products of yeast fermentation. Not only lipids, but also acid compounds, containing amino and organic acids, showed a strong positive correlation (r > 0.7) with alcohols, aldehydes, esters, ketones, and volatile acids. This could be because amino acids are substrates in the Ehrlich pathway and alanine, aspartate and glutamate metabolism, which produce alcohols and volatile acids [28]. These data may point to the interaction of both metabolites and flavor during the fermentation process of Khao-Mak. Significant metabolites would indeed imply that they were in charge of the development of flavor compounds with a fragrant aroma during Khao-Mak production. To create Khao-Mak from other rice varieties with unique flavors and elevated concentrations of bioactive compounds, the results may provide knowledge for controlling these variables and Khao-Mak conditions.
The lower triangular heat map represents a pairwise correlation analysis between metabolites and flavor compounds during the fermentation of sweet fermented rice. Each square represents Spearman’s rank correlation coefficient at a significance level of P ≤ 0.05. An orange-red, strong positive correlation (r > 0.7); green, strong negative correlation (r < – 0.7)
Conclusion
Khao-Mak from the rice varieties W, KD, KDG, LP, and LPG was successfully prepared in the laboratory. The changes in the physico-chemical properties, metabolite profile, and flavor profile were investigated to determine the overall properties of the sweet fermented rice during fermentation. The biomarkers of Khao-Mak fermentation were identified as: lactic acid, 3-methyl-1-butanol, 2-methyl-1-propanol and 1-propanol. Understanding TPCs, antioxidant activity was higher in the fermentation period. LP is the best variety in this research for producing Khao-Mak because it had the highest TAC, TPC and %DPPH. The best time of fermentation is 2 days, because after day 2 all the physico-chemical properties slightly changed. In addition, the interactions of both the metabolites and flavor compounds of Khao-Mak during fermentation were simply understood by multivariate and correlation analysis. This technique could be used to identify important bioactive and aroma compounds as well as other characteristics of Khao-Mak. This crucial information could be applied in the future to enhance the processing quality of fermented beverage. The combination of metabolomic and flavoromic techniques resulted in a powerful alternative tool for comprehensively determining the dynamic changes that occur during Khao-Mak fermentation.
Data availability
The author confirm that the data supporting the findings of this study are available within the article. The rest of them are available upon request from the corresponding author.
References
Alves JA, Lima LCDO, Dias DR, Nunes CA, Schwan RF (2010) Effects of spontaneous and inoculated fermentation on the volatile profile of lychee (Litchi chinensis Sonn) fermented beverages. Int J Food Sci Technol 45(11):2358–2365
AOAC (2005) Official methods of analysis of the association of official analytical chemists, 18th edn. Association of Official Analytical Chemist, p 1
Belda I, Ruiz J, Esteban-Fernández A, Navascués E, Marquina D, Santos A et al (2017) Microbial contribution to wine aroma and its intended use for wine quality improvement. Molecules 22(2):189
Cevallos-Cevallos JM, Reyes-De-Corcuera JI, Etxeberria E, Danyluk MD, Rodrick GE (2009) Metabolomic analysis in food science: a review. Trends Food Sci Technol 20(11–12):557–566
Dela Rosa JGL, Medina PMB (2021) Philippine rice wine (Tapuy) made from Ballatinao black rice and traditional starter culture (Bubod) showed high alcohol content, total phenolic content, and antioxidant activity. Food Science and Technology 42:5120
Garriga M, Almaraz M, Marchiaro A (2017) Determination of reducing sugars in extracts of Undaria pinnatifida (harvey) algae by UV-visible spectrophotometry (DNS method). DESARROLLO E INNOVACIÓN EN INGENIERÍA
Hazelwood LA, Daran J-M, Van Maris AJ, Pronk JT, Dickinson JR (2008) The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl Environ Microbiol 74(8):2259–2266
Ivanova V, Dörnyei Á, Márk L, Vojnoski B, Stafilov T, Stefova M et al (2011) Polyphenolic content of Vranec wines produced by different vinification conditions. Food Chem 124(1):316–325
Jin M, Zheng W, Zhang Y, Gao B, Yu L (2020) Lipid compositions and geographical discrimination of 94 geographically authentic wheat samples based on UPLC-MS with non-targeted lipidomic approach. Foods 10(1):10
Jin Z, Cai G, Wu C, Hu Z, Xu X, Xie G et al (2021) Profiling the key metabolites produced during the modern brewing process of Chinese rice wine. Food Res Int 139:109955
Kong J-M, Chia L-S, Goh N-K, Chia T-F, Brouillard R (2003) Analysis and biological activities of anthocyanins. Phytochemistry 64(5):923–933
Lambrechts M, Pretorius I (2000) Yeast and its importance to wine aroma-a review. S Afr J Enol Vitic 21(1):97–129
Lamçe F, Gozhdari K, Kongoli R, Meta B, Kyçyk O (2018) Evaluation of the content of polyphenols and flavonoids during the fermentation of white wines (cv. Pulëz and Shesh i bardhë) with and without skins. Alban j agric sci 17:564–571
Lee B-J, Kim J-S, Kang YM, Lim J-H, Kim Y-M, Lee M-S et al (2010) Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem 122(1):271–276
Mannazzu I, Angelozzi D, Belviso S, Budroni M, Farris GA, Goffrini P et al (2008) Behaviour of Saccharomyces cerevisiae wine strains during adaptation to unfavourable conditions of fermentation on synthetic medium: cell lipid composition, membrane integrity, viability and fermentative activity. Int J Food Microbiol 121(1):84–91
Manosroi A, Ruksiriwanich W, Kietthanakorn B, Manosroi W, Manosroi J (2011) Relationship between biological activities and bioactive compounds in the fermented rice sap. Food Res Int 44(9):2757–2765
Mongkontanawat N, Lertnimitmongkol W (2015) Khoa Mak from germinated native black glutinous rice. J Agric Technol 11(2):501–515
More T, RoyChoudhury S, Gollapalli K, Patel SK, Gowda H, Chaudhury K et al (2015) Metabolomics and its integration with systems biology: PSI 2014 conference panel discussion report. J Proteom 127:73–79
Oliveira MdS, Feddern V, Kupski L, Cipolatti E, Badiale-Furlong E, de Souza-Soares L (2010) Physico-chemical characterization of fermented rice bran biomass Caracterización fisico-química de la biomasa del salvado de arroz fermentado. CyTA J Food 8(3):229–236
Saerens SM, Delvaux FR, Verstrepen KJ, Thevelein JM (2010) Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb Biotechnol 3(2):165–177
Samappito J, Kubola J, Supakot P, Sitthitrai K (2019) Study on production of sweet fermented rice (Khao-Mak) from black glutinous rice using look pang varieties in Buriram. Rajabhat Agric 18(1):47–55
Styger G, Prior B, Bauer FF (2011) Wine flavor and aroma. J Ind Microbiol Biotechnol 38(9):1145
Taweekasemsombut S, Tinoi J, Mungkornasawakul P, Chandet N (2021) Thai rice vinegars: production and biological properties. Appl Sci 11(13):5929
Tochampa W, Jittrepotch N, Kongbangkerd T, Kraboun K, Rojsuntornkitti K (2011) The study of microwave heating time on chemical and microbiological properties and sensory evaluation in sweet fermented glutinous rice (Khao-Mark). Int Food Res J 18(1):239–248
Wattanakul N, Morakul S, Lorjaroenphon Y, Na Jom K (2020) Integrative metabolomics-flavoromics to monitor dynamic changes of ‘Nam Dok Mai’ mango (Mangifera indica Linn) wine during fermentation and storage. Food Biosci 35:100549
Wongsa J, Hamontree C, Rungsardthong V, Yasutomo T (2018) Production and analysis of volatile flavor compounds in sweet fermented rice (Khao Mak). In: MATEC Web of Conferences, p 192
Yu H, Xie T, Xie J, Ai L, Tian H (2019) Characterization of key aroma compounds in Chinese rice wine using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem 293:8–14
Zhang J, Xu L, Xu X, Wu X, Kuang H, Xu C (2022) Profiles of Sterigmatocystin and its metabolites during traditional chinese rice wine processing. Biosensors 12(4):212
Zhao C, Su W, Mu Y, Jiang L, Mu Y (2020) Correlations between microbiota with physicochemical properties and volatile flavor components in black glutinous rice wine fermentation. Food Res Int 138:109800
Zheng R-L, Ren T, Niu C-T, Zheng F-Y, Wang J-J, Liu C-F et al (2022) Anthocyanins composition and antioxidant activity of purple rice and color degradation under sunlight exposure of purple rice wine. J Food Meas Charact 16(3):1889–1900
Acknowledgements
The authors are greatly thankful to the Faculty of Agro-Industry, Kasetsart University for their research support. Kasetsart University Research and Development Institute's English proofreading service and support are acknowledged.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
YR: writing–original draft, investigation, and formal analysis. KNJ: conceptualization, methodology, supervision, writing–review and editing. Y. Lorjaroenphon: writing–review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruangchaisirawet, Y., Lorjaroenphon, Y. & Na Jom, K. Combined metabolomics and flavoromics to follow the fermentation process in sweet fermented rice (Khao-Mak). Eur Food Res Technol 250, 511–520 (2024). https://doi.org/10.1007/s00217-023-04403-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04403-w