Abstract
Isotope ratios of methylmercury (MeHg) within organisms can be used to identify sources of MeHg that have accumulated in food webs, but these isotopic compositions are masked in organisms at lower trophic levels by the presence of inorganic mercury (iHg). To facilitate measurement of MeHg isotope ratios in organisms, we developed a method of extracting and isolating MeHg from fish and aquatic invertebrates for compound-specific isotopic analysis involving nitric acid digestion, batch anion-exchange resin separation, and pre-concentration by purge and trap. Recovery of MeHg was quantified after each step in the procedure, and the average cumulative recovery of MeHg was 93.4 ± 2.9% (1 SD, n = 28) for biological reference materials and natural biota samples and 96.9 ± 1.8% (1 SD, n = 5) for aqueous MeHgCl standards. The amount of iHg impurities was also quantified after each step, and the average MeHg purity was 97.8 ± 4.3% (1 SD, n = 28) across all reference materials and natural biota samples after the final separation step. Measured MeHg isotopic compositions of reference materials agreed with literature values obtained using other MeHg separation techniques, and MeHg isotope ratios of aqueous standards, reference materials, and natural biota samples were reproducible. On average, the reproducibility associated with reference material process replicates (2 SD) was 0.10‰ for δ202MeHg and 0.04‰ for Δ199MeHg. This new method provides a streamlined, reliable technique that utilizes a single sample aliquot for MeHg concentration and isotopic analysis. This promotes a tight coupling between MeHg concentration, %MeHg, and Hg isotopic composition, which may be especially beneficial for studying complex food webs with multiple isotopically distinct sources of iHg and/or MeHg.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The speciation of mercury (Hg) influences its mobility, bioavailability, and toxicity. In natural environments, oxidized inorganic mercury (iHg) may be chemically reduced to elemental mercury (Hg(0)) by microbial, photochemical, or dark abiotic reactions, after which it may be evaded to the atmosphere [1]. A variety of microorganisms can also convert bioavailable iHg into methylmercury (MeHg), an organic form of mercury that is highly toxic and bioaccumulative [2]. This MeHg may then be partially degraded by microbial and/or photochemical reactions [3] before bioaccumulating in organisms and biomagnifying in food webs. Each of these reactions and processes, along with many others, can induce mercury isotope fractionation, resulting in distinct isotopic compositions within various environmental compartments and between different mercury species. Mercury stable isotope ratios, which can be altered by both mass-dependent and mass-independent isotope fractionation (MDF and MIF) mechanisms [4], can be used to study the biogeochemical cycling of mercury in ecosystems and to aid in the identification of sources of mercury contamination.
Typically, isotopic measurements are made on total mercury (THg) within environmental samples, but recently, isotopic measurements on individual mercury pools or species within individual samples have become possible with the use of various sequential extraction [5,6,7,8,9,10,11,12,13] or compound-specific extraction [14,15,16,17,18,19,20,21,22,23,24,25,26,27] techniques. Various chemical species of mercury within a sample can have distinct isotopic compositions, and when the specific mercury species of interest, often MeHg, makes up only a small fraction of the THg, its isotopic composition can be masked [27]. For example, while the MeHg fraction in fish occupying higher trophic levels often makes up the majority of the THg, the MeHg fraction in other environmental samples such as sediment, biofilm, and lower-trophic-level organisms is typically small, meaning that their MeHg isotopic compositions are masked when only the THg isotopic composition is measured. Estimation approaches using linear regression and mass balance techniques have been developed to approximate the isotopic composition of MeHg within a food web while only measuring the THg and MeHg concentrations and the THg isotopic composition of each organism [28,29,30]. This approach works well when there is only one primary source of iHg and one primary source of MeHg to a food web, each with a narrow and consistent isotopic composition. However, in some ecosystems, there may be multiple isotopically distinct sources of iHg and/or MeHg, in which case the ability to directly analyze the MeHg isotopic composition within organisms and basal resources is especially beneficial. Assessing the isotopic composition(s) of MeHg within a food web, either directly or by estimation, is useful for identifying potential sources of MeHg to the organisms, as well as tracking biogeochemical transformations within bioavailable mercury prior to its incorporation into the food web.
A number of techniques have been developed to separate MeHg from iHg for isotopic analysis [31]. One example is the toluene extraction method [15, 17, 21, 22, 25, 26], which has been used successfully for a wide variety of sample types including plant and animal tissue and human hair. However, this method has been found to be unsuitable for biota samples with a high lipid content, such as plankton and some fish samples, due to the formation of a thick emulsion layer during the extraction [22, 26]. Another recently developed method is an alkaline digestion followed by reduction and volatilization of iHg by stannous chloride (SnCl2), which can be accomplished in a shorter amount of time compared to some of the other MeHg separation methods and has been shown to work well for fish and plankton reference materials [26]. However, dissolved organic matter (DOM) in a non-brominated sample matrix can potentially cause incomplete reduction and removal of iHg by SnCl2 [32]. Additionally, while SnCl2 is generally considered to be nonreactive toward MeHg [33,34,35], a recent study found that MeHg can be reduced by SnCl2 under conditions of low sulfate concentration [36], though this issue was not observed when the alkaline digestion/SnCl2 reduction method was applied to aqueous MeHg standards [26]. Other techniques for separating MeHg from iHg for isotopic analysis have involved the use of gas chromatography (GC), either offline [16, 20, 21] or coupled directly to a multi-collector inductively coupled plasma mass spectrometer [14, 18, 37,38,39,40]. Variations of these GC separation methods have been used for sediment, soil, and animal tissue samples, as well as bacterial cultures and aqueous solutions with high MeHg concentrations. However, incomplete recovery of MeHg by variable species derivatization (i.e., ethylation or propylation) efficiency can cause artificial shifts in the mercury isotopic composition, leading to inaccurate isotopic measurements and high uncertainty [41]. Additional challenges for the online GC separation method involve drifting isotope ratios during transient peak elution [14, 37, 38], and even when sample pre-concentration strategies (extended elution times) and species-specific bracketing schemes are employed, reported uncertainty in isotopic measurements has remained high relative to other offline MeHg separation methods [18]. Offline high-performance liquid chromatography (HPLC) has also recently been used for MeHg separation for isotopic analysis [19]. This method does not require species derivatization and has resulted in isotope data with lower levels of uncertainty. Other methods of separating MeHg have involved the use of anion-exchange chromatography, which has been used for MeHg isotopic analysis of aqueous solutions with high MeHg concentrations [42, 43]. More recently, methods employed to separate MeHg for isotopic analysis have involved the use of distillation followed by either GC separation [16, 38] or anion-exchange chromatography [22,23,24, 27], the latter of which has been used successfully for a wide variety of biological and abiotic sample types with both high and low levels of MeHg relative to iHg (%MeHg). Distillation can be a particularly useful pre-treatment method for samples with a high organic matter content, though this method requires careful optimization to achieve complete recovery of MeHg and prevent the artificial formation of MeHg during the process, which can be an issue for samples with low %MeHg [44, 45].
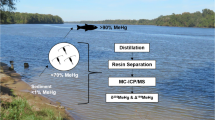
In this study, we developed a method for extracting and isolating MeHg from biota samples for isotopic analysis that utilizes a widely used hot nitric acid digestion followed by a batch anion-exchange resin separation procedure (Fig. 1). This new combination of procedures is relatively simple and avoids some of the challenges involved with optimizing distillation and GC separation methods. A nitric acid digestion may also be more suitable for certain sample types than a toluene extraction or alkaline digestion. Additionally, by using this method, the MeHg concentration and MeHg isotope ratios can be measured on the same sample aliquot, and both MeHg and THg concentrations can be measured at the end of the anion-exchange resin separation procedure to verify MeHg recovery and check for iHg impurities prior to isotopic analysis.
To assess accuracy and precision, we performed this new method on aqueous MeHg and iHg standards, four different biological reference materials, and five different natural biota samples. Each sample type was processed and analyzed multiple times. The MeHg isotopic compositions of the reference materials were compared to results from several other studies that used different MeHg separation techniques. In this paper, we also present the results of a holding test in which aqueous MeHgCl standards were exposed to different acidic matrices (with and without nitric acid) and to different bottle types (borosilicate glass and polyethylene terephthalate glycol (PETG)) to verify that the materials used throughout the procedure were sufficient for maintaining consistent MeHg concentrations.
Methods
Description of reference materials and biota samples
Standard reference materials used for THg and MeHg concentration and isotopic analysis included DORM-3 fish protein, TORT-2 lobster hepatopancreas, DOLT-2 dogfish liver, and DOLT-5 dogfish liver (National Research Council Canada; DOLT-5 used for MeHg analyses only). Total Hg and MeHg concentration and isotopic analyses were also performed on five biota samples collected from East Fork Poplar Creek, a point-source contaminated stream in Oak Ridge, TN, USA. These samples, in order of increasing proportion of THg as MeHg (%MeHg), include Asian clams (soft tissue; 6.3% MeHg), megaloptera larvae (whole body; 12% MeHg), mayfly larvae (whole body; 13% MeHg), crayfish (muscle tissue; 62% MeHg), and shiner (skinless fillet; 104% MeHg). Except for the shiner fillets, all samples were multi-individual composites. All samples were placed in either clean plastic bags or clean centrifuge tubes, placed on dry ice in the field, and then frozen the same day upon arrival at the laboratory. Biota samples were later freeze-dried and ground using a SPEX 8000 Mixer/Mill with an alumina grinding cylinder and ball. To avoid cross contamination, Ottawa Sand (quartz, Fisher Scientific) was ground between each sample, and the grinding cylinder and ball were rinsed thoroughly with deionized water and isopropanol. Prepared samples were stored in trace-metal clean borosilicate glass vials in the dark.
Total mercury extraction by combustion
Standard reference materials (Table S1) and natural biota samples (Table S2) were prepared for analysis of THg concentration and isotopic composition following a previously described combustion procedure [46]. Sample aliquots (250 to 460 mg for reference materials; 25 to 100 mg for natural biota samples) were combusted in a two-stage furnace, and volatilized Hg(0) was trapped in a 24-g oxidizing solution of 1% KMnO4 (w/w) in 10% H2SO4 (v/v) (hereafter, 1% KMnO4). Trap solutions of 1% KMnO4 were later reduced with hydroxylamine hydrochloride (HONH3Cl), and a small aliquot was analyzed for THg concentration using cold vapor atomic fluorescence spectrometry (CVAFS; RA-3F, Nippon Instruments) following EPA Method 1631 [47]. Samples were analyzed in batches with quality control that included analysis of calibration verification standards, secondary standards, and blanks (see Section S1 in the Electronic supplementary material).
To eliminate matrix effects from combustion residues, aliquots of the 1% KMnO4 combustion trap solutions were reduced with SnCl2, and Hg(0) was transferred to secondary 1% KMnO4 trap solutions [46]. These secondary solutions were later reduced with HONH3Cl, and a small aliquot was analyzed for THg concentration by CVAFS. This was done to assess the percent recovery of the transfer process and to allow matching of standard and sample concentrations for isotopic analysis. Recovery of mercury after the transfer process was 98.0 ± 2.7% (1 SD, n = 19 including reference materials and natural biota samples) (Table S1, Table S2).
Procedural blanks and standard reference materials were combusted to monitor combustion performance. Average procedural blank 1% KMnO4 solutions yielded 0.12 ng Hg (± 0.10 ng Hg, 1 SD, n = 18) prior to transfer, and 0.10 ng Hg (± 0.08 ng Hg, 1 SD, n = 16) after transfer, representing < 0.2% of the sample solution mercury mass. Standard reference materials (with certified THg concentrations provided in parentheses) included DORM-3 (382 ± 60 ng g−1 THg) with an average recovery of 97.5 ± 2.4% (1 SD, n = 7), TORT-2 (270 ± 60 ng g−1 THg) with an average recovery of 100.5 ± 1.7% (1 SD, n = 5), and DOLT-2 (1990 ± 100 ng g−1 THg) with an average recovery of 106.5 ± 3.0% (1 SD, n = 2) relative to certified values (Table S1).
Methylmercury extraction by nitric acid digestion
Standard reference materials and natural biota samples were prepared for analysis of MeHg concentration (Table S3) following a modified version of a previously described 12-h nitric acid digestion procedure [48, 49]. The digestion was done in a stainless steel water bath placed inside a drying oven (Fisher Scientific, Isotemp Oven, Model 625G) (Fig. S1a). Two thermocouples were threaded through a hole in the top of the oven, one of which was also threaded through a small hole drilled through the lid of the water bath (Fig. S1a–b). These thermocouples were used to monitor air and water temperature prior to and during the digestion.
On the morning of the digestion, sample aliquots (25 to 75 mg for reference materials; 40 to 60 mg for natural biota samples; aiming for a minimum of 6 ng MeHg) were weighed into pre-weighed 15-mL borosilicate glass centrifuge tubes, and then 7.5 mL of 30% (v/v) HNO3 was added to each vial. Separate sample aliquots weighed for matrix spike tests were immersed in the nitric acid, as described above, and then dosed with small volumes (120 to 800 µL) of a 50- or 1000-ng g−1 MeHgCl spike solution in 0.5% (v/v) acetic acid + 0.2% (v/v) HCl. Additional centrifuge tubes for procedural blanks and aqueous MeHgCl standard tests, to which no dry sample material was added, were included with each digestion batch. Aqueous MeHgCl standard tests received 400 µL of a ~ 50-ng g−1 MeHgCl spike solution. Each tube was shaken by hand and then centrifuged for 15 min at 3300 revolutions per minute (1380 relative centrifugal force (RCF)) which helped prevent the sample material from floating to the top of the solution during the digestion. After centrifuging, the tubes were shaken vigorously either by hand or with a touch mixer (Fisher Scientific, Model 232) to dislodge the plug of sample material from the tip of the centrifuge tube and to thoroughly remix contents. Just before the centrifuge tubes were placed into the hot water bath, each tube was shaken again and then rolled while being held at an angle, which helped prevent the sample material from sticking to the glass in the top half of the tube. Each tube was then placed in a plastic vial rack in the hot water bath inside the oven. The air and water temperatures were monitored to maintain a water temperature of 59.5 to 60.5 °C throughout the 12-h digestion. At the end of the digestion, the sample tubes were removed from the water bath and shaken by hand, then the tube contents were diluted with 7.5 mL of deionized water [49] and the sample tubes were shaken again. The sample tubes were placed in a refrigerator to lower the temperature of the digestion samples and stored there overnight (maximum of 2 days) prior to MeHg analysis, at which time they were brought to room temperature and weighed to determine the total solution mass for calculation of the solid-sample MeHg concentration.
Aliquots of the digestion samples were analyzed for their MeHg concentration by gas chromatography coupled with cold vapor atomic fluorescence spectroscopy (GC-CVAFS) (MERX-M, Brooks Rand Instruments) following a modified version of EPA Method 1630 [50] (Table S3). Sample and standard aliquots were added to 40-mL amber glass vials containing deionized water and 300 µL of 2 M acetate buffer. The pH was adjusted to 4.5 using small amounts of 20% (w/v) KOH [49], and the amber vials were inverted to mix. Freshly thawed 1% (w/v) sodium tetraethylborate (NaBEt4) in 2% KOH was slowly inverted five times to gently mix the solution, and then 50 µL was added to each of the amber vials, which were then re-capped and shaken to mix before starting the analysis. After removing aliquots for MeHg concentration analysis, digestion samples in their original glass tubes were returned to a refrigerator overnight before performing the batch anion-exchange resin separation procedure.
Samples were analyzed for MeHg concentration in batches with quality control that included calibration verification standards, secondary standards, and blanks, and each digestion sample was analyzed in duplicate (see Section S2 in the Electronic supplementary material). The exact concentration, or titer, of the aqueous MeHgCl standard (Brooks Rand) used for calibration standards, digestion matrix spike tests, and aqueous standard tests was determined following EPA Method 1630 [50]. On average, the nitric acid digestion procedural blank solutions yielded 0.01 ng MeHg (± < 0.01 ng MeHg, 1 SD, n = 13), representing < 0.2% of the sample solution MeHg mass. Standard reference materials (with certified MeHg concentrations provided in parentheses) included DORM-3 (355 ± 56 ng g−1 MeHg; 93% MeHg), TORT-2 (152 ± 13 ng g−1 MeHg; 56% MeHg), DOLT-2 (693 ± 53 ng g−1 MeHg; 35% MeHg), and DOLT-5 (119 ± 58 ng g−1 MeHg; 27% MeHg). Duplicate nitric acid digestion matrix spike tests were performed on each of the reference materials and natural biota samples, with an average recovery of 101.4 ± 2.4% (1 SD, n = 22) (Table S3). Nitric acid digestion aqueous MeHgCl standard tests had an average recovery of 99.9 ± 2.7% (1 SD, n = 8).
Isolation of methylmercury by resin separation for isotopic analysis
The nitric acid digestion releases not only MeHg, but also a large portion of the iHg from biota samples. To isolate the MeHg from the iHg for isotopic analysis, we utilized an anion-exchange resin (Bio-Rad AG 1-X4 resin, analytical grade, 200–400 mesh, chloride form), which under acidic and chlorinated conditions removes negatively charged iHg species (e.g., HgCl3− and HgCl42−) while leaving neutral MeHg species (e.g., MeHgCl0) in solution [51,52,53]. For this study, reference materials and natural biota samples with greater than ~ 60% of THg as MeHg underwent one resin separation step, and those with less than ~ 60% of THg as MeHg underwent two consecutive resin separation steps. This protocol was based on testing an initial set of reference materials.
Prior to the nitric acid digestion, resin was cleaned and conditioned [54, 55] so that it was ready for use immediately after the MeHg concentration analysis. Approximately 2.5 g of resin was weighed into 20-mL glass vials, which were then filled to the shoulder with 4 M HNO3. Resin vials were loaded onto a tube rotator (Fisher Scientific) and rotated for 15 min at 25 rotations per minute (Fig. S1c). Resin vials were then removed from the rotator, and after waiting ~ 10 min with them sitting upright, the liquid layer was pipetted out and discarded, and the vials were again filled with 4 M HNO3. This HNO3 cleaning process was repeated four times, except that after rotating the fourth time, the resin vials were not decanted, but rather they sat upright filled with HNO3 overnight. The next day, this same process was used to condition the resin, first with one round of 4 M HNO3, then three rounds of deionized water, then three rounds of 0.1 M HCl. After rotating a third time with HCl, the resin vials were not decanted, but rather they sat upright filled with HCl for a few days until it was time to perform the resin separation procedure.
After the MeHg concentration analysis was complete, acid digestion sample tubes were removed from the refrigerator, brought to room temperature, and weighed (total solution mass was required for calculating the syringe filtering percent recovery). Digested samples were syringe filtered using 50-mL polypropylene syringes (no rubber on plunger) that had been cleaned with 5% HCl, and 0.45 µm polypropylene syringe filters. Just before filtering each digestion sample, the filter was cleaned by pipetting 15 mL of 15% HNO3 into the syringe barrel and filtering into a waste container. The digestion sample was then shaken and carefully poured into the syringe barrel and filtered into either a 125-mL PETG bottle containing 50 mL of 1.1% HCl or a 250-mL PETG bottle containing 150 mL of 1.1% HCl. All PETG bottles were pre-weighed and had previously been filled completely with 1% HCl, which was discarded just prior to syringe filtering without rinsing the bottles. After each digestion sample was syringe filtered into its PETG bottle, 15 mL of 1.1% HCl was pipetted into the glass digestion sample tube, which was shaken and then poured into the syringe barrel and filtered into the PETG bottle. This rinse step was repeated a total of four times. At this point, samples filtered into 125-mL PETG bottles would have an acid content of 1.8% HNO3 + 1% HCl, and samples filtered into 250-mL PETG bottles would have an acid content of 1% HNO3 + 1% HCl. The PETG bottles were weighed (total solution mass was required for calculating the syringe filtering percent recovery), then a small aliquot was transferred from each bottle into a pre-weighed trace-metal clean borosilicate vial for MeHg and THg concentration analysis, and then the PETG bottles were weighed again (total solution mass was required for calculating the resin separation recovery). Next, the 0.1 M HCl was pipetted out of each of the 20-mL resin conditioning vials and discarded, and the vials were filled with a 1% HNO3 + 1% HCl solution, shaken by hand, and poured into each of the PETG bottles. The PETG bottle caps were secured with parafilm and attached to the tube rotator which was tilted backward to 129° to accommodate the height of the bottles (Fig. S1d–e). The bottles were rotated for 2 h at 25 rotations per minute, based on the mixing time used previously by Štrok et al. [54] for their batch resin mercury pre-concentration procedure.
At the end of the rotation period, the contents of the PETG bottles were filtered using 0.45-µm cellulose nitrate filter cups (Thermo Scientific, #130-4045) and a vacuum pump (Fig. S1f). Each filter cup was first conditioned with ~ 150 mL of a 1% HNO3 + 1% HCl solution, which was filtered and discarded. For samples undergoing one resin separation step, the contents of the PETG bottle were filtered into a 500-mL Pyrex glass bottle that had been previously trace-metal cleaned in a hot 10% HNO3 bath for at least 8 h and then filled with 1% HCl at room temperature for at least 8 h, then rinsed and set to dry. For samples undergoing two consecutive resin separation steps, the contents of the PETG bottle were filtered into a secondary PETG bottle (either into a 250-mL bottle if the sample was originally in a 125-mL bottle or into a 500-mL bottle if the sample was originally in a 250-mL bottle). As with the first set of PETG bottles, these Pyrex and secondary PETG bottles were also pre-weighed and had previously been filled completely with 1% HCl, which was discarded just prior to filtering the samples. After each sample was filtered, 40 mL of a 1% HNO3 + 1% HCl solution was poured into the original PETG bottle, which was shaken, filtered, and poured into the new Pyrex or PETG bottle. This rinse step was repeated with two rounds of 40 mL of 0.1 M HCl and then 40 mL of deionized water. The Pyrex and PETG sample bottles were weighed (total solution mass was required for calculating the resin separation recovery), then a small aliquot was transferred from each PETG bottle into a pre-weighed trace-metal clean borosilicate vial for MeHg and THg concentration analysis, and then the PETG bottles were weighed again (total solution mass was required for calculating the second resin separation recovery). The resin separation process was repeated a second time for those samples in PETG bottles. After the second resin separation step, the samples were filtered (using new filter cups) into 500-mL Pyrex bottles. Pyrex sample bottles were refrigerated for up to 2 days before MeHg concentration analysis.
After the resin separation procedure was complete, sample aliquots were analyzed for their MeHg concentration following EPA Method 1630 [50] as described in the previous section. Samples representing the final resin step (in Pyrex bottles) were analyzed in duplicate, and sample aliquots used to calculate recovery of MeHg after syringe filtering and the first of two resin separation steps (in glass vials) were each analyzed once. Following the MeHg concentration analysis, all Pyrex bottles and glass vials containing samples were weighed, and then the samples were oxidized with 5% BrCl for the final resin step samples in Pyrex bottles, or 10% BrCl for sample aliquots in glass vials, and weighed again. It is important while performing the resin separation procedure to not expose samples to any BrCl, including fumes, as this could inadvertently oxidize MeHg and convert it into iHg. Brominated sample bottles and vials were placed on a covered hot plate at 70 °C for a minimum of 2 days, and then aliquots of syringe-filtered and resin separation samples were transferred into 15-mL Teflon vials, after which the sample bottles were immediately returned to the hot plate. Teflon sample vials were exposed to ultraviolet (UV) light for 7 to 16 days to break down dissolved organic matter—this was a conservative approach based on convenience and previous experience, but was not specifically optimized to maximize efficiency. Sample aliquots were then analyzed for their THg concentration by CVAFS following EPA Method 1631 [47]. Samples were analyzed in batches with quality control including calibration verification standards, secondary standards, blanks, and matrix spike recovery tests (see Section S1 in the Electronic supplementary material). The MeHg and THg concentrations were used to calculate MeHg recovery and purity after each of the syringe filtering and resin separation steps (Table S3).
Prior to isotopic analysis, each of the final resin separation samples was chemically reduced, and the resulting Hg(0) was purged from solution and re-oxidized in a 1% KMnO4 trapping solution following previously described methods [46]. In preparation for the purge-and-trap procedure, brominated samples were placed on a covered hot plate for 2 weeks to break down dissolved organic matter—again, this was a conservative approach based on convenience and previous experience but was not specifically optimized to maximize efficiency. Samples were then diluted to 1 L with deionized water and then further acidified (0.5% HCl). Additional BrCl (to 1% BrCl) was typically not necessary, as the samples had already been brought to 5% BrCl prior to dilution. Samples were then pre-reduced with 2 mL HONH3Cl per 1 L of 5% BrCl solution and allowed to react for ~ 1 h. Within the closed purge-and-trap system, samples were reduced with ~ 100 mL of 10% SnCl2 (in 10% HCl), and the resulting Hg(0) was purged from solution with clean-laboratory air (passed through a gold filter) and subsequently trapped in a 5–10-g oxidizing solution of 1% KMnO4. The 1% KMnO4 trap solutions were later reduced with HONH3Cl, and a small aliquot was analyzed for THg concentration using CVAFS following EPA Method 1631 [47] as previously described for combustion solutions. Purge-and-trap recovery of mercury from resin separation samples was 101.4 ± 1.8% (1 SD, n = 30 including aqueous MeHgCl standards, biological reference materials, and natural biota samples) (Table S4). Purge-and-trap procedural blanks and standards (7.5, 15, and 35 ng Hg; NIST SRM 3133) were used to monitor analytical performance. Procedural blank 1% KMnO4 solutions, yielding 0.03 ng Hg (± 0.01 ng Hg, 1 SD, n = 3), represented < 0.5% of sample solution mercury mass. Procedural standard recovery was 98.5 ± 0.7% (1 SD, n = 3), and procedural standards were not significantly fractionated isotopically relative to NIST SRM 3133 bracketing standards (Table S1).
Mercury isotopic analysis
Following the transfer (for combustion analysis) and purge-and-trap (for resin separation) pre-concentration procedures, the mercury isotopic composition of each 1% KMnO4 trap solution (Table S4) was measured using cold vapor multiple collector inductively coupled plasma mass spectrometry (CV-MC-ICP-MS; Nu Instruments) using previously described methods [56, 57]. Thallium (NIST SRM 997) was used as an internal standard to correct for instrumental mass bias, along with sample-standard bracketing with mercury standard NIST SRM 3133. On-peak zero corrections were applied to all masses.
Mass-dependent isotope fractionation (MDF) is reported as the permil (‰) deviation from the average of NIST SRM 3133 bracketing standards [57] using delta notation:
where xxx is the mass of each mercury isotope between 199Hg and 204Hg. Mass-dependent fractionation is reported as δ202Hg values. Mass-independent isotope fractionation (MIF) is reported as the difference between the measured δxxxHg value and that which is theoretically predicted by the kinetic mass-dependent fractionation law [57], using capital delta notation:
where xxx is the mass of each mercury isotope 199Hg, 200Hg, 201Hg, and 204Hg, and β is a constant for each isotope (0.252, 0.502, 0.752, 1.493, respectively) [57].
To characterize the analytical uncertainty and reproducibility associated with isotope ratio measurements, each analytical session included 5 to 14 analyses of a secondary standard (UM-Almadén) at representative mercury concentrations (1 to 5 ng g−1). We also measured the isotopic composition of each combustion reference material two to four times within an analytical session. To evaluate the accuracy and reproducibility of our results, we calculated the mean isotopic composition (± 2 SE) for the collection of independent preparations of UM-Almadén and each reference material type (Table S1), and compared those means to the long-term average isotopic composition measured at the University of Michigan [58]. We represent the analytical uncertainty in the THg isotopic composition of natural biota samples (via combustion) with the average uncertainty (2 SD) across combustion reference material analyses (Table S1, Table S2). Because each reference material process replicate was analyzed only once for MeHg isotopic composition, we represent the analytical uncertainty in the MeHg isotopic composition of reference materials and natural biota samples (via resin separation) with the average uncertainty (2 SD) across UM-Almadén analyzed alongside resin separation materials within each session (Table S1, Table S4). Additionally, in order to provide a direct comparison of the MeHg isotopic composition of our reference materials to other published values, we also report the reproducibility associated with complete process replicates (reported as 2 SD) for each of the four biological reference materials (each with 4 or 5 replicates), and also for each of the five natural biota samples (each with 2 replicates) (Table S4).
Results and discussion
Nitric acid digestion validation
Standard reference materials were used to assess the performance of the nitric acid digestion procedure. Reference materials included DORM-3 with an average MeHg recovery of 94.7 ± 0.8% (1 SD, n = 4), TORT-2 with an average recovery of 108.1 ± 2.4% (1 SD, n = 4), DOLT-2 with an average recovery of 114.4 ± 1.6% (1 SD, n = 5), and DOLT-5 with an average recovery of 108.2 ± 0.9% (1 SD, n = 5) relative to certified values (Table S3). The average measured MeHg concentrations for three of the four reference materials were within the uncertainty of their certified values, with the exception of DOLT-2 for which the measured MeHg concentration was within two times the uncertainty of its certified value (Table S3). The exact concentration, or titer [50], of the Brooks Rand 1 ppm aqueous MeHgCl standard solution was found to be 1.076 µg g−1 MeHg (as Hg). When this titer was accounted for in the calibration curve used for MeHg analyses, this resulted in sample concentrations that were 7.6% higher than if the MeHgCl standard was assumed to be exactly 1.000 µg g−1. It is unknown whether the titer of the MeHg standard solution was accounted for in the calibration curves of the analyses performed to certify each of the reference materials. If it was not, then this might explain why our nitric acid digestion recoveries were ≥ 108% for three of the standard reference materials. In general, a difference in measured MeHg concentrations of 7.6% would have a very small effect on interpreting the results of environmental samples; however, this difference has a relatively large effect on calculating the percent purity (MeHg/THg) of resin separation samples (Table S3). Overall, we believe that it is important to use the exact titer of the MeHgCl standard solution in the calibration curves of all MeHg concentration analyses, even though it shifts the nitric acid digestion percent recovery for one of the reference materials (DOLT-2) just beyond its certified range.
The average percent relative standard deviation (%RSD) associated with the measured MeHg concentration of the nitric acid digestion sample replicates for both reference materials and natural biota samples was 1.2 ± 0.8% (1 SD, n = 9), demonstrating consistency in the extraction of MeHg during the nitric acid digestion. Aqueous MeHgCl standard tests (containing no solid material) had an average recovery of 97.9 ± 3.1% (1 SD, n = 7) after the nitric acid digestion. Duplicate matrix spike tests were performed on each of the reference materials and natural biota samples, in which a small aliquot of the aqueous MeHgCl standard solution was added to the glass sample tube prior to the start of the nitric acid digestion. The MeHg concentration of each of the matrix spike tests was ~ 2 to 3 times higher than the background concentration of the sample, with the exception of the mayfly larvae samples in which the matrix spike tests were ~ 6 times higher. Across all reference materials and natural biota samples, the average recovery for the matrix spike tests was 101.4 ± 2.4% (1 SD, n = 22) (Table S3). Complete recovery of nitric acid digestion matrix spike tests suggested that our MeHg concentrations were not influenced by matrix interferences, and therefore, we did not make independent measurements of MeHg concentrations of natural biota samples using another mercury extraction technique. As individual laboratories develop the use of this or other MeHg separation techniques, it will be important to evaluate QA/QC metrics to determine whether independent verification of MeHg concentrations in natural samples is warranted. Overall, complete recovery of MeHg for the reference materials and matrix spike tests in this study, along with small %RSD values for sample replicates, demonstrates that the nitric acid digestion procedure was reliable for extracting MeHg from biota samples, which could then be subsequently separated from iHg for isotopic analysis using the resin separation procedure.
Separation efficiency of methylmercury in aqueous standard solutions
In order to follow a nitric acid digestion with a resin separation procedure, samples need to be diluted to a lower nitric acid content because MeHg degrades over time under high nitric acid content [59, 60]. Additionally, high acidity prevents iHg from adsorbing to the resin [61]. We aimed to dilute the digestion sample solutions to 1–2% HNO3 based on other studies which showed that MeHg solutions maintained their concentration for at least 2 weeks in a 1% HNO3 matrix [62] and demonstrated complete adsorption of iHg to the same anion-exchange resin used in our study in a 1% HNO3 + seawater matrix [54]. We also added ~ 1% HCl to form negatively charged iHg species (HgCl3− and HgCl42−) [53] that would be retained on the resin [51], and to prevent MeHg from adsorbing to the PETG and glass bottles and vials throughout the process [60].
To test whether a 1% HNO3 + 1% HCl matrix was sufficient for maintaining consistent MeHg concentrations throughout the resin separation process without either degrading the MeHg or allowing the MeHg to be adsorbed to the walls of the container, we conducted a long-term holding test. This involved the use of 13-, 45-, and 135-pg g−1 aqueous MeHgCl standards in either a 1% HCl or a 1% HNO3 + 1% HCl matrix, in both PETG and glass bottles. Each of the solutions was analyzed for its MeHg concentration after 0, 7, 14, 28, 49, and 83 days (Table S5). Aqueous MeHgCl standards at all three solution concentrations in a 1% HCl matrix in glass bottles maintained > 97% recovery over the entire testing period. For the other three matrix + bottle type combinations (1% HCl in PETG; 1% HNO3 + 1% HCl in glass; and 1% HNO3 + 1% HCl in PETG), MeHg recovery across all three solution concentrations declined to ≤ 95% either between 7 and 14 days or between 14 and 28 days (Fig. S2). For MeHgCl standards in a 1% HNO3 + 1% HCl matrix, the 13-pg g−1 solutions were more susceptible to loss of MeHg than solutions at higher concentrations, reaching minimum recoveries of 90.4% and 88.6% in glass and PETG bottles, respectively, though this difference in recovery at lower concentrations was not observed for MeHgCl standards in a 1% HCl matrix (Fig. S2). Despite the observed losses of MeHg over time, potentially due to degradation by nitric acid and/or adsorption to PETG bottles, as long as the nitric acid digestion, syringe filtering, and resin separation procedures are performed in less than a week, the use of PETG bottles and a 1% HNO3 + 1% HCl matrix should not influence MeHg recoveries.
We tested the syringe filtering and resin separation procedures using 3, 10, and 30 ng aqueous MeHgCl standards (~ 200, 670, and 2000 pg g−1 in 15 mL of 15% HNO3 in glass centrifuge tubes prior to syringe filtering and dilution with 1.1% HCl). The average recovery of MeHg after syringe filtering was 98.3 ± 1.1% (1 SD, n = 5), and the average recovery of the resin separation was 98.6 ± 1.3% (1 SD, n = 5), resulting in an average cumulative recovery of 96.9 ± 1.8% (1 SD, n = 5) (Table S3). The average isotopic composition of the post-resin separation aqueous MeHgCl standards (Table S4) was within error of the average THg isotopic composition of the source MeHgCl (Table S1). Note that different batches of Brooks Rand MeHgCl standards may be isotopically unique, as they are not certified for mercury isotopic composition, with recorded δ202Hg values of different batches ranging from − 1.21 to − 0.48‰ [16, 20, 22, 26, 27]. Average nitric acid digestion procedural blank solutions yielded 0.01 ng MeHg (± 0.02 ng MeHg, 1 SD, n = 3) after syringe filtering, and 0.03 ng MeHg (± 0.03 ng MeHg, 1 SD, n = 3) after the resin separation, representing < 0.6% of the sample solution MeHg mass.
We also tested the resin separation procedure using 10, 100, and 250 ng aqueous iHg standards (NIST 3133 in 2% BrCl reduced with HONH3Cl) and found that on average, 98.6 ± 0.5% (1 SD, n = 4) of the iHg had adsorbed to the resin. We found that using 5 g of resin only had a slightly higher iHg adsorption efficiency (99.4%, n = 1) compared to using 2.5 g of resin (98.6%, n = 1) for 250 ng aqueous iHg standards, and so we opted to use 2.5 g of resin for all resin separations to minimize resin usage. Average nitric acid digestion procedural blank solutions yielded 0.03 ng THg (± < 0.01 ng THg, 1 SD, n = 3) after syringe filtering, and 0.06 (± 0.03 ng THg, 1 SD, n = 3) after the resin separation, representing < 1.2% of the sample solution THg mass.
Overall, nearly complete recovery of MeHg and adsorption of iHg to the resin from aqueous standard solutions, along with the apparent lack of procedurally induced isotope fractionation, suggests that the batch resin separation procedure is a reliable method of separating MeHg from iHg in a 1–2% HNO3 + 1% HCl matrix following a nitric acid digestion.
Separation efficiency of methylmercury for biological standard reference materials and natural biota samples
Across all four standard reference materials (DORM-3, TORT-2, DOLT-2, and DOLT-5), the average recovery of MeHg after syringe filtering the nitric acid digestion samples was 95.5 ± 2.3% (1 SD, n = 18). Similarly, across all five natural biota samples (shiner fillet, crayfish, megaloptera larvae, mayfly larvae, and Asian clams), the average recovery of MeHg after syringe filtering the nitric acid digestion samples was 96.9 ± 1.1% (1 SD, n = 10). The small losses of MeHg during syringe filtering could be explained by several factors, such as degradation and conversion into iHg by the ~ 15% HNO3, re-adsorption to solid particles, or adsorption to the glass centrifuge tube after the initial MeHg concentration analysis but before syringe filtering, which were typically done 1 day apart. Other possible explanations include physical losses while filtering, adsorption to the 0.45-µm polypropylene syringe filter, or adsorption to the PETG bottle after filtering but before removing an aliquot for analysis and beginning the resin separation procedure, which was typically done the day after syringe filtering. To determine which of these potential explanations were more likely, we performed a test using replicate nitric acid digestion samples for DOLT-2, the shiner fillet, and the megaloptera larvae. We found that after 3 days in a refrigerator, the unfiltered nitric acid digestion samples still had an average MeHg recovery of 100.2 ± 0.4% (1 SD, n = 3), suggesting that the MeHg was not degraded by the ~ 15% HNO3, nor had it re-adsorbed to solid particles or adsorbed to the glass centrifuge tubes over a 3-day holding time. These digestion samples were syringe filtered into PETG bottles, and aliquots were transferred into trace-metal clean glass vials immediately after filtering and again after ~ 12 and ~ 60 h, which were then analyzed for their MeHg concentration. Across these three samples, recovery of MeHg immediately after syringe filtering was 98.1 ± 0.7% (1 SD, n = 3), suggesting that ~ 2% of the MeHg was lost due to either physical losses or adsorption to the 0.45-µm polypropylene syringe filter. This result aligns with the MeHg recovery of 98.3 ± 1.1% (1 SD, n = 5) after syringe filtering the aqueous MeHgCl standards (Table S3). For DOLT-2 and the megaloptera larvae, recovery of MeHg after ~ 12 and ~ 60 h in a PETG bottle was 96.0 ± 1.2% (1 SD, n = 4), suggesting that another ~ 2% of the MeHg in these samples had adsorbed to the PETG bottles. For the shiner fillet, recovery of MeHg after ~ 12 and ~ 60 h was 98.4 ± 1.1% (1 SD, n = 2), suggesting that the shiner fillet sample did not experience a significant amount of MeHg adsorption to the PETG bottle. It should be noted that the DOLT-2 and megaloptera larvae digestion samples each contained ~ 20 ng MeHg, whereas the shiner fillet digestion sample contained ~ 70 ng MeHg, and so a larger mass of MeHg in the shiner fillet sample would need to be adsorbed to reflect a 2% adsorption loss. Thus, samples containing lower masses of MeHg may be more susceptible to decreases in recovery by adsorption. Overall, to minimize the loss of MeHg during and after syringe filtering, care must be taken to avoid physical losses, and it may be beneficial to use a different type of filter (e.g., 0.45-µm polyethersulfone filters). It is also important to begin the resin separation procedure as soon as possible after syringe filtering into PETG bottles, which is more easily achieved by working with smaller sample sets so that syringe filtering and the resin separation can be done on the same day.
Across the four standard reference materials, average recovery of MeHg after the first and second resin separation steps was 98.6 ± 2.5% (1 SD, n = 18) and 99.7 ± 2.3% (1 SD, n = 14), respectively. The average cumulative recovery of MeHg after syringe filtering and the resin separation procedure was 94.0 ± 2.6% (1 SD, n = 18) (Table S3). The resin separation recoveries of MeHg were similar among the four reference materials, but for the natural biota samples, recovery of MeHg after the first resin step was lower for the Asian clams than for the other four sample types. For the clam sample (6.3% MeHg in the solid material), the average recovery of MeHg was 90.1 ± 3.1% (1 SD, n = 2), and for the other four sample types (12, 13, 62, and 104% MeHg in the solid material), the average recovery of MeHg was 98.7 ± 1.3% (1 SD, n = 8) after the first resin separation step. Unlike the first resin step, there was not a significant difference in MeHg recovery between natural biota sample types for the second resin separation step, with an average recovery of 97.9 ± 1.2% (1 SD, n = 8 excluding shiner fillets which only underwent one resin step). The average cumulative recovery of MeHg after syringe filtering and the resin separation procedure was 87.1 ± 1.2% (1 SD, n = 2) for clams and 93.7 ± 2.1% (1 SD, n = 8) for the other four sample types (Table S3). The reason for the lower MeHg recovery for the clam samples after the first resin separation step is unclear. It is possible that lower recovery during the first resin step for Asian clams could have been due to losses of MeHg by adsorption to the resin or binding to DOM or other compounds in the digestion sample matrix that were retained by the resin, which would have the greatest impact on recovery for samples containing the lowest mass of MeHg. However, the TORT-2, DOLT-5, and mayfly larvae digestion samples, as well as the 3 and 10 ng MeHgCl standard tests, each contained less MeHg than the clam digestion samples, but none of those other samples had major losses of MeHg to the resin or other surfaces, as demonstrated by their high recovery after the first resin step. Additionally, since high levels of DOM would be ubiquitous across all nitric acid digestion samples of biological material, the lower MeHg recovery for the clam samples alone, relative to all other biological reference materials and natural biota samples, is unlikely to have uniquely resulted from binding to DOM retained on the resin. While the explanation remains unclear, the lower MeHg recovery for the clam samples after the first resin separation step may be more related to the high level of iHg relative to MeHg within the digestion samples. Additional study is required to further explain the reason for lower MeHg recovery after the first resin separation for the clam samples and to potentially modify the MeHg separation procedure to achieve higher cumulative MeHg recovery for samples with low %MeHg.
Based on measured MeHg concentrations relative to THg concentrations, MeHg purity was calculated for each of the standard reference materials and natural biota samples after syringe filtering and after each of the resin separation steps. These values indicate how much iHg remained in the samples after each step. For both the reference materials and natural biota samples, materials with > 60% MeHg (DORM-3, shiner fillet, and crayfish) had ~ 100% MeHg purity after the first resin separation step, whereas materials with ≤ 60% MeHg had ~ 53–93% MeHg purity after the first resin step (Table S3). Although our aqueous iHg standard tests, which were not in the presence of DOM, demonstrated that 250 ng iHg could be adsorbed in a single resin step, this was not reflected in our reference materials and natural biota samples. For example, TORT-2, DOLT-2, and DOLT-5 each contained ~ 8–30 ng iHg, but these samples had ~ 85–93% MeHg purity after the first resin step and required a second resin separation step to further diminish the iHg impurities, bringing their MeHg purity up to ~ 94–99% (Table S3). Likewise, the megaloptera larvae, mayfly larvae, and Asian clam samples each contained ~ 85 to 240 ng iHg, and had ~ 53–69% MeHg purity after the first resin step and ~ 91–98% MeHg purity after the second resin step. Across all reference materials and natural biota samples, the average MeHg purity at the end of the resin separation procedure (whether they underwent one or two resin separation steps) was 97.8 ± 4.3% (1 SD, n = 28), with Asian clams having the lowest MeHg purity of 91.2 ± < 0.1% (1 SD, n = 2) (Table S3). These high-purity values demonstrate that a majority of the iHg was removed from each of the sample solutions by the resin.
For reference materials and natural biota samples that underwent two resin separation steps, the solid material left in the cellulose nitrate filter cup was typically a darker yellow color after the first resin step than after the second resin step, and the aqueous solution became clearer and less foamy each time it was filtered. This could be due to negatively charged DOM within the digestion sample adsorbing to the anion-exchange resin along with the iHg. Though the exact mechanism is unknown, the presence of DOM may be the reason why iHg in the reference material and natural biota digestion samples was not adsorbing to the resin to the same extent as the aqueous iHg standard tests. Washburn et al. [55] similarly found that for surface water samples with high levels of DOM, only 4.1 to 58.8% of the THg (n = 3) was adsorbed when the samples were passed through a column containing the same anion-exchange resin used in our study, compared to an average of 91.6 ± 3.1% (1 SD, n = 7) for other surface water samples with lower levels of DOM. For our samples, once a substantial amount of the DOM was apparently removed from solution during the first resin step, the remaining iHg was more easily adsorbed to the resin during the second step. The slightly lower MeHg purity (91.2%) for the clam sample after the second resin separation step (Table S3) may have been due to both its low %MeHg (6.3%) as well as its organic-rich sample matrix. For samples with < 10% MeHg and those with complex organic-rich matrices, additional modifications to this MeHg separation procedure may be necessary to achieve higher MeHg purity, such as utilizing a third resin separation step, using a larger amount of resin, or employing a resin column separation method rather than a batch resin separation method.
One strength of this study is our rigorous analysis of both MeHg and THg concentrations after each step in the process. The ability to calculate not only MeHg recovery, but also MeHg purity after syringe filtering and after each of the resin separation steps, provides validation that each of the resin-separated mercury samples truly represents MeHg in the organism and allows for the quantification of iHg impurities. Some previous studies using other MeHg separation methods describe measuring both the MeHg and THg concentrations after the final separation step so that both MeHg recovery and purity can be determined [15, 17, 21, 25]. Other studies describe measuring only the THg concentration after the final separation step [22,23,24, 26, 27] and assume that this is equal to the MeHg concentration because, theoretically, all of the iHg should have been removed. However, THg concentration analysis alone could be misleading if MeHg recovery is low and if iHg impurities are present after the final separation step. A good example of this scenario involves the Asian clam sample in our study. Based on the MeHg concentration after the second resin separation step, the calculated cumulative recovery of MeHg was only 87.1% (Table S3). However, because there were iHg impurities in the sample at the end of the procedure, the apparent cumulative recovery of MeHg based on the THg concentration alone after the second resin step would have been 95.5%, making it seem like the procedure performed much better on the clam sample than it actually did. Achieving high MeHg recovery and purity is important for ensuring accurate measurements of MeHg isotopic composition, as losses of MeHg during the separation procedure could potentially lead to isotope fractionation, and the presence of iHg impurities would shift the measured MeHg isotopic composition toward that of the iHg. Therefore, regardless of which MeHg separation procedure is used, we recommend measuring both the MeHg and THg concentrations after the final separation step, prior to isotopic analysis, to verify the actual MeHg recovery and purity for each sample.
For this method of isolating MeHg from biological samples involving nitric acid extraction coupled with batch anion-exchange resin separation, we recommend acceptable separation thresholds of ≥ 90% cumulative MeHg recovery and ≥ 90% MeHg purity, and suggest that recovery and purity values are clearly reported for each sample. We note that the impact of iHg impurities on the measured isotopic composition of a resin-separated mercury sample depends not only on the amount of impurities, but also on the offset in isotopic composition between iHg and MeHg within the organism. For example, if the iHg and MeHg isotopic compositions are ~ 1‰ apart from one another, as is the case for TORT-2 and DOLT-2 (with δ202iHg values calculated via mass balance using measured δ202THg and δ202MeHg values), then the isotopic composition of a resin-separated mercury sample with 90% MeHg purity would theoretically be shifted toward that of the iHg by ~ 0.1‰. In this study, the offsets in δ202Hg and Δ199Hg values of iHg and MeHg within the natural biota samples were never more than ~ 0.3‰, and so the measured MeHg isotope ratios of even the Asian clam samples with only ~ 91% MeHg purity (Table S3) would theoretically be shifted by only ~ 0.03‰. Shifts in the measured MeHg isotopic composition caused by the presence of < 10% iHg impurities are unlikely to influence interpretations based on these measured values, though this in part would depend on the overall range in measured MeHg and THg isotopic compositions of organisms (and basal resources) within the study.
Based on high MeHg recovery and purity (Table S3), nitric acid digestion paired with a batch resin separation procedure is a reliable method for isolating MeHg from iHg for compound-specific isotopic analysis. We recommend using two batch resin separation steps (each with 2.5 g of resin) for biota samples with less than ~ 60% MeHg in the solid material, and only one resin separation step for biota samples with greater than ~ 60% MeHg. However, for biota samples near this ~ 60% MeHg threshold, sample type may also be an important variable to consider when deciding whether to use one or two resin separation steps. For example, TORT-2 lobster hepatopancreas and the natural crayfish muscle tissue sample both had 56–62% MeHg, but only TORT-2 benefitted from a second resin separation step whereas the crayfish had already reached ~ 100% MeHg purity after the first step (Table S3). This suggests that protein-rich samples (e.g., muscle tissue) with moderate %MeHg values may require only one resin step, whereas more complex sample matrices (e.g., organ tissue or whole-body homogenates) with moderate %MeHg values may require two resin steps, potentially due to the quantity and/or quality of organic matter within different sample types. Further investigation may be needed to address differing results for different sample matrices.
Methylmercury isotopic composition of biological standard reference materials and natural biota samples
The MeHg isotopic compositions of the biological reference materials in our study are similar to the values obtained in other studies for the same materials (Fig. 2). Because the reference materials are not certified for their MeHg isotopic composition, we do not know the “true” isotopic composition or whether there are slight differences between batches. The reproducibility associated with complete process replicates (2 SD, n = 4 or 5) for each of the four types of reference materials ranged from 0.04 to 0.15‰ for δ202MeHg and from 0.03 to 0.04‰ for Δ199MeHg (Table S4). These values showed better reproducibility than those associated with the online GC method [18] and were comparable to those associated with offline GC separation [20], toluene extraction [15, 17, 21, 22, 25, 26], distillation and resin separation [22,23,24, 27], and KOH digestion and purge and trap [26] methods (Fig. 2). Similarly, the reproducibility of the MeHg isotopic composition associated with complete process replicates (2 SD, n = 2) for each of the five natural biota samples ranged from < 0.01 to 0.15‰ for δ202MeHg and from 0.01 to 0.03‰ for Δ199MeHg (Fig. 3A, Table S4), also indicating high reproducibility for our nitric acid digestion and resin separation method. Note that the analytical uncertainty in the isotopic composition of MeHg in resin separation samples is represented by the average uncertainty (2 SD) across UM-Almadén analyses (0.05‰ for δ202MeHg and 0.04‰ for Δ199MeHg) (Table S1, Table S4).
Methylmercury isotopic compositions of biological reference materials including (A) DORM-3 and DORM-4, (B) DOLT-5, (C) DOLT-2, and (D) TORT-2, as reported for this study and in the literature using other MeHg separation techniques [15, 18, 20,21,22,23,24,25,26,27]. Note that for (D), partially obscured symbols include an orange square, green triangle, and yellow circle. In order to compare our data with previously published values, the error bars in this figure show the reproducibility associated with complete process replicates (2 SD) for each reference material (Table S4)
THg (open symbols) and MeHg (filled symbols) isotopic compositions of natural biota samples. Shown are (A) Δ199Hg vs. δ202Hg for both THg and MeHg, (B) Δ199THg vs. %MeHg, and (C) δ202THg vs. %MeHg. Error bars on each of the filled symbols (MeHg) show the reproducibility associated with complete process replicates (2 SD, n = 2 for each sample type) (Table S4), which in most cases are the same size or smaller than the size of the symbol. Analytical uncertainty in THg delta values is shown as the average uncertainty (2 SD) across combustion reference material analyses (Table S2). Analytical uncertainty in MeHg delta values is shown as the average uncertainty (2 SD) across UM-Almadén analyses (Table S4)
The MeHg isotopic compositions of TORT-2 (56% MeHg) and DOLT-2 (35% MeHg) differed from their THg isotopic composition, both having δ202MeHg and Δ199MeHg values that were higher than their respective δ202THg and Δ199THg values (Table S1, Table S4). The MeHg isotopic composition of DORM-3 (93% MeHg) was more similar to its THg isotopic composition, as expected. Additionally, some natural biota samples had MeHg isotopic compositions that differed from their THg isotopic compositions, while others had isotopically similar MeHg and THg isotopic compositions. In comparison to the THg isotopic composition of each of the biota samples, mayfly larvae (13% MeHg) and clams (6.3% MeHg) had δ202MeHg values that were lower than their respective δ202THg values, whereas the shiner fillet (104% MeHg), crayfish (62% MeHg), and megaloptera larvae (12% MeHg) each had δ202MeHg and δ202THg values that were similar (Fig. 3A). Each of the biota samples had Δ199MeHg values that were higher than their respective Δ199THg values, with the exception of the shiner fillet for which the Δ199MeHg and Δ199THg values were similar (Fig. 3A).
One previously developed method of estimating the isotopic compositions of iHg and MeHg within a food web involves performing linear regressions for δ202THg vs. %MeHg and Δ199THg vs. %MeHg [30, 63, 64]. The points at which δ202THg and Δ199THg intercept with 100% MeHg indicate the approximate MeHg isotopic composition, and the points at which δ202THg and Δ199THg intercept with 0% MeHg indicate the approximate iHg isotopic composition of the food web. Although the five natural biota samples used in this study were collected from the same stream, their δ202THg and %MeHg results do not conform to a linear relationship (Fig. 3C). Three of the natural biota samples have relatively low %MeHg (6.3 to 13%) but have a wide range in δ202THg (− 0.76 to − 0.14‰) (Fig. 3C), potentially due to the presence of multiple isotopically distinct sources of iHg to the food web. Without a linear relationship between δ202THg and %MeHg, the MeHg isotopic composition of each of these three samples could not be estimated using the regression method. Additionally, although there appears to be a relatively linear relationship between Δ199THg and %MeHg for these five natural biota samples (Fig. 3B), the extracted MeHg isotopic compositions revealed a range in Δ199MeHg values (0.13 to 0.33‰), whereas an ordinary least-squares regression provides only a single value for Δ199MeHg (0.25 ± 0.04‰, 1 SE).
Another previously developed method of estimating the MeHg isotopic composition of organisms involves assigning a consistent iHg isotopic composition to all organisms in a food web, and then calculating the MeHg isotopic composition of each organism using mass balance [28]. However, this approach relies on the assumption that there is only one primary source of iHg to the food web, and that the iHg source has been correctly identified. While linear regression and isotopic mass balance approaches for estimating MeHg isotopic compositions of organisms may work well for food webs with only one primary source of iHg and one source of MeHg [63], the ability to extract and isolate MeHg for direct isotopic analysis is especially beneficial for food webs with multiple isotopically distinct sources of iHg and/or MeHg.
Conclusion
In this study, the use of a nitric acid digestion combined with a batch anion-exchange resin separation procedure has been shown to successfully separate MeHg from iHg within fish and aquatic invertebrate samples, allowing for accurate and precise measurements of MeHg isotopic composition that compare well with other offline separation methods. This new method has some distinct advantages over other MeHg separation methods. For example, nitric acid digestion and batch resin separation procedures are relatively simple methods that can be done without investing in the set-up and careful optimization of distillation procedures, which can be an obstacle for labs that do not already have experience with this technique. Nitric acid digestion is a well-established, widely used method of determining MeHg concentrations of biological samples, and the resin separation procedure can be added as a secondary step without repeating or performing a different mercury extraction technique on a separate sample aliquot. Not only does this eliminate additional procedures for independently measuring MeHg concentrations, but it also conserves sample mass, which is often limited. Moreover, this streamlining of MeHg concentration and separation techniques promotes a tight coupling between MeHg concentration, %MeHg, and isotopic composition, which will likely benefit studies of complex food webs. Additionally, for certain sample types, a nitric acid digestion may be a more suitable method than an alkaline digestion or a toluene extraction. Alkaline digestions can sometimes cause excessive foaming during the MeHg concentration analysis [49], and toluene extractions have been shown to produce a lipid emulsion for some fish [65,66,67] and plankton [22] samples, and are also more toxic. However, the nitric acid digestion and resin separation procedure also has some limitations, such as the total amount of time elapsed between digestion and isotopic analysis, as well as the apparent challenges of achieving high MeHg purity in the presence of organic-rich matrices with low %MeHg, as demonstrated with Asian clams. With additional optimization of UV treatment and hot plate digestion steps, it is likely that the amount of time required for the nitric acid digestion and resin separation method could be substantially decreased. Also, given the success of this method with biological samples ranging from 6 to 100% MeHg, follow-up studies that extend the use of nitric acid digestion and resin separation procedures to isolate MeHg from basal resources with < 10% MeHg are warranted.
By measuring both the MeHg and THg concentrations at several points throughout the MeHg separation process, we were able to independently calculate MeHg recovery and purity for each sample prior to isotopic analysis. We recommend adopting this practice regardless of which MeHg separation method is chosen, because measuring the THg concentrations alone at the end of the procedure has the potential to mask incomplete MeHg recovery if iHg impurities are present. This may be especially important for unknown samples that have low %MeHg, a high organic matter content, and/or a particularly complex matrix, as these types of samples may have an increased risk of being affected by loss of MeHg and incomplete removal of iHg.
We have also shown that for some of the biological standard reference materials and natural biota samples, the isotopic composition of MeHg within the organism can be different from its THg isotopic composition, and that these MeHg isotopic compositions may not be accurately estimated by the regression method when the relationship between THg isotope ratios and %MeHg is not linear. In such cases, directly measuring the isotopic composition of MeHg within organisms, as well as within basal resources, will aid in determining the sources of MeHg production within the ecosystem.
References
Selin NE. Global biogeochemical cycling of mercury: a review. Annu Rev Environ Resour. 2009;34:43–63.
Regnell O, Watras CJ. Microbial mercury methylation in aquatic environments: a critical review of published field and laboratory studies. Environ Sci Technol. 2019;53:4–19.
Barkay T, Gu B. Demethylation–the other side of the mercury methylation coin: a critical review. ACS Environ Au. 2022;2:77–97.
Blum JD, Sherman LS, Johnson MW. Mercury isotopes in earth and environmental sciences. Annu Rev Earth Planet Sci. 2014;42:249–69.
Stetson SJ, Gray JE, Wanty RB, Macalady DL. Isotopic variability of mercury in ore, mine-waste calcine, and leachates of mine-waste calcine from areas mined for mercury. Environ Sci Technol. 2009;43:7331–6.
Wiederhold JG, Smith RS, Siebner H, Jew AD, Brown GE Jr, Bourdon B, et al. Mercury isotope signatures as tracers for Hg cycling at the New Idria Hg Mine. Environ Sci Technol. 2013;47:6137–45.
Yin R, Feng X, Wang J, Bao Z, Yu B, Chen J. Mercury isotope variations between bioavailable mercury fractions and total mercury in mercury contaminated soil in Wanshan Mercury Mine, SW China. Chem Geol. 2013;336:80–6.
Wiederhold JG, Skyllberg U, Drott A, Jiskra M, Jonsson S, Björn E, et al. Mercury isotope signatures in contaminated sediments as a tracer for local industrial pollution sources. Environ Sci Technol. 2015;49:177–85.
Brocza FM, Biester H, Richard J-H, Kraemer SM, Wiederhold JG. Mercury isotope fractionation in the subsurface of a Hg(II) chloride-contaminated industrial legacy site. Environ Sci Technol. 2019;53:7296–305.
Grigg ARC, Kretzschmar R, Gilli RS, Wiederhold JG. Mercury isotope signatures of digests and sequential extracts from industrially contaminated soils and sediments. Sci Total Environ. 2018;636:1344–54.
Crowther ER, Demers JD, Blum JD, Brooks SC, Johnson MW. Use of sequential extraction and mercury stable isotope analysis to assess remobilization of sediment-bound legacy mercury. Environ Sci: Process Impacts. 2021;23:756–75.
Huang S, Zhao Y, Lv S, Wang W, Wang W, Zhang Y, et al. Distribution of mercury isotope signatures in Yundang Lagoon, Xiamen, China, after long-term interventions. Chemosphere. 2021;272:129716.
McLagan DS, Schwab L, Wiederhold JG, Chen L, Pietrucha J, Kraemer SM, et al. Demystifying mercury geochemistry in contaminated soil-groundwater systems with complementary mercury stable isotope, concentration, and speciation analyses. Environ Sci: Process Impacts. 2022;24:1406–29.
Epov VN, Rodríguez-González P, Sonke JE, Tessier E, Amouroux D, Maurice Bourgoin L, et al. Simultaneous determination of species-specific isotopic composition of Hg by gas chromatography coupled to multicollector ICPMS. Anal Chem. 2008;80:3530–8.
Masbou J, Point D, Sonke JE. Application of a selective extraction method for methylmercury compound specific stable isotope analysis (MeHg-CSIA) in biological materials. J Anal At Spectrom. 2013;28:1620–8.
Janssen SE, Johnson MW, Blum JD, Barkay T, Reinfelder JR. Separation of monomethylmercury from estuarine sediments for mercury isotope analysis. Chem Geol. 2015;411:19–25.
Li P, Du B, Maurice L, Laffont L, Lagane C, Point D, et al. Mercury isotope signatures of methylmercury in rice samples from the Wanshan mercury mining area, China: environmental implications. Environ Sci Technol. 2017;51:12321–8.
Bouchet S, Bérail S, Amouroux D. Hg compound-specific isotope analysis at ultratrace levels using an on line gas chromatographic preconcentration and separation strategy coupled to multicollector-inductively coupled plasma mass spectrometry. Anal Chem. 2018;90:7809–16.
Entwisle J, Malinovsky D, Dunn PJH, Goenaga-Infante H. Hg isotope ratio measurements of methylmercury in fish tissues using HPLC with off line cold vapour generation MC-ICPMS. J Anal At Spectrom. 2018;33:1645–54.
Qin C, Chen M, Yan H, Shang L, Yao H, Li P, et al. Compound specific stable isotope determination of methylmercury in contaminated soil. Sci Total Environ. 2018;644:406–12.
Qin C, Du B, Yin R, Meng B, Fu X, Li P, et al. Isotopic fractionation and source appointment of methylmercury and inorganic mercury in a paddy ecosystem. Environ Sci Technol. 2020;54:14334–42.
Rosera TJ, Janssen SE, Tate MT, Lepak RF, Ogorek JM, DeWild JF, et al. Isolation of methylmercury using distillation and anion-exchange chromatography for isotopic analyses in natural matrices. Anal Bioanal Chem. 2020;412:681–90.
Manceau A, Brossier R, Janssen SE, Rosera TJ, Krabbenhoft DP, Cherel Y, et al. Mercury isotope fractionation by internal demethylation and biomineralization reactions in seabirds: Implications for environmental mercury science. Environ Sci Technol. 2021;55:13942–52.
Poulin BA, Janssen SE, Rosera TJ, Krabbenhoft DP, Eagles-Smith CA, Ackerman JT, et al. Isotope fractionation from in vivo methylmercury detoxification in waterbirds. ACS Earth Space Chem. 2021;5:990–7.
Yang S, Wang B, Qin C, Yin R, Li P, Liu J, et al. Compound-specific stable isotope analysis provides new insights for tracking human monomethylmercury exposure sources. Environ Sci Technol. 2021;55:12493–503.
Zhang W, Sun G, Yin R, Feng X, Yao Z, Fu X, et al. Separation of methylmercury from biological samples for stable isotopic analysis. J Anal At Spectrom. 2021;36:2415–22.
Rosera TJ, Janssen SE, Tate MT, Lepak RF, Ogorek JM, DeWild JF, et al. Methylmercury stable isotopes: new insights on assessing aquatic food web bioaccumulation in legacy impacted regions. ACS EST Water. 2022;2:701–9.
Tsui MTK, Blum JD, Kwon SY, Finlay JC, Balogh SJ, Nollet YH. Sources and transfers of methylmercury in adjacent river and forest food webs. Environ Sci Technol. 2012;46:10957–64.
Kwon SY, Blum JD, Chen CY, Meattey DE, Mason RP. Mercury isotope study of sources and exposure pathways of methylmercury in estuarine food webs in the northeastern U.S. Environ Sci Technol. 2014;48:10089–97.
Kwon SY, Blum JD, Nadelhoffer KJ, Timothy Dvonch J, Tsui MT-K. Isotopic study of mercury sources and transfer between a freshwater lake and adjacent forest food web. Sci Total Environ. 2015;532:220–9.
Zhang L, Yin Y, Li Y, Cai Y. Mercury isotope fractionation during methylmercury transport and transformation: a review focusing on analytical method, fractionation characteristics, and its application. Sci Total Environ. 2022;841:156558.
Stoichev T, Rodríguez Martín-Doimeadios RC, Amouroux D, Molenat N, Donard OFX. Application of cryofocusing hydride generation and atomic fluorescence detection for dissolved mercury species determination in natural water samples. J Environ Monit. 2002;4:517–21.
Magos L. Selective atomic-adsorption determination of inorganic mercury and methylmercury in undigested bioogical samples. Analyst. 1971;96:847–53.
Gao Y, Liu R, Yang L. Application of chemical vapor generation in ICP-MS: a review. Chin Sci Bull. 2013;58:1980–91.
Balarama Krishna MV, Karunasagar D. Robust ultrasound assisted extraction approach using dilute TMAH solutions for the speciation of mercury in fish and plant materials by cold vapor atomic absorption spectrometry (CVAAS). Anal Methods. 2015;7:1997–2005.
Li D, Li Y, Wang X. Study on the simultaneous reduction of methylmercury by SnCl2 when analyzing inorganic Hg in aqueous samples. J Environ Sci (China). 2018;68:177–84.
Krupp EM, Donard OFX. Isotope ratios on transient signals with GC-MC-ICP-MS. Int J Mass Spectrom. 2005;242:233–42.
Dzurko M, Foucher D, Hintelmann H. Determination of compound-specific Hg isotope ratios from transient signals using gas chromatography coupled to multicollector inductively coupled plasma mass spectrometry (MC-ICP/MS). Anal Bioanal Chem. 2009;393:345–55.
Rodríguez-González P, Epov VN, Bridou R, Tessier E, Guyoneaud R, Monperrus M, et al. Species-specific stable isotope fractionation of mercury during Hg(II) methylation by an anaerobic bacteria (Desulfobulbus propionicus) under dark conditions. Environ Sci Technol. 2009;43:9183–8.
Epov VN, Bérail S, Jiménez-Moreno M, Perrot V, Pecheyran C, Amouroux D, et al. Approach to measure isotopic ratios in species using multicollector-ICPMS coupled with chromatography. Anal Chem. 2010;82:5652–62.
Yang L, Sturgeon RE. Isotopic fractionation of mercury induced by reduction and ethylation. Anal Bioanal Chem. 2009;393:377–85.
Malinovsky D, Latruwe K, Moens L, Vanhaecke F. Experimental study of mass-independence of Hg isotope fractionation during photodecomposition of dissolved methylmercury. J Anal At Spectrom. 2010;25:950–6.
Malinovsky D, Vanhaecke F. Mercury isotope fractionation during abiotic transmethylation reactions. Int J Mass Spectrom. 2011;307:214–24.
Bloom NS, Colman JA, Barber L. Artifact formation of methyl mercury during aqueous distillation and alternative techniques for the extraction of methyl mercury from environmental samples. Fresenius J Anal Chem. 1997;358:371–7.
Hintelmann H, Falter R, Ilgen G, Evans RD. Determination of artifactual formation of monomethylmercury (CH3Hg+) in environmental samples using stable Hg2+ isotopes with ICP-MS detection: calculation of contents applying species specific isotope addition. Fresenius J Anal Chem. 1997;358:363–70.
Demers JD, Blum JD, Zak DR. Mercury isotopes in a forested ecosystem: implications for air-surface exchange dynamics and the global mercury cycle. Global Biogeochem Cycles. 2013;27:222–38.
U.S. EPA. Method 1631, Revision E: mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry. Washington, D.C.: U.S. Environmental Protection Agency, Office of Water; 2002. Report No.: EPA-821-R-02-019.
Hammerschmidt CR, Fitzgerald WF. Methylmercury in mosquitoes related to atmospheric mercury deposition and contamination. Environ Sci Technol. 2005;39:3034–9.
Brooks Rand Instruments. Application note: nitric acid digestion of biological tissue for methylmercury analysis. 2013.
U.S. EPA. Method 1630, methyl mercury in water by distillation, aqueous ethylation, purge and trap, and CVAFS. Washington, D.C.: U.S. Environmental Protection Agency Office of Water; 2001. Report No.: EPA-821-R-01-020.
Korkisch J. Handbook of ion exchange resins: their application to inorganic analytical chemistry. Boca Raton: CRC Press; 1989.
Alderighi L, Gans P, Midollini S, Vacca A. Co-ordination chemistry of the methylmercury(II) ion in aqueous solution: a thermodynamic investigation. Inorg Chim Acta. 2003;356:8–18.
Powell KJ, Brown PL, Byrne RH, Gajda T, Hefter G, Sjöberg S, et al. Chemical speciation of environmentally significant heavy metals with inorganic ligands. Part 1: The Hg2+– Cl–, OH–, CO32–, SO42–, and PO43– aqueous systems. Pure Appl Chem. 2009;77:739–800.
Štrok M, Hintelmann H, Dimock B. Development of pre-concentration procedure for the determination of Hg isotope ratios in seawater samples. Anal Chim Acta. 2014;851:57–63.
Washburn SJ, Blum JD, Donovan PM, Bliss SM. Isotopic evidence for mercury photoreduction and retention on particles in surface waters of Central California, USA. Sci Total Environ. 2019;674:451–61.
Lauretta DS, Klaue B, Blum JD, Buseck PR. Mercury abundances and isotopic compositions in the Murchison (CM) and Allende (CV) carbonaceous chondrites. Geochim Cosmochim Acta. 2001;65:2807–18.
Blum JD, Bergquist BA. Reporting of variations in the natural isotopic composition of mercury. Anal Bioanal Chem. 2007;388:353–9.
Blum JD, Johnson MW. Recent developments in mercury stable isotope analysis. Rev Mineral Geochem. 2017;82:733–57.
Horvat M. Determination of mercury and its compounds in water, sediment, soil and biological samples. In: Pirrone N, Mahaffey KR, editors. Dynamics of mercury pollution on regional and global scales: atmospheric processes and human exposures around the world. New York: Springer Science+Business Media Inc.; 2005. p. 153–90.
Parker JL, Bloom NS. Preservation and storage techniques for low-level aqueous mercury speciation. Sci Total Environ. 2005;337:253–63.
Chen J, Hintelmann H, Dimock B. Chromatographic pre-concentration of Hg from dilute aqueous solutions for isotopic measurement by MC-ICP-MS. J Anal At Spectrom. 2010;25:1402–9.
Leermakers M, Lansens P, Baeyens W. Storage and stability of inorganic and methylmercury solutions. Fresenius J Anal Chem. 1990;336:655–62.
Xu X, Zhang Q, Wang W-X. Linking mercury, carbon, and nitrogen stable isotopes in Tibetan biota: implications for using mercury stable isotopes as source tracers. Sci Rep. 2016;6:25394.
Meng M, Sun R-Y, Liu H-W, Yu B, Yin Y-G, Hu L-G, et al. Mercury isotope variations within the marine food web of Chinese Bohai Sea: implications for mercury sources and biogeochemical cycling. J Hazard Mater. 2020;384:121379.
Maggi C, Berducci MT, Bianchi J, Giani M, Campanella L. Methylmercury determination in marine sediment and organisms by direct mercury analyser. Anal Chim Acta. 2009;641:32–6.
Watanabe T, Kikuchi H, Matsuda R, Hayashi T, Akaki K, Teshima R. Performance evaluation of an improved GC-MS method to quantify methylmercury in fish. Shokuhin Eiseigaku Zasshi. 2015;56:69–76.
Valsecchi L, Roscioli C, Schiavon A, Marziali L. Methylmercury determinatino in freshwater biota and sediments: static headspace GC-MS compared to direct mercury analyzer. MethodsX. 2021;8:101581.
Acknowledgements
We would like to thank Teresa Mathews, senior scientist and group leader of the Biodiversity and Ecosystem Health Group within the Environmental Sciences Division at Oak Ridge National Laboratory, for helping facilitate our collection of aquatic invertebrate samples from East Fork Poplar Creek and for supplying the fish tissue sample used in this study. Patrick Donovan, Spencer Washburn, and Aaron Kurz also provided valuable assistance with the collection of benthic invertebrates used in this study. We would also like to thank Paul Drevnick for the use of the Brooks Rand automated MERX MeHg analyzer. This manuscript was improved substantially thanks to the thoughtful comments of two anonymous reviewers.
This research was supported by the U.S. Department of Energy (DOE), Office of Science, Biological and Environmental Research (BER), Subsurface Biogeochemical Research (SBR) program under Award No. DE-SC0016489 and is also a product of the Critical Interfaces Science Focus Area (SFA) at Oak Ridge National Laboratory (ORNL). ORNL is managed by UT-Battelle, LLC for the DOE under Contract No. DE-AC05-00OR22725. Additional funding was provided by the Geological Society of America Graduate Student Research Grant (2019), the University of Michigan (UM) Rackham Graduate Student Research Grant (2019), the UM Scott Turner Award (2020 and 2021), the Anchor QEA Scholarship (2021), and the John D. MacArthur Professorship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Crowther, E.R., Demers, J.D., Blum, J.D. et al. Coupling of nitric acid digestion and anion-exchange resin separation for the determination of methylmercury isotopic composition within organisms. Anal Bioanal Chem 415, 759–774 (2023). https://doi.org/10.1007/s00216-022-04468-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04468-8