Abstract
This study has been designed to examine incorporation of 7, 10 and 15% olive oil in milk-based media by using ultrasound (US) at 24 kHz, for 3 min in order to form new dairy-based, emulsifier-free emulsions. The prepared emulsions were characterized in terms of the emulsion capacity and stability, creaming index, zeta potential, droplet size, color and rheological analysis. The morphology of the emulsions was examined under the fluorescence microscope to evaluate the changes caused by US. The peroxide value and fatty acid composition were determined. After the treatment, application of the US increased the stability of emulsions. A decrease in the creaming index and droplet size and an increase in the zeta potential were observed. All the emulsions exhibited a weak gel structure. The fluorescence microscope images showed that the US decreased the size of oil droplet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, it has become essential to develop new food-processing technologies with increasing awareness of functional and healthy food preference of the public. Further, growing awareness about healthy lifestyles has prompted research on novel techniques for prolonging the shelf life of food products without the necessity of using preservatives [21]. Ultrasound, as a novel treatment, has the potential to be important for food processing, especially for the purpose of formulating the emulsions’ systems. Emulsions are heterogeneous mixtures consisting of two immiscible liquids, in which one liquid is dispersed within the other as droplets [16, 37]. Emulsion systems are thermodynamically unstable, so they tend to be destabilized by several mechanisms such as flocculation, aggregation, coalescence and phase inversion [17]. The emulsion systems are commonly used in food, agriculture, cosmetic and pharmaceutical industries. Additionally, the emulsions are utilized for the preparation of commercially available food products, such as sauces, condiments, spreads, dressings and desserts [33]. In literature, the emulsion-based delivery systems have been studied thoroughly. The role of emulsion-based delivery systems in food industry is used to protect bioactive compounds from environmental conditions, increase the stability and bioavailability of the prepared emulsions and mask unpleasant odor and taste. The stable emulsions are preferred which does not show phase separation throughout the shelf life and they should take their first version with little agitation [18, 37]. Since the emulsion systems are prone to instability and phase separation due to the interfacial tension, food industry has to overcome the challenge of instability [41, 55, 58]. For the purpose of preventing phase separation and instability of emulsion, emulsifiers and additives are used or various emulsification techniques are employed. Conventional emulsification techniques such as piston homogenizers and high shear mixer are used to deliver bioactive in liquid foods and in a variety of dairy systems [44, 52]. In recent years, low frequency high intensity ultrasound (US) has been started to be tested by researchers in order to increase the solubility of bioactive compounds which possesses poor solubility, reduce the particle size, get more amorphous structure, increase the surface area of components and deliver high-value nutrients [54]. US technology has many advantages as compared to conventional methods of emulsion formation and they can be listed as follows (a) the produced emulsions have sub-micron range, (b) more stable emulsions can be generated with US, (c) there is no need for surfactants to produce and stabilize the emulsion, (d) the energy required to produce an emulsion which is less than that needed in conventional methods [11, 12]. US have been focus of numerous studies in both laboratory and industrial scale in the production process of milk, yoghurt and ice-cream [38, 49, 64]. Chemat and Khan [11] pointed out that the droplet size of the dispersed phase of an emulsion formed with US is smaller than the emulsion created with conventional methods. Kaci et al. [26] evaluated the properties of surfactant free emulsions created by US which comprise of 5, 10 and 15% sunflower oil. Also, in a study done by Abismaıl et al. [1], it was observed that the smaller droplet size could be obtained through US treatment, less energy consumption could be achieved and the surfactant free emulsions can be generated as compared to mechanical agitation performed at 10,000 rpm with ultra-turrax. In the same study, more stable and less poly-disperse emulsions were obtained with US as compared to the mechanical methods. The literature data for the usage of US in the field of food mostly focused on simple food matrices such as an emulsion of sunflower oil in water. However there is a research gap regarding the usage of US for creating complex food emulsions [28, 30, 32]. The major aim of this study is to develop milk-based novel emulsions by the application of US and containing three different ratios of olive oil (7, 10, and 15%). To this end, the ξ-potential and droplet size characterization of prepared emulsions were employed. The stability and rheological tests were conducted. In addition, morphological characteristics of the samples were examined under a fluorescence microscope.
Materials and methods
The extra virgin olive oil (Kristal brand, Izmir, Turkey contains 18 g/100 g of saturated fat, 71 g/100 g of monounsaturated fat and 11 g/100 g of polyunsaturated fat) used in this study was purchased from a local supermarket and immediately stored at dark and in a cooled place until further use. Skimmed milk powder (contains 3.6% protein and 0.01% fat) and the emulsifiers (50% monoglycerides, 25% diglycerides and 25% triglycerides) were obtained from Pinar Dairy Co., Izmir and Dairy Processing Vocational School, Erciyes University, respectively. Xanthan gum used as a stabilizer was from Sigma-Aldrich Co.
Application of the US in model food emulsions
In this study, mellorine mixes was prepared as a good example of emulsion systems. Mellorine is similar to ice cream except that all or a proportion of milk fat is replaced by plant-based oils [27]. In previous researches, it was obvious that one of the most important factors that affected the stability of an emulsion was the amount of oil added into the emulsion system [43, 51, 57]. Therefore, the end product was contained 7, 10, 15% of olive oil and 14% sucrose, 11% skimmed milk powder, 0.2% stabilizer (xanthan gum) and 0.3% emulsifier (mono-diglycerides). The ingredients used to prepare the emulsions were added to the water in the following temperature and order and at the specified temperature olive oil at 30 °C, skimmed milk powder at 40 °C, dry mixture (emulsifier, sucrose, stabilizer) at 70 °C. The mixture was heated to 85 °C and held 30 s at this temperature. After the heating process, the samples were subjected to the mechanical stirring and US application. Mechanically stirred samples were considered as control and an experimental process was done via US (Hielscher Ultrasound Technology, UP400S, Germany). As for the conditions of the US applications a titanium probe, 22 cm long (H22D, Hielscher, Germany) was used at 24 kHz for 3 min. The maximum energy density and amplitude of the probe was 85 W/cm2 and 120 µm, respectively. The probe was immersed up to the 3 cm of the emulsions container. The experimental design of the study was explained in Table 1. All of the samples were prepared in duplicate.
Emulsification activity index and emulsion stability index
The emulsion activity and stability of prepared emulsions were determined with the method proposed by Manoi and Rizvi [36] with some modifications in order to test the flocculation and coalescence. For this purpose, 10 µl prepared emulsions were diluted in 5 ml of 0.1 M buffer containing 0.1% SDS (sodium dodecyl sulfate). Then, the absorbance of diluted emulsions was determined at 500 nm in a 1 cm path length cuvette in a spectrophotometer (Agilent 8453 E UV–Visible Spectroscopy System, USA). The turbidity (T) of emulsion samples and the emulsion activity index (EAI) are calculated by the following formula:
where A is the absorbance at 500 nm, l is the path length of cuvette, T is turbidity, D is dilution factor, \( {\oint } \) is oil fraction, 10,000 is the correction factor for m2 and the C is the weight of protein per unit volume of aqueous phase before the emulsion was formed (g/ml). EAI of emulsions was recorded after a storage period of 0, 1, 24 h.
After the emulsions held at 4 °C for 24 h, the turbidity values were measured and ESI (emulsion stability index) of emulsions were calculated by the following formula:
where the where T is the initial turbidity, Δt is the time interval and ΔT is the change in turbidity during the storage periods [36].
Creaming index
In order to analyze the tendency of emulsions to the creaming, the creaming index analysis was performed. For this reason, 10 ml of emulsion sample was filled into a test tube (1.5 cm inner diameter × 12 cm height) and monitored 14 days at 4 °C. The height of the serum (H s) and the total height of emulsions (H t) were recorded for 1, 7, and 14 days [20]. The creaming index value was calculated by following formula:
Droplet size and zeta potential
The emulsions were diluted 1/100 (v/v) with distilled water for the measurements of ζ-potential and droplet size measurements using a Malvern zetasizer (Zetasizer ZS90, Malvern Instruments, England). The diluted emulsions were placed in the disposable capillary cell of the zetasizer at 25 °C. The data were evaluated by Zetasizer Software program (Malvern, England). Each measurement was replicated three times.
Color value
The color parameters of prepared emulsions were measured by a colorimeter (Lovibond RT Series Reflactance Tintometer, England). After the white and black calibration of the colorimeter, the L *, a * and b * values were recorded at room temperature.
Rheological characterization
Steady shear properties of emulsions
The rheological measurements of emulsions were performed by a rheometer (Thermo-HAAKE, Mars III, Karlsruhe, Germany) equipped with a Peltier temperature-controlled system. The temperature was kept constant at 20 °C during analyses. The measurements were made with a parallel-plate configuration (P35TiL, radius = 35 mm). Special care was taken to avoid bubbles in the emulsions during loading. Measurements were carried out at a shear rate range of 1–100 /s. During the shearing, a total of 25 data points were recorded at 0.1–100 /s intervals. The apparent viscosities of emulsions were determined as a function of the shear rate. Data obtained were analyzed using the RheoWin data manager (RheoWin Pro V 4.0, HAAKE, Karlsruhe, Germany). The flow behavior index (n) and consistency coefficient (K) values were calculated according to the Ostwald de Waele (power law) model that was fitted to the measured data. The power law model is the most widely used model for describing the flow behaviors of non-Newtonian fluids [4]. The following model was used to describe flow behavior of the emulsion samples:
where σ is shear stress (Pa), K is consistency coefficient (Pa sn), γ̇ is shear rate (/s) and n is flow behavior index which is dimensionless.
Small-amplitude oscillatory shear data
In order to determine the linear viscoelastic region, the stress sweep test was performed at 1 Hz in the range of 0.1–10 Pa. The frequency sweep test was run in the range of 0.1–10 Hz in the linear viscoelastic region (0.2 Pa) at 25 °C. The storage modulus (G′) and loss modulus (G″) values were calculated using RheoWin Data Manager (RheoWin Pro V 4.0, HAAKE, Karlsruhe, Germany).
Peroxide value
The peroxide values of samples were determined whether US application causes any changes in oxidation levels of the emulsion. In order to extract oil from the emulsion system, isooctane/isopropanol (3:2 v/v) solution was used and AOAC [3] method was employed for the peroxide values of extracted oil.
Determination of fatty acid composition
100 mg of each of the extracted oil samples was mixed 100 ml 2 N KOH, and 3 ml hexane for saponification. Then, the mixture was strongly shaken with a vortex for 1 min and then centrifuged at 5000 rpm for 5 min (Nuve NM 110 model, Turkey). 1 ml of the upper phase was put into GC vials and injection to the GC (Agilent 6890) was started immediately [2].
The operating conditions of the GC were as follows column 100 m × 0.25 mm id HP-88; detector flame ionization detector (FID); The carrier gas Helium, flow rate of 2 ml/min; injection block temperature 230 °C; injection volume 1 μl; split rate 1/50.
The fatty acids were identified according to retention time of the standard of fatty acids and the results were expressed as g fatty acid/100 g total fatty acid.
Fluorescence microscopy images
The fluorescence microscopy images of prepared emulsions were taken using a fluorescence microscopy (Nicon-Eclipse Ti-S, Japan). In order to make the fat phase visible under fluorescence light, the emulsion was irritated with Nile Blue A (a fluorescence dye; Alfa Aesar GmbH & Co. KG, Karlsruhe, Germany). The fluorescence images were taken at 20× magnification.
Statistical analysis
The statistical analysis of the obtained data was performed using SigmaPlot 11.0 software package. ANOVA was used to compare the means of results and the Tukey’s multiple comparison test was used to separate means.
Results and discussions
Emulsification activity index and emulsion stability index
Figure 1 shows the emulsification activity index (EAI) of the emulsion samples, which were monitored at 0 h (start time, freshly prepared sample), at 1 h and at 24 h. According to the results of EAI analysis, the highest EAI value (107 m2/g) was observed in sample ZUSE7, which comprised 7% oil. In a previous study, the EAI of the samples was found to be 230 m2/g when the micro fluidization method was used. 1% of protein concentration and φ = 0.2–0.6 of oil [34]. In another study performed by Manoi and Rizvi [36], with high speed homogenization, and 3% protein, EAI values were 22 m2/g. The differences between the results may stem from the protein concentration or the method used for the preparation of the emulsions.
In general, the EAI values of the US-treated samples were higher than those of the samples which were only prepared by mechanical stirring. Further, there were significant differences between the EAI values of US-treated emulsion samples and mechanically stirred emulsion samples (p < 0.05).
On the other hand EAI value was related to turbidity measurements. Turbidity of an emulsion is a function of concentration and particle size of the particles which forms the emulsion. The stability of an emulsion may determine the changes in the turbidity occurring over time [50]. In this monitoring work, the method of Pearce and Kinsella [48], which was based on turbidity changes, was used in order to determine the stability and activity of the prepared emulsions. Also, the EAI value was related to the surface area of a unit of protein that stabilizes the emulsion and therefore it referred to the ability of water absorption and interfacial area of oil and protein [48]. The result of EAI analysis showed that the most drastic changes were observed in mechanically prepared samples. Dalgleish [14] claimed that the emulsion instability occurred due to the insufficient surfactant present in an emulsion system to cover the entire water–oil interface or the presence of interfacial cavities that causes reduction in the total absorbed layer. In another study, it was found that the soluble aggregates generated by ultrasound treatment are likely to be adsorbed at the oil–water interface and this increase leads to a better potential adsorption of proteins at the oil–water interface [23]. Therefore, it can be speculated that US application can contribute to the stabilization of emulsions by closing the interfacial cavities at the water and oil interface.
In order to test the stability of prepared emulsions, the emulsion stability analyses were conducted and results are shown in Fig. 2. The highest ESI value was observed in the ZUSE7 sample, and effect of the US treatment and oil concentration on the ESI values was statistically significant (p < 0.05). The most stable emulsions were ZUSE7, ZUS7 and ZUSE15, which were prepared with US application. ESI values of an emulsion are the measure of resistance against the coalescence [47]; thus it can be concluded that the emulsions, which are generated only by mechanical stirring are more susceptible to coalescence. Emulsions with longer shelf life can be prepared with US treatment.
Creaming index
Creaming index is another parameter for the characterization of emulsions. In order to evaluate the creaming index values of prepared emulsions, the samples were monitored throughout 14 days and the volume of total emulsion and the separated serum layer was recorded in 1st, 7th and 14th days and the creaming index value was calculated. The creaming index values of emulsion samples are illustrated at Fig. 3. In general, while serum separation was observed in mechanically prepared emulsions on the first day of the preparation, there was no serum separation in the US-treated samples. At the same time, the increase in the creaming index value of US-treated samples was lower than that of the mechanically stirred emulsion samples. Creaming index values are also used to assess of stability against coalescence. The coalescence had a reduced the shelf life of oil in water (o/w) emulsion by promoting oil separation. The creaming index causes membrane ruptures due to the gravity, colloidal and mechanical forces and for this reason, it expresses the sensitivity to the coalescence [42, 48].
Khan et al. [29] prepared emulsions without stabilizer by using ultra high pressure homogenization and stated that the 50% of the prepared emulsion creamed within 2 h. In the same work, the researchers observed that the increase in the homogenization pressure reduced the creaming rate. In our study, when compared to the mechanically stirred samples, the creaming index values of the US-treated emulsion samples were lower. For this reason, US treatment may be preferred by the food industry for products with long mechanical shelf life and good stability.
Zeta (ζ) potential and droplet size of emulsions
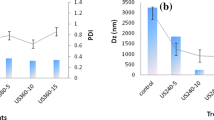
When the absolute ζ-potential values of samples examined in detail, it was seen that the ζ-potential of US-treated emulsion samples had higher value than the mechanically prepared emulsions. Figure 4 illustrates the zeta (ζ) potential of the prepared emulsion samples. As seen, the maximum ζ value was observed in ZUSE7 sample (−51.10 ± 0.85 mV) and an increase in the oil fraction may have led to decrease in the ζ-potential values of emulsions. Zeta potential value is an important parameter to assess the stability of emulsions. The ζ-potential may be related to the stability of emulsions and these values can be defined as the potential difference between the dispersion medium and the stationary layer of fluid attached to the dispersed droplet. Although the emulsions with low zeta potential (negative or positive) are prone to flocculate or coagulate, the emulsions with high zeta potential are electrically stabilized [63]. Julio et al. [25] claimed that there is a strong correlation between the ζ-potential and droplet sizes. When the zeta potential and creaming index values of the samples were considered together, it was observed that as the creaming index values increased, the zeta potential values decreased. It may be concluded that the droplet of oil can be dispersed into emulsion system and other components such as protein, lactose and sucrose may be well distributed with US application. Thus, the phase separation in the emulsion system might be prevented. From our results it may speculated that the high zeta potential value is associated with low value of creaming index. When the obtained results were examined in detail, it was observed the US treatment decreased droplet size and polydispersity of the emulsions significantly. The droplet size of prepared emulsions was presented in Fig. 5 and also Fig. 6 shows the average distributions of the droplet of samples. US treatment formed smaller droplets. It was attempted to reduce the droplet size of an emulsion using US, high pressure homogenization or by adding more emulsifier to the emulsion system in order to have more stable emulsions [53]. It was found that the emulsion which had smaller droplet sizes was more stable and the creaming index values were low. According to the results of this study, it can be concluded that there is an association between the creaming index and droplet size and the smaller droplets may be created by US treatment for resistance to the coalescence.
Color
The color measurements of the prepared emulsion samples were performed in order to investigate the influence of US application and oil fraction and the results are shown in Table 2. According to the CIE (Commission Internationale de l‟Eclairage), L * is a measure of the lightness–darkness, a * redness–greenness and b * yellowness–blueness, respectively [31]. US application caused to whiter color (Table 2). The added oil fraction showed significant differences between the samples (p < 0.05). Also, the US application affected the L * , a * and b * values of emulsion samples significantly (p < 0.05). When compared with the mechanically stirred samples, US treatment affected the L * values of samples positively. In a study conducted by Paradiso et al. [46], it was highlighted that there were significant changes in L * values of emulsion filled gels containing different ratios of oil. The color properties of a complex food system are influenced not only by one factor but also several variables. In an emulsion system, the color values are affected by both the droplet size and their concentrations. The decrease in droplet concentration and size causes lower chromaticness (a * and b * indices) and higher lightness [46]. McClements [40] also claimed that reduction in the droplet size causes augmentation in L * values. Smaller oil droplets may increase the surface-to-volume ratio of the oily phase and the absorbance of the light. Further, because of the localized temperature peaks potential browning may contribute to color values and may result in lower brightness and higher chromaticness [46]. In our study, an increase was observed in L * values of the US-treated samples. The reason for this result may be due to the reduction in droplet size of the oil which found in the structure. The oil fraction and applied process affected color parameters statistically significant (p < 0.05). US application lead to increase in b * values of emulsions. In literature, similar studies indicate US application increases b * values [46]. Especially, the color properties of some dairy-based foods such as ice cream, mellorine, infant formula and salad dressing are important for consumer preferences. US application may cause whiter color in products and so it can be considered in dairy products’ processing (Table 3).
Fatty acid composition and peroxide values
The fatty acid composition of emulsions was slightly changed by US application (Table 4). Samples before US application were pure olive oil which was used for emulsion preparation. After US application, oils were the extracted from US-treated emulsion samples. In the results, oleic acid was found as the dominant fatty acid in extracted oil from emulsions and the other fatty acids were in the following order: linoleic, palmitic, stearic and α-linolenic fatty acids. The data obtained after analyzing the fatty acid composition showed that there were no remarkable changes after the US application. There are contradictory results in literature. Kaci et al. [26] prepared sunflower oil emulsion by the piezoelectric high frequency ultrasound and they claimed that there were no differences in samples with the ultrasound application. However, in another work conducted by Chemat et al. [9, 10], it is stated that after the US treatment, linoleic acid amount in sunflower oil increased. It can be speculated that the contradictory results may be a result of different processing conditions of US such as frequency and time of application.
In order to investigate the whether the US application changed the oxidation levels in the oil which was used to prepare emulsions, peroxide values of untreated olive oil, and oils extracted from the US-treated emulsion samples were determined. The results of peroxide values in units of meq O2/kg oil are presented at Table 3. The results obtained from the peroxide value analyses indicated that there was a slight but statistically significant difference between the US-treated emulsion samples and the mechanically stirred emulsion samples (p < 0.05). The slight differences may be due to the heating process and extraction process. Peroxide value is one of the commonly used parameters for the determination of oxidation and rancidity in fats and oils [65]. Peroxides formed at the beginning of the oxidation reaction, degrade into other oxidation products at the later stages of oxidation. According to the Turkish food codex, the peroxide value of refined and cold pressed olive oil should not exceed 10 and 15 meq O2/kg oil, respectively [13]. In this study, it was observed that the peroxide values of samples were within the allowed limits of Turkish food codex. In different works, the effect of US on peroxide value of samples was investigated. Chemat et al. [9, 10] reported that the peroxide value of sunflower oil was 5.38 meq O2/kg before the US treatment and 5.69 meq O2/kg after the US application. Researchers stated that there were no major changes induced by the US application in the peroxide value of oil. In another study, it was claimed that there were slight but statistically significant changes in the peroxide value of samples with the US treatment [46]. Likewise, Chemat et al. [9, 10] reported that the US might cause oxidation in oils.
Rheological characterization of emulsions
Steady shear rheological properties of emulsion samples
The rheological data are utilized for product development, process calculations and quality control of a product. US processing used in this study was considered as an innovative technique to replace the use of an emulsifier agent. Therefore, the determination of the rheological characterization may be important for new dairy-based emulsion systems.
Figure 7 shows the apparent viscosity versus shear rate plots of the emulsions containing 7, 10, 15% of virgin olive oil, respectively. As it is seen in these figures, the apparent viscosity values of the emulsions decreased with the increase in shear rate which indicates the shear thinning behavior (Fig. 7). The US treatment caused a reduction in apparent viscosity of the emulsions. The increased oil ratio in samples had a statistically significant effect on the apparent viscosity of all samples (p < 0.05). The flow behavior index values of samples were lower than 1 indicating that the flow behavior of samples was non-Newtonian pseudo plastic behavior. In literature, various studies indicate that emulsions show shear thinning behavior [6, 15, 36]. After evaluation of the physical behavior of emulsion samples and R 2 of the Ostwald de Waele, Herschel-Bulkley, Casson and Bingham model, it was decided that the samples were in accordance with the Oswald–de Waele (R 2 > 0.998). The consistency coefficient (K) and the flow behavior index of samples were determined with the Oswald–de Waele model and the results are shown in Table 5. While US application created better results for stability and mechanical properties of prepared oil in water emulsion, it negatively affected the rheological properties of them. The findings of this study showed that the K values of US-treated samples were lower than those of the mechanically stirred samples. It was observed from the literature on similar studies that US application had a reduced effect on the viscosity of foods. Vercet et al. [60] indicated that the sonication reduced the consistency coefficient of tomato juices and that the apparent viscosity of US-treated tomato juices was lower than that of the non-treated tomato juices. In another research investigating the effect of US on the viscosity of pure pectin solution, it was pointed out that the US caused a decrease in the particle size of molecules and thus the apparent viscosity of solution was reduced [56]. Tiwari et al. [59] reported that US application had a substantial reduction on the viscosity of pectin solution of 2%. In a food system which consists of several food components, the consistency index, K value, depends on the particle size of food components. During US application, the particle size of the emulsion components was decreased by the effect of cavitations. Therefore, significant reductions were observed in the consistency coefficient and the apparent viscosity values of US-treated emulsions. Another reason for the reduction occurred in US-applied emulsions may be due to the effective dispersion of protein and oil particles in a total emulsion system. Since there are other studies indicating that US has a stimulating effect of protein denaturation due to the production of strong eddies within the liquid that is subjected to the ultrasound [8, 24, 61], it can be also speculated that the protein denaturation caused the reduction in the apparent viscosity and consistency coefficient values. In this study, it was also observed that the increase in the oil fraction in the emulsion system caused increase in the consistency coefficient of samples. Manoi and Rizvi [36] investigated the rheology data of emulsion prepared with different oil fractions ranging from 0.2 to 0.8%. They observed that with the increasing ratio of oil the viscosity also increased. In literature, it is emphasized that with the increasing rate of oil, the oil droplets come together and interact with each other and thus a network consisting of oil droplets is formed [34, 39]. The data obtained in this study regarding steady shear rheological were in agreement with the literature.
Dynamic oscillatory shear test
Figure 8 showed the change of elastic or storage modulus (G′) and viscous or loss modulus (G″) at the applied frequency range. At a frequency of 1 Hz, G′ values of ZK7, ZUSE7 and ZUS7 samples was 1.99 ± 0.38, 4.52 ± 0.12, 1.51 ± 0.18 Pa, respectively. Similar trend of G′ and G″ was reported often in previous studies regarding food emulsions [19, 35, 62]. Throughout this study, the magnitude of the storage modulus was higher than the loss modulus (G′ > G″) and no crossover was observed. This behavior demonstrated that elastic behavior dominated over viscous component. The larger value of G′ indicates strong particle–particle interactions and a network like structure in a stabilized form [7, 22].
According to the result of this study, it was interesting to find that the US-treated and mechanically stirred emulsion samples showed a similar G′ and G″ trends at applied frequencies. The G″ values of ZK7, ZUSE7 and ZUS7 samples at a frequency of 1 Hz were 2.19 ± 0.39, 4.96 ± 0.20, and 1.24 ± 0.14 Pa, respectively. As a result of frequency sweep measurements, the three types of systems which are concentrated solutions dilute solutions and gels could be identified. For gel systems, G′ is always greater than G″ in frequency range. In dilute solutions, G″ modulus is greater than G′ that is close to each other at higher frequencies. For concentrated systems, G′ value is smaller than G″ at low frequency and crossover can be seen in middle of the frequency ranges [45]. In this experiment, over the entire frequency range, the loss modulus values were lower than storage modulus values for all samples. Not only the G′ values but also the G″ values showed small increase with frequency (Fig. 8). Hence, it will not be wrong to say that weaker structures in emulsion were created by US application; namely, the rheological deformation was elastic and the emulsion samples can be considered as weak gels.
Fluorescence microscopy images
The fluorescence microscopy images of emulsions taken under 20× magnifications are shown in Fig. 9. Different fluorescence microscopy images of the samples were taken. Figure 9 was preferred since it showed the best distributions of particles of the oil droplets in emulsions. Nile blue was used to dye the oil phase of emulsion to take the fluorescence images of samples under fluorescence microscopy. From these images, it was clear that the oil particles treated with US were smaller than the particles of oil in emulsion samples treated by mechanical stirring (Fig. 9c). This might be caused by the US application which created much smaller and dispersed oil fraction in emulsion system. Figure 9a shows control sample which was not treated with US. It can be seen from this figure that the size of oil droplets was bigger than the emulsions obtained from US application. Figure 9b shows the images of the emulsion samples made by using an emulsifier agent and also applied with the US process. According to the results of this study, US application process formed fine particles of oil droplets in emulsion systems. The findings of this study are consistent with the other studies in the literature. Behrend et al. [5] also used US for the purpose of emulsification and they claimed that the US formed smaller particles as compared with other emulsification techniques.
Conclusions
The conclusion of this study is that US treatment could enhance the stability and efficiency of dairy-based emulsion systems. According to the result of this work, US can be considered as a safe method to prepare stable emulsions, since it does not form peroxides nor change fatty acid composition. The results show that in US treatment, the food additives like emulsifiers could be eliminated from the preparation of food emulsions. The findings of this study could be beneficial for both economic and commercial aspects. This knowledge can then be used for the ice-cream mixes in order to extend shelf life of the products. US application can be used in different food systems for the prevention of phase separation. As such, future work may focus on the freezing rate, melting rate and sensory characterization of the product produced with ultrasonic application. Ultimately, we hope to understand the interfacial rheology of the US-treated emulsions and as to how US affects the heat-mass transfers in food processing.
References
Abismaıl B, Canselier JP, Wilhelm AM, Delmas H, Gourdon C (1999) Emulsification by ultrasound: drop size distribution and stability. Ultrason Sonochem 6(1):75–83
Anonymous (1987) International union of pure and applied chemistry. Pergamon Press, Oxford
AOAC (2000) Official methods of analysis. Association of official analytical chemists (vol 2)
Barnes HA, Hutton JF, Walters K (1989) An introduction to rheology, 3rd edn. Elsevier, Amsterdam
Behrend O, Ax K, Schubert H (2000) Influence of continuous phase viscosity on emulsification by ultrasound. Ultrason Sonochem 7(2):77–85
Boutin C, Giroux HJ, Paquin P, Britten M (2007) Characterization and acid-induced gelation of butter oil emulsions produced from heated whey protein dispersions. Int Dairy J 17(6):696–703
Carreau PJ, Cotton F (2002) Rheological properties of concentrated suspensions. In: Kee D, Chhabra RP (eds) Transport processes in bubbles, drops and particles. Taylor & Fransis, Boca Raton
Chandrapala J, Zisu B, Palmer M, Kentish S, Ashokkumar M (2011) Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason Sonochem 18(5):951–957
Chemat F, Grondin I, Costes P, Moutoussamy L, Sing ASC, Smadja J (2004) High power ultrasound effects on lipid oxidation of refined sunflower oil. Ultrason Sonochem 11(5):281–285
Chemat F, Grondin I, Sing ASC, Smadja J (2004) Deterioration of edible oils during food processing by ultrasound. Ultrason Sonochem 11(1):13–15
Chemat F, Khan MK (2011) Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason Sonochem 18(4):813–835
Chendke P, Fogler HS (1975) Macrosonics in industry: 4. Chemical processing. Ultrasonics 13(1):31–37
Codex TF (2001) Bitki Adıyla Anılan Yemeklik Yağlar Tebliği
Dalgleish DG (1997) Adsorption of protein and the stability of emulsions. Trends Food Sci Technol 8(1):1–6
Demetriades K, McClements DJ (1998) Influence of pH and heating on physicochemical properties of whey protein-stabilized emulsions containing a nonionic surfactant. J Agric Food Chem 46(10):3936–3942
Dickinson E, Stainsby G (1982) Colloids in food. Applied Science Publishers, London
Dickinson E (1997) Properties of emulsions stabilized with milk proteins: overview of some recent developments. J Dairy Sci 80(10):2607–2619
Eccleston GM (2007) Emulsions and microemulsions. Encycl Pharm Technol 3:1548–1565
Erçelebi EA, Ibanoğlu E (2009) Rheological properties of whey protein isolate stabilized emulsions with pectin and guar gum. Eur Food Res Technol 229(2):281–286
Firebaugh J, Daubert C (2005) Emulsifying and foaming properties of a derivatized whey protein ingredient. Int J Food Prop 8(2):243–253
Galus S, Kadzińska J (2015) Food applications of emulsion-based edible films and coatings. Trends Food Sci Technol 45(2):273–283. doi:10.1016/j.tifs.2015.07.011
Hirashima M, Takahashi R, Nishinari K (2004) Effects of citric acid on the viscoelasticity of cornstarch pastes. J Agric Food Chem 52(10):2929–2933
Jambrak AR, Lelas V, Mason TJ, Krešić G, Badanjak M (2009) Physical properties of ultrasound treated soy proteins. J Food Eng 93(4):386–393
Jambrak AR, Mason TJ, Lelas V, Herceg Z, Herceg IL (2008) Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J Food Eng 86(2):281–287
Julio LM, Ixtaina VY, Fernández MA, Sánchez RMT, Wagner JR, Nolasco SM, Tomás MC (2015) Chia seed oil-in-water emulsions as potential delivery systems of ω-3 fatty acids. J Food Eng 162:48–55
Kaci M, Meziani S, Arab-Tehrany E, Gillet G, Desjardins-Lavisse I, Desobry S (2014) Emulsification by high frequency ultrasound using piezoelectric transducer: formation and stability of emulsifier free emulsion. Ultrason Sonochem 21(3):1010–1017
Keeney PG (2012) Ice cream and frozen desserts. In: Bellusi G, Bohnet M, Bus J, Drauz K, Greim H, Jäckel KP, Karst U, Kleeman A, Kreysa G, Laird T et al (eds) Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH, Weinheim, pp 543–550
Kentish S, Wooster T, Ashokkumar M, Balachandran S, Mawson R, Simons L (2008) The use of ultrasonics for nanoemulsion preparation. Innov Food Sci Emerg Technol 9(2):170–175
Khan NM, Mu TH, Zhang M, Chen JW (2013) Effects of high hydrostatic pressure on the physicochemical and emulsifying properties of sweet potato protein. Int J Food Sci Technol 48(6):1260–1268
Krasulya O, Bogush V, Trishina V, Potoroko I, Khmelev S, Sivashanmugam P, Anandan S (2016) Impact of acoustic cavitation on food emulsions. Ultrason Sonochem 30:98–102. doi:10.1016/j.ultsonch.2015.11.013
Lee S-M, Lee K-T, Lee S-H, Song J-K (2013) Origin of human colour preference for food. J Food Eng 119(3):508–515
Leong T, Wooster T, Kentish S, Ashokkumar M (2009) Minimising oil droplet size using ultrasonic emulsification. Ultrason Sonochem 16(6):721–727
Lett AM, Norton JE, Yeomans MR (2016) Emulsion oil droplet size significantly affects satiety: a pre-ingestive approach. Appetite 96:18–24. doi:10.1016/j.appet.2015.08.043
Liu F, Tang C-H (2011) Cold, gel-like whey protein emulsions by microfluidisation emulsification: rheological properties and microstructures. Food Chem 127(4):1641–1647
Lorenzo G, Zaritzky N, Califano A (2008) Modeling rheological properties of low-in-fat o/w emulsions stabilized with xanthan/guar mixtures. Food Res Int 41(5):487–494
Manoi K, Rizvi SSH (2009) Emulsification mechanisms and characterizations of cold, gel-like emulsions produced from texturized whey protein concentrate. Food Hydrocoll 23(7):1837–1847. doi:10.1016/j.foodhyd.2009.02.011
Martin A, Busmante P, Chun A (1993) Coarse dispersions. In: Febiger LA (ed) Physical pharmacy, Fourth edn. Lea & Febiger, Philadelphia, pp 477–511
Mason TJ, Paniwnyk L, Lorimer JP (1996) The uses of ultrasound in food technology. Ultrason Sonochem 3(3):S253–S260. doi:10.1016/S1350-4177(96)00034-X
McClements DJ (1999) Emulsion stability. Food emulsions: principles, practices, and techniques. CRC Press, Boca Raton, London, New York, Washington, DC, pp 185–233
McClements DJ (2002) Colloidal basis of emulsion color. Curr Opin Colloid Interface Sci 7(5):451–455
McClements DJ (2004) Protein-stabilized emulsions. Curr Opin Colloid Interface Sci 9(5):305–313
McClements DJ (2015) Food emulsions: principles, practices, and techniques. CRC Press, Boca Raton
Mirhosseini H, Tan CP, Hamid NSA, Yusof S (2008) Effect of Arabic gum, xanthan gum and orange oil contents on ζ-potential, conductivity, stability, size index and pH of orange beverage emulsion. Colloids Surf A 315(1–3):47–56. doi:10.1016/j.colsurfa.2007.07.007
Moore R, Duncan S, Rasor A, Eigel W, O’Keefe S (2012) Oxidative stability of an extended shelf-life dairy-based beverage system designed to contribute to heart health. J Dairy Sci 95(11):6242–6251
Naji-Tabasi S, Razavi SMA (2017) New studies on basil (Ocimum bacilicum L.) seed gum: part III—steady and dynamic shear rheology. Food Hydrocoll 67:243–250. doi:10.1016/j.foodhyd.2015.12.020
Paradiso VM, Giarnetti M, Summo C, Pasqualone A, Minervini F, Caponio F (2015) Production and characterization of emulsion filled gels based on inulin and extra virgin olive oil. Food Hydrocoll 45:30–40
Patel MT, Kilara A (1990) Studies on whey protein concentrates. 2. Foaming and emulsifying properties and their relationships with physicochemical properties. J Dairy Sci 73(10):2731–2740
Pearce KN, Kinsella JE (1978) Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem 26(3):716–723
Pingret D, Fabiano-Tixier A-S, Chemat F (2013) Degradation during application of ultrasound in food processing: a review. Food Control 31(2):593–606
Reddy S, Fogler HS (1981) Emulsion stability: determination from turbidity. J Colloid Interface Sci 79(1):101–104
Rousseau D (2000) Fat crystals and emulsion stability—a review. Food Res Int 33(1):3–14. doi:10.1016/S0963-9969(00)00017-X
Saga LC, Kristinova V, Kirkhus B, Jacobsen C, Skaret J, Liland KH, Rukke E-O (2013) Characterization of oxidative stability of fish oil-and plant oil-enriched skimmed milk. J Am Oil Chem Soc 90(1):113–122
Schubert H, Engel R (2004) Product and formulation engineering of emulsions. Chem Eng Res Des 82(9):1137–1143
Shanmugam A, Ashokkumar M (2014) Ultrasonic preparation of stable flax seed oil emulsions in dairy systems—physicochemical characterization. Food Hydrocoll 39:151–162. doi:10.1016/j.foodhyd.2014.01.006
Silva EK, Gomes MTM, Hubinger MD, Cunha RL, Meireles MAA (2015) Ultrasound-assisted formation of annatto seed oil emulsions stabilized by biopolymers. Food Hydrocoll 47:1–13
Sun D-W (2014) Emerging technologies for food processing. Elsevier, Philadelphia
Sun C, Gunasekaran S (2009) Effects of protein concentration and oil-phase volume fraction on the stability and rheology of menhaden oil-in-water emulsions stabilized by whey protein isolate with xanthan gum. Food Hydrocoll 23(1):165–174. doi:10.1016/j.foodhyd.2007.12.006
Tadros TF (2009) Emulsion science and technology: a general introduction. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Tiwari B, Muthukumarappan K, O’Donnell C, Cullen P (2010) Rheological properties of sonicated guar, xanthan and pectin dispersions. Int J Food Prop 13(2):223–233
Vercet A, Sánchez C, Burgos J, Montañés L, Buesa PL (2002) The effects of manothermosonication on tomato pectic enzymes and tomato paste rheological properties. J Food Eng 53(3):273–278
Villamiel M, de Jong P (2000) Influence of high-intensity ultrasound and heat treatment in continuous flow on fat, proteins, and native enzymes of milk. J Agric Food Chem 48(2):472–478
Wang M, Feng M-Q, Jia K, Sun J, Xu X-L, Zhou G-H (2017) Effects of flaxseed gum concentrations and pH values on the stability of oil-in-water emulsions. Food Hydrocoll 67:54–62. doi:10.1016/j.foodhyd.2017.01.004
Wang B, Li D, Wang LJ, Özkan N (2010) Effect of concentrated flaxseed protein on the stability and rheological properties of soybean oil-in-water emulsions. J Food Eng 96(4):555–561
Wu H, Hulbert GJ, Mount JR (2000) Effects of ultrasound on milk homogenization and fermentation with yogurt starter. Innov Food Sci Emerg Technol 1(3):211–218
Zhang Y, Yang L, Zu Y, Chen X, Wang F, Liu F (2010) Oxidative stability of sunflower oil supplemented with carnosic acid compared with synthetic antioxidants during accelerated storage. Food Chem 118(3):656–662
Acknowledgements
The authors would like to thank the TUBITAK (programme code 2210-C) for scholarship of the MSc and to Erciyes University Scientific Research Project Unit (BAP). It was supported by project number of FYL-2015-586.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
This work does not contain any study with animal or human subjects.
Rights and permissions
About this article
Cite this article
Aslan, D., Dogan, M. The influence of ultrasound on the stability of dairy-based, emulsifier-free emulsions: rheological and morphological aspect. Eur Food Res Technol 244, 409–421 (2018). https://doi.org/10.1007/s00217-017-2966-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-2966-3