Abstract
This study was conducted to investigate the impact of wheat gluten protein degradation during storage in a dough and bread system due to different levels of fungal contamination with F. culmorum. Therefore, artificially infected grains were stored in a model system and analysed for their gluten content and quality. This revealed a substantial loss in wet gluten content and a weakening of the gluten network in the infected samples during storage. Subsequently, the samples were used to bake wholegrain breads and the obtained dough and breads were analysed. Reduced quality as a consequence of storage was found for all samples, but in the infected samples to a greater extent than in the healthy grains. The breads presented a reduced quality, mainly due to their high bake loss, deformed loaf shape and open crumb structure. The reason for these results was mainly found to be due to the increased enzymatic activities originating from the fungal metabolism. Furthermore, the deterioration of the bread quality after storage was independent of the level of initial infection. Consequently, this study demonstrates the importance of optimal storage conditions and the development of efficient methods to suppress fungal proliferation during storage, in order to reduce economic losses and ensure a consistent high quality of the resulting products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over thousands of years, cereals have played an essential role in human nutrition. Wheat in particular is one of the most produced and consumed cereals. Its high prominence is mainly due to the fact that wheat represents the main raw ingredient for a large variety of products. By far, the most important of these are bakery products, mostly bread, but also cakes, biscuits and others [1]. Bread, in all of its variations, is an essential part of human nutrition all over the world and most commonly it is produced from wheat flour [2]. Consequently, it is crucial to ensure a high and continuous grain quality, being essential for a product of the highest quality.
One of the main reasons for poor product quality of cereal-based foods is fungal contamination and spoilage. A very common cereal disease is Fusarium head blight (FHB), caused by different Fusarium spp. [3]. It is responsible for significant losses relating to the wheat quality and yield. Although fungal contamination usually occurs in the field, it was shown in a previous study [4] that even low levels of initial infection can spread during storage if conditions are suitable. Pitt and Hocking [5] reported that the post-harvest economic losses of wheat due to fungal spoilage and mycotoxins exceed $300 million annually, just in the USA alone. In particular, less developed countries face even bigger damage due to post-harvest fungal spoilage [5]. However, although this is such an important topic, both from safety and grain quality points of view, there are just very few publications investigating this topic.
Subsequently, this study investigated the impact of post-harvest fungal spoilage on wholemeal dough and bread quality. The impact of the enzymatic activities of infected wheat samples on storage proteins and polysaccharides, such as starch and different fibres, was evaluated previously [4]. In this study, the impact on the dough characteristics and final bread quality was examined. Therefore, different gluten quality parameters, in particular the development time and maximal network strength, were determined. Furthermore, important quality characteristics of the dough and baked breads, produced from the infected and stored samples, were analysed. Thus, the impact of fungal spoilage during storage on the final bread quality could be illustrated.
Materials and methods
Materials
Commercial hard winter wheat (Triticum aestivum), harvested in 2013, was supplied by Doves Farm Foods Ltd. (Hungerford, UK). Wheat grains were stored in barrels at 20 ± 2 °C and were regularly aerated. Fusarium culmorum strain TMW 4.2043 was originally isolated from barley and provided by the Lehrstuhl für Technische Mikrobiologie, TU-München Weihenstephan.
For the baking trials, dry yeast was purchased from Puratos, Belgium; salt from Glacia British Salt Limited, UK; and commercial vegetable oil and sugar from Nordzucker, Ireland. All reagents used were at least of analytical grade and sourced from Sigma-Aldrich.
Preparation of fungal spore suspension and grain infection
The spore solution of Fusarium culmorum was prepared according to the method described by Oliveira et al. [6]. Briefly, fungus was cultivated on potato dextrose agar (PDA) plates. After 5 days at 25 °C, six small fragments of inoculated PDA were transferred to 800 mL synthetic nutrient-poor bouillon. To induce spore production, fungal suspensions were kept at room temperature under continuous stirring. Prior to use, the suspensions were filtered through a 30-mm filter paper. The concentration of spores was determined to be 105 spores/mL, using a haemocytometer.
Preceding the artificial fungal infection, grains were disinfected using hydrogen peroxide (10%, w/v) and ultraviolet light as described by Oliveira et al. [6]. In brief, the grains were disinfected by washing for 10 min in a 10% (w/v) hydrogen peroxide (H2O2) solution (using 4 L per 600 g of grains). After rinsing the grains for 5 min in distilled water (4 L), the procedure was repeated once again. Subsequently, the grains were transferred into sterile plastic boxes and dried under vertical sterile laminar flow for 24 h at room temperature. After drying, the grains were subjected to ultraviolet light (10 min) and collected aseptically for further use.
Infected wheat grains were prepared using the following procedure. Disinfected wheat grains were mixed with 2% (v/w) sterile filtered spore suspension of F. culmorum. Subsequently, the grains were incubated for 10 days at 25 °C with 75% relative humidity to allow fungal proliferation. The infected grains produced were defined as 100% infected. After homogenisation, moisture content of the samples was determined.
Mixing and storage trials
Infected and disinfected grains were mixed together to a total sample size of 4.5 kg (dry matter) with specific infection levels of 0, 5, 10 and 20% and stored in lab-scale model systems under conditions generally suitable for fungal growth. This allowed to study the fungal impact in relation to the level of initial infection. Each mixture and the control sample was divided into nine portions and filled into sterile plastic bags. Every bag was sealed and perforated with two pipette tips with barrier filter to allow gas exchange. The bags were stored for 6 weeks at room temperature. After 0, 3 and 6 weeks, 3 portions of each sample were taken and milled (Laboratory Disk Mill DLFG, Bühler AG, Uzwil, Switzerland) to a wholegrain flour (particle size <0.5 mm). The flours obtained from the three portions of each sample were combined, homogenised and stored at −20 °C until further use.
Gluten characterisation
Wheat flour samples were analysed using the Glutomatic (Perten Instruments GmbH, Hamburg, Germany) and the GlutoPeak (Brabender GmbH & Co. KG, Duisburg, Germany). The Glutomatic provides essential information regarding the amount of gluten (wet and dry gluten contents) but also about the quality (water-binding capacity and gluten index). The determination of wet and dry gluten contents, water-binding capacity and gluten index was carried out according to the AACC method 38-12.02 for wholemeal wheat flour [7].
For further characterisation of the gluten quality, in terms of its development time and maximum strength, the GlutoPeak was used. Therefore, the sample was mixed with water under high shear force until the gluten network formed and broke down again. The viscosity was recorded graphically as a function of time (Fig. 1). Hence, strong flours, as desired for bread making, developed fast, showing high peaks with short peak times [8]. The test was performed according to the manufacturer’s recommendations [8]. Briefly, 9 g of sample (14.0% moisture content) and 9 g of distilled water were weighed into the mixing chamber and equilibrated. Sample and water were mixed with 2500 rpm at 27 °C. After the maximal mixing resistance was detected, or latest after 10 min, the measurement was stopped. Peak time and height were recorded to evaluate the gluten quality.
Analysis of dough quality
Important quality characteristics of the bread dough were analysed using a TA-XT2i texture analyser (Stable Micro Systems, Surrey, UK), equipped with a 5-kg load cell. Firstly, the stickiness of the freshly prepared dough was measured using the SMS Chen-Hoseney dough stickiness cell, equipped with a 25-mm Perspex spherical probe with a uniform adherence surface. The probe first compressed the dough (test speed 2.0 mm/s) until the test force (40 g) was reached. This force was maintained for 0.1 s until the probe moved upwards (100 mm at 10 mm/s). The maximum force measured was used to evaluate the stickiness.
In addition, the dough strength was determined for all samples, using the Kieffer cell dough extensibility rig (Stable Micro Systems, Surrey, UK). The freshly prepared dough was rolled by hand into a cylindrical shape, placed in the lubricated Teflon mould and compressed with the lubricated top Teflon plate. The sample was allowed to rest for 25, 50 and 75 min, in the proofer to investigate the development of the dough strength during proofing. Subsequently, by clamping the sample between the Kieffer rig plates, analysis was performed according to the method described by Dunnewind et al. [9] and the maximum force in tension was recorded. Five replicates of each dough formulation were measured.
Baking procedure
Baking trials were carried out with the wholegrain flour obtained from the stored samples (particle size <0.5 mm). The percentage of water (based on flour weight) required to yield a dough consistency of 500 Brabender units (BU) (determined using a Brabender Farinograph according to the AACC method 54-21.02 [10]) was equal to 83% for the natural sample at week 0. This amount of water was used for all the wholemeal wheat flour samples baked.
Additionally, 2% yeast, 3% oil, 3% sugar and 2% salt (each based on flour weight) were used in the bread recipe. Prior to dough preparation, yeast was reactivated, using the whole amount of water (30 °C) of the recipe, and placed in a proofer (KOMA sunriser, Roermond, Netherlands) at 30 °C and 80% relative humidity for 10 min. Afterwards, the yeast suspension and the dry ingredients were combined in a mixer (Kenwood Chef Classic KM336). Mixing was carried out with a dough hook at speed I for 1 min, followed by scraping down the sides of the bowl and further mixing at speed II for 7 min. After mixing, the dough was divided into portions of 65 g, manually rounded, placed in non-stick baking tins (dimensions-top inside, 50 mm × 90 mm; bottom outside, 45 mm × 85 mm; inside depth, 30 mm; Sasa UK, Enfield Middlesex, UK) and proofed for 75 min (30 °C, 85% RH). Subsequently, the tins were transferred to the oven (Belling, Prescott, UK) and baked for 27 min at 175 °C (top and bottom). Afterwards, the bread loaves were immediately removed from the tins and allowed to cool to room temperature for 120 min before further analysis.
Analysis of bread quality
After cooling to room temperature, the breads were weighed to determine the bake loss (weight reduction during baking). The specific volume was measured using a Volscan profiler (Stable Micro Systems, UK). After slicing the breads (25 mm width), the four slices from the centre of each loaf were used for further analysis. First, the crumb colour was analysed using a chroma meter CR-400 (Konica Minolta Inc., Tokyo, Japan) with CIE standard illuminant D65. The L * value, as calculated by the software, was used to evaluate the crumb lightness. The C-cell Bread Imaging System (Calibre Control International Ltd., UK) was used to characterise the crumb structure. The following parameters were evaluated: number of cells per slice, total area of cells as a percentage of the total slice area and average diameter of the cells.
The crumb texture was characterised by texture profile analysis (TPA), using a TA-XT2i texture analyser (Stable Micro Systems, Surrey, UK), equipped with a 25-kg load cell and a 20-mm aluminium cylindrical probe. A speed of 5 mm/s and a force of 0.98 N were applied to compress the middle of the crumb to 50% of its original height. Twelve slices per batch were analysed on the baking day and the crumb hardness was evaluated.
Statistical analysis
Samples were baked in two batch replicates. All analyses were run in triplicate, unless otherwise stated. Statistical analysis was performed using Minitab 17 software. Data were checked for outliers (Grubb’s test) and the evaluation of significant differences was performed using one-way analysis of variance (ANOVA). All differences were considered significant at p < 0.05. Where F-values were significant, pairwise comparisons were carried out with the help of Tukey’s Post Hoc test to describe the statistical significance between the infected and uninfected samples over the time of storage.
Results and discussion
The impact of a fungal contamination on wheat quality criteria was investigated in our previous study [4]. Therefore, the grains infected with F. culmorum were stored in a model system to investigate the impact of post-harvest fungal spoilage. Subsequently, this study was carried out to examine the impact of this infection on the grains’ technological performance during bread making. Previously, the activities of different enzymes, in relation to the level of initial fungal infection and the time of storage, were determined and discussed [4]. As this study refers to the same samples, these results were used to understand how F. culmorum and its metabolism influenced the bread quality. A table summarising the enzymatic activities of all samples analysed is also provided as supplemental material (S1).
Gluten characterisation
Results of the gluten characterisation for the infected and stored wheat samples, as well as for the uninfected controls, are summarised in Table 1. The wet and dry gluten contents in natural and 0% infected grains did not change significantly during the 6 weeks of storage (p < 0.05). The wet gluten contents of the uninfected samples ranged between 38.6 ± 3.3 and 46.3 ± 2.0 g/100 g and the dry gluten contents between 14.2 ± 1.6 and 18.1 ± 0.4 g/100 g. In contrast, the infected samples revealed a significant decrease in both wet and dry gluten contents, over time of storage. In particular, the wet gluten content was found to decrease rapidly, due to the fungal infection. A reduction of up to 30% in wet gluten content (20% infected sample) was measured during the 6 weeks. No substantial differences between the infected and uninfected samples were found at week 0, while after 6 weeks remarkably reduced values were determined for the infected grains (p < 0.05). Consequently, the loss of wet gluten during storage is related to the fungal infection. However, also the reduction in dry gluten content was significant (p < 0.05) but notably less severe. The 5% infected sample even showed no significant loss over time at all (p < 0.05). Furthermore, after 6 weeks no substantial differences, regarding the dry gluten content, were found between the three levels of initial infection. Consequently, the water-binding capacity, calculated from wet and dry gluten contents [11], also decreased over time in the infected grains, but not in the uninfected ones. A reduction of up to 50% (20% infected sample) was found during the storage. After 6 weeks, a clear indirect proportionality between water-binding capacity and the level of initial infection was apparent, as the 20 and 0% infected sample showed the lowest and the highest water-binding capacity, respectively. Since the binding of water is one of the main purposes of gluten during bread making, this finding resembles a quality loss in terms of technological performance [12].
The gluten index (GI) measures the strength of the gluten network and therefore represents a key parameter for the bread-making performance of a flour [11]. In this regard, the uninfected samples revealed no significant changes during storage (p < 0.05). Values of 97 ± 3 to 99 ± 0 were determined, indicating a very strong flour [11]. On the contrary, the infected samples showed a significantly reduced GI value, indicating an evident loss of gluten network strength due to the fungal infection. While at week 0 the lowest GI value was found for the highest infection level, after 6 weeks no noteworthy differences between the 10 and 20% infected samples could be detected anymore (p < 0.05). The biggest reduction (from 94 ± 0 to 48 ± 6) was determined for the 10% infected sample, indicating that even small contamination levels can result in remarkable damage.
Table 1 further shows the results for peak time and height obtained from the GlutoPeak. Both values were used to evaluate the gluten development time and network strength. Therefore, strong flours present short peak times with high intensities. Weak flours show longer peak times with lower intensities. In extreme cases, no peak is detected at all (Fig. 1). The results obtained for peak time and height correlate well with each other. Samples with long development times also present low peak maxima, both indicative of a weak gluten network. Furthermore, the samples found to be the weakest by the GlutoPeak also presented the lowest GI values. The uninfected samples were found to be strong flours without significant changes in peak height or development time throughout the storage (p < 0.05). In contrast, the infected ones showed a decrease in gluten network strength over time of storage.
After 3 and 6 weeks, the 5 and 10% infected samples presented more than a threefold prolonged peak development time compared to week 0. Consequently, the peak intensities were also substantially decreased. The biggest decrease was found for the 20% infected sample which, after 6 weeks, produced no detectable peak anymore (Fig. 1). Consequently, the fungal damage in this sample was so severe that no gluten network could form. This correlates well with the electrophoretic analysis of the storage proteins [4], where the 20% infected sample after 6 weeks presented a total degradation of the gliadin fraction.
This loss of gluten quality is most likely due to the substantially increased proteolytic activity in the infected samples during storage (S1). According to Wang et al. [13], fungal proteases primarily attack the storage protein fractions. Furthermore, this degradation of the glutenins and gliadins also improves their solubility due to the reduced molecular weight. Consequently, the water-binding capacity, as determined by the Glutomatic, decreases similarly. The proteolytic activity of the infected samples at week 6 revealed no substantial differences between the infection levels. In contrast, the gluten quality parameters were affected more by the higher contamination levels. The reason behind is in the increased lipase and xylanase activities after 0 and 3 weeks. Both enzymes are released to overcome some of the grains’ physical protection barriers, namely the cuticle (lipase) and cell walls (xylanases). Thus, increased activities of these enzymes indicate a faster penetration of the fungus into the endosperm due to higher initial infection. Consequently, this resulted in more damage after 6 weeks. Thus, the highest loss in gluten strength occurred in the 20% infected sample (shown by the GI and GlutoPeak), while no noteworthy changes occurred to the gluten network of the uninfected ones.
These results show that even minor levels of F. culmorum contamination (5%) during storage, if conditions are suitable, result in a substantially reduced quality of the gluten network.
Dough characterisation
To characterise the dough obtained from the stored samples, the stickiness of the freshly prepared formulation and its strength after 25, 50 and 75 min of proofing were determined. These analyses assessed the impact of fungal contamination and storage time on the most important dough characteristics. The results are summarised in Table 2.
Regarding dough stickiness, the samples from week 0 show the expected trend; in the natural and the three infected samples, the stickiness increased with the initial level of fungal contamination. The significantly higher stickiness of the disinfected sample (p < 0.05) is potentially due to denaturation of the proteins during the initial sanitation, which led to a decreased water-binding ability. After 3 weeks of storage, a substantially increased stickiness was found for all samples, including the uninfected ones. In fact, the biggest increase was found for the natural sample (from 20.1 ± 5.5 to 51.1 ± 3.8 N/cm2), whereas the 20% infected sample showed just a minor increase from 43.3 ± 4.9 to 53.8 ± 5.5 N/cm2. From week 3 to 6, however, the stickiness of all samples increased only slightly, which was not found to be significant (p < 0.05). Finally, after 6 weeks of storage no substantial differences between the infected and uninfected samples could be determined anymore. However, the outcome for the dough stickiness analysis was generally in good correlation with the results of gluten characterisation, as the samples that were shown to contain a poor-quality gluten network also resulted in more sticky dough. As shown above (“Gluten characterisation”), the fungal infection reduced the water-binding capacity of gluten during storage significantly. Thus, the water-binding ability of the dough is also lower, leading to an increased dough stickiness for the respective samples. In addition, the increased enzymatic activities of amylases (α and β), xylanase and glucanase in the infected samples [4] (S1) promote the degradation of various polysaccharides, which would otherwise also absorb water [14]. Thus, the amount of free water in the dough was further increased. The higher amylase activity also caused a delay in starch gelatinisation, which reduced the water-binding capacity of the dough even further [15].
Interestingly, the stickiness of the dough produced from uninfected grains was not constant during the storage period either. A potential conclusion would be that the stickiness primarily depends on the time of storage, not the rate of fungal infection. On the other hand, for the week 0 samples a direct correlation between dough stickiness and the level of infection was visible. This also confirms the results of Dexter et al. [16] who reported a more sticky dough as a result of Fusarium damaged kernels. It also has to be mentioned that after 3 and 6 weeks the infected dough samples produced a batter, rather than a proper dough. Thus, differences in viscosity were more evident than in stickiness. This caused certain difficulties during the measurement, which are likely to have influenced the results of the analysis.
Consequently, differences between the samples could not be determined properly. However, a comparable study of Nightingale et al. [17] used the endosperm flour of 20% F. graminearum-infected wheat kernels without storage. The authors reported that the degree of fungal infection had just a little impact on the water absorption ability of the dough. In addition, the present study used wholemeal flour, containing a high amount of fibre from the husk layers. The water absorption of these fibres in the dough served to mask the effects of the gluten degradation further. Thus, just minor differences in terms of dough stickiness were observed.
Overall, the impact of F. culmorum on the dough properties resulted in a reduced dough quality, ultimately leading to a decreased marketability. In particular, from the technological point of view, a stickier and less elastic dough is more difficult to handle. Furthermore, the high amounts of free water and weakness of the dough would be expected to cause problems regarding the final product quality, such as high bake loss and poor crumb structure. In order to investigate this topic further, baking trials were performed using the dough samples discussed in this paragraph.
Bread characterisation
The wholegrain breads produced from the grain samples were analysed for the following quality parameters: bake loss, specific loaf volume, crumb lightness, crumb structure and physical crumb texture. This allowed essential information to be obtained in order to evaluate the fungal impact on the final product quality. Images of the breads produced from the natural, 5% infected and 20% infected samples, after 0 and 6 weeks of storage, are shown in Fig. 2.
The natural sample after week 0 and week 6 is shown in Fig. 2a, b, respectively. Although the sample in 2B displays a more open crumb structure, overall it maintained a good bread-making quality during storage. However, the breads obtained from the infected flours after storage were found to have a much wider and irregular crumb structure, indicating a more substantial quality deterioration due to the fungal infection. In particular, the 5% infected one shows significant differences between week 0 (2 C) and week 6 (2D). While at week 0 the crumb presented a closed and dense structure with pale colour, after 6 weeks it appeared to be very open with big holes and a much darker, brown colour. Furthermore, the shape of the breads after 6 weeks resembled the shape of the tins used, indicating a weak gluten structure. In contrast to this, the loaf in 2C had an oval shape, due to the strong gluten network retaining the produced gas and maintaining the original loaf shape. In addition, some of the slices in 2D indicate that the breads slightly collapsed, visible by the raised edges. Images 2E and 2F show the 20% infected sample after 0 and 6 weeks, respectively. These breads had an irregular shape even in week 0. Regarding the crumb structure and colour, no significant differences to the uninfected ones are visible. In contrast, the stored samples led to breads with a dark brown colour, huge holes and a very irregular loaf shape. The reason for this is the degradation of gluten and polysaccharides by fungal enzymes, such as proteases, amylases and xylanases [4] (S1). This breakdown weakened the dough so much, such that it could not hold its shape and the expanding gas during baking. The release of reducing sugars and amino acids as enzymatic degradation products caused the dark crumb colour, due to Maillard reaction during baking [20].
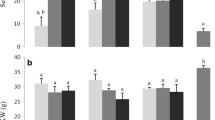
Selected important bread quality parameters were quantified and the results are summarised in Table 3. Firstly, the bake loss was found to be significantly higher for the infected samples, compared to the healthy ones. This reduced the quality but also the marketability as bread is sold by weight. However, none of the samples revealed a substantial change in bake loss during storage (p < 0.05). Furthermore, no noteworthy differences were found between the three initial infection levels. These findings propose that the bake loss increased due to the fungal infection but widely independent of the degree of infection. This correlates just partially with the results obtained for the gluten characterisation. One would expect the bake loss to be in direct correlation with the reduction in water-binding capacity, which decreased with time of storage. However, the bake loss was found to be independent of the time of storage for all samples. That is primarily because wholegrain flour was used for baking. Thus, it is not exclusively the gluten that is binding water but also the polysaccharides, reducing the influence of the gluten. Thus, the infected samples provided more free water for the remaining starch, enhancing its gelatinisation and so partly reducing the bake loss further [14]. Besides, the increased lipase activity due to the infection improves the starch gelatinisation by degrading starch lipids [21]. The complex interactions of all these factors determine the final bake loss measured.
At the same time, the specific loaf volumes were found to increase due to the fungal infection. Although the volumes of all breads were significantly increasing due to the storage, the infected samples resulted in noteworthy higher volumes after 6 weeks, independent of the degree of initial infection. The increased volume is most likely due to the gluten proteolysis, leading to higher amounts of free water and a reduced network strength. This resulted in an increased gas pressure of the expanding water combined with a reduced ability of the gluten to restrain this expansion and maintain the shape. In addition, the higher bake loss in the infected samples reduced the loaf weight, while the total volume remained constant, hence leading to a bigger specific volume. Furthermore, higher amounts of free sugars compared to the uninfected samples [4] were available for the yeast during proofing. In consequence, the gas production increased and led to a bigger rise of the dough. Nonetheless, this rise of the dough requires a certain gas-holding capacity, provided through the improved gelatinisation of the remaining starch. Thus, the weakening of gluten and gelatinisation of starch created a balance, leading to the increased volume in the infected samples. Nightingale et al. [17] reported decreasing bread volume due to fungal proteases when using the endosperm flour. This further supports the assumption that in the present study primarily starch and fibres are responsible for the water and gas holding in the dough.
To evaluate the fungal impact on the bread crumb characteristics, first the crumb lightness was determined. As the results in Table 3 show, there were no significant differences between the samples of week 0 (p < 0.05). After 3 and 6 weeks, all five samples presented a significantly reduced lightness but the reduction in the three infected samples was notably bigger compared to the uninfected ones. Similar to the loaf characteristics, no substantial differences were found between the three initial infection levels. The darkening indicates higher amounts of free amino acids and reducing sugars, leading to a higher rate of Maillard reaction during baking and thus the darker crumb colour. This also correlates with the increased protease and amylase activities in the infected samples after the storage period [4] (S1). Likewise, the uninfected samples showed lower but still quantifiable enzymatic activities, explaining the slight darkening of the breads obtained from the stored grains. In addition, the proteolytic activities and free sugar contents of the infected samples were found to be widely independent of the level of infection [4]. Consequently, the rate of Maillard reaction was also independent of the contamination level. This increase in Maillard reaction due to F. culmorum can cause a further loss in bread quality. As shown by Bråthen and Knutsen [22], increased levels of free amino acids (i.e. asparagine) in a bread system are likely to increase the formation of acrylamide, a neurotoxic by-product of the Maillard reaction [20].
The crumb structure was analysed using the C-cell bread imaging system. The results of the selected parameters are shown in Table 3. At week 0, no significant difference between the disinfected and natural samples regarding the number of cells per slice area was evident. In contrast, the infected ones presented a decreasing number of cells with increasing level of infection. During storage, all five samples revealed a substantial decline of cells per slice area (p < 0.05). However, the reduction in the infected samples, due to the fungus, is notably bigger than in the controls. In addition, after 6 weeks the number of cells per slice on the infected breads was found to be independent of the initial level of infection. In order to fully understand this parameter, it has to be evaluated in connection with the average area of cells and the average cell diameter (Table 3). While the number of cells was found to decrease in the uninfected samples, the percentile area of cells increased significantly over time of storage. Consequently, the cells on these slices got fewer but bigger. Likewise, the area of cells per slice in the infected samples was found to decrease substantially during storage, while the percentile area of cells increased. Nonetheless, the percentile area of cells after storage was noticeably larger, compared to the uninfected flours. Likewise, the cell diameters showed a notable increase due to the fungus during the 6 weeks. While no significant differences between the cell diameters of the five samples were found in week 0, after 6 weeks the infected samples led to substantially bigger cells than the uninfected ones.
Since a fine structure with many small cells is desirable for most types of commercial bread, the F. culmorum infection results in reduced bread quality and marketability. In particular, the increased cell size of the infected samples equates to a substantial quality loss, which is also visible in Fig. 2d, f, respectively. These breads were found to have a visibly more open and irregular crumb structure. This correlates well to the increased activity of hydrolytic enzymes and the weakening of the gluten network, as discussed above. Due to the higher moisture content and yeast activity, the infected dough contained more gas, expanding while baking, and the weakened gluten was unable to retain the gas in small cells. Furthermore, the degradation of polysaccharides by fungal enzymes is known to cause poor crumb structure [13]. Similar to other quality parameters, the damage after 6 weeks was found to be independent of the degree of initial infection.
Finally, the physical crumb texture of the breads was analysed by TPA and the results are summarised in Table 3. The results reveal that no significant differences regarding the crumb hardness were found between the samples of week 0, except for the 20% infected sample which had a significantly softer crumb than the other breads. The storage period resulted in a substantially decreased crumb hardness for all samples (p < 0.05), excluding the disinfected one. However, in correlation to other quality parameters, the decrease was noticeably larger in the infected samples, compared to the uninfected ones. In addition, the softening of the infected breads appeared widely independent of the level of initial infection. This is yet another consequence of the improved starch gelatinisation in the infected samples, as discussed above. Although a soft crumb is generally desired, the crumbs of the infected samples were too soft for easy handling, such as for buttering a slice of the bread. Consequently, the softening of the crumb has to be considered as another point representing reduced quality and marketability due to the fungal infection of the grains.
Conclusion
In conclusion, this study clearly demonstrates that using wheat grains, infected with F. culmorum, has a major impact on the final bread quality. During the 6 weeks of storage the fungus substantially reduced both gluten quantity and network strength. This resulted in a substantial loss in dough and bread quality, primarily expressed by the high dough stickiness, bake loss, very open crumb structure and a softer bread crumb. Furthermore, the quality deterioration was found to a similar extent for all three infection levels (5, 10 and 20%) and thus was concluded to be widely independent of the initial degree of contamination. This shows how even minimal field contamination can, due to poor storage practices, lead to substantial post-harvest economic losses. Furthermore, it was shown previously that the infected samples used for baking contained substantial amounts of mycotoxins [4]. Hence, in combination with the quality deterioration, the breads are also likely to impose a potential consumer health hazard. Although the model system investigated here applied a relatively high initial fungal contamination and very poor storage conditions, the time of storage (6 weeks) was very short. Therefore, it is likely that under better conditions but with a more realistic storage time similar results would be achieved. Thus, the results illustrate the importance of good storage practices and grain decontamination strategies to prevent post-harvest fungal spoilage, to reduce economic losses and ensure a consistently high product quality.
References
USDA (2013) Agricultural marketing service: grain and oilseed shipment sizes and distances hauled by rail. United States Department of Agriculture. Foreign Agricultural Service, Washington, DC
McGee H (2004) On food and cooking: the science and lore of the kitchen. Scribner, New York, p 517
Clark B, Bryson R, Tonguç L, Kelly C, Jellis G (2012) The encyclopedia of cereal diseases. 889 HGCA/BASF. http://www.hgca.com. Accessed 05 Dec 2014
Schmidt M, Horstmann S, DeColli L, Danaher M, Speer K, Zannini E, Arendt EK (2016) Impact of fungal contamination of wheat on grain quality criteria. J Cereal Sci 69:95–103
Pitt J, Hocking AD (2009) Fungi and food spoilage, 3rd edn. Springer, London
Oliveira PM, Mauch A, Jacob F, Waters DM, Arendt EK (2012) Fundamental study on the influence of Fusarium infection on quality and ultrastructure of barley malt. Int J Food Microbiol 156:32–43
AACC International. Approved methods of analysis (1995) Method 38-12.02. Wet gluten, dry gluten, water-binding capacity, and gluten index. Approved November 3, 1999, 11th edn. AACC International, St. Paul. doi:10.1094/AACCIntMethod-38-12.02
GlutoPeak Test procedure (2016) Brabender, Duisburg, Germany http://www.brabender.com/english/food/products/quality-control/baking-conditions/glutopeakr.html. Accessed 01 Nov 2016
Dunnewind B, Janssen AB, Van Vliet T, Weenen H (2004) Relative importance of cohesion and adhesion for sensory stickiness of semisolid foods. J Texture Stud 35:603–630
AACC International (1995) Approved Methods of analysis. Method 54-21.02. Rheological behavior of flour by farinograph: constant flour weight procedure. Approved November 3, 1999, 11th edn. AACC International, St. Paul. doi:10.1094/AACCIntMethod-54-21.02
Perten Glutomatic “About Gluten” (2016) Perten instruments. http://www.perten.com/Products/Glutomatic/About-gluten/. Accessed 01 Nov 2016
Kieffer R, Wieser H, Henderson MH, Graveland A (1998) Correlations of the breadmaking performance of wheat flour with rheological measurements on a micro-scale. J Cereal Sci 27:53–60
Wang J, Wieser H, Pawlezik E, Weinert J, Keutgen AJ, Wolf GA (2005) Impact of the fungal protease produced by Fusarium culmorum on the protein quality and breadmaking properties of winter wheat. Eur Food Res Technol. doi:10.1007/s00217-004-1112-1
Biliaderis CG, Izydorczyk MS, Rattan O (1995) Effect of arabinoxylans on bread-making quality of wheat flours. Food Chem 53:165–171
Tester RF, Qi X, Karkalas J (2006) Hydrolysis of native starches with amylases. Animal Feed Sci Technol 130:39–54
Dexter JE, Clear RM, Preston KR (1996) Fusarium head blight: effect on the milling and baking of some Canadian wheats. J Cereal Chem 73(6):695–701
Nightingale MJ, Marchylo BA, Clear RM, Dexter JE, Preston KR (1999) Fusarium head blight: effect of fungal proteases on wheat storage proteins. J Cereal Chem 76(1):150–158
Koehler P, Hartmann G, Wieser H, Rychlik M (2007) Changes of folates, dietary fibre, and proteins in wheat as affected by germination. J Agric Food Chem 55:4678–4683
Meyer D, Weipert D, Mielke H (1986) Effects of Fusarium culmorum infection on wheat quality. Getreide Mehl Brot 40:35–39
Mei N, McDaniel L, Dobrovolsky V, Guo X, Shaddock JG, Mittelstaedt RA, Azuma M, Shelton S, McGarrity L, Doerge D, Heflich R (2010) The genotoxicity of acrylamide and glycinamide in big blue rats. Toxicol Sci 115(2):412–421
Koehler P, Wieser H (2013) Chemistry of cereal grains. In: Gobetti M, Gaenzle M (eds) Handbook on sourdough technology. Springer, New York, pp 11–46
Bråthen E, Knutsen SH (2005) Effect of temperature and time on the formation of acrylamide in starch-based and cereal model systems, flat breads and bread. J Food Chem 92:693
Acknowledgements
Financial support for this research was awarded by the Irish Government under the National Development Plan 2007–2013 through the research programme FIRM/RSF/CoFoRD. This research was also partly funded by the Irish Department of Agriculture, Food and the Marine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmidt, M., Zannini, E. & Arendt, E.K. Impact of post-harvest degradation of wheat gluten proteins by Fusarium culmorum on the resulting bread quality. Eur Food Res Technol 243, 1609–1618 (2017). https://doi.org/10.1007/s00217-017-2869-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-2869-3