Abstract
Fragaria pentaphylla, one of several wild strawberry species, produces white or red fruits. The white fruits have a stronger aroma than the red. In this study, solid-phase microextraction was used in combination with gas chromatography–mass spectrometry to compare volatiles during fruit development and maturation from the two fruit types of F. pentaphylla and the cultivated F. × ananassa. A total of 38 volatile compounds were identified in F. × ananassa, while 61 and 53 volatile compounds were identified in the white and red fruits of F. pentaphylla, respectively. The predominant volatiles in white ripe fruits of F. pentaphylla were 3(2H)-furanone 4-methoxy-2,5 methyl (24.71%), butanoic acid, 2-methyl, methyl ester (10.43%), trans-2-hexenal (9.23%). The main volatiles in red ripe fruits of F. pentaphylla were 2-hexenal (21.23%), 1-hexanol (13.29%) and 2-hexen-1-ol acetate (13.00%). While the main volatiles in ripe fruits of F. × ananassa were butanoic acid, ethyl ester (25.80%), 2-hexenal (23.47%) and butanoic acid, 2-methyl (10.09%). In addition, cyclopropane propyl was first found in the white fruits of wild F. pentaphylla at high levels (4.83%). As the intense aroma of the white fruits of F. pentaphylla is characteristic of high 3(2H)-furanone 4-methoxy-2,5 methyl production. RNA-seq was used for quantitative analysis of volatiles-related gene expression. Integrative analysis of GC–MS data and RNA-seq data from fruits of F. pentaphylla indicated that reduction of sugar in red fruits of F. pentaphylla might lead to a relatively lower DMF and higher aldehydes and alcohols compared with that in white fruits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Strawberry (Fragaria × ananassa) is a popular fruit crop worldwide [1]. In 2012, global strawberry production reached 4.5 million tons, two times more than the sum of all other berries (http://faostat.fao.org/). Cultivated strawberries, however, often lack flavour and fragrance [2], and production of berries with diverse aroma patterns requires breeding of strawberry cultivars. Compared to cultivated strawberries, wild strawberries (Fragaria species) produce smaller fruits and lower yields, but more diverse aroma patterns, which are directly related to the production of volatile compounds. Comparisons of the volatile compounds of wild Fragaria species and cultivated strawberries may provide valuable information for future breeding efforts to produce more appealing cultivars.
Different patterns of volatiles in cultivated strawberries and other wild strawberries have been reported [3, 4]. More than 360 compounds have been found in cultivated strawberry (F. × ananassa), predominantly esters, aldehydes, furanones, sulfuric and terpenic compounds [5,6,7,8,9]. Among them, the most abundant aroma compounds are methylbutanoate, ethyl butanoate, ethyl hexanoate and methyl 2-methylbutanoate [10, 11]. However, the major flavour compounds in wild F. vesca are methyl anthranilate, butyl formate, octyl acetate, decyl acetate, benzyl acetate, carveyl acetate, decyl butanoate, methyl nicotinate and methyl N-formylanthranilate [12]. Furthermore, the monoterpene linalool is more abundant in cultivated strawberries, while terpenoids and ketones are present at higher levels in wild berries [3, 4]. However, with the exception of F. vesca [2], F. moschata L. [2], and F. virginiana Mill. [13], little focus has been placed on the volatile chemical patterns of the other wild Fragaria spp.
Fragaria pentaphylla, one of the wild strawberry species, produces white or red fruits. The white fruits have a stronger aroma than the red [14] and therefore provide a good model to study volatility patterns and their impact on aroma and flavour. In this study, solid-phase microextraction was used in combination with solid phase microextraction and gas chromatography–mass spectrometry (SPME–GC/MS) and RNA-seq techniques for quantitative analysis of volatile compounds and their related gene expression, respectively. Volatile compounds from two types of F. pentaphylla fruits were compared to the cultivated F. × ananassa during fruit development and maturation. The results expand our knowledge of the biosynthesis and regulation of volatiles, which play important roles in the breeding of cultivated strawberries.
Materials and methods
Fruit collection
Wild strawberry F. pentaphylla with unripened fruit were collected in July 2012 in Mao County (31°41′16″N, 103°52′41″E), Chengdu City, Sichuan Province, China. The plants were transplanted to a walk-in growth chamber at Taizhou University, Zhejiang Province, China. The plants were grown at day/night temperatures of 20/15 °C, with a photoperiod of 10/14 h, with 75% constant humidity. When the fruits were ripened, 18 red fruits of F. pentaphylla were random collected and six fruits were mixed as one sample, three samples of fruits were used for the analysis. And the same was repeated for white fruits. The fruits were immediately frozen in liquid nitrogen and stored at −80 °C for RNA-seq.
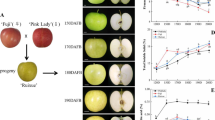
The cultivated strawberry F. ananassa Duch. cv ‘Benihoppe’ was bought in November 2012 from a cooperative society in Linhai City, Zhejiang Province, China, and transplanted to the walk-in growth chamber under the same conditions noted above. In June 2015, when the cultivated and wild strawberries were flowering, hand cross-pollination was conducted to produce fruits. Two fruits per plant were collected at three ripening stages [14]: the unripe stage (S1), the intermediate stage (S2), and the ripe stage (S3), which corresponded to 22, 28, and 36 days after full bloom, respectively. Six fruits from different plants were mixed as one sample. The fruits were immediately frozen in liquid nitrogen and stored at −80 °C until chemical analysis.
Analysis of aroma volatiles
Strawberry fruit flesh was ground using a mortar, and 5 g was placed into a 15-mL vial which was flushed with nitrogen, then, the sample was equilibrated with stirring at 35 °C for 30 min in a water bath. After equilibration, a solid-phase microextraction (SPME) fibre (Supelco, Bellefonte, PA, USA), coated with an absorbent phase of polydimethylsiloxane/carboxen/divinylbenzene (PDMS/CAR/DVB) was exposed to the headspace of the vial for 30 min, after which the fibre was inserted into a GC–MS (Agilent Technologies, Inc., Palo Alto, CA, USA) desorption 3 min for analysis. All the samples were prepared in triplicate.
Volatiles analysis was done with reference to method described previously with some modification [15]. GC–MS analysis was performed on the Agilent 7890B (GC) coupled with a 5975C mass spectrometer (EI mode, 70 eV) (Agilent Technologies, Inc., Palo Alto, CA, USA). Helium was used as the carrier gas at a flow rate of 1 mL/min with a splitless injection. Volatile compounds were separated using a DB-5 MS column (30 m × 0.32 mm, 0.25 μm) under the following conditions: 1 min at 50 °C, followed by an increase from 50 to 320 °C at a rate of 5 °C/min, where the temperature was maintained until the procedure was manually stopped. The injector and the interface temperature were 220 and 280 °C, respectively. The m/z range was from 29 to 450. Compound identification was performed using the data system library (NIST 11).
Volatile compounds was confirmed by comparing the mass spectra of the samples with the data system library (NIST 11), retention index (RI) and authentic references. Retention index (RI) of volatiles was calculated using the mixture of n-alkanes (C7–C40, purchased from Sigma-Aldrich, St. Louis, MO, USA) as standards. The standards compounds (hexanal, (E)-2-hexenal, 3(2H)-furanone,4-methoxy-2,5 methyl) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Expression analysis of aroma-volatile genes by RNA-seq
Total RNA was extracted from six red and six white fruit samples of ripe stage, and then quantified. Total RNA was extracted in the TRIzol reagent (Life Technologies) according to the manufacturer’s protocol. RNA concentration was quantified using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA) and the ratio of absorbance at 260 and 280 nm was calculated to evaluate the quality of RNA. Subsequently, mRNA was purified using NEXTflex™ Poly(A) Beads and cDNA libraries were prepared. The cDNA libraries were quantified using a Qubit 2.0 (Thermo Fisher Scientific Inc., Wilmington, DE, USA), and the size distribution was assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc. Santa Clara, CA, USA). The cDNA libraries were sequenced on an Illumina NextSeq 500 sequencing instrument (Illumina Inc., San Diego, CA, USA). All experiments were conducted at Tianke Hi-New Technology Co. Ltd. in Zhejiang Province, China. Raw RNA-seq reads were trimmed for low quality, clean reads were determined by their error rate, Q20, Q30, and GC-contents. Then the clean reads were mapped to the F. vesca genome using Bowtie software, the number of mapped clean reads for each UniGene was counted and then normalized into RPKM value (reads per kb per million reads, which was widely used to calculate the UniGene expression) [16], and then the gene expression was represented as log2 (fold-change) with the gene expression level in white ripe fruits as reference. Expression fold change of genes was calculated using DESeq [17]. Any gene with an adjusted p value of <0.05 and |log2 (fold change)| ≥ 1 was determined to be differentially expressed gene (DEGs) [18].
KEGG enrichment analysis was conducted as the following. Firstly, the number of genes per pathway was calculated by mapping all of DEGs to the KEGG database (www.Genome.jp/keg/), then the significantly enriched DEGs were found using the binormal test. The pathway with the Q value ≤0.05 is defined as pathway that is significantly enriched in the differentially expressed genes.
Statistical analysis
The significance of the different volatile profiles was determined by one-way analysis of variance (ANOVA) using SPSS, version 17.0 (SPSS Inc., 2009). Principal component analysis (PCA) of the volatile compounds was performed using Soft Independent Modelling of Class Analogies (SIMCA-P, V.11.5) in Unscrambler (Camo Process AS, Oslo, Norway). Heat map was obtained using the Cluster Software package and the Multi Experiment Viewer.
Results
Qualitative analyses of volatile compounds
A total of 61 volatile compounds were identified in the white fruits of F. pentaphylla, of which 29, 26, and 38 compounds were detected in the S1, S2 and S3 stages, respectively. Fifty-three volatile compounds were identified in the red fruits of F. pentaphylla, of which 17, 20, and 35 compounds were detected in the S1, S2 and S3 stages, respectively. Thirty-eight volatile compounds were detected in cultivated fruits of F. × ananassa, of which 10, 8, and 33 compounds were detected in the S1, S2 and S3 stages, respectively (Table 1). In the ripened white fruits of F. pentaphylla, the profile was as follows (Fig. 1a): aldehydes (five compounds), 15.86%; alcohols (seven compounds), 7.41%; hydrocarbons (three compounds), 7.82%; acids (two compounds), 5.58%; ketones (one compound), 24.71%; esters (16 compounds), 37.72%; and other compounds (four), 0.90%. In the ripened red fruits of F. pentaphylla, the profile of the volatiles was as follows: aldehydes (four compounds), 30.71%; alcohols (five compounds), 21.97%; hydrocarbons (three compounds), 1.30%; acids (two compounds), 1.61%; ketones (two compounds), 9.50%; esters (13 compounds), 31.65%; and other compounds (six), 3.26%. In the ripened fruits of F. × ananassa, the profile of the volatiles was as follows: aldehydes (two compounds), 23.62%; alcohols (three compounds), 5.02%; hydrocarbons (one compounds), 0.35%; acids (three compounds), 11.80%; ketones (four compounds), 6.46%; esters (18 compounds), 51.85%; and other compounds (two), 0.89%. As shown in Fig. 1b, the total amounts of volatile compounds in both the red and white fruits of F. pentaphylla was higher than that in F. × ananassa (p < 0.01), while no significant difference occurred between the red and white fruits of F. pentaphylla.
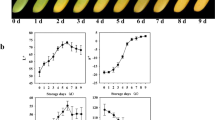
The main volatile compounds in the white ripe fruits of F. pentaphylla were 3(2H)-furanone 4-methoxy-2,5 methyl (DMF) (24.71%), butanoic acid, 2-methyl, methyl ester (10.43%), trans-2-hexenal (9.23%), 2-hexen-1-ol, acetate (6.28%), and hexanal (5.72%). The content of DMF declined during the early stages and then rose rapidly, reaching its highest value (24.71%) at maturity (Fig. 2; Table 1). 2-Methyl butanoic acid methyl ester was not detected in the early stages, but reached a relative content of 10.43% at maturity. The content of 2-hexenal and hexanal continuously decreased with fruit maturity; 2-hexenal dropped from 41.77% in the unripe stage to 9.23% at maturity, while hexanal fell from 13.10 to 5.72%. The content of 2-hexen-1-ol acetate was slightly lower during early development and then sharply increased to a maximum value of 6.28% at maturity. In addition, cyclopropane propyl was found in the white fruits of wild F. pentaphylla at high levels (4.83%), which is the first report of this compound in white fruit strawberries.
Heat map analysis of all strawberries and volatile compounds during maturation. Red colour represents low levels, and green colour represents high levels. WS1 indicates the white fruits of F. pentaphylla at the unripe stage; WS2 at the intermediate stage; and WS3 at the ripe stage. RS1 indicates the red fruits of F. pentaphylla at the unripe stage; RS2 at the intermediate stage; and RS3 at the ripe stage. CS1 indicates the fruits of F. × ananassa at the unripe stage; CS2 at the intermediate stage; and CS3 at the ripe stage
In the red ripe fruits of F. pentaphylla, the main volatiles detected were 2-hexenal (21.23%), 1-hexanol (13.29%), 2-hexen-1-ol acetate (13.00%), hexanal (8.94%), 2-hexenol (7.71%), and DMF (7.19%). 2-Hexenal increased from 13.32 to 21.23%, while the content of 1-hexanol and 2-hexenol increased to 12.82 and 7.44%, respectively. 2-Hexen-1-ol acetate increased early and then decreased to 13.00% at the ripe fruit stage. The maximum amount of DMF appeared in unripe fruit (S1), decreased rapidly to the lowest value (S2) and then increased slightly to 7.19% at the ripe fruit stage.
The main components in ripe fruits of F. × ananassa were butanoic acid ethyl ester (25.80%), 2-hexenal (23.47%), 2-methyl butanoic acid (10.09%), and hexanoic acid ethyl ester (8.75%), DMF (5.31%). The two main esters (butanoic acid ethyl ester and hexanoic acid ethyl ester) were observed only in ripe fruits. The highest value of 2-hexenal occurred in the unripe fruit stage (S1) at 80.41% and then gradually decreased to 23.47% at maturity.
PCA and heat map analysis showed that the volatile compounds of the wild and cultivated species were well differentiated in CS2 and CS3 (Figs. 2, 3).
PCA score plot of the main sources of variability between strawberry samples. WS1 indicates the white fruits of F. pentaphylla at the unripe stage; WS2 at the intermediate stage; and WS3 at the ripe stage. RS1 indicates the red fruits of F. pentaphylla at the unripe stage; RS2 at the intermediate stage; and RS3 at the ripe stage. CS1 indicates the fruits of F. × ananassa at the unripe stage; CS2 at the intermediate stage; and CS3 at the ripe stage
RNA-seq analysis and aroma-related gene expression in the red and white fruits of F. Pentaphylla
An averaged 9,705,633 and 10,179,395 raw reads were produced from red fruit and white fruit, respectively. After the filtering out of the low-quality reads, an average of 9,646,731 and 10,102,495 clean reads remained for red fruit and white fruit, respectively. The reads from red fruit and white fruit were mapped approximately 53.93 and 53.28% of the reference genome (F. vesca), respectively (Table 2).
Totally, 2271 DEGs were found between the red and white fruits of F. pentaphylla, of which 1164 up-regulated and 1107 down-regulated in red fruits of F. pentaphylla compared to the white fruits (Fig. 4a). In total, 20 categories of biological processes were enriched in the DEGs, and the most enriched pathway was “Biosynthesis of secondary metabolites” (Fig. 4b).
Analysis of the differentially expression genes (DEGs) between the red fruits and white fruits of F. pentaphylla. a Histogram of different expressed genes (p < 0.05). b Statistics of pathway enrichment; Total means total numbers of DEGs between the red ripe fruits and white ripe fruits of F. pentaphylla; Up means up-regulated DEGs; Down means down-regulated DEGs; Red vs White means the white fruits were used as the reference; White vs Red means the red fruits were used as the reference
To elucidate the genetic regulation of the volatile biosynthesis, the genes were filtered for these involved in the volatile biosynthesis pathway of F. pentaphylla (Supplementary Table 1). As fatty acid derivatives, aldehyde and alcohol are derived from the degradation of C18 unsaturated fatty acids (linoleic or linolenic acids) through “the lipoxygenase pathway” (Fig. 5). The key step for producing aldehyde is the deoxygenation of unsaturated fatty acids, catalysed by lipoxygenase (LOX). Among nine identified LOXs, four genes were significantly up-regulated in wild red fruits, resulting in the content of hexanal, the product of LOX, in red fruits was higher than that in white fruits as expected (red: 235.01, white: 112.04) (Supplementary Table 1). The aldehydes produced from the unsaturated fatty acid can be further degraded by alcohol dehydrogenases (ADHs). Among 15 identified ADHs, five genes were significantly expressed with two significantly up-regulated genes and three significantly down-regulated genes in the red fruits. As the product of ADH, the content of hexenol in red and white fruits was 162.6 and 27.67, respectively (Supplementary Table 1). Finally, an ester can be generated from an alcohol by the catalysis of acyl transferases (AATs). Among two identified AATs, only one gene was found to be significantly down-regulated in the red fruits (Fig. 5). As the product of AAT, the content of hexen-1-ol, acetate in red and white fruits was 162.6 and 27.67, respectively (Supplementary Table 1). Interestingly, a glyoxysome malate dehydrogenase was found to be significantly up-regulated in the red fruits.
Features of carbon and lipid metabolism in F. pentaphylla. Log2 (fold change) of differentially expressed genes between the red and white strawberries of F. pentaphylla (Red vs White, p < 0.05) are presented as bars. The bar above zero means the gene expression level was up-regulated in red fruits compared with that in white fruits. The bar below zero means the gene expression level was down-regulated in red fruits compared with the white that in white fruits. The compounds marked in gray box means it was higher in red fruits than that in white fruits, while the compounds marked in white box means it was lower in red fruits than that in white fruits. R wild red fruit, W wild white fruit, LOX lipoxygenase, ADH alcohol dehydrogenase, AAT alcohol acyl transferases, UFGT flavonoid-3-O-glucosyltransferase, F1,6P fructose 1,6-diphosphate, DMF 3(2H)-furanone 4-methoxy-2,5 methyl, 3-PGA 3-phosphoglycerate, PEP phosphoenolpyruvic acid, ShikA shikimate, Phe phenylalanine, Tyr tyrosine, Glc glucose, Pg pelargonidin, Pg3glc 3-O-β-glucopyranosides of pelargonidin, Cy cyanin, Cy3glc 3-O-β-glucopyranosides of cyanidin
Discussion
Composition of aroma
Although more than 360 compounds have been previously reported in strawberries [19], less than 20 compounds contribute significantly to strawberry flavour in a proportional way [11, 20]. According to the threshold value [20], the top 10 key aroma compounds (parts per billion) were β-ionone (0.007), DMF (0.03), DHF (0.04), ethyl butanoate (0.13), (Z)-3-hexenal (0.25), methyl anthranilate (3), linalool (6), eugenol (11), (E)-2-hexenal (16), and heptanone-2 (50).
Aroma furanones, which always present as DMF and DHF in fruit, are considered to be important volatile compounds with a caramel-like odour [21]. They always exist at very low levels in fruits; however, we found DMF to be the highest single compound in the white fruits, representing 24.71% of the total volatiles, far greater than the percentage observed in the wild red and cultivated fruits. In addition to DMF, 2-hexenal, hexanal, 2-hexen-1-ol, 2-hexen-1-ol acetate, and acetic acid hexyl ester were found at high levels in F. pentaphylla. Although all these compounds confer a positive, sweet flavour to the aroma, only 2-hexenal has a relatively low threshold aroma value (17 ppb), contributing a green odour. The other compounds were considered to offer little contribution to flavour because the threshold aroma value of these compounds is far more than that of the key aroma compounds mentioned above. Although the relative abundance of 2-hexenal in the wild white fruits was approximately 0.44 times less than that in the wild red fruits and cultivated fruits, considering the highest total amount of volatiles and highest level of DMF in the white fruits, we inferred that the aroma of the wild white fruits of F. pentaphylla is far stronger than that of the wild red and cultivated fruits, which is consistent with the description in “flora of China” and report by Risser and Navatel [14], more than that, it even better than those of other wild strawberries, such as F. vesca, characterized by high levels of methyl anthranilate [20].
The characteristic compounds (DMF and 2-hexenal) in the wild white fruits may confer a positive, pleasant character to their flavour and possess important properties, such as attractant, anti-carcinogenic, and fungicidal properties [22, 23].
For the first time, we report the identification of cyclopropane propyl and cyclohexanol in strawberries. Although alkanes have little impact on flavour [19], the extent to which these novel compounds contribute to flavour still needs to be studied.
Synthetic pathway of aroma
Furanones are unique group of flavour molecules with extremely low odour thresholds. It has been suggested that furanones are synthesized from d-fructose 1,6-bisphosphate. Although it is not clear how the furanone ring is synthesized and which enzymes are involved [24], studies in yeast and tomato suggest that phosphorylated carbohydrates serve as potential precursors of furanones [25, 26]. Hexose and pentose are primary photosynthetic products and may serve as excellent aroma precursors of furanones without degradation of the carbon skeleton [6]. They originate from the degradation of starch through glycolytic pathway, so the total soluble sugar content in fruits may be strongly influenced by the biosynthesis of furanones; the lower sugar content found in wild red fruits and corresponding low DMF level supports this speculation. Furthermore, DMF decreases dramatically from unripe to ripened wild red fruits. Availability of carbohydrates for DMF synthesis reduces because a large amount of carbohydrates are needed for the biosynthesis of anthocyanins at the colour-changing stage in red fruits [27].
Aldehydes are synthesized via the lipoxygenase (LOX) pathway from C18 unsaturated fatty acids, which undergo deoxygenation catalysed by LOX and hydroperoxide lyase (HPL) [28]. The aldehydes are further reduced by alcohol dehydrogenase and then by alcohol acyl transferases (AATs) to esters [29]. These compounds are associated with fresh green fragrances [30] and are known as “green-leaf volatiles” (GLV) [29]; their content always decreases as fruits ripen, and they are often produced in response to wounding and pest attack [31, 32]. We observed a continuous decrease in “GLV” content in the white fruits of F. pentaphylla and fruits of F. × ananassa. In contrast, in the red fruits of F. pentaphylla, GLVs showed a continuously increasing abundance during fruit development. Moreover, significantly up-regulated LOX expression and higher content of hexanal were found in the red fruits compared with the white fruits of F. pentaphylla. These results imply that red fruit-bearing plants experience selection pressures, although there was little difference in wounding between the wild white and red fruits. At the end of LOX pathway, only one significantly down-regulated AAT and lower product conversion rate (Supplementary Table 2) in red fruits compared with the white fruits, suggesting that hexenol might have other degradation pathways. Feussner et al. [33] reported an alternative pathway associated with the expression of LOX and lipid metabolism. Unsaturated fatty acids are catalysed to their corresponding hydroxides, which are later degraded via glyoxysomal β-oxidation. Recently, Fan et al. [34] also suggested that lipids may metabolize to starch via glyoxysomal β-oxidation in oleaginous Chlorella spp. The significant up-regulation of glyoxysomal malate dehydrogenase and 3-ketoacyl-CoA thiolase in wild red fruits supports the metabolic flux of unsaturated fatty acids to the tricarboxylic acid cycle via a LOX-dependent pathway in red fruits. Although energy use is less effective in this pathway [35], it may be sufficient to compensate for the energy loss as glucose is metabolized during the synthesis of anthocyanin in red fruits. Therefore, we speculated that the reduction of sugar and correspondingly low DMF leads to increased LOX activity, ultimately resulting in a high level of aldehydes and alcohols (Fig. 5). Having known that the glyoxylate cycle plays a central role in the use of stored oil in oilseeds [36], more studies should be conducted to verify the presence of the glyoxylate cycle in fruits.
Abbreviations
- DMF:
-
3(2H)-Furanone 4-methoxy-2,5 methyl
- DHF:
-
2,5-Dimethyl-4-hydroxy-3(2H)-furanone
- LOX:
-
Lipoxygenase
- ADH:
-
Alcohol dehydrogenase
- AAT:
-
Alcohol acyl transferases
- UFGT:
-
Flavonoid-3-O-glucosyltransferase
- F1,6P:
-
Fructose 1,6-diphosphate
- 3-PGA:
-
3-Phosphoglycerate
- PEP:
-
Phosphoenolpyruvic acid
- ShikA:
-
Shikimate
- Phe:
-
Phenylalanine
- Tyr:
-
Tyrosine
- Glc:
-
Glucose
- Pg:
-
Pelargonidin
- Pg3glc:
-
3-O-β-Glucopyranosides of pelargonidin
- Cy:
-
Cyanin
- Cy3glc:
-
3-O-β-Glucopyranosides of cyanidin
- RI:
-
Retention indices
- ID:
-
Identification method
- SPME–GC/MS:
-
Solid phase microextraction and gas chromatography–mass spectrometry
References
Hancock JF (1999) Strawberries. CAB International, Oxford
Negri AS, Allegra D, Simoni L, Rusconi F, Tonelli C, Espen L, Galbiati M (2015) Comparative analysis of fruit aroma patterns in the domesticated wild strawberries “Profumatadi Tortona” (F. moschata) and “Reginadelle Valli” (F. vesca). Front. Plant Sci 6(56):1–13
Ulrich D, Olbricht K (2013) Diversity of volatile patterns in sixteen Fragaria vesca L. accessions in comparison to cultivars of Fragaria × ananassa. J Appl Bot Food Qual 86:37–46
Ulrich D, Olbricht K (2014) Diversity of metabolite patterns and sensory characters in wild and cultivated strawberries. J Berry Res 4:11–17
Menager I, Jost M, Aubert C (2004) Changes in physicochemical characteristics and volatile constituents of strawberry (Cv. Cigaline) during maturation. J Agric Food Chem 52:1248–1254
Bood KG, Zabetakis I (2002) The biosynthesis of strawberry flavor (II): biosynthetic and molecular biology studies. J Food Sci 67(1):2–8
Forney CF (2001) Horticultural and other factors affecting aroma volatile composition of small fruit. Horttechnology 11:529–538
Azodanlau R, Darbellay C, Luisier JL, Villettaz JC, Amado R (2003) Quality assessment of strawberries (Fragaria species). J Agric Food Chem 51:715–721
Azodanlau R, Darbellay C, Luisier JL, Villettaz JC, Amado R (2004) Changes in flavour and texture during the ripening of strawberries. Eur Food Res Technol 218:167–172
Larsen M, Poll L (1992) Odour thresholds of some important aroma compounds in strawberries. Z Lebensm Unters FA 195:120–123
Schieberle P, Hofmann T (1997) Evaluation of the character impact odorants in fresh strawberry juice by quantitative measurements after sensory studies on model mixtures. J Agric Food Chem 45:227–232
Pyysalo T, Honkanen E, Hirvi T (1979) Volatiles of wild strawberries, Fragaria vesca L., compared to those of cultivated berries, Fragaria ananassa cv. Senga Sengana. J Agric Food Chem 27:19–22
Ulrich D, Komes D, Olbricht K, Hoberg E (2007) Diversity of aroma patterns in wild and cultivated Fragaria accessions. Genet Resour Crop Evol 54:1185–1196
Risser G, Navatel JC (1997) Phenologic stages of strawberry plant. In strawberry: plant and varieties. Ctifl, Paris
Cheng H, Chen JL, Li X, Pan JX, Xue SJ, Liu DH, Ye XQ (2015) Differentiation of the volatile profiles of Chinese bayberry cultivars during storage by HS–SPME–GC/MS combined with principal component analysis. Postharvest Biol Technol 100:59–72
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5(7):621–628
Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol. doi:10.1186/gb-2010-11-10-r106
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq 2. Genome Biol 15:550–570
Schwab W, Davidovich-Rikanati R, Lewinsohn E (2008) Biosynthesis of plant-derived flavor compounds. Plant J 54:712–732
Ulrich D, Hoberg E, Rapp A, Kecke S (1997) Analysis of strawberry flavour discrimination of aroma types by quantification of volatile compounds. Z Lebensm Unters FA 205:218–223
Latrasse A (1991) Fruits III. In: Maarse H (ed) Volatile compounds in food and beverages. Marcel Dekker, Inc., New York, USA
Farine JP, Le Quere JL, Duffy J, Everaerts C, Brossut R (1994) Male sex pheromone of cockroach Eurycotis floridana (Walker) (Blattidae, Polyzosteriinae): role and composition of tergites 2 and 8 secretions. J Chem Ecol 20:2291–2306
Slaughter JC (1999) The naturally occurring furanones: formation and function from pheromone to food. Biol Rev 74:259–276
Roscher R, Bringmann G, Schreier P, Schwab W (1998) Radiotracer studies on the formation of 2,5-dimethyl-4-hydroxy-3(2H)-furanone in detached ripening strawberry fruits. J Agric Food Chem 46:1488–1493
Sasaki M, Nunomura N, Matsudo T (1991) Biosynthesis of 4-hydroxy-2(or5)-ethyl-5(or 2)-methyl-3(2H)-furanone by yeasts. J Agric Food Chem 39:934–938
Hauck T, Hubner Y, Bruhlmann F, Schwab W (2003) Alternative pathway for the formation of 4,5-dihydroxy-2,3-pentanedione, the proposed precursor of 4-hydroxy-5-methyl-3(2H)-furanone as well as autoinducer-2, and its detection as natural constituent of tomato fruit. BBA 1623:109–119
Petroni K, Tonelli C (2011) Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci 181(3):219–229
Feussner I, Wasternack C (2002) The lipoxygenase pathway. Annu Rev Plant Biol 53:275–297
Olias JM, Sanz C, Rios JJ, Perez AG (1995) Substrate specificity of alcohol acyltransferase from strawberry and banana fruit. In: Rouseff RL, Leahy MM (eds) Fruit flavors: biogenesis, characterization and authentication. American Chemical Society, Washington, DC, USA
Poll L, Lewis MJ (1986) Volatile components of elderberry juice. Lebensm Wiss Technol 19:258–262
Matsui K (2006) Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol 9:274–280
Hamilton-Kemp TR, Archbold DD, Collins RW, Yu KS (2003) Emission patterns of wound volatile compounds following injury of ripe strawberry fruit. J Sci Food Agric 83:283–288
Feussner I, Kuhn H, Wasternack C (2001) The lipoxygenase dependent degradation of storage lipids. Trends Plant Sci 6:268–273
Fan JH, Ning K, Zeng XW, Luo YC, Wang DM, Hu JQ, Li J, Xu H, Huang JK, Wan MX, Wang WL, Zhang DJ, Shen GM, Run CL, Liao JJ, Fang L, Huang S, Jing XY, Su XQ, Wang AH, Bai LL, Hu ZM, Xu J, Li YG (2015) Genomic foundation of starch-to-lipid switch in oleaginous Chlorella spp. Plant Physiol 169:2444–2461
Gerhardt B, Fischer K, Balkenhohl TJ, Pohnert G, Kühn H, Wasternack C, Feussner L (2005) Lipoxygenase-mediated metabolism of storage lipids in germinating sunflower cotyledons and β-oxidation of (9Z,11E,13S)-13-hydroxy-octadeca-9,11-dienoic acid by the cotyledonary glyoxysomes. Planta 220:919–930
Peter JE, Ian AG (2001) Re-examining the role of the glyoxylate cycle in oil seeds. Trends Plant Sci 6(2):72–77
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 31261120580).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical requirements
This research does not contain any studies with human or animal subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duan, W., Sun, P., Chen, L. et al. Comparative analysis of fruit volatiles and related gene expression between the wild strawberry Fragaria pentaphylla and cultivated Fragaria × ananassa . Eur Food Res Technol 244, 57–72 (2018). https://doi.org/10.1007/s00217-017-2935-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-2935-x