Abstract
A series of alkylpyrazines, contributing to the overall flavor, are described in tea and tea infusions. Their low concentrations and moderate water solubility are facing analytical trouble when it comes to typical applied headspace analyses. Accordingly, a stir bar sorptive extraction methodology (SBSE) was established. It was found that immersion-based techniques (direct-immersion SBSE (DI-SBSE) and multiple SBSE (mSBSE)) showed higher extraction efficiency compared to headspace sorptive extraction (HSSE). In the end, a DI-SBSE methodology was established with a calibration range of 0.1–50 µg/L for six alkylpyrazines to systematically quantify their presence in tea and tea-like infusions. With determined LOQs between 21 and 118 ng/L, the method was sensitive enough to detect the expected low amounts. Application of the established DI-SBSE methodology for six alkylpyrazines in seven green, three white, one oolong, two black teas, as well as two tea-like infusions (nettle and raspberry leaf) revealed great variations in total concentration of alkylpyrazines between 0.36 ± 0.05 µg/L (raspberry leaf) and 24.20 ± 0.41 µg/L (oolong tea). Furthermore, concentration levels in between the same tea type ranged drastically, with 14.19 ± 0.62 µg/L for Longjing green tea and 0.74 ± 0.02 µg/L for Warwick green tea. Most abundant alkylpyrazines in the samples were 2-methylpyrazine and 2,5-dimethylpyrazine. Overall good similarities in compositional distribution of alkylpyrazines in the same tea type and strong correlations of the alkylpyrazines over all types indicating distinct fingerprints being present and the types are differentiable based on their alkylpyrazine pattern.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alkylpyrazines are character impact compounds of many heat-processed foods such as coffee, tea, cocoa, chocolate, roasted barley, meat products, and baked goods since they are typical products from the Maillard reaction [1,2,3,4]. During heat treatment of tea to stop enzymatic browning and to dry the processed leaves, the formation of Maillard products including alkylpyrazines contribute to the nutty and/or roasted aroma in the resulting products. Multiple studies demonstrated that alkylpyrazines are important aroma compounds for the overall flavor impression of especially pan-fried teas [5,6,7] as they likely exhibit higher considerable concentrations of alkylpyrazines [8]. Hence, their presence and quantity may serve as indicator for the extent and type of heat-treatment. Furthermore, the alkylpyrazine patterns of the tea plant would be of interest towards differentiation of geographic origin, cultivar, and further processing. Until now, a total of 21 different alkylpyrazines were identified in several green and stem teas [9,10,11], including 2-methylpyrazine, 2-ethylpyrazine, 2,3, 2,5-, and 2,6-dimethylpyrazine, 2-ethyl-3,5(6)-dimethylpyrazine, 2,3,5-trimethylpyrazine as well as 2-ethyl-5-methylpyrazine as key compounds. Besides, trace amounts of other alkylpyrazines were identified. To date, no systematical quantification and comparison of present alkylpyrazines in tea is available.
The choice of a suitable isolation methodology for volatile isolation is the most crucial step in analysis of complex food matrices. In the last years, solvent-free extraction techniques such as headspace—solid phase microextraction (HS-SPME), dynamic headspace extraction (DHS), or stir bar sorptive extraction (SBSE) has displaced the classical solvent isolation techniques such as liquid—liquid—extraction (LLE) or distillation [12]. No need of solvent is undisputed a big advantage for environment and handling. Besides, sorptive techniques shows higher sensitivity compared to solvent-based techniques and no need for long and high distillation temperatures are gentle for matrix and aroma compounds. As Maillard products, alkylpyrazines are produced during heating processes. High temperatures during volatile extraction such as in distillation therefore might increase the total alkylpyrazine concentration in some food matrices and influence the authenticity. This phenomenon is already reported for cocoa [13]. To date, alkylpyrazines are often analyzed via headspace—solid phase microextraction (HS-SPME) from solid substrates, like coffee powder [14, 15]. However, headspace analyses brings serval drawbacks for liquid samples due to good water solubility and high boiling points of alkylpyrazines (Table 1). In contrast, stir bar sorptive extraction (SBSE) as an advanced solvent-free technique has been designed in 1999 and can be used as both direct immersion (DI) and headspace (HS) extraction method due to its robustness. Its large sorbent surface area (> 50 µL) and a connection to a cooled injection system (CIS) results in SBSEs’ multifaceted properties such as high analyte recovery, less competition effect, decreased thermal artifact formation, and even subsequently high resolution of GC system by generation of sharp peaks in combination with CIS [16]. So far, SBSE represents a powerful tool for trapping key odorants, especially trace odorants present in the highly varied and complex matrices of samples (gas, solid, aqueous, and oily) [17, 18]. Currently, flavor analysis of tea by means of SBSE is still rare [16, 19, 20] and no focus on systematical elucidation on presence of alkylpyrazines were made.
We, therefore, developed a sensitive stir bar sorptive extraction (SBSE) method for the quantification of various alkylpyrazines in various tea (green, white, oolong, and black) and tea-like (nettle and raspberry leaf) infusions to elucidate similarities and differences in the alkylpyrazine patterns of the different tea types.
Material and methods
Materials and chemicals
2-Methylpyrazine (99%), 2,5-dimethylpyrazine (99%) and 2-ethyl-3,5(6)-dimethylpyrazine (99%, mix of isomers) were purchased from Alfa Aesar (Kandel, Germany). 2-Ethylpyrazine (98%) was purchased from Sigma-Aldrich (Darmstadt, Germany). 2,3,5-Trimethylpyrazine (98%) was purchased from J&K Scientific Ltd. (Karlsruhe, Germany). Sodium chloride (99%) was purchased from Carl Roth (Karlsruhe, Germany).
Seven green teas (Sencha from Japan; Seogwang from South Korea; Himalaya View from Nepal; Warwick Special Green from South India; Gunpowder, Longjing, and Lung Ching from China), three white teas (Pai Mu Tan White, as well as one high- and low-quality tea from China), one oolong tea (Formosa Fancy Superior Oolong from Taiwan), two black teas (Darjeeling Nr. 9 Himalaya First Flush from India and English Breakfast) and two tea-like products (nettle and raspberry leaf from Germany) were analyzed for their alkylpyrazine pattern. All teas were purchased from Tee Gschwendner GmbH (Meckenheim, Germany) except Longjing (directly imported form Zheijang province, China), Lung Ching (J.T. Ronnefeldt KG, Frankfurt am Main, Germany), as well as high- and low-quality white tea (Fuzhou University, China).
Method development for quantifying alkylpyrazines in aqueous environment
Stir bar sorptive extraction for isolation of alkylpyrazines
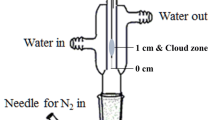
Three different approaches were tested in this study: direct immersion—stir bar sorptive extraction (DI-SBSE, one PDMS-coated stir bar stirring in the liquid phase), headspace sorptive extraction (HSSE, one PDMS coated stir bar attached in the headspace using a magnetic cuff and one non-coated stir bar stirring in the liquid), and multiple stir bar sorptive extraction (mSBSE, one PDMS-coated twister stirring in the liquid phase and one PDMS-coated stir bar attached to the headspace using a magnetic cuff) (Fig. 1). To evaluate the efficiency of the applied methods to extract alkylpyrazines from an aqueous matrix, a standard containing 2-methylpyrazine, 2-ethylpyrazine, 2,5-dimethylpyrazine, 2,3,5-trimethylpyrazine, and 2-ethyl-3,5(6)-dimethylpyrazine in water was prepared. 5 mL alkylpyrazine standard (concentration c = 1 µg/L, 50 µg/L, and 100 µg/L of each pyrazine) was transferred into a 20-mL headspace vial. 1.6 g NaCl (saturated solution) and 50 µL of internal standard (IS, thymol, c = 4.2 µg/L) were added to each sample. For each method, extraction time was set to 2 h and 1000 rpm stirring at room temperature (~ 24 °C). After extraction, the coated stir bars were removed with forceps, rinsed with deionized water, dried, and placed together in a conditioned thermal desorption unit (TDU) liner (OD 6 mm, ID 4 mm, L 60 mm) (Gerstel, Mühlheim an der Ruhr, Germany). For mSBSE, both Twisters were transferred to the same TDU liner. Desorption started at a temperature of 40 °C (1 min) and then ramped at 120 °C/min to 220 °C, and held for 10 min. The cryofocusing was performed in a cooled injection system (CIS) (Gerstel) equipped with a glass wool liner (OD 3 mm, ID 2 mm, L 71 mm) with liner-in-liner principle with the TDU liner (Gerstel) in solvent-vent mode (40 mL/min). The analytes were released to a gas chromatography system equipped with a mass spectrometry detector and an olfactory detection port (GC–MS-O) system in the CIS with a temperature program starting at -100 °C (1 min), then increased with 12 °C/s to 230 °C, and finally held for 5 min [16, 19].
Establishment of external calibration curves
A calibration curve with ten concentrations between 0.1 and 200 µg/L (0.1 µg/L, 0.5 µg/L, 1 µg/L, 5 µg/L, 10 µg/L, 20 µg/L, 30 µg/L, 50 µg/L, 100 µg/L and 200 µg/L) were established using DI-SBSE. 5 mL of each standard were transferred into a HS-vial and 1.6 g salt (saturated solution) and 50 µL of IS were added. The standards were extracted under same conditions as presented in “Stir bar sorptive extraction for isolation of alkylpyrazines bar sorptive extraction for isolation of alkylpyrazines”. Calibration curves were established by plotting the normalized peak area (peak area of alkylpyrazine/peak area of internal standard) against the concentration.
Linearity of the calibration curves for every single alkylpyrazines was determined by means of calculated determined R2 values and residual analysis. Concentrations of upper limit measurement points were varied between 50 and 200 µg/L and then linearities were compared. Precision of measurements were calculated by means of relative standard deviation (RSD) of the five replicates of each concentration measured for the calibration. Limit of detection (LOD) was calculated as three times the standard deviation from repeated analyses (n = 6) of the lowest level of the calibration curve. Limit of quantification (LOQ) was defined as ten times standard deviation of the lowest level of the calibration curve (n = 6).
Alkylpyrazine fingerprinting in different tea and tea-like infusions
Preparation of tea infusions
All tea infusions were prepared with the same method: 10 g/L tea leaves put into tea bags (size 3, Edeka, Hamburg, Germany) were steeped in boiling water for 15 min. After removing the tea bags, the infusions were cooled down to room temperature in an ice bath and stored at – 20 °C prior analysis. 5 mL of each tea infusion was transferred into HS-vials. 1.6 g salt (saturated solution) and 50 µL of IS were added. The samples were extracted under the same conditions as standards in water (see “Stir bar sorptive extraction for isolation of alkylpyrazines bar sorptive extraction for isolation of alkylpyrazines”).
Identification and quantification of six alkylpyrazines in tea and tea-like infusions
The alkylpyrazines were identified by the retention indices (RI) on a polar column (DB-WAXms), the mass spectra in comparison with those of authentic standards and data published in the literature and MS database (NIST17). Six alkylpyrazines were quantified using established external calibration curves in water between 0.1 and 50 µg/L.
Gas chromatography—mass spectrometry (GC–MS)
Gas chromatography (GC) was performed with an Agilent 7890B gas chromatograph connected to a 5977B mass spectrometry detector (Agilent Technologies, Waldbronn, Germany) equipped with TDU, CIS as well as an olfactometry detection port (ODP 3, Gerstel). An Agilent J&W DB-WAXms column (30 m × 0.25 mm ID × 0.25 µm film thickness) (Agilent Technologies) was installed. Helium (5.0) (Westfalen, Muenster, Germany) served as carrier gas with a constant flow rate of 1.62 mL/min. The GC oven temperature was held at 40 °C (3 min) and then ramped with 5 °C/min to 240 °C (10 min). The following parameters were applied: MS mode, scan; scan range, m/z 40–330; electron ionization energy, 70 eV; source temperature, 230 °C; quadrupole temperature, 150 °C; ODP 3 transfer line temperature, 250 °C; ODP mixing chamber temperature, 150 °C; ODP 3 makeup gas, N2 (5.0) (Westfalen) [16, 19]. The data were collected using Agilent Mass Hunter B07.06 combined with Gerstel Maestro.
Results and discussion
SBSE-based method development for isolation and quantification of alkylpyrazines in aqueous environment
Headspace—solidphase microextraction (HS-SPME) is currently the most used approach to analyze pyrazines from foods. HS-SPME can be fully automatized and therefore enables a high sample throughput. However, knowing that especially alkylpyrazines are moderate water-soluble, a headspace approach brings several drawbacks for analyzing liquid samples. Stir bar sorptive extraction (SBSE) can be applied in various immersion- or headspace-based methods and therefore enables a tailor-made use for the analyzed sample. Multiple SBSE (mSBSE) can improve the sensitivity of volatile extraction for rather middle or non-polar compounds while extracting in both headspace and liquid and increase the polarity range when two different coating materials (polydimethylsiloxane (PDMS) and PDMS-EG (ethylene glycol) copolymer) are used [21, 22]. But van Lancker, et al. [23] already proved the PDMS coating as more suitable material for extraction of alkylpyrazines from watery solutions than PDMS-EG copolymer. Therefore, in our approach, direct-immersion SBSE (DI-SBSE), headspace sorptive extraction (HSSE), and multiple SBSE (mSBSE) using PDMS-coated stir bars were compared to find the most suitable approach for analyzing expected low amounts of alkylpyrazines in tea and tea-like beverages. In our study, we focused on the six most abundant alkylpyrazines 2-methylpyrazine, 2-ethylpyrazine, 2,5-dimethylpyrazine, 2,3,5-trimethylpyrazine, as well as 2-ethyl-3,5-dimethylpyrazine and 2-ethyl-3,6-dimethylpyrazine (Table 1).
In the first step, three levels of alkylpyrazines in water (1 µg/L, 50 µg/L, and 100 µg/L) were compared to find the most suitable SBSE method. At a low concentration of 1 µg/L, DI-SBSE and mSBSE showed a clearly higher extraction yield in comparison to HSSE (Fig. 1). Higher extraction performance with immersion techniques can be explained by the water solubility of alkylpyrazines at low concentrations like 1 µg/L. The low log Kow (logarithm of octanol/water partitioning coefficient) of the selected alkylpyrazines (log Kow = 0.21 to 2.07, Table 1) attesting their rather hydrophilic behavior in aqueous environment. Conversely, at high concentrations—in this case 100 µg/L—extractions performance of DI-SBSE and mSBSE was similar, while HSSE showed again a lower efficiency towards the other two methods (Fig. 1). Measurement series containing 50 µg/L analytes showed similar results. A slightly higher recovery of mSBSE at higher concentrations was observed, but considering the expected low alkylpyrazine concentrations in tea, the DI-SBSE methodology is satisfying and was finally chosen also due to easier handling.
In the next steps, external calibration curves using DI-SBSE with aqueous alkylpyrazine solutions were set. In the beginning a calibration range between 0.1 and 200 µg/L for each alkylpyrazine was established. Subsequently, a graphical residual analysis (data not shown) was performed to improve the linear regression and to adjust the method to the expected concentrations. Calibration curves with a linear range of 0.1–50 µg/L showed the highest R2 (R2 > 0.99, Table 2) in comparison to upper calibration limits of 100 µg/L and 200 µg/L (0.95 < R2 < 0.97) (data not shown).
Results of precision are shown in Table 2. For all selected pyrazines, a precision of > 90% was obtained with the selected method. Recovery was calculated with values between 22.8 and 90.8% for 0.1 µg/L and 97.2% to 100.1% for 50 µg/L (Table 2). An upward trend was found with increase of concentration and log Kow. LOD and LOQ were calculated between 6.2 and 35.5 ng/L for the single alkylpyrazines in the LOD and 20.6 ng/L to 118.2 ng/L for the LOQ (Table 2).
Alkylpyrazine fingerprinting in tea infusions
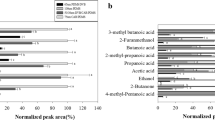
Differences in the chemical composition of various teas are not only attributed to the manufacturing methods, but also to growth, harvesting time, climate, cultivars, soil, and geography [24]. The effects of processing on chemical composition are widely reported, such as for the polyphenol content [25]. In addition, intensive research has been done to reveal the relationship between the metabolome and climate [26, 27]. Gulati et al., [28] studied the influence on the harvest time and found massive differences in the flavor of the corresponding tea infusion. Especially early flush harvest was found to have much higher concentrations of important tea compounds such as linalool, geraniol or β-ionone compared to main flush harvest. However, no systematic research on alkylpyrazines in tea, which play an important role to the overall flavor, were done so far. Therefore, in our research, we focused on the systematic presence six alkylpyrazines in different tea and tea-like infusions. Fifteen different teas and tea-like products were analyzed for their alkylpyrazine concentration and pattern infusions using DI-SBSE in combination with an established external calibration. The results showed huge differences in concentration and proportion (Table 3, Fig. 2, 3). Highest total alkylpyrazine concentration was found for the semi-oxidized oolong tea sample with 24.20 ± 0.41 µg/L. Main alkylpyrazine in oolong is 2-methylpyrazine with around 50% of total alkylpyrazine concentration, besides 2,5-dimethylpyrazine and 2-ethylpyrazine. Almost 90% of the total alkylpyrazine concentration were attributed to these three. Only minor proportions were detected for higher substituted alkylpyrazines, in detail 2,3,5-trimethylpyrazine, as well as 2-ethyl-3,5(6)-dimethylpyrazine.
Second highest total alkylpyrazine concentration was found for pan-fried Longjing green tea with 2,5-dimethylpyrazine as leading alkylpyrazine. Interestingly, huge concentration differences between the different green tea types were found: the total concentration ranged between 0.74 ± 0.02 µg/L for Indian Warwick green tea and 14.19 ± 0.62 µg/L for Longjing (Table 3, Fig. 2). Even though absolute concentrations of alkylpyrazines varied drastically between the analyzed green tea infusions, a similar proportioning of the analyzed alkylpyrazines was observed (Fig. 3). All seven green teas analyzed showed extraordinary similar patterns with 2-methlypyrazine and 2,5-dimethylpyrazine making up around 80–90% of total alkylpyrazines analyzed. Interestingly, in the Warwick green tea, exceptional high proportion of 2-ethyl-3,5-dimethylpyrazine (15%) was detected compared to the other green tea types, nevertheless the total concentration was comparable to the others (c = 0.10–0.13 µg/L). But, 2-ethyl-3,5-dimethylpyrazine has an extremely low odor threshold (0.04 µg/L) [29] and therefore plays an important role to the overall roasted aroma of different tea infusions even in low concentration. Mizukami et al. [30], already proved the importance of low concentration alkylpyrazines as key odorants in roasted green tea. The two analyzed Dragon Well teas (Longjing and Lung Ching) showed an almost identical fingerprint even though overall concentrations differ majorly with 14.19 ± 0.62 µg/L for Longjing and 4.61 ± 0.16 µg/L for Lung Ching. Interestingly, also Sencha green tea, the typical steam treated green tea from Japan [31], showed a high total alkylpyrazine concentration with 13.28 ± 0.49 µg/L which is in similar concentration to the roasted Longjing green tea (Table 3). This suggests that also, the initial alkylpyrazine concentration in the tea leaves is important and not all pyrazines are formed during manufacturing. The gentle steam treatment (around 100 °C, indirect heat) in comparison to high temperature roasting in pans (up to 250 °C, direct heat), would expect a lower alkylpyrazine concentration. Seogwang, Himalaya View, and Gunpowder green tea showed similar alkylpyrazine levels around 4 µg/L and a similar pattern to other green teas.
White tea is characterized by a fine note and is produced like green tea but using only leaves with fine silvery-white hairs on the unopened buds [24]. In the three analyzed white teas concentrations of 2-ethyl-3,6-dimethylyprazine were all below the LOQ. Besides, 2,5-dimethylpyrazine was only identified in the high-quality white tea and was found in second highest concentration after 2-methylpyrazine (Fig. 3). 2-Methylpyrazine was also found as major pyrazine in the other two analyzed white teas. Low quality white tea and Pai Mu Tan showed similar quantities of alkylpyrazines with around 2 µg/L, whereas the high-quality tea had an almost five times fold concentration with 12.12 ± 0.57 µg/L (Table 3, Fig. 2). Nevertheless, in the overall pattern, all analyzed white teas showed huge similarities and were clearly distinguishable from the other tea types.
The two analyzed black tea infusions showed similar total alkylpyrazine concentrations around 2 µg/L (Table 3), but slight differences in the proportions: in Indian Darjeeling 2,5-dimethylpyrazine shared the highest proportion (37%) besides 2-methyl- (29%) and 2-ethylpyrazine (16%). In the English Breakfast infusion, clearly 2-methylpyrazine shared the highest proportion with 51% of the total alkylpyrazine concentration, followed by 2,5-dimethylpyrazine with 23% and 2-ethylpyrazine with 11% (Fig. 3). Proportions of higher substituted alkylpyrazines shared similar values for the analyzed black teas. Total concentrations of total alkylpyrazines in black tea were comparable to white tea and some green teas. Interestingly, the 2-ethylpyrazine proportions is much higher in white and black tea types compared to green tea (Table 3). Black tea often comes from India or Sri Lanka and less from other tea producing countries like China or Japan. Because of different climatic realities, black tea is often produced from Camellia sinensis var. assamica, whereas green teas from China and Japan are produced from Camellia sinensis var. sinensis [24]. Both varieties have different physiological properties regarding climate, growing area, and constitutions regarding different chemical classes, e.g., the caffeine content [32, 33]. Therefore, it seems reasonable that a different precursor pattern and therefore a different alkylpyrazine pattern might be present in the original leaves from the two varieties. Besides, black tea is produced via an enzymatically induced oxidation catalysed by catechol oxidases [34]. However, not only catechol oxidases are active during the oxidation but also a series of other enzymes and reactions that finally form the desired black tea flavor [35]. Therefore, it might also be possible that the pyrazine pattern is also changing during the oxidation process. This effect could not be cleared up in this study since no corresponding fresh leaves were analyzed.
Besides the classical teas from the tea plant, there are also a series of other teas available on the market. To distinguish between the different types, teas produced from herbs, other leaves or even fruits are referred as tea-like products. Two examples of this are raspberry leaf tea and nettle tea. Both teas are quite common in Germany as they are reported to have certain health effects [36, 37]. In this study, the two tea-like samples were chosen to also gain hints on the alkylpyrazine pattern in other tea-like products. It could clearly be shown, that both samples had a unique pyrazine pattern and were clearly differentiable from the other samples. Both tea-like infusions together with Warwick green tea also shared the lowest detected total alkylpyrazine concentrations among all analyzed samples (c < 1.30 µg/L). In the raspberry leaf infusion, only the higher substituted alkylpyrazines were detected: 2,3,5-trimethylpyrazine and 2-ethyl-3,5(6)-dimethylpyrazines were found in similar relative concentrations (Fig. 3). No single or double substituted pyrazines were detected and therefore a completely different pattern to the Camellia-teas could be observed. For nettle tea 2-ethyl-3,6-dimethylpyrazine was found with a proportion about 23%, besides 2-methylpyrazine and 2-ethlypyazine as major pyrazines. In all other analyzed tea infusions 2-ethyl-3,5-dimethylpyrazine and 2-ethyl-3,6-dimethylpyrazine were found as minor compound with maximum 10% in the whole proportion, except for Warwick green tea. In addition, for nettle tea, a characteristic pyrazine pattern could be established which differed from the Camellia teas. Overall, we could show that it is possible to distinguish types of tea and tea-like infusion types by its selected alkylpyrazine pattern, even though the total alkylpyrazine quantity does not show any similarities.
When comparing the overall proportioning some trends for the alkylpyrazines in each tea infusion could be observed as well. Especially when comparing single alkylpyrazines, distinct dependencies were found. For instance, the 2,5-dimethylpyrazine and the 2-ethyl-3,6-dimethylpyrazine concentration (R2 = 0.85), the 2-methylpyrazine and the total alkylpyrazine concentration (R2 = 0.84), and the 2-methylpyrazine and 2-ethylpyrazine content (R2 = 0.83) seemed to be highly correlated in the analyzed infusions (Fig. 4 A-C). But also, good correlations (R2 > 0.62) were found for 2,5-dimethylpyrazine and 2,3,5-trimethylpyrazine concentration, 2-ethylpyrazine and 2,3,5-trimethylpyrazine concentration, 2-ethylpyrazine and total alkylpyrazine concentration, 2-methylpyrazine and 2,3,5-trimethylpyrazine concentration, 2,3,5-trimethylpyrazine and 2-ethyl-3,6-dimethylpyrazine concentration, as well as 2-ethyl-3,6-dimethylpyrazine and the total alkylpyrazine concentration (Fig. 4 D–I). This shows that despite typical patterns for different teas also some strong overall correlations between different alkylpyrazines are existing. Since these correlations were found for all tea infusions, we assume that the manufacturing processes do not only determine the alkylpyrazine pattern, but also a defined intrinsic precursor pattern in the leaves. The exact effects could not be determined in this study, since no detailed information on geographic origin, harvest time, or processing were available for most of the samples. Despite only the final teas were analyzed and no monitoring while processing from the fresh leaves to the final products was performed.
Conclusion
Alkylpyrazines are character compounds in many heated and processed foods. In tea they play an important role for the overall roasted or nutty aroma of several types. With help of a reliable and sensitive DI-SBSE method, we could show that overall quantity of alkylpyrazines can drastically differ but show a similar pattern for each tea type. In addition, distinct dependencies between single alkylpyrazines were observed for all individual analyzed samples. Whether the effects only come from processing methods or also are given by the intrinsic pyrazine precursor ratio and concentration present in the harvested leaves could not be clarified in total here since no detailed information on exact geographic origin or harvest time were available. Further studies need to be conducted with comparing also non-processed fresh tea leaves to the final tea products. Besides, the effects of infusion preparing were not examined here and are worth further investigations.
References
Cheeseman GW, Werstiuk ES (1972) Recent advances in pyrazine chemistry. Adv Heterocycl Chem 14:99–209
Maga JA, Sizer CE, Myhre DV (1973) Pyrazines in foods. Crit Rev Food Sci Nutr 4:39–115
Rizzi GP (1988) The biogenesis of food-related Pyrazines. Food Rev Intl 4:375–400
Müller R, Rappert S (2010) Pyrazines: occurrence, formation and biodegradation. Appl Microbiol Biotechnol 85:1315–1320
Kumazawa K, Masuda H (2002) Identification of potent odorants in different green tea varieties using flavor dilution technique. J Agric Food Chem 50:5660–5663
Gong X, Han Y, Zhu J et al (2017) Identification of the aroma-active compounds in Longjing tea characterized by odor activity value, gas chromatography-olfactometry, and aroma recombination. Int J Food Prop 20:S1107–S1121
Flaig M, Qi SC, Wei G et al (2020) Characterisation of the key aroma compounds in a Longjing green tea infusion (Camellia sinensis) by the sensomics approach and their quantitative changes during processing of the tea leaves. Eur Food Res Technol 246:2411–2425
Hara T (1989) Studies on the firing aroma and off-flavor components of green tea. Bulletin of the National Research Institute of Vegetables, Ornamental Plants and Tea. Series B.(Japan)
Yamanishi T, Shimojo S, Ukita M et al (1973) Aroma of roasted green tea (Hoji-cha). Agr Biol Chem 37:2147–2153
Sasaki T, Koshi E, Take H et al (2017) Characterisation of odorants in roasted stem tea using gas chromatography–mass spectrometry and gas chromatography-olfactometry analysis. Food Chem 220:177–183
Yang Y, Zhang M, Hua J et al (2020) Quantitation of pyrazines in roasted green tea by infrared-assisted extraction coupled to headspace solid-phase microextraction in combination with GC-QqQ-MS/MS. Food Res Int 134:109167
Sides A, Robards K, Helliwell S (2000) Developments in extraction techniques and their application to analysis of volatiles in foods. TrAC Trends Analyt Chem 19:322–329
Hashim L, Chaveron H (1994) Extraction and determination of methylpyrazines in cocoa beans using coupled steam distillation-microdistillator. Food Res Int 27:537–544
Rocha S, Maeztu L, Barros A et al (2004) Screening and distinction of coffee brews based on headspace solid phase microextraction/gas chromatography/principal component analysis. J Sci Food Agric 84:43–51
Petisca C, Pérez-Palacios T, Farah A et al (2013) Furans and other volatile compounds in ground roasted and espresso coffee using headspace solid-phase microextraction: effect of roasting speed. Food Bioprod Process 91:233–241
Rigling M, Yadav M, Yagishita M et al (2021) Biosynthesis of pleasant aroma by enokitake (Flammulina velutipes) with a potential use in a novel tea drink. LWT 140:110646
Trapp T, Jäger DA, Fraatz MA et al (2018) Development and validation of a novel method for aroma dilution analysis by means of stir bar sorptive extraction. Eur Food Res Technol 244:949–957
Rigling M, Fraatz MA, Trögel S et al (2019) Aroma investigation of Chios mastic gum (Pistacia lentiscus variety chia) using headspace gas chromatography combined with olfactory detection and chiral analysis. J Agric Food Chem 67:13420–13429
Rigling M, Liu Z, Hofele M et al (2021) Aroma and catechin profile and in vitro antioxidant activity of green tea infusion as affected by submerged fermentation with Wolfiporia cocos (Fu Ling). Food Chem 361:130065
Wang MQ, Ma WJ, Shi J et al (2020) Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC–MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res Int 130:108908
Ochiai N (2015) The twister sisters pick up the flavors. Gerstel Solut Worldw 15:9–12
Ochiai N, Sasamoto K, Ieda T et al (2013) Multi-stir bar sorptive extraction for analysis of odor compounds in aqueous samples. J Chrom A 1315:70–79
van Lancker F, Adams AN, de Kimpe N (2010) Formation of pyrazines in Maillard model systems of lysine-containing dipeptides. J Agric Food Chem 58:2470–2478
Chow KB, Kramer I (1990) All the tea in China. China Books
Zuo Y, Chen H, Deng Y (2002) Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta 57:307–316
Lee LS, Choi JH, Son N et al (2013) Metabolomic analysis of the effect of shade treatment on the nutritional and sensory qualities of green tea. J Agric Food Chem 61:332–338
Lee JE, Lee BJ, Chung JO et al (2015) Metabolomic unveiling of a diverse range of green tea (Camellia sinensis) metabolites dependent on geography. Food Chem 174:452–459
Gulati A, Ravindranath SD (1996) Seasonal variations in quality of Kangra tea (Camellia sinensis (L) O Kuntze) in Himachal Pradesh. J Sci Food Agric 71:231–236
Buttery RG, Ling LC (1997) 2-Ethyl-3, 5-dimethylpyrazine and 2-ethyl-3, 6-dimethylpyrazine: odor thresholds in water solution. LWT 30:109–110
Mizukami Y, Sawai Y, Yamaguchi Y (2008) Changes in the concentrations of acrylamide, selected odorants, and catechins caused by roasting of green tea. J Agric Food Chem 56:2154–2159
Graham PJ (1999) Tea of the Sages: the Art of Sencha. University of Hawaii Press
Robertson A (1992) The chemistry and biochemistry of black tea production—the non-volatiles. Tea. Springer, pp 555–601
Harbowy ME, Balentine DA, Davies AP et al (1997) Tea chemistry. Crit Rev Plant Sci 16:415–480
Tanaka T, Kouno I (2003) Oxidation of tea catechins: chemical structures and reaction mechanism. Food Sci Technol Res 9:128–133
Tan J, Dai W, Lu M et al (2016) Study of the dynamic changes in the non-volatile chemical constituents of black tea during fermentation processing by a non-targeted metabolomics approach. Food Res Int 79:106–113
Hodroj MH, Bast A, Al Hoda N et al (2020) Nettle tea inhibits growth of acute myeloid leukemia cells in vitro by promoting apoptosis. Nutrients 12:2629
Cheang KI, Nguyen TT, Karjane NW et al (2016) Raspberry leaf and hypoglycemia in gestational diabetes mellitus. Obstet Gynecol 128:1421–1424
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MR project administration; methodology; conceptualization; writing—original draft; J-PK data curation; formal analysis; writing—original draft; YZ supervision, project administration; writing—review and edit.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rigling, M., Kanter, JP. & Zhang, Y. Application of a direct immersion—stir bar sorptive extraction (DI-SBSE) combined GC–MS method for fingerprinting alkylpyrazines in tea and tea-like infusions. Eur Food Res Technol 248, 1179–1189 (2022). https://doi.org/10.1007/s00217-021-03954-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03954-0