Abstract
“Hidden” peanut allergens in commercial foods pose a potential risk for peanut-allergic individuals. Sensitive and reliable analytical methods are urgently needed for detecting peanut in processed foods at low levels. We developed a peanut sandwich ELISA test kit by pairing two polyclonal sera against peanut proteins obtained from different spices. Its analytical performance of sensitivity, specificity, accuracy, trueness, and precision were evaluated, respectively. The limit of detection (LOD) was defined as 0.001 mg/kg, and no cross-reactivity was observed in 25 spices including legumes, tree nuts, and seeds. Besides, the mean recoveries in four spiked food matrixes ranged from 74.067 to 122.953%, and the recoveries of the three model foods incurred with peanut proteins were within the range of 80–120%. Acceptable results of repeatability and reproducibility were obtained referring to the AOAC standard. Moreover, it was verified to be capable of applying for detecting peanut residues in commercially available food products in the market and evaluating the food labels effectively. The study provides powerful technical support for the sensitive detection of peanut products for both food manufacturers and regulatory agencies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, peanut allergy (PA) has emerged as the poster girl in the food allergy world due to its high sensitization rate, increasing prevalence, as well as reaction severity [1]. It has increased significantly in the past few decades in both developed and developing countries, affecting 1%–3% of children [2, 3]. The reactions of PA are often severe, and occasionally fatal, which become a leading cause of food-induced allergic reactions, anaphylaxis, and death [4, 5]. However, PA is less likely to be outgrown compared with milk as well as egg allergy, which is lifelong in most cases and places a profound burden on millions of peanut-allergic sufferers worldwide [6]. Therefore, effective management of this major public health concern is highly essential for the health-related quality of life of patients with PA.
Up to now, avoidance of food is mainly in practice the only approach to prevent allergic individuals from allergenic substances ingestion, which highlights the importance of requirement for accurate and complete information of ingredients on food labels [7]. Peanuts are one of the “big eight” major food allergens declared by the World Health Organization Codex Alimentarius Commission and are mandated labeling in most developed countries as well as some developing countries [8]. However, peanut-allergic individuals are still at risk of unintended allergenic ingredients exposure due to labeling errors caused by false detection and even fraud, or labeling vagaries, such as precautionary allergen labeling (PAL) of “may contain,” “may contain traces,” “produced in a factory with,” “produced on the same line,” which warns consumers about the potential presence of “hidden” allergens (trace of allergens) from cross-contact/contamination during manufacturing [8,9,10]. Hence, reliable analytical methods are urgently needed for detecting the presence of any unintentional or intentional allergenic ingredients in foods to protect peanut-allergic individuals from accidental exposure by aiding the enforcement of allergen labeling regulations.
Currently, ELISA is the most widely used analytical method for screening the presence of allergens in food products by food industries as well as food control authorities owing to its outstanding sensitivity and simplicity [11]. There are a certain number of commercially available peanut sandwich ELISA (sELISA) test kits for food safety control with LOD typically ranging between 2.5 and 0.1 μg/mL declared by the manufacturers [12]. It is worthwhile to remark that trace amounts (30–100 μg) of peanut protein may trigger peanut-allergic reactions [13] and no regulatory threshold exists for allergenic contents in food [7]. As a result, VITAL (Voluntary Incidental Trace Allergen Labelling) allergen reference doses have been proposed for the application of PAL [14], which makes it important to know how much unintended trace amount of peanut allergen is present in food products. However, it is difficult to detect peanut allergens in food, since they can be masked by food matrixes and are often present in trace amounts [15]. Thus, the LOD is expected to be as low as possible for the developed analytical methods and the pursuit for lower LOD is of substantial value for more sensitive detection as well as the protection of highly sensitive peanut-allergic individuals.
Moreover, recalls of food products with undeclared peanut due to false detection in recent years underscore the significance of verifying the performance of analytical methods [16, 17]. Nevertheless, there are few published reports on the validation, development, and application in detecting peanut residues in processed foods of the developed peanut sELISA test kits. Comprehensive validation of sensitivity, specificity, accuracy, trueness, and precision for the developed peanut sELISA test kits is necessary for increasing the reliability of food labels and reducing the unnecessary economic losses caused by recalls.
Therefore, the purpose of the present study is to develop a peanut sELISA test kit with the lowest possible LOD, and evaluate its sensitivity, specificity, accuracy, trueness, precision, as well as applicability for detecting peanut residues in processed foods.
Materials and methods
Extraction of peanut proteins
Conditions for extracting peanut proteins were optimized for the improvement of its quality. The optimal method was developed for the preparation of peanut proteins as follows. First, the peeled raw peanuts (Arachis Hypogaea, Baisha, China) purchased from a local market in Qingdao, China were crushed into powder and defatted by N-hexane in a ratio of 1:7 (w/v), with continually stirring at 4 °C for 8 h. After being defatted four times, the peanut powder was recovered by centrifuging at 9000 rpm for 10 min at 4 °C and was air-dried. Next, the peanut powder was extracted with Tris–HCl (50 mM, pH 8.0) in a ratio of 1:10 (w/v) at 4 °C, and centrifuged at 9000 rpm for 15 min, followed by dialysis at 4 °C for 36 h as well as suction filtration. Finally, the obtained peanut proteins solution was freeze-dried and stored at − 20 °C for use.
Polyclonal antisera preparation
The polyclonal rabbit anti-peanut serum was obtained from Luoyang Bai Aotong Experimental Materials Center (Luoyang, Henan Province, China) and the polyclonal rat anti-peanut serum was obtained from Hubei Prokin Technology Co., Ltd (Wuhan, Hubei Province, China). The peanut proteins solution mixed with Freund’s adjuvant was injected to immunize a New Zealand rabbit as well as a Wistar rat, and their negative sera were collected before immunization. After several immunizations at appropriate intervals, the whole blood was collected by centrifugation. Finally, the sera were separated.
The titer and specificity of peanut-specific sera were monitored by indirect ELISA and the evaluation was conducted by IgG immunoblotting according to the previous study [18].
SDS-PAGE and Western blot
Proteins of seven species with high homology to peanut were extracted by different buffers regarding the method in 2.1 with minor modifications, which were electrophoresized together with peanut proteins with reference to the method in a previous study [19] by the Bio-Rad electrophoresis system (Powerpac Universal, Bio-Rad Laboratories, Inc., USA). The SDS-PAGE gel was prepared with 5% stacking gel and 12% separating gel, and samples (1.0 mg/mL) were mixed with 4 × loading buffer in the ratio of 3:1 (v/v), boiled for 7 min at 100 °C, and loaded into the gel (10 μL). Then, the electrophoresis of all the allergens was performed in the stacking gel at 80 V for 0.5 h and in the separating gel at 120 V for 1.5 h.
The gel was stained with Coomassie brilliant blue R-250 and destained in a de-staining solution (10% HAc [v/v], 10% methanol [v/v] in water). Afterward, the result of electrophoresis was analyzed by the BIO-RAD Universal Hood II gel imaging system (Bio-Rad Laboratories, Inc., CA, USA).
Protein samples from identical sample gel were electrophoretically transferred to polyvinylidene difluoride (PVDF, Millipore, USA) membranes by iBlot® 2 Gel Transfer Device (Life Technologies Co., Israel) for 7 min and the membranes were washed with PBST (PBS containing 0.05% Tween-20 [Beijing Solarbio Science & Technology Co., Ltd., Beijing, China], pH 7.4). Next, the membranes were blocked with 5% non-fat dry milk (BD Company, USA) in PBST for 2 h at room temperature, followed by incubation with the polyclonal rabbit anti-peanut serum (diluted 1:20,000 [v/v]) or the polyclonal rat anti-peanut serum (diluted 1:20,000 [v/v]) for 2 h at room temperature. Next, the membranes were washed thrice in PBST and incubated with HRP-labeled goat anti-rabbit IgG antibody (diluted 1:20,000, Luoyang Bai Aotong Experimental Materials Center, Luoyang, Henan Province, China) or HRP-labeled rabbit anti-rat IgG antibody (diluted 1:20,000, Luoyang Bai Aotong Experimental Materials Center, Luoyang, Henan Province, China) for 1 h at room temperature. After three washes in PBST, the results were captured by the BIO-RAD Universal Hood II gel imaging system (Bio-Rad Laboratories, Inc., CA, USA).
Development of peanut sELISA test kit
The polyclonal rabbit anti-peanut serum and the polyclonal rat anti-peanut serum were used as capture and detection antibodies, respectively, which displayed the best detection performance in the development of the peanut sELISA method demonstrated by substantial preliminary tests. Polystyrene 96-well microtiter plates (Corning, NY, USA) were coated with 100 μL polyclonal rabbit anti-peanut serum diluted (1:50,000) with 50 mM sodium carbonate buffer (0.05 M NaHCO3, 0.05 M Na2CO3, pH 9.6) each well, and then incubated overnight at 4 °C. Washed thrice by PBST, each well was then blocked with 150 μL 1%BSA (in PBST), and incubated at 37 °C for 2 h, to avoid the unspecific protein-binding sites. Afterward, the plate was washed thrice with PBST and added 100 μL/well of peanut proteins solution of 50, 10, 2.5, 0.5 0.125, 0.025, 0.01, 0.005, and 0.001 μg/mL, followed by incubation at 37 °C for 1 h. After three washes with PBST, the polyclonal rat anti-peanut serum was diluted 1:10,000 with detection antibody dilution buffer before the addition of 100 μL/well, followed by incubation at 37 °C for 1 h. Subsequently, after three washes by PBST, 100 μL HRP-labeled rabbit anti-rat IgG (diluted 1:20,000, Luoyang Bai Aotong Experimental Materials Center, Luoyang, Henan Province, China) was added to each well. The plates were again incubated at 37 °C for 1 h and washed three times with PBST followed by adding 100 μL/well of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), and were incubated in the dark for 10 min at 37 °C prior to the stop of the reaction by the addition of 50 μL/well of 2 M sulphuric acid. Eventually, the absorbance of light was obtained at 450 nm main wavelength and a 630 nm reference wavelength by a microplate reader (800TM TS, BioTek Instruments Inc., Winooski, Vt, USA).
Pre-coated polystyrene 96-well microtiter plates, detection antibody solution, HRP-labeled antibody solution, and peanut proteins solution were prepared and optimized for the development of the peanut sELISA test kit. To verify the stability of the kit, the standard curves before and after 6 months were tested and compared.
Evaluation of detection sensitivity
Limit of detection (LOD) is determined as the lowest concentration of the analyte in a test sample that can be distinguished from a true blank sample at a specified probability level, and limit of quantitation (LOQ) is the lowest level of the analyte in a test sample that can be reasonably quantified at a specified level of precision [20]. According to the validation guidance of EURACHEM [21], LOD and LOQ were calculated as the mean of the measured values of ten blank samples plus three and ten times the standard deviation (SD), respectively. LOD was calculated by extracts from 5, 50, 250, 500 g food materials using the method above to compare with the action levels reported by Holzhauser et al. [12].
Study of cross-reactivity
Species botanically related to peanut were focused on, which would be expected to share similar amino acid sequence and protein structure with peanut, including legumes (soybean), tree nuts (hazelnut, walnut, almond, and pistachio), and seeds (sesame, wheat) [22, 23]. 25 different species were chosen for evaluating the specificity of the developed peanut sELISA test kit. All the proteins of 25 different species, as well as peanut, were extracted and detected by the developed peanut sELISA test kit at the concentration of 5 mg/kg.
Evaluation of detection accuracy
Food matrixes including milk, beef sauce, noodles, and chocolate were spiked by peanut proteins for the accurate evaluation of the developed peanut sELISA test kit. First, four food samples were homogenized. Next, they were spiked with peanut proteins solution of 0, 50, 200, and 1000 ng/g, followed by extraction by Tris–HCl (50 mM, pH 8.0) for 1 h. Afterward, the solution was centrifuged at 10,000 g for 10 min and the collected supernatants of each food sample were detected by the developed kit in triplicate. The mean values for the recoveries were calculated for the evaluation of recovery rates.
Analysis of model foods
Model processed food samples would be the best source of information on the method performance for allergen detecting methods [24]. Therefore, three kinds of model processed foods (biscuit, rice gruel, and chocolate) were prepared. The food materials for the preparation of model foods were purchased at a supermarket in Qingdao, China.
Model biscuits consist of butter, sugar, low-gluten flour, and water. 6 g butter, 2 g sugar, and 10 g low-gluten flour were all added into three bowls, respectively, followed by the addition of water containing peanut powder and thoroughly mix. The final peanut proteins contents of the three mixtures were 5, 50, and 500 mg/kg. Then, the three model biscuits were baked in a conventional oven (Haier, Qingdao, China) at 180 °C for 20 min after refrigerating at – 80 °C for 10 min.
Rice and water were prepared for the model rice gruel, which was boiled in a high-pressure cooker (Midea, Guangzhou, China) for 30 min and homogenized. The rice gruel was incurred by peanut proteins of 5, 50, and 500 mg/kg, respectively.
Model chocolates with peanut proteins contents of 5, 50, and 500 mg/kg were melted and maintained at 40 °C during the whole procedure. 5 mg of peanut proteins were added to 10 g of melted chocolate for the preparation of the model chocolate containing 500 mg/kg of peanut proteins and the other two model chocolates were obtained by adding the melted chocolate successively.
Each model food was extracted by Tris–HCl (50 mM, pH 8.0) for 1 h and detected following the procedure described in the previous sections. The mean values for the recovery rates of peanut proteins from the model foods were calculated for further analysis.
Evaluation of detection precision
The precision of the peanut sELISA test kit was examined by analysis of repeatability and reproducibility. Biscuit model food incurred with 50 mg/kg of peanut proteins, and rice gruel, as well as chocolate spiked with 50 mg/kg of peanut proteins, were analyzed. Extracts of the incurred and spiked foods were measured in ten replicates on one plate to calculate the repeatability and were measured in three replicates on 3 different days to calculate the reproducibility.
Detection of peanut in commercially processed foods
To verify the performance of the developed peanut sELISA test kit in detecting the commercially processed foods and evaluate the food labels, we simulated the detection scene of the commercial foods. The commercially processed foods used for the analysis were purchased from the local markets in Qingdao, China, which were divided into three categories: foods labeled with peanut allergens, foods labeled with the potential presence of peanut allergens, and foods labeled without peanut allergens, according to the food labels. Twenty different commercially processed foods were analyzed using the developed peanut sELISA test kit. Each commercially processed food was extracted by Tris–HCl (50 mM, pH 8.0) for 1 h and detected following the procedure described in the previous sections.
Statistical analysis
Each sample was tested in duplicate, and each experiment was repeated at least thrice. Results were processed by Office Excel (Microsoft, USA) and Origin 8 (Origin Lab, Northampton, MA, USA).
Results
Antisera production and binding specificity to peanut proteins
The polyclonal rabbit anti-peanut serum and the polyclonal rat anti-peanut serum were obtained by immunizing rabbit and rat with peanut proteins as the immunogen, respectively. To determine the performance of the two polyclonal anti-peanut sera, we evaluated their titers as well as the binding specificity to both peanut and its botanically related spices by indirect ELISA and IgG immunoblotting.
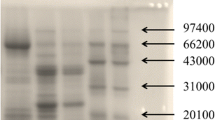
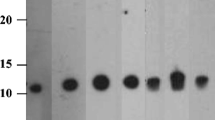
As the results were shown, the titers of rabbit and rat polyclonal anti-peanut sera were 16,384,000 and 1,280,000, respectively, which were of high levels. SDS-PAGE of eight extracts is shown clearly in Fig. 1a and the protein bands of peanut extracts were at molecular masses ranging roughly from 14 to 63.5 kDa. By IgG immunoblotting analysis in Fig. 1b and c, we found that both of the two polyclonal anti-peanut sera can strongly bind to main peanut extracts in SDS-PAGE (Fig. 1a) ranging roughly from 14 to 63.5 kDa despite some minor differences. Besides, none of the other extracts exhibited specific binding to polyclonal rat anti-peanut serum, while extracts of the kidney bean, walnut, and wheat showed weak binding to polyclonal rabbit anti-peanut serum. Thus, the two polyclonal anti-peanut sera verified with high titers and specificity were utilized in the development of the peanut sELISA test kit.
SDS-PAGE and binding specificity of rat and rabbit polyclonal anti-peanut sera to eight different species with loading concentration of 1 mg/mL. M marker, Lane 1 peanut, Lane 2 soybean, Lane 3 kidney bean, Lane 4 hazelnut, Lane 5 walnut, Lane 6 pistachio, Lane 7 sesame, Lane 8 wheat. a SDS-PAGE pattern of eight protein extracts. b Western blot analysis of rat polyclonal anti-peanut serum (diluted 1:20,000). c Western blot analysis of rabbit polyclonal anti-peanut serum (diluted 1:20,000)
Standard curve and stability assessment
A calibration curve was obtained with peanut proteins at a concentration ranging from 1 to 50,000 ng/mL in Fig. 2. A seven-point standard curve, the mid-linear portion of the calibration curve, was drawn with log fitting using peanut proteins at a concentration ranging from 5 to 10,000 ng/mL (insert to Fig. 2), and the R2 was higher than 0.99 (y = 0.1901ln(x) − 0.2273, R2 = 0.9929). The results of the stability tests are shown in Fig. 3, indicating that the peanut sELISA test kit stored for 6 months was able to provide a high linear fitting degree of the standard curve and the detection OD values reduction was less than 15%. Thus, our peanut sELISA test kit was proved to be able to store at 4 °C for 6 months.
Sensitivity and cross-reactivity evaluation
The LOD and LOQ of the calibration curve were defined as 0.001 and 0.05 mg/kg, respectively. After experimental verification, the LOD of our developed kit obtained from the four food matrixes was below the action levels in serving sizes of 5, 50, 250, and 500 g, respectively.
The specificity of the peanut sELISA test kit was evaluated by specificity evaluation with 25 species (Fig. 4) including species that show high homology to peanut, such as legumes (soybean), tree nuts (hazelnut, walnut, almond, and pistachio), and seeds (sesame, wheat). No cross-reactivity was observed, indicating that the developed peanut sELISA test kit is of high specificity for detecting peanut proteins in a variety of foods.
Analysis of peanut proteins spiked samples for accuracy evaluation
The recovery rates of peanut from four foods (peanut allergens free) are summarized in Table 1. The recovery rates of peanut proteins from spiked milk, beef sauce, noodles, and chocolate we found were ranged from 99.296 to 118.686%, 98.455 to 105.899%, 90.417 to 112.233%, and 74.067 to 122.953%, respectively, and control samples without peanut proteins gave results below 0.05 mg/kg.
Trueness and precision of the developed sELISA test kit for detecting peanut proteins
As listed in Table 2, the peanut contents in the model foods were detected by the developed peanut sELISA test kit. The recovery rates were ranged between 86.644 and 105.880% for biscuits, between 87.878 and 107.316% for rice gruel, and between 81.030 and 86.660% for chocolate. Results of the precision measurement are listed in Table 3. CV ranged from 5.467 to 8.671% for repeatability, and from 7.893 to 10.456% for reproducibility, which were both within the limits recommended by the AOAC [25].
Detection of peanut in commercial food
To verify the capacity for practical application of the developed peanut sELISA test kit, 20 commercially processed foods were detected following the method established above, and the results are listed in Table 4. The detection results were considered to be positive if the peanut levels detected were higher than the limit of quantification (LOQ), which was 0.05 mg/kg. In all of the seven foods labeled with peanuts in the ingredients, peanut proteins were detected. Four of six foods labeled with the potential presence of peanut allergens were detected peanut positive, with peanut proteins ranging between 0.026 and 0.352 mg/kg. No peanut protein has been detected in the seven foods labeled without peanut components.
Discussion
In this study, we successfully developed a reliable peanut sELISA test kit using rabbit and rat polyclonal antisera and also verified its performance. Our results demonstrated that this peanut sELISA test kit could provide brilliant analytical performance and applicability. Besides, it can be stably stored at 4 °C for 6 months indicating high stability. This study is of great significance, because we have made an improvement in the performance, especially the sensitivity of the developed peanut sELISA test kit compared with the most commercially available ones. Moreover, although some studies have reported the development of sELISA for the detection of peanut [26, 27], there were few reports on the development of peanut sELISA test kits. In our study, we introduced the method of developing the peanut sELISA test kit including long-term stability evaluation of the kit.
A crucial factor for the high performance of the developed kit was high-titer polyclonal antiserum, which was attributed to the high-quality antigen obtained by optimized extraction procedures. A lower LOD of 0.001 mg/kg was achieved compared with most of the commercially available peanut sELISA test kits. Furthermore, LOD obtained in food matrixes met the requirement of protein reference dose verification in serving size of 5–500 g, indicating that our developed peanut sELISA test kit is of high sensitivity. Since sensitivity is an important factor to be considered in the judgment of whether a method is suitable for the detection of allergen in food and LOD is expected to be as low as possible, the LOD at a fairly low level is bound to become an advantage for further application of the developed kit. The range of quantification of the developed peanut sELISA test kit was determined to be 5–10,000 ng/mL (Fig. 2), which showed a wider range compared with six commercially available peanut sELISA test kits calculated in the previous report [28]. No cross-reactivity occurred with the other 25 species including most of those that were in high homology with peanut (Fig. 4), although polyclonal rabbit anti-peanut serum showed weak binding to kidney bean, walnut, and wheat (Fig. 1c), which may be due to the homology of the proteins. The results indicate that the developed kit is of high specificity to detect peanut proteins in various kinds of food products.
Besides, the accuracy of the developed peanut sELISA test kit was evaluated in recovery experiments, adding peanut proteins at four different concentration levels to four different blank food matrixes (milk, beef sauce, noodles, and chocolate). As results are listed in Table 1, an ideal recovery of 80–120% by AOAC has been reached by milk, beef sauce, and noodles, indicating that the developed kit is suitable to detect peanut in these three food matrixes [20]. Despite the influence caused by matrix interference [29], the recovery rates in chocolate were between 74.067 and 122.953%, which were within the acceptable recovery of 50–150% by AOAC [20]. The accurate detection of peanut in chocolate is necessary, since the undeclared traces of peanut are likely to present in chocolate [30]. Therefore, the developed peanut sELISA test kit is sufficiently accurate and is able to be utilized for detecting a wide range of food matrixes, including liquid, pasty, and solid matrixes. Acceptable recoveries ranging from 80 to 120% were also obtained from three incurred foods of biscuit, rice gruel, and chocolate, as listed in Table 2. The repeatability and reproducibility (Table 3) were ranged from 5.467 to 8.671% and 7.893 to 10.456%, respectively, which were within the limits recommended by the AOAC for food allergens [25]. The results were controlled at low levels, indicating that the developed kit is of high precision.
Furthermore, we examined the commercially processed foods to evaluate the reliability of the food labels as well as the applicability of the developed peanut sELISA test kit (Table 4). The detection results of foods labeled with or without peanuts in the ingredients were all in compliance with the information listed on the labels, respectively. Four of six foods labeled with the potential presence of peanut proteins were detected peanut positive with peanut proteins ranging between 0.026 and 0.352 mg/kg, implying that the cross-contact/contamination of peanuts in production lines or raw materials does occur, which highlights the importance of reliable analytical methods in processed food for the application of PAL. In general, the developed sELISA test kit is capable of detecting peanut residues in processed food.
Conclusions
The newly developed peanut sELISA test kit by pairing two peanut-specific polyclonal antisera in this study exhibited excellent sensitivity, specificity, accuracy, trueness, and precision. The LOD was identified as 0.001 mg/kg, which ensures that the developed kit is sensitive enough to detect peanut in the presence of a variety of processed foods at low levels. These results demonstrated that our peanut sELISA test kit could contribute to the effective management of PA and ensure the compliance of food labeling, thus helping to protect the health as well as increase the quality of life of peanut-allergic individuals.
References
Shah F, Shi A, Ashley J, Kronfel C, Wang Q, Maleki SJ, Adhikari B, Zhang J (2019) Peanut allergy: characteristics and approaches for mitigation. Compr Rev Food Sci F 18(5):1361–1387
Gray CL (2020) Current controversies and future prospects for peanut allergy prevention, diagnosis and therapies. J Asthma Allergy 13:51–66
Sicherer SH, Sampson HA (2018) Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immun 141(1):41–58
Flinn A, Hourihane JO (2013) Allergic reaction to peanuts: can we predict reaction severity in the wild? Curr Allergy Asthm R 13(6):645–650
Bock SA, Munoz-Furlong A, Sampson HA (2007) Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immun 119(4):1016–1018
Savage J, Sicherer S, Wood R (2016) The natural history of food allergy. J Aller Cl Imm-Pract 4(2):196–203
Sena-Torralba A, Pallás-Tamarit Y, Morais S, Maquieira Á (2020) Recent advances and challenges in food-borne allergen detection. Trac-Trend Anal Chem 132:116050
Allen KJ, Turner PJ, Pawankar R, Taylor S, Sicherer S, Lack G, Rosario N, Ebisawa M, Wong G, Mills ENC (2014) Precautionary labelling of foods for allergen content: are we ready for a global framework? World Allergy Organ 7:10
Venter C, Sicherer SH, Greenhawt M (2019) Management of peanut allergy. J Aller Cl Imm-Pract 7(2):345-355.e2
Campuzano S, Montiel VR, Serafin V, Yanez-Sedeno P, Pingarron JM (2020) Cutting-edge advances in electrochemical affinity biosensing at different molecular level of emerging food allergens and adulterants. Biosensors-Basel 10(2):10
Prado M, Ortea I, Vial S, Rivas J, Calo-Mata P, Barros-Velazquez J (2016) Advanced DNA- and protein-based methods for the detection and investigation of food allergens. Crit Rev Food Sci 56(15):2511–2542
Holzhauser T, Johnson P, Hindley JP, O’Connor G, Chan CH, Costa J, Faeste CK, Hirst BJ, Lambertini F, Miani M, Robert MC, Roder M, Ronsmans S, Bugyi Z, Tomoskozi S, Flanagan SD (2020) Are current analytical methods suitable to verify VITAL(R) 2.0/3.0 allergen reference doses for EU allergens in foods? Food Chem Toxicol 145:111709
Wensing M, Penninks AH, Hefle SL, Koppelman SJ, Bruijnzeel-Koomen CA, Knulst AC (2002) The distribution of individual threshold doses eliciting allergic reactions in a population with peanut allergy. J Allergy Clin Immun 110(6):915–920
Allen KJ, Remington BC, Baumert JL, Crevel RW, Houben GF, Brooke-Taylor S, Kruizinga AG, Taylor SL (2014) Allergen reference doses for precautionary labeling (VITAL 20): clinical implications. J Allergy Clin Immun 133(1):156–164
Pomes A, Vinton R, Chapman MD (2003) Peanut allergen (Ara h 1) detection in foods containing chocolate. J Allergy Clin Immun 111(2):S194–S194
Vandekerckhove M, Droogenbroeck BV, Loose MD, Taverniers I, Daeseleire E, Gevaert P, Lapeere H, Poucke CV (2017) Development of an LC-MS/MS method for the detection of traces of peanut allergens in chili pepper. Anal Bioanal Chem 409(22):5201–5207
Marsh JT, Jayasena S, Gaskin F, Baumert JL, Johnson P (2020) Thermal processing of peanut impacts detection by current analytical techniques. Food Chem 313:126019
Hefle SL, Jeanniton E, Taylor SL (2001) Development of a sandwich enzyme-linked immunosorbent assay for the detection of egg residues in processed foods. J Food Protect 64(11):1812–1816
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Abbott M, Hayward S, Ross W, Godefroy SB, Delahaut P (2010) Validation procedures for quantitative food allergen elisa methods: community guidance and best practices. J AOAC Int 93(2):442–450
Magnusson B, Örnemark U (2014) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics. 2nd ed. ISBN 978-91-87461-59-0. http://www.eurachem.org
Sicherer SH, Sampson HA (2000) Peanut and tree nut allergy. Curr Opin Pediatr 12(6):567–573
Mennini M, Dahdah L, Mazzina O, Fiocchi A (2016) Lupin and other potentially cross-reactive allergens in peanut allergy. Curr Allergy Asthm R 16(12):84
Morishita N, Kamiya K, Matsumoto T (2008) Reliable enzyme-linked immunosorbent assay for the determination of soybean proteins in processed foods. J Agr Food Chem 56(16):6818–6824
Guidelines for standard method performance requirements. Appendix F, 16
Stephan O, Vieths S (2004) Development of a real-time PCR and a sandwich ELISA for detection of potentially allergenic trace amounts of peanut (Arachis hypogaea) in processed foods. J Agr Food Chem 52(12):3754–3760
Hefle SL, Bush RK, Yunginger JW, Chu FS (1994) A sandwich enzyme-linked immunosorbent assay (ELISA) for the quantitation of selected peanut proteins in foods. J Food Protect 57(5):419–423
Jayasena S, Smits M, Fiechter D, Jong AD, Nordlee J, Baumert J, Taylor SL, Pieters RH, Koppelman SJ (2015) Comparison of six commercial ELISA kits for their specificity and sensitivity in detecting different major peanut allergens. J Agr Food Chem 63(6):1849–1855
Khuda S, Slate A, Pereira M, Al-Taher F, Jackson L, Diaz-Amigo C, Bigley EC 3rd, Whitaker T, Williams KM (2012) Effect of processing on recovery and variability associated with immunochemical analytical methods for multiple allergens in a single matrix: sugar cookies. J Agr Food Chem 60(17):4195–4203
Pele M, Brohee M, Anklam E, Van Hengel AJ (2007) Peanut and hazelnut traces in cookies and chocolates: relationship between analytical results and declaration of food allergens on product labels. Food Addit Contam 24(12):1334–1344
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 32072338).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Compliance with ethics requirements
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ji, Y., Lin, H., Zhao, J. et al. Development of a sensitive sandwich enzyme-linked immunosorbent assay test kit for reliable detection of peanut residues in processed food. Eur Food Res Technol 248, 273–282 (2022). https://doi.org/10.1007/s00217-021-03879-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03879-8