Abstract
In the present study, navy bean and chickpea seeds were tempered to 20% or 30% moisture content and exposed to infrared heating at surface temperatures 120 °C or 140 °C. The resulting flours were evaluated in terms of their physicochemical, functional, color and morphological properties. Proximate composition of flours was not affected by infrared heating unlike α-amylase and gelatinized starch content which were determined highly susceptible to infrared heating due to inhibitory effects of high surface temperatures on enzyme activity and increase in starch gelatinization with high temperatures. The surface hydrophobicity results from chickpea and navy bean increased up to 3 folds after the treatment. Scanning electron microscope (SEM) results showed that high surface temperature (140 °C) caused more significant changes to the starch structure in chickpea flours than navy bean flours. Water hydration capacity of flours increased; however, solubility, oil emulsification and foaming ability of flours were adversely affected by the infrared treatment. The protein quality and starch digestibility of each pulses were enriched using infrared heating with tempering. Overall, the effect of infrared heating on pulse seeds might be related to surface temperature, tempering moisture, final moisture content of seeds, seed size, and seed composition. Our results will be useful for designing processes to enhance the properties of pulse-derived functional materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Legumes contribute to our daily diet with their high nutrition profile rich in complex carbohydrates, vitamins, minerals, fiber and proteins even though the presence of anti-nutritional factors exist [1, 2]. Pulses belong to the legume family and can be consumed in different forms, such as whole/flour form or protein/starch rich fractions. In addition to their possible health benefits including reducing the risk of obesity, cardiovascular diseases, cancer, etc., pulses have a low glycaemic index and slowly promotes postprandial blood glucose increases [3,4,5]. Recently, changing consumption habits of customers due to health concerns and increasing trends on vegetarian/vegan products lead the food industry to seek new ingredients. Hence, the usage of pulse flours in food formulations is becoming popular and contributes to new gluten-free product formulations [1].
However, the usage of pulses is negatively affected by their long cooking time at atmospheric pressures. Pulses are prepared using various cooking methods for legumes, such as roasting, boiling, frying, steaming, sautéing, sous vide, microwave, and pressure-cooking [6]. And, often involve soaking, precooking, and usage of alkaline salts to decrease cooking time and enhance the nutritional quality of chickpea and navy bean [7,8,9]. Among all cooking methods, infrared heating (micronization) can provide short-time high-temperature process to alter the functional and nutritional quality of pulses [10, 11]. Basically, micronization exposes food materials to electromagnetic radiation ranging between wavelengths of 1.8 and 3.4 µm to raise the internal temperature and cause uniform heat, lower cost, and short processing time [12]. The heating mechanism of infrared heating involves inducing the molecular movement of water molecules at a frequency of 60,000–150,000 MHz [13]. On the other hand, tempering is performed as a pretreatment in milling processes of pulse flours to easily dehull and increase flour yield [14, 15].
In previous studies, the effect of micronization on cooking time and functional properties of cowpeas [16,17,18] and bambara groundnut [19]; lipoxygenase activity of soybeans [20]; the physical, functional, and mechanical properties of kidney beans, green peas, black beans, lentil, pinto beans, and bambara groundnut were evaluated [21, 22]. Tempering and micronization negatively affected the solubility of desi chickpea flours, but increased the emulsion activity at high surface temperature with tempering [23]. Also, the effects of tempering level and surface temperature on protein quality were found different according to the pulse type by Bai et al. [11]. The study showed that desi chickpeas applied to high surface temperature (135 °C) with 20% moisture content had significant increase in in vitro protein digestibility corrected amino acid scores (IV-PDCAAS); however, IV-PDCAAS of barley samples increased at 135 °C regardless of moisture content. Moreover, effects of tempering with water/mixture of salts/mixture of acids (28%) and micronization at 112 °C on physicochemical properties were evaluated in navy bean samples [24].
Evaluation of the effect of surface temperature together with the seed moisture content is essential to improve the functional and nutritional properties of the pulses. It was hypothesized that partial protein denaturation and starch gelatinization at different seed moisture content and surface temperatures may affect physicochemical, functional, and nutritional properties of chickpea and navybean flours. Navy bean and Kabuli chickpea flours were chosen to present the interactive effect of tempering and micronization according to different initial physicochemical properties of these flours (such as lipid content, protein content, grain size, etc.). In this study, a comprehensive evaluation of the effect of surface temperature (120 °C or 140 °C) and tempering (20% or 30%) of seeds on the physicochemical and functional properties of chickpea and navy bean flours was examined.
Materials and methods
Materials
Certified seeds of Kabuli chickpea (var: CDC Orion) and navy bean (var: Nautica) were purchased from Reisner Farms (Limerick, SK, Canada) and Hensall District Co-op (Hensall, ON, Canada), respectively. All seeds were stored in large sealed containers at room temperature. Total starch assay, α-amylase assay, and starch damage assay kits were purchased from Megazyme International Ltd. (Co. Wicklow, Ireland). All other chemicals were at least reagent grade and purchased from Sigma-Aldrich (Oakville, ON, Canada). In this research, water was provided by a Milli-Q™ water purification system (Millipore Corp., Milford, MA, USA).
Tempering and infrared heating of seeds
Prior to infrared heating, samples were tempered to 20% or 30% moisture content according to AACC international Approved Method of 26-95.01 [25]. Infrared heating of tempered seeds was performed by InfraReady Products Ltd (Saskatoon, SK, Canada) using a laboratory-scale micronizer with 152 cm long magnetic feeder (Model A 566379-B0, FMC Syntron®: Bulk Handling Equipment, Homer City, PA, USA). The infrared radiators was 19 cm above the conveyor. The surface temperature of seeds was adjusted to 120 °C or 140 °C and checked with a handheld IR thermometer (Oakron, Vernon Hills, IL, USA). Tempering moisture content and infrared heating temperatures were selected based on preliminary experiments. Approximately 2 kg of each tempered seeds were processed under the same conditions three times. Untempered and unprocessed samples were chosen as control samples.
Milling of seeds
Control and processed samples were milled into flours using a laboratory disc mill (Model 3310, Perten Instruments Canada, Winnipeg, MB, Canada) equipped with a type-1 medium grinding disc at setting 5 with a 0.5 mm mesh size. The obtained flours were kept in polyethylene bags in a cold room (4 °C) until the subsequent analyses.
Physicochemical properties
Compositional analyses
Moisture, ash, and crude fat analyses of control and processed flours were performed according to the AOAC method 925.10, 923.03, and 920.85, respectively [26]. Nitrogen content was determined according to the Dumas combustion method by a Nitrogen/Protein Analyzer (CN628, LECO Corp., St. Joseph, MI, U.S.A), which was converted to protein content (%N × 6.25) following AACC Method 46-30.01 [25]. Alpha-amylase activity of flours was measured according to the AACC method 22-02.01 using Megazyme α-Amylase SD Assay Kit [25]. The total starch content of flours was determined according to the AACC method 76-13.01 using Megazyme Total Starch Kit [25]. Gelatinized starch content of flours was analyzed using Megazyme Starch Damage Assay Kit following AACC method 76-31.01 [25]. The compositional data were reported on a dry basis (db) of the flours.
Morphology of flours
Morphology of control and processed flours was examined using a scanning electron microscope (SEM, SU8010, Hitachi High Technologies Canada Inc., Rexdale, ON, Canada). Samples were coated with 10 nm of gold using Q150T ES coater (Quorum Technologies Inc., Puschlinch, ON, Canada). The images were captured at 500X, 800X, and 1000X.
Zeta potential
The zeta potential (ζ) of samples was determined using a Zetasizer Nano (Malvern Instruments, Westborough, MA, USA) according to method of Chang et al. [27]. Flour samples were dispersed in water at a concentration of 0.05% (w/w) protein. The dispersion was adjust to pH 7.0 with 0.1 M HCl or NaOH and stirred overnight. The pH of dispersions was checked and adjusted to pH 7.0 prior to analysis.
Surface hydrophobicity
Surface hydrophobicity was determined according to the method of Kato, Nakai [28]. Briefly, all flours (0.025% w/w, protein basis) were dissolved in 10 mM sodium phosphate buffer (pH 7.0) and stirred overnight at 4 °C. Next day, 0.005%, 0.010%, 0.015%, and 0.020% dilutions were prepared in 10 mM sodium phosphate buffer (pH 7.0). For each concentration (0.005–0.025%), 1.6 mL of sample were mixed with 20 µL of 8 mM 8-Anilino-1-naphthalenesulfonic acid (ANS) solution (dissolved in 10 mM sodium phosphate buffer (pH 7.0)), afterwards, samples were vortexed for 10 s and kept in the dark for 5 min. Fluorescence intensity (FI) was measured using a FluoroMax-4 spectrofluorometer (Horiba Jobin Yvon Inc., Edison, NJ, USA) with excitation and emission wavelengths of 390 and 470 nm, respectively. FI values of ANS blank and protein blanks were substracted from the FI of the protein solutions containing ANS. The initial slope (S0) of FI against protein concentration was calculated by linear regression analysis and used as an index of the protein surface hydrophobicity.
Colour
The colour of flour samples was measured using a Colorimeter (ColorFlex EZ 45/0, Hunter Associates Laboratory, Inc., Reston, VA, USA) as L* [lightness], a* [red ( +)- green (-))] and b* [yellow ( +)- blue (-)]. Colour change between control and processed samples was evaluated by \(\Delta E\) using the following equation:
Functional properties
Protein solubility
Protein solubility of control and processed flours was determined according to the modified method of Morr et al. [29]. Briefly, 1% (w/w) of flour (protein basis) was dispersed in distilled water. The pH of suspensions was adjusted to 7.0 with 0.1 M HCl or NaOH and stirred overnight at cold room (4 °C). Next morning, the pH of suspensions were checked and readjusted to pH 7.0. The suspension was then centrifuged at 4430 × g for 10 min at room temperature. After the centrifugation, 5.0 g of the supernatant was used for nitrogen and protein (N% × 6.25) determination AOAC method 960.52 [26]. Protein solubility of samples was determined using Eq. 2.
Water hydration and oil holding capacities
Water hydration capacity (WHC) and oil holding capacity (OHC) of all flours were determined according to AACC method 56-30.01 [25] and the method of Nidhina and Muthukumar [30] with slight modifications, respectively. In brief, suspensions were prepared with 0.5 g of flour in 5 g of distilled water (or 5 g canola oil for OHC) in a 10 mL centrifuge tube. The centrifuge tube was vortexed for 10 s every 5 min in total 30 min. Afterwards, centrifuge tubes were centrifuged at 1000 × g for 15 min. The weight of sediment was recorded as wet flour weight. WHC and OHC values were calculated in g/g using the following equation:
Foaming properties
Foaming properties of all flours were determined as foaming capacity (FC) and stability (FS) using the method of Bai et al. [23]. Briefly, 1% (w/w) flour suspensions were prepared at pH 7.0 and stirred overnight. The solution pH was adjusted again to 7.0 prior to analysis and 15 ml of this solution was transferred to 400 mL beaker for homogenization using Omni Macro Homogenizer (Omni International, Marietta, GA, U.S.A) fitted with a sawtooth probe at speed 4 for 5 min. Then, the sample was transferred to 50 mL graduated cylinder, and the volume of the foam was recorded as V1. After 30 min, the volume of the foam was measured and recorded as V2. FC and FS were calculated based on Eq. 4, and Eq. 5, respectively.
Oil emulsion capacity (OEC)
OEC was measured according to method of Wang, Maximiuk [31]. In brief, pulse flour suspension, 0.40% (w/v, DM) in 75 mL deionized water, was homogenized for 30 s in a 500 mL glass jar using a PowerMax AHS 250 homogenizer (VWR International LLC, Mississauga, ON, Canada) at setting 1, equipped with an 10 × 105 mm saw tooth generator probe. The glass jar containing sample suspension was then attached onto a BF-30 homogenizer. Canola oil (25 mL) was delivered into the glass jar using a Masterflex pump (Cole-Parmer, Vernon Hills, IL, USA). The suspension, together with 25 mL oil, was blended at a speed in the range of 6000 rpm using the BF-30 homogenizer for 30 s at which time oil was delivered continuously into the chamber with the pump at a rate of 1.0 mL/s. The entire process for emulsion formation and collapse was recorded by measuring the electrical resistance with a digital multimeter, where the emulsion break point was indicated by a sudden increase in electrical resistance. At break point, oil addition was stopped, and the total amount of oil emulsified was calculated. OEC was expressed as mL oil/g sample.
Pasting properties
Pasting properties of all flours were measured by suspending flours in distilled water as a total weight of 28.0 g (8% dry solids content) analyzed using a Rapid Visco-Analyzer (RVA Super 3, Newport Scientific, Sydney, Australia) with Standard Method 2 in the Thermocline Software (Version 2.2, Newport Scientific, Sydney, Australia) [4].
Protein quality
Amino acid composition and amino acid scores
The amino acid contents of the samples were determined by 6 N hydrochloric acid hydrolysis using the AOAC Official Method 982.30 [26]. Whereas, methionine and cysteine were determined by the performic acid oxidized hydrolsis procedure according to AOAC Official Method 985.28 [26]. And Tryptophan was determined by alkaline hydrolysis using ISO protocol 13,904 (I 2016). Amino acid score was calculated by dividing the amino acid pattern of the sample (mg/g protein) by the FAO 1991 amino acid pattern reference for children from age 2 to age 5 (threonine (34 mg/g protein), valine (35 mg/g protein), methionine + cysteine (25 mg/g protein), isoleucine (28 mg/g protein), leucine (66 mg/g protein), phenylalanine + tyrosine (63 mg/g protein), histidine (19 mg/g protein), lysine (58 mg/g protein), tryptophan (11 mg/g protein) [32]. The limiting amino acid (LAA) was determined by the lowest ratio.
In vitro protein digestibility—and in vitro protein digestibility corrected amino acid score (IV-PDCAAS)
In vitro protein digestibility (IVPD) of samples was determined using the modified method of Hsu et al. [33]. The flour suspensions (6.25 mg protein/mL) were prepared in water at pH 8.0. and incubated in a water bath at 37 °C for 1 h. Trypsin (1.6 mg/mL; porcine pancreas ≥ 13,000–20,000 BAEE units/mg protein), chymotrypsin (3.1 mg/mL; bovine pancreas ≥ 40 units/mg protein), and protease (1.3 mg/mL; Streptomyces griseus ≥ 15 units/mg) was prepared in 10 mL water as a multi-enzyme solution and the solution was adjusted to pH 8.0 and stored in water bath at 37 °C. Multi-enzyme solution (1 mL) was added to flour suspension (10 ml) in an agitated water bath at 37 °C. The pH drop (ΔpH) for each sample was recorded every 30 s for 10 min. IVPD was calculated using Eq. 6:
where ΔpH10 min indicates to the change in pH from initial 8.0 to the end of 10 min.
IV-PDCAAS was calculated by multiplying IVPD by LAA scores [11].
In vitro starch digestibility
In vitro starch digestibility in infrared treated chickpea and navy bean flours was measured according to the method of Englyst et al. [34]. The analysis was carried out under controlled enzymatic hydrolysis followed by colorimetric measurement of the glucose released. Rapidly digestible starch (RDS) and slowly digestible starch (SDS) were calculated following incubation with porcine pancreatic alpha-amylase and amyloglucosidase in a water bath (37 °C) in terms of glucose released at 20 min and 120 min incubation, respectively. Resistant starch (RS) was determined indirectly by calculating the starch that is not hydrolysed after 120 min incubation.
Data analysis
Infrared treatment at 120 °C and 140 °C of navy bean and Kabuli chickpea seeds were performed in triplicate (three independent batches for each treatment). For each batch of processed samples, analyses were performed in duplicate. Statistical analyses were performed within the triplicates (average of duplicates) using a one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test at a significance level of 0.05 using SPSS software (version 21.0, SPSS, Chicago, IL, USA).
Results and discussion
Physicochemical properties
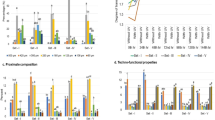
Proximate compositions of all flours are presented in Table 1. Infrared treatment and tempering affected the final moisture content of flours. The protein contents of flours for each pulse were not affected significantly by the treatments and were found 25–26% for navy bean and 19–20% for chickpea. Lipid content of navy bean (1.7–2%) was not significantly affected by the treatments; however, after the treatments, lipid contents of chickpea flours (7.1–7.2%) were found significantly higher than the control (6.6%). Similar effect on lipid content of desi chickpeas tempered to 20% moisture and infrared heated to 115 ℃ and 135 ℃ was observed [23]. After infrared heat application, the increase in lipid content could be related to increase in extractability of lipids due to altered cell–matrix permeability [35]. The processing effect on lipid content of chickpeas might be related to change in bound lipid content due to heating by moist [36]. Infrared radiation is efficient in the inhibition of enzymes such as α-amylase and lipase in rice [13]. Inhibitory effects of high temperatures (≈75 °C) on α-amylase activity was also determined by Apar, Özbek [37]. In the same manner, α-amylase activity in processed samples was found significantly (p < 0.05) lower than control samples in both chickpea and navy bean flours. Also, the reduction in α-amylase activity of processed pulse seeds was found independent from tempering moisture content and heating temperature, as seen in Table 1. Total starch values for each pulse did not change significantly after infrared treatment and were determined as 37.5–39.1% and 43.2–46.1% for navy bean and chickpea flours, respectively. Contrarily, gelatinized starch values evaluated from damaged starch content were significantly affected by tempering and infrared heating conditions as presented in Table 1. Furthermore, gelatinized starch contents of control samples of chickpea and navy bean flours were not significantly different (p > 0.05). However, process conditions affected the gelatinized starch content varyingly. The highest gelatinized starch content was determined at 140 °C surface temperature and 30% seed moisture content as 19.7% and 29.8% for navy bean and chickpea flours, respectively. The difference of gelatinized starch content between navy bean and chickpea samples can be explained with the initial protein content of each flour. According to a previous study, proteins could create a wall-like structure around starch molecules; thus protecting the starch granule from gelatinization [38]. In the present study, at the same tempering conditions, higher surface temperatures increased the starch gelatinization significantly in all processed flours (p < 0.05). Increasing seed moisture content was also found to significantly increase gelatinized starch content (p < 0.05) in navy bean samples at the same surface temperatures due to the higher amount of available water for the gelatinization; however, there was no significant difference (p > 0.05) among chickpea samples with different tempering level at the same processing temperatures which may be explained with suppressing effect of their higher lipid content.
Morphological changes
The morphology of control and processed chickpea and navy bean flours were examined by scanning electron microscopy (Fig. 1). SEM micrographs of control samples of each pulse flour (Fig. 1 A, B) showed the smooth starch molecules surrounded by proteins or fragments of the protein matrix. In addition, starch granules were seen in different sizes which might be due to the presence of starch granules from various biosynthesis levels [39]. The micrographs were found corresponding to the gelatinized starch content of samples. The morphological difference between chickpea and navy bean flours at low surface temperature (120 °C) was presented in Fig. 1C–F. SEM micrographs of chickpea samples showed larger protein–starch aggregations at 120 °C and high final moisture content. Similarly, the SEM micrographs of chickpea flours after soaking and heat treatment of chickpea seeds showed larger starch granules together with protein molecules as big aggregates [2]. However, navy bean flours at the same conditions were composed of starch granules and small aggregates of protein molecules. Furthermore, SEM micrographs of pre-soaked (53% moisture) and micronized bambara groundnut seeds indicated disruption in starch granules and coating of starch granules with proteinaceous and/or other detached cell components [22]. In addition, starch fractions of navy bean and chickpea seeds formed irregular and amorphous aggregates after autoclaving due to swelling and leaching [40]. On the other hand, microstructures of boiled green lentil, Kabuli chickpea, and yellow peaflours were detected as a homogeneous network with flat surface consisted of cross-linked protein and starch molecules different than raw and roasted flours [41]. These studies showed that the effect of water content during heat processing might cause different morphological changes regarding the processed material. Furthermore, higher surface temperature (140 °C) caused more significant damage such as dents and hollows at the starch structure in chickpea flours (Fig. 1 G, I) than navy bean samples (Fig. 1H, J). In general, micrographs of navy bean flours showed smoother starch surface; however, swelling of starch and damaged structures could be observed at high temperature (140 °C) and at high tempering levels (30%).
Surface characteristics and colour properties
The surface characteristics of chickpea and navy bean flours at pH 7.0 were evaluated (see Table 2). Change in zeta potential and surface hydrophobicity of flours alter molecular interactions which may effect the functionality of flours such solubility and emulsification. In general, zeta potential values of both chickpea and navy bean flours were determined higher than ± 30 mV which may indicate moderate colloidal stability for values between ± 31–40 mV and good colloidal stability for ± 41–60 mV [42]. A slight decrease in zeta potential values was observed in navy bean flours tempered to 20% seed moisture content at all surface temperatures; however, lower moisture contents caused slightly higher zeta potential values in chickpea flours. Overall, zeta potential values of navy bean and chickpea flours were measured − 38- − 43 mV and − 40- − 43 mV, respectively. On the other hand, infrared treatment on native black gram protein improved the zeta potential significantly from − 18.7 mV up to − 25.0 mV [43].
The surface hydrophobicity results of control and processed flours were presented in Table 2. Possible conformational changes due to infrared heating lead to an increase in surface hydrophobicity results. As stated in previous studies, buried hydrophobic sites in tertiary structures of proteins may be exposed during the conformational changes of the protein followed by denaturation cause an increase in surface hydrophobicity [44, 45]. The surface hydrophobicity results from chickpea and navy bean increased after infrared heating up to 3 folds. This showed the partial denaturation of proteins and could be used to predict the denaturation level [45]. However, surface hydrophobicity results for processed samples did not follow the same pattern in all samples. In navy bean samples, surface hydrophobicity decreased from 73 to 65 with increasing surface temperature at 30% tempering level; however, at 20% tempering levels, increasing temperature increased the hydrophobicity from 52 to 62. On the other hand, for chickpea samples, at the same tempering level, the increasing surface temperature increased the surface hydrophobicity to 1.4 fold and 1.8 fold at 20% and 30% tempering level, respectively. This can be explained as excessive heating might lead to aggregation and structural collapse which reduced the exposing of hydrophobic parts [45, 46].
The colour properties of chickpea and navy bean flours were evaluated as L*, a*, and b*color values in control and processed samples. CIE L*a*b* colour space includes 3 dimensions: L* shows the level of lightness (L* = 0, black; L* = 100, white), a* represents green (negative values) and red (positive values), and b* indicates blue (negative values) and yellow (positive values). L* values indicated high processing temperatures (140 °C) caused darker colour in all flours. The a* values of navy bean flours were found close to zero and slightly increased at 140 °C. Similarly, b* values of navy bean flours increased at 140 °C and were not changed at 120 °C. In the literature, colour properties of Kabuli chickpea was reported as 85.4, − 0.9, and 15.5 for L*, a*, and b*, respectively [47]. Although L* of chickpea flours was found at lowest at 140 °C, b* were found at highest (28.5–30.6) at 120 °C and a* were not affected from the heating. Overall, the tempering process did not affect the colour values significantly (p > 0.05). Similar to our findings, soaking and thermal processing of chickpea such as pressure cooking and roasting decreased the a*, b* and color change (ΔE) of protein isolates [48]. ΔE of navy bean flours increased with increasing surface temperature; however, there was no significance difference in chickpea flours based on surface temperature at the same tempering condition. This could be explained by the difference in seeds size of navy bean and chickpea. Darker colours of infrared heated preconditioned cowpeas was related to Maillard browning reaction which resulted in roasted flavour notes in cowpea samples [18].
Functional properties
Water hydration capacity (WHC), oil holding capacity (OHC), solubility, foaming capacity (FC), foaming stability (FS), emulsification capacity (OEC), and pasting properties were evaluated under functional properties of control and processed flours and are presented in Tables 3 and 4. WHC values showed the amount of water (g) was absorbed per g of pulse flours. The results indicated that infrared heating increased the water hydration capacity of chickpea and navy bean flours (see Table 3). WHC were found similar between control samples of chickpea (1.06 g/g) and navy bean (0.84 g/g) (p > 0.05). The highest increase in WHC was observed as to twofold at 140 °C surface temperature and 30% tempering moisture and to 2.8 fold at 120 °C surface temperature and 20% tempering moisture for navy bean and chickpea, respectively. In a similar manner, thermal processes such as cooking, and industrial dehydration (75 °C for 6 h.) on chickpeas and lentil seeds raised WHC to 4.80–4.90 g/g [2]. In addition, hydration capacity of infrared heated navy beans were found negatively correlated with surface temperature: however, positively correlated with moisture content [49]. Moreover, infrared heating of various legume seeds have increased WHC due to protein denaturation and starch gelatinization [21]. This was also confirmed with the present gelatinized starch values. For instance, at 120 °C and 20% tempering moisture, navy bean flour had the lowest gelatinized starch concentration and WHC among all processed samples. Pulse flours with high WHC could be preferred in bakery applications due to their higher absorption capacity of water during dough preparations leading to enhanced bread characteristics [41].
OHC values were around approximately 1 g/g in the control samples for both seeds. Tempering and infrared heating did not significantly affect OHC values in navy bean flours (p > 0.05). However, OHC slightly increased after processing of chickpea seeds at 120 °C with each moisture level and 140 °C with a moisture content of 20%. OHC values of chickpea and lentil were not altered after soaking, soaking-cooking, or soaking-cooking-dehydration processes [2]. Similarly, there were no significant differences (p > 0.05) between OHC of raw and processed (tempered to 20% moisture content and infrared heated at 115 °C or 135 °C) Desi chickpea and hull-less barley flours [23]. Besides, higher OHC values were reported for boiled pulse flours that might be related to exposing non-polar amino acid sides and increasing porosity letting higher oil entrapment after the treatment [41].
The solubility values of flours were significantly decreased after infrared heating down to 0.9% for navy bean and 6.6% for chickpea flours. Overall results found that chickpea flours had higher solubility than navy bean flours. This might be related to size differences of seeds which was affect the heat amount transferred to inside of the seeds [21]. The results showed that increasing surface temperatures caused higher reduction in all tempering moisture contents for both chickpea and navy bean flours. Similarly, protein solubility results of roasted chickpea isolates were reported lower than raw samples at different pHs (2.0, 4.5, 7.0, and 9.0) [48]. Higher surface hydrophobicity and lower nitrogen solubility index of tempered (41% moisture) and infrared heated cow peas at 130 °C and 170 °C indicated alteration of protein conformation of cowpeas due to infrared heating [17]. Furthermore, protein solubility of navy bean with 30% moisture content processed at 120 °C was found lower than navy bean samples with 20% moisture content at the same temperature. This agrees with the findings reported by Arntfield et al. [50], where higher tempering moisture caused soft texture which was related to increasing starch gelatinization, protein denaturation, and aggregation during infrared heating. The decrease in protein solubility due to thermal processing could be explained as (1) exposure of hydrophobic groups (2) denaturation of proteins by intermolecular disulphide bond formation (3) aggregation of unfolded proteins [48, 51, 52].
Foaming capability of flours is required to form aeration for textural properties and leavening purposes [53]. Foaming capacity (FC) and foaming stability (FS) parameters were evaluated in control and processed chickpea and navy bean flours. Flours from processed chickpea seeds could not generate foam. Furthermore, the decreasing effect of heat application was also determined in navy bean flours. During foaming, proteins position at air–water interface due to their hydrophilic and hydrophobic sides [23]. Decreased foaming capacities under increasing processing temperatures might be related to higher protein denaturation which can be supported by decreased protein solubility. Therefore, protein availability for air–water interface might be lower with increasing processing temperatures. Similar to solubility results, infrared heating had less effect on navy bean seeds than chickpea seeds due to the size difference of seeds. Although foaming capacity was found 80% at 120 °C with 30% tempering moisture, foaming stability was found at the lowest among processed navy bean seeds together with seeds processed at 140 °C with 20% tempering moisture. Foaming capacity values of Desi chickpeas were found between 128 and 215% followed by higher stability 79–93% [23]. Besides, adverse effects of cooking and industrial dehydration processes on foaming properties of chickpea and lentil flours were reported by [2] who determined the highest reduction on chickpea flours as 67%. Similar to present findings, 60% reduction in foaming capacity was detected in cowpea flours after infrared drying at 130 °C [17].
OEC of infrared treated seeds was found lower than raw seeds. Similar to our findings, heat treatments such as roasting and boiling decreased emulsification activity in legumes [41, 54]. Surface hydrophobicity of infrared treated samples was higher than untreated samples, however, process conditions affected the degree of hydrophobicity. In pulse flours, interactions between proteins and carbohydrates may also effect emulsion capacity [41]. In addition, higher emulsion capacity may be achieved with increased balanced surface ability of hydrophobic and hydrophilic sites of proteins [55].
Pasting properties of all flours are presented in Table 4. Pasting properties of chickpea samples processed at 140 °C and 120 °C with lower tempering moisture content (20%) could not be determined. However, control samples of chickpea showed higher peak and trough viscosities than navy bean flours. In addition, peak viscosity of navy bean samples could not be affected by thermal processing significantly (p > 0.05). The highest final viscosity was found in unprocessed chickpea samples. After that, the highest final and setback viscosity among navy bean samples were found in samples processed at 120 °C with 20% moisture content. Similarly, infrared heating (130 °C) of pre-soaked Bambara groundnut seeds (53% moisture) resulted decrease in pasting viscosity with increasing processing time [22]. The differences in pasting properties might be related to the lower solubility of processed chickpea and navy bean flours, high starch–protein interactions, and starch molecules surrounded with denatured protein molecules which may diminish granular selling.
Protein quality
The amino acid composition (g/100 g flour) of infrared treated and control samples of navy bean and chickpea are given in Tables 5, 6. Amino acid composition of the control samples of chickpea was not significantly affected by surface temperature and tempering moisture content. However, tryptophan, phenylalanine, arginine, glycine, valine, isoleucine, and leucine contents were slightly increased with higher surface temperature for navy bean samples. Similarly, the effect of boiling of faba beans for a short time increased leucine, tyrosine, threonine, and histidine content regarding raw samples [56].
The concentrations of essential amino acids for control and treated navy bean and chickpea flour are given in Table 7 along with the FAO/WHO (1991) nutrition reference pattern for children (2–5 years). In the case of navy bean flours, only sulfur containing amino acids (methionine and cysteine) were found lower than the reference pattern. It was expected, as pulses are deficient sulfur containing amino acids [57]. However, infrared treated and control samples of chickpea flours had lower tryptophan than the reference pattern. The results showed that the limiting amino acid (LAA) scores for navy bean (methionine and cysteine) were statistically constant, and ranged between 0.83 and 0.86. The LAA scores (tryptophan) for Kabuli chickpea were found similarly constant and ranging between 0.83 and 0.89. The results indicated that LAA scores for both navy bean and Kabuli chickpea remained constant and were not affected by tempering moisture content and surface temperature. In general, amino acid compositions of chickpea were found equal or higher than the reference pattern, except tryptophan, which was at most 2 mg/g lower than the reference level. This finding was in accordance with the chickpea results of Bai et al. [11] and Wang et al. [58]. The unexpected deficiency in tryptophan rather than sulfur amino acids could be the result of the use of sulfur containing fertilizers [58]. Also, after sulfur containing amino acids, tryptophan was reported as the second limiting amino acids in legumes [59].
In vitro protein digestibility results for treated and control samples of both navy bean and chickpea are given in Table 7. For all flours were affected significantly (p < 0.05) by both infrared treatment and tempering moisture content regardless of seed type. In detail, higher surface temperature (140 °C) increased protein digestibility from 74% up to 84% for navy bean and from 74% up to 85% for chickpea samples due to higher protein exposure [11]. Moreover, at the same surface temperature, an increase in tempering moisture levels increased the protein digestibility. As mentioned before, higher tempering moisture levels caused softer texture which may provide an easy matrix for enzyme digestion and also increase protein exposure [50].
In vitro protein digestibility corrected amino acid scores (IV-PDCAAS), an overall term for protein quality of flours, enhanced with high surface temperature (140 °C) and high tempering moisture (30%) content for navy bean; however, it did not follow a definitive pattern for chickpea. IV-PDCAAS for navy bean increased from 64 to 71% after infrared treatment at 140 °C with 30% moisture content. The IV-PDCAAS of navy bean for all samples agreed with IVPD results. The positive effect of high temperature and high moisture content on IV-PDCAAS of navy beans can be explained with higher protein denaturation and decrease in anti-nutritional compounds with increased temperature and increased availability of proteins to digestive enzymes due to both high temperature and moisture [11, 60]. On the other hand, IV-PDCAAS was found 65% for control samples of chickpea and infrared treatments at 120 °C with 20% moisture level and 140 °C with 30% moisture level increased IV-PDCAAS to 74% and 72% significantly (p < 0.05), respectively. However, IV-PDCAAS of all surface temperature levels and tempering moisture contents were not found statistically different than each other. The IV-PDCAAS of chickpea samples reflects LAA scores and IVPD values. The higher IV-PDCAAS at 120 °C surface temperature with 20% moisture level might be affected LAA score of 0.88 and relatively high IVPD (84%). Rather than this, the IV-PDCAAS of chickpea samples also supported the results of navy bean samples, which showed the more significant effect of high temperature on IV-PDCAASs of samples. In this study, IV-PDCAAS of control samples of navy bean (64%) were found lower than the reported PDCAAS for navy bean (67%); however, IV-PDCAAS of chickpea (65%) were found higher than the reported PDCAAS (52%) [61]. Moreover, navy bean samples treated at 140 °C regardless of moisture content had IV-PDCAAS above 67%.
In vitro starch digestibility
As an important nutritional property, in vitro starch digestibility of navy bean and chickpea flours were determined in terms of rapidly digestible starch (RDS), slowly diestible starch (SDS), and resistant starch (RS) contents (Table 8). Untreated navy bean and chickpea flours had higher RS proportion compared to other starch fractions suggesting high levels of enzymatic resistance of starch. This is also supported in previous studies, due to association of starch and densed protein and fiber matrices, RS percentage was reported higher than RDS and SDS contents of raw pulse flours [62, 63]. Infrared heating provided positive effect on starch digestibility of both navy bean and chickpea flours. Infrared heating at 140℃ led to higher increase in RDS content of navy bean flours. Increase in starch digestibility after infrared heating could be due to partial elimination of antinutrients suggesting possible space in dense matrix which eases enzyme interaction with starch granules [64]. However, both steam heating and high pressure heating was reported as responsible for higher RS contents in legumes [65]. This indicates extereme conditions during heat treatments might cause atypical glyosidic bond formation as well as carbohydrate side reactions which decrease enzymatic susceptibility [65, 66]. Infrared heating at low surface temperature (120 °C) and low tempering moisture content (20%) didn’t affect starch digestibility of navy beans. Besides, infrared treatment of chickpeas led to higher RDS and lower RS content.
Conclusions
Infrared heating did not change the proximate composition; however, α-amylase and gelatinized starch content were found highly susceptible to thermal processing. Surface characteristics were affected by both moisture content and surface temperature during infrared heating. Color properties were altered according to surface temperature. Among functional properties, WHC, OHC, solubility, FC, FS, OEC, and pasting properties evaluated. WHC increased in all samples except navy bean processed at 120 °C with 20% moisture content. In addition, OHC values of control and processed navy bean flours were not significantly different (p > 0.05). Solubility, OEC, and foaming abilities adversely affected by infrared heating. IV-PDCAASs and starch digestibility of pulses were positively affected by infrared treatment. The comprehensive evaluation of infrared heating and tempering on navy bean and Kabuli chickpea seeds with different initial physicochemical characteristics were performed. The findings will help to design new processes to obtain pulse flours with desired chemical and functional properties for future food applications. Future studies should evaluate the protein and starch digestibility after cooking the processed flours.
References
Maphosa Y, Jideani VA (2017) The role of legumes in human nutrition. Funct Food Improve Health Through Adequate Food 1:13
Aguilera Y, Esteban RM, Benítez V, Molla E, MaA M-C (2009) Starch, functional properties, and microstructural characteristics in chickpea and lentil as affected by thermal processing. J Agric Food Chem 57(22):10682–10688
García-Alonso A, Goni I, Saura-Calixto F (1998) Resistant starch and potential glycaemic index of raw and cooked legumes (lentils, chickpeas and beans). Zeitschrift für Lebensmitteluntersuchung und-Forschung A 206(4):284–287
Ai Y, Jin Y, Kelly JD, Ng PKW (2017) Composition, functional properties, starch digestibility, and cookie-baking performance of dry bean powders from 25 Michigan-grown varieties. Cereal Chem 94(3):400–408
Goñi I, Valentı́n-Gamazo C (2003) Chickpea flour ingredient slows glycemic response to pasta in healthy volunteers. Food Chem 81(4):511–515
Fabbri ADT, Crosby GA (2016) A review of the impact of preparation and cooking on the nutritional quality of vegetables and legumes. Int J Gastron Food Sci 3:2–11
Haileslassie HA, Henry CJ, Tyler RT (2019) Impact of pre-treatment (soaking or germination) on nutrient and anti-nutrient contents, cooking time and acceptability of cooked red dry bean (Phaseolus vulgaris L.) and chickpea (Cicer arietinum L.) grown in Ethiopia. Int J Food Sci Technol 54(8):2540–2552
Bidkhori P, Mohammadpour Karizaki V (2021) Diffusion and kinetic modeling of water absorption process during soaking and cooking of chickpea. Legume Sci. https://doi.org/10.1002/leg3.116
Wainaina I, Wafula E, Sila D, Kyomugasho C, Grauwet T, Van Loey A, Hendrickx M (2021) Thermal treatment of common beans (Phaseolus vulgaris L.): Factors determining cooking time and its consequences for sensory and nutritional quality. Compr Rev Food Sci Food Saf. https://doi.org/10.1111/1541-4337.12770
Liu S, Yin H, Pickard M, Ai Y (2020) Influence of infrared heating on the functional properties of processed lentil flours: a study focusing on tempering period and seed size. Food Res Int 136:109568
Bai T, Nosworthy MG, House JD, Nickerson MT (2018) Effect of tempering moisture and infrared heating temperature on the nutritional properties of desi chickpea and hull-less barley flours, and their blends. Food Res Int 108:430–439
Shariati-Ievari S, Ryland D, Edel A, Nicholson T, Suh M, Aliani M (2016) Sensory and physicochemical studies of thermally micronized chickpea (Cicer arietinum) and green lentil (Lens culinaris) flours as binders in low-fat beef burgers. J Food Sci 81(5):S1230–S1242
Aboud SA, Altemimi AB, Rs Al-HiIphy A, Yi-Chen L, Cacciola F (2019) A comprehensive review on infrared heating applications in food processing. Molecules 24(22):4125
Wood JA, Malcolmson LJ (2021) Chapter 10—Pulse milling technologies. In: Tiwari BK, Gowen A, McKenna B (eds) Pulse foods, 2nd edn. Academic Press, pp 213–263
Pulivarthi MK, Nkurikiye E, Watt J, Li Y, Siliveru K (2021) Comprehensive understanding of roller milling on the physicochemical properties of red lentil and yellow pea flours. Processes 9(10):1836
Ndungu KE, Emmambux MN, Minnaar A (2012) Micronisation and hot air roasting of cowpeas as pretreatments to control the development of hard-to-cook phenomenon. J Sci Food Agric 92(6):1194–1200
Mwangwela AM, Waniska RD, Minnaar A (2007) Effect of micronisation temperature (130 and 170° C) on functional properties of cowpea flour. Food Chem 104(2):650–657
Kayitesi E, Duodu KG, Minnaar A, de Kock HL (2013) Effect of micronisation of pre-conditioned cowpeas on cooking time and sensory properties of cooked cowpeas. J Sci Food Agric 93(4):838–845
Ogundele OM, Emmambux MN (2018) Effect of infrared heating of pre-soaked whole and dehulled bambara groundnut (Vigna subterranea) seeds on their cooking characteristics and microstructure. LWT 97:581–587
Žilić S, Šobajić S, Mladenović-Drinić S, Kresović B, Vasić MG (2010) Effects of heat processing on soya bean fatty acids content and the lipoxygenase activity. J Agric Sci 55(1):55–64
Fasina O, Tyler B, Pickard M, Zheng GH, Wang N (2001) Effect of infrared heating on the properties of legume seeds. Int J Food Sci Technol 36(1):79–90
Ogundele OM, Minnaar A, Emmambux MN (2017) Effects of micronisation and dehulling of pre-soaked bambara groundnut seeds on microstructure and functionality of the resulting flours. Food Chem 214:655–663
Bai T, Stone AK, Nickerson MT (2018) Effect of tempering moisture and infrared heating temperature on the functionality of Desi chickpea and hull-less barley flours. Cereal Chem 95(4):508–517
Bellido G, Arntfield SD, Cenkowski S, Scanlon M (2006) Effects of micronization pretreatments on the physicochemical properties of navy and black beans (Phaseolus vulgaris L.). LWT-Food Science and Technology 39(7):779–787
AACC (2000) Approved methods of analysis (10th edn) AACC International, St. Paul. American Association of Cereal Chemists
AOAC (2003) Official method of analysis, 17th edn. Association of Official Analytical Chemists Inc., Virginia
Chang C, Tu S, Ghosh S, Nickerson MT (2015) Effect of pH on the inter-relationships between the physiochemical, interfacial and emulsifying properties of pea, soy, lentil and canola protein isolates. Food Res Int 77:360–367
Kato A, Nakai S (1980) Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochimica et biophysica acta (BBA)-Protein Structure 624(1):13–20
Morr CV, German B, Kinsella JE, Regenstein JM, Buren JV, Kilara A, Lewis BA, Mangino ME (1985) A collaborative study to develop a standardized food protein solubility procedure. J Food Sci 50(6):1715–1718
Nidhina N, Muthukumar SP (2015) Antinutritional factors and functionality of protein-rich fractions of industrial guar meal as affected by heat processing. Food Chem 173:920–926
Wang N, Maximiuk L (2015) Development of an improved electrical resistance method for determining emulsifying capacity of pulse and soy materials 1. Cereal Chem 92(3):253–257
FAO/WHO (1991) Protein Quality Evaluation-Report of the Joint FAO/WHO Expert Consultation; Food and Nutrition Paper 51. Food and Agriculture Organization of the United Nations, The World Health Organization, Rome
Hsu HW, Vavak DL, Satterlee LD, Miller GA (1977) A multienzyme technique for estimating protein digestibility. J Food Sci 42(5):1269–1273
Englyst HN, Kingman SM, Cummings JH (1992) Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 46:S33
Pathiratne SM, Shand PJ, Pickard M, Wanasundara JPD (2015) Generating functional property variation in lentil (Lens culinaris) flour by seed micronization: effects of seed moisture level and surface temperature. Food Res Int 76:122–131
Singh U (1985) Nutritional quality of chickpea (Cicer arietinum L.): current status and future research needs. Plant Foods Hum Nutr 35(4):339–351
Apar DK, Özbek B (2005) α-Amylase inactivation during rice starch hydrolysis. Process Biochem 40(3–4):1367–1379
Chandrashekar A, Kirleis AW (1988) Influence of protein on starch gelatinization in sorghum. Cereal Chem 65(6):457–462
Ravi R, Harte JB (2009) Milling and physicochemical properties of chickpea (Cicer arietinum L.) varieties. J Sci Food Agric 89(2):258–266
Xu J, Ma Z, Ren N, Li X, Liu L, Hu X (2019) Understanding the multi-scale structural changes in starch and its physicochemical properties during the processing of chickpea, navy bean, and yellow field pea seeds. Food Chem 289:582–590
Ma Z, Boye JI, Simpson BK, Prasher SO, Monpetit D, Malcolmson L (2011) Thermal processing effects on the functional properties and microstructure of lentil, chickpea, and pea flours. Food Res Int 44(8):2534–2544
Du Plessis J, Ramachandran C, Weiner N, Müller DG (1996) The influence of lipid composition and lamellarity of liposomes on the physical stability of liposomes upon storage. Int J Pharm 127(2):273–278
Kamani MH, Semwal J, Meera MS (2021) Functional modification of protein extracted from black gram by-product: effect of ultrasonication and micronization techniques. LWT 144:111193
Sharma P, Oey I, Everett DW (2016) Thermal properties of milk fat, xanthine oxidase, caseins and whey proteins in pulsed electric field-treated bovine whole milk. Food Chem 207:34–42
Moro A, Gatti C, Delorenzi N (2001) Hydrophobicity of whey protein concentrates measured by fluorescence quenching and its relation with surface functional properties. J Agric Food Chem 49(10):4784–4789
Alizadeh-Pasdar N, Li-Chan ECY (2000) Comparison of protein surface hydrophobicity measured at various pH values using three different fluorescent probes. J Agric Food Chem 48(2):328–334
Kaur M, Singh N (2005) Studies on functional, thermal and pasting properties of flours from different chickpea (Cicer arietinum L.) cultivars. Food Chem 91(3):403–411
Xu Y, Obielodan M, Sismour E, Arnett A, Alzahrani S, Zhang B (2017) Physicochemical, functional, thermal and structural properties of isolated Kabuli chickpea proteins as affected by processing approaches. Int J Food Sci Technol 52(5):1147–1154
Bellido GG, Arntfield SD, Scanlon MG, Cenkowski S (2003) The effect of micronization operational conditions on the physicochemical properties of navy beans (Phaseolus vulgaris L.). J Food Sci 68(5):1731–1735
Arntfield SD, Scanlon MG, Malcolmson LJ, Watts B, Ryland D, Savoie V (1997) Effect of tempering and end moisture content on the quality of micronized lentils. Food Res Int 30(5):371–380
Pérez-Gago MB, Nadaud P, Krochta JM (1999) Water vapor permeability, solubility, and tensile properties of heat-denatured versus native whey protein films. J Food Sci 64(6):1034–1037
Avanza MV, Chaves MG, Acevedo BA, Añón MC (2012) Functional properties and microstructure of cowpea cultivated in north-east Argentina. LWT 49(1):123–130
Kaur M, Sandhu KS (2010) Functional, thermal and pasting characteristics of flours from different lentil (Lens culinaris) cultivars. J Food Sci Technol 47(3):273–278
Obatolu VA, Fasoyiro SB, Ogunsunmi L (2007) Processing and functional properties of yam beans (Sphenostylis stenocarpa). J Food Process Preserv 31(2):240–249
Ashraf S, Saeed SMG, Sayeed SA, Ali R (2012) Impact of microwave treatment on the functionality of cereals and legumes. Int J Agric Biol 14(3):365–370
Khalil AH, Mansour EH (1995) The effect of cooking, autoclaving and germination on the nutritional quality of faba beans. Food Chem 54(2):177–182
Benito O, Galvez AF, Revilleza MJ, Krenz DC (1999) Molecular strategies to improve the nutritional quality of legume proteins. In: Chemicals via higher plant bioengineering. Springer, pp 117–126
Wang S, Nosworthy MG, House JD, Ai Y, Hood-Niefer S, Nickerson MT (2019) Effect of barrel temperature and feed moisture on the physical properties of chickpea–sorghum and chickpea–maize extrudates, and the functionality and nutritional value of their resultant flours—Part II. Cereal Chem 96(4):621–633
Bressani R, Elias LG (1979) Post-Harvest Food Conversation: Improvement of the nutritional quality of food legumes. Food Nutr Bull 1(4):1–14
Guldiken B, Yovchev A, Nosworthy MG, Stone AK, House JD, Hood-Niefer S, Nickerson MT (2019) Effect of extrusion conditions on the physical properties of desi chickpea-barley extrudates and quality attributes of their resulting flours. J Texture Stud. https://doi.org/10.1111/jtxs.12470
Nosworthy MG, House JD (2017) Factors influencing the quality of dietary proteins: implications for pulses. Cereal Chem 94(1):49–57
Guldiken B, Konieczny D, Wang N, Hou A, House JD, Tu K, Rosendahl S, Lavier M, Nickerson MT (2021) Effect of variety and environment on the physicochemical, functional, and nutritional properties of navy bean flours. Eur Food Res Technol. https://doi.org/10.1007/s00217-021-03745-7
Setia R, Dai Z, Nickerson MT, Sopiwnyk E, Malcolmson L, Ai Y (2019) Impacts of short-term germination on the chemical compositions, technological characteristics and nutritional quality of yellow pea and faba bean flours. Food Res Int 122:263–272
Rehman Z-u, Shah WH (2005) Thermal heat processing effects on antinutrients, protein and starch digestibility of food legumes. Food Chem 91(2):327–331
Tovar J, Melito C (1996) Steam-cooking and dry heating produce resistant starch in legumes. J Agric Food Chem 44(9):2642–2645
Siljeström M, Björck I, Westerlund E (1989) Transglycosidation reactions following heat treatment of starch–Effects on enzymic digestibility. Starch-Stärke 41(3):95–100
Funding
Financial support was provided through the AgriScience Program (Canadian Pulse Science Research Cluster) under the Canadian Agricultural Partnership.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical statement
This research article had no ethical considerations, as animal and human subjects were not used.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guldiken, B., Konieczny, D., Franczyk, A. et al. Impacts of infrared heating and tempering on the chemical composition, morphological, functional properties of navy bean and chickpea flours. Eur Food Res Technol 248, 767–781 (2022). https://doi.org/10.1007/s00217-021-03918-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03918-4