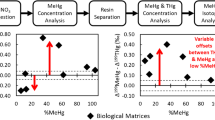
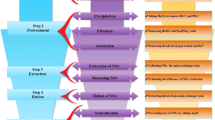
Abstract
The development of mercury (Hg) stable isotope measurements has enhanced the study of Hg sources and transformations in the environment. As a result of the mixing of inorganic Hg (iHg) and methylmercury (MeHg) species within organisms of the aquatic food web, understanding species-specific Hg stable isotopic compositions is of significant importance. The lack of MeHg isotope measurements is due to the analytical difficulty in the separation of the MeHg from the total Hg pool, with only a few methods having been tested over the past decade with varying degrees of success, and only a handful of environmentally relevant measurements. Here, we present a novel anion-exchange resin separation method using AG 1-X4 that further isolates MeHg from the sample matrix, following a distillation pretreatment, in order to obtain ambient MeHg stable isotopic compositions. This method avoids the use of organic reagents, does not require complex instrumentation, and is applicable across matrices. Separation tests across sediment, water, and biotic matrices showed acceptable recoveries (98 ± 5%, n = 54) and reproducible δ202Hg isotope results (2 SDs ≤ 0.15‰) down to 5 ng of MeHg. The measured MeHg pools in natural matrices, such as plankton and sediments, showed large deviations from the non-speciated total Hg measurement, indicating that there is an important isotopic shift during methylation that is not recorded by typical measurements, but is vital in order to assess sources of Hg during bioaccumulation.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemical speciation of mercury (Hg) affects its mobility and toxicity in the environment [1]. Within the aquatic ecosystem, inorganic Hg (iHg) can be transformed into the organometallic form, methylmercury (MeHg), by a variety of microorganisms in the sediments [2,3,4] and the water column [5,6,7]. Methylmercury can then rapidly bioaccumulate and biomagnify within the aquatic food web, leading to Hg content in fish that are mostly (> 90%) MeHg and often elevated a million-fold higher than surrounding waters [8, 9]. Human health concerns arise from the direct consumption of fish with elevated MeHg and have led to fish consumption advisories [10]. Tracing the reactivity and bioavailability of discrete sources of Hg continues to challenge scientists, yet this information is crucial for resource managers.
The application of Hg stable isotope measurements has been leveraged to examine bioaccumulation of Hg from specific sources and in elucidating specific transformation pathways [11]. Mercury has seven stable isotopes that undergo mass-dependent fractionation (MDF) and mass-independent fractionation (MIF) processes [12]. Mercury MDF, most commonly discussed as δ202Hg, and MIF, most commonly depicted as Δ199Hg, Δ200Hg, Δ201Hg, and Δ204Hg, have been observed in a variety of biotic and abiotic processes [11, 12]. The main drivers of Hg MDF are kinetic and equilibrium fractionation [13,14,15], while MIF is largely caused by the magnetic isotope effect and the nuclear volume effect [16, 17]. These isotope tracers have been used to examine the process of Hg methylation and demethylation in pure bacterial culture [18, 19], but it remains unclear how this fractionation affects ambient pools of MeHg. Previous efforts [20] also used the total Hg (HgT) isotopic composition of biotic tissues to mathematically calculate the MeHg isotopic composition of sediments, but no direct measurement of the ambient MeHg pool was performed to validate the estimation. In order to properly track and understand the processes of methylation and bioaccumulation, quantitative measurements of MeHg isotopic composition in natural settings are crucial, yet highly limited.
Despite the interest in compound-specific measurements of Hg isotopes, the few published methods present several challenges related to the precision, reliability, and cost. For example, offline techniques, such as toluene extraction, have been explored [21] but incur large reagent costs, in addition to previous data being limited to biological certified reference materials (CRMs). Offline gas chromatographic (GC) separation of ethylated derivatives has also been attempted in sediment matrices [22]. These approaches suffer from variable efficiencies of the ethylating agents (e.g., sodium tetraethylborate, NaTEB) and incomplete recoveries that cause large MDF shifts of Hg isotopes > 1.0‰ [22, 23]. When coupled to a multicollector inductively coupled plasma mass spectrometer (MC-ICP-MS), online GC shows improvements in uncertainties of species-specific measurements despite the use of ethylating and propylating reagents [24,25,26,27,28]. However, the precision of chromatographic separation can result in large variation in δ202Hg during peak elution [24], potentially affecting the reconstruction of isotope ratios from the transient signal [18]. More recent applications of GC/MC-ICP-MS, which aim to overcome these issues with a temperature vaporization injector, were still only able to precisely measure MeHg isotopes at concentrations > 300 ng g−1, which is higher than most environmental measurements of sediments and lower trophic level biota [27]. Lastly, high-performance liquid chromatography (HPLC) separations help circumvent the use of NaTEB but are currently limited to fish CRMs with limited application to natural matrices [29].
To address the challenges associated with derivatization, online techniques, and organic extractions, we developed a technique that uses ion-exchange resins for separation of MeHg [30, 31]. For dissolved Hg species, the chemical forms of MeHg+ and iHg in acidified aqueous solutions containing chloride are neutral (MeHgCl) and chlor-anionic complexes (HgCl3− and HgCl42−), respectively [32, 33], which allow for the charged inorganic species to be retained on the resin and the neutral organic species to pass through. Ion-exchange resins have been previously used to purify MeHg from bulk Hg for isotope analysis under laboratory conditions and with stock standards [34] but have not been further adapted for natural matrices. In this study, we present a novel resin separation method that allows for the quantitative recovery of as little as 5 ng of MeHg that can be applied across biological matrices, waters, and sediments. To do so, we use distillation pretreatment steps to liberate matrix-bound MeHg and remove chemical interferences, which is subsequently followed by resin separation of the distillate to purify the MeHg pool. This method has the additional benefit of not requiring additional equipment or mathematical corrections as observed in other previous species-specific methods. Improving the reproducibility and practicality of measuring the isotopic composition of MeHg is vital in the future application of isotope measurements to elucidate Hg sources and bioaccumulation within the food web.
Materials and methods
Materials and reagents
All experiments used ultra-high-purity (18.2 MΩ.) reagent water, referred to as Milli-Q (MQ). Concentrated hydrochloric acid (HCl) and nitric acid (HNO3) (OmniTrace, EMD Millipore) were used for digestions and acid additions. A sub-stock of MeHg standard solution was prepared from a 1 μg mL−1 stock from Brooks Rand (99% purity). The AG 1-X4 (200–400 mesh, chloride form, Bio-Rad) anion-exchange resin was used for Hg species separation. For column pretreatment and elution, a 0.05% (w/v−1) l-cysteine (≥ 98%, Aldrich) was prepared, in addition to a 1% citric acid trisodium salt dihydrate (VWR), similar to Chen et al. [30]. Additional reagents utilized for preparation of bromine monochloride (BrCl) and Hg analysis were ACS grade. Stannous chloride (SnCl2) (ACS grade, Fisher) was used during concentration, purge and trap preconcentration, and isotopic measurements.
Two biological CRMs were used during method development: DOLT-2 dogfish liver (National Research Council Canada; HgT 1990 ± 100 ng g−1, MeHg 693 ± 53 ng g−1, 35% HgT as MeHg; Electronic Supplementary Material (ESM) Table S1) and TORT-2 lobster hepatopancreas (National Research Council Canada; HgT 270 ± 60 ng g−1, MeHg 152 ± 13 ng g−1, 56% HgT as MeHg; ESM Table S1). These CRMs were analyzed to ensure method precision and accuracy, compared to published values, using their respective methods [21, 27] (ESM Table S1). It is important to note that published MeHg isotopic compositions for DOLT are only available for the DOLT-4 CRM, though the two iterations of the CRM are from the same source and expected to exhibit the same chemical characteristics. Toluene extractions were performed according to Masbou et al. [21] for biological CRMs to ensure that direct comparison could be made between CRM batches for MeHg isotopes (SI methods). Large batches of local freshwater plankton (Lake Mendota, Madison, Wisconsin) were also collected, size-fractioned (> 500 μm and > 243 μm), lyophilized, and homogenized to serve as a low-Hg, high-organic carbon in-house MeHg reference (ESM Table S1).
In addition, five natural surface sediments with varying HgT and MeHg concentrations (HgT and MeHg ranging from 2210 to 6960 ng g−1 and from 9.1 to 15.8 ng g−1, respectively) and organic carbon content were collected, lyophilized, and homogenized for use during method development (ESM Table S1). Sediments from a stream site in New York (SCNY) and a Wisconsin lake sediment (BRI-1), which had low MeHg content, were used for MeHg-amended spike experiments. Sediments from the Fox River, Wisconsin (Sed-1, Sed-2, Sed-3), were analyzed for the ambient isotopic composition of MeHg (ESM Table S1). To test our method’s ability to quantitatively recover MeHg in difficult matrices, three natural surface waters and one pure culture medium were collected, filtered (0.45 μm), and amended with 30 ng of the MeHg standard: Pheasant Branch (PBSW; Middleton, Wisconsin), Fox River (FRSW; Green Bay, Wisconsin), and Lake Michigan (LMSW; Milwaukee, Wisconsin) (ESM Table S1), and an iron-reducing growth medium inoculated with Geobacter sulfurreducens.
MeHg isotope separation
Typically, 20–30 ng MeHg amendments of the 1 μg mL−1 stock standard, from now on referred to as MeHg matrix spikes, were added to waters and sediments prior to distillation. CRMs and natural samples were weighed out to produce approximately 15–80 ng of MeHg. Sample mass was limited in each Teflon distillation vial to 0.5 g of material to prevent issues with foaming and back reactions, often resulting in multiple distillate vials being composited for a single sample following distillation, when > 0.5 g of material was necessary to obtain ~ 15 ng of MeHg. Solid samples were diluted in 30 mL of reagent water and 2 mL of acidified potassium chloride/copper sulfate (KCl/CuSO4) solution prior to distillation.
Distillation vials were heated to ~ 120 °C in a custom aluminum heating block, purged with nitrogen gas (flow rate 65–75 mL min−1), and stopped when ~ 15% of the solution remained in the Teflon distillation vessels. ESM Fig. S3 shows an example distillation setup. Distillates were transferred to PETG bottles, diluted, and acidified to a final 0.1 M HCl solution. All distillations were performed with blanks (0.002 ± 0.001 ng mL−1, n = 10) and method spikes (reagent lab water, 30 ng and 60 ng MeHg spikes) to assess recovery and potential fractionation [35]. Following the distillation, 1–2-mL aliquots were taken from the receiving vial and analyzed for concentration to allow for an assessment of the quantitative recovery of the MeHg [36].
Previous application of ion-exchange resins for Hg utilized the now discontinued Dowex M-41 resin [37, 38] or the cation-exchange resin Chelex 100 [34, 39]. The Chelex resin requires a high pH level (> 5) for loading which becomes problematic due to enhanced loss of Hg to vessel walls via adsorption and/or diffusion at higher pH, resulting in poor recoveries of Hg spikes [30, 40]. Thus, the anion-exchange resin AG 1-X4 was chosen for these experiments. The AG 1-X4 resin (20 g) was prepared according to previous washing and conditioning protocols [30] (SI methods). Following column conditioning, a distilled sample was introduced to the resin at ~ 10.1 mL min−1 and the column outlet Teflon tubing was inserted into a receiving 100–250 mL bottle for collection of the MeHg. Post-sample rinsing solutions (40 mL of 0.1 M HCl and ~ 80 mL of reagent water) were also collected in the receiving bottle to ensure complete recovery.
Once separated, the receiving bottle containing the uncharged MeHg was oxidized with BrCl (2% by volume) and placed in the oven for ~ 12 h at 55 °C. After oxidation, an aliquot of the isolated MeHg sample was neutralized with hydroxylamine hydrochloride and analyzed for HgT concentration via SnCl2 reduction, gold amalgamation, and cold vapor atomic fluorescence spectrometry (CVAFS) [36]; detailed information about concentration analysis can be found in the SI methods. Percent MeHg separation recovery was calculated after each resin separation to ensure a quantitative yield of 90–110% was achieved. If acceptable yields were achieved and the sample was too dilute for direct stable isotope analysis, additional preconcentration steps were used. The additional preconcentration step was a purge and trap method, coupled to a semi-rapid thermal desorption into a 40% 3HNO3:BrCl oxidant trap [41]. The trap solutions were then analyzed for HgT concentration, and percent recovery was calculated to ensure total recovery of the sample during preconcentration steps. For both the resin and preconcentration steps, only yields between 90 and 110% were determined to be acceptable for MC-ICP-MS.
Mercury stable isotope analysis
Stable Hg isotope ratios were measured using a Thermo Scientific Neptune Plus MC-ICP-MS. A custom gas-liquid separator (GLS) was used for Hg sample introduction into the MC-ICP-MS [42]. Briefly, tin chloride (3% SnCl2 in 10% HCl) was mixed in-line with Hg solution and introduced to the GLS at 0.9 mL min−1. Inorganic Hg is reduced to gaseous Hg0 within the GLS and carried to the plasma with argon (Ar) gas (0.1–0.2 L min−1). Thallium (40 ng mL−1) is simultaneously introduced to the GLS using an Apex-Q desolvating nebulizer (20 μL min−1, Ar gas = 0.8–0.9 L min−1) for mass bias correction [42, 43]. Eight Faraday cups were used to monitor six Hg isotopes (198Hg, 199Hg, 200Hg, 201Hg, 202Hg, 204Hg) and two thallium isotopes (203Tl, 205Tl). Daily instrumental parameters (gases, lenses, torch settings, etc.) were tuned for stability and intensity of Hg (1 V, ~ 1 ng mL−1). In between samples, the GLS was rinsed for 6 min using a 3% (v/v−1) HNO3 wash solution to achieve a < 0.03 V background signal. For each measurement, 180 ratios were collected over three blocks with an integrations time of 2.096 s, and ratios were rejected if they exceeded 2 standard deviations (SDs) of the average ratio for the sample.
Samples were analyzed using standard-sample bracketing (SSB) relative to the National Institute of Standards and Technology (NIST) 3133 Standard Reference Material (SRM); standards and samples were concentrated (≤ 10% difference) and matrix matched. Isotope data were presented according to convention [43] where MDF was expressed as δXXXHg:
where XXX represents the Hg isotope and NIST 3133 is the normalizing standard [43].
MIF was reported in ΔXXXHg notation, which describes the difference between the measured δXXXHg and the theoretically predicted δXXXHg value, in units of per mil (‰), and is calculated according to
where β represents a mass scaling factor [43].
A secondary standard solution (NIST RM 8610) was regularly measured every five samples to evaluate instrument accuracy and precision. Average values for the RM 8610 standard were in range with certified [44] values (δ202HgT − 0.52 ± 0.07‰; Δ199HgT − 0.02 ± 0.07‰; Δ200HgT 0.01 ± 0.06‰; Δ201HgT − 0.04 ± 0.06‰; 2 SDs, n = 124; ESM Table S1). Measurements of the RM 8610 standard were shown to be robust across a wide range of working concentrations (0.5 ng mL−1 to 1 ng mL−1) allowing for the detection down to 5 ng of HgT or MeHg during sample analysis (ESM Fig. S1). To assess preconcentration method performance, process samples of SRM NIST 3133 were also analyzed within each run. Average NIST 3133 values were comparable to the stock standard indicating no fractionation during preconcentration (δ202HgT − 0.03 ± 0.12‰; Δ199HgT 0.03 ± 0.08‰; Δ200HgT 0.00 ± 0.06‰; Δ201HgT 0.01 ± 0.07‰; 2 SDs, n = 43; ESM Table S1). All measured data that follows can be found in the published data release [45].
Results and discussion
Separation efficiency of MeHg in aqueous matrices using resin preconcentration
To test the viability and performance of the AG 1-X4 resin for separation and quantitative recovery of MeHg, spiked samples (30 ng MeHg stock standard) denoted as resin checks, were prepared in reagent water, preserved to a final 1% HCl concentration, and passed through the resin. These tests yielded acceptable % MeHg recoveries (101 ± 2%, n = 5; ESM Table S2), similar to previous experiments using the Chelex resin [34, 39], but at lower concentration ranges. Comparably, spikes of MeHg stock mixed with iHg (RM 8610) also had acceptable recoveries of 102 ± 4% (n = 3; ESM Table S2). When the resin separation step was coupled to distillation, MeHg standards and MeHg/iHg spikes (MQ: MeHg and MQ: MeHg + iHg) showed an average % MeHg recovery of 95 ± 5% (n = 12; ESM Table S2) and 96 ± 5% (n = 8; ESM Table S2). These coupled distillation and resin experiments were also expanded to filtered natural waters, demonstrating the method’s ability to process a more complex matrix. The natural waters had low MeHg concentrations (< 0.05 ng L−1) and moderate dissolved organic carbon (DOC) contents (PBSW 9.2 mg L−1, FRSW 9.5 mg L−1, and LMSW 2.6 mg L−1; ESM Table S1). Natural waters required the distillation step, rather than resin separation alone, due to the presence of DOC, which could create a neutral complex with iHg and increase MeHg recovery due to artifact formation. Distillation and column separations of MeHg-amended natural waters (LMSW, PBSW, and FRSW) resulted in an average % MeHg recovery of 97 ± 4% (n = 13; ESM Table S2).
The applicability of resin methods for the direct determination of the ambient MeHg isotopes in water requires more development in order to minimize sample volumes and avoid large volume distillations. However, for aqueous samples with higher MeHg content, the distillation and resin approach are preferred over other methods including direct ethylation of distillates [22] and direct GC/MC-ICP-MS [27], which introduce the potential of isotope fractionation during derivatization steps. One potential sample matrix that is ideal for this resin separation approach is experimental media, which often contains higher Hg concentrations but complex organic matter and trace metals. This type of matrix was tested via distillation and column separation using a microbial growth medium containing ferric citrate, fumarate, and culture (Geobacter sulfurreducens). Spikes of MeHg to this culture medium, that were subject to distillation pretreatment and resin separation, had recoveries (97 ± 5%, n = 3; ESM Table S2) like those of reagent water and distilled natural waters.
Isotopic recoveries of MeHg amendments in aqueous solutions
The MeHg standard used for standard amendments was oxidized with BrCl and analyzed directly (no processing) to establish an isotope baseline for method validation (δ202MeHg − 0.48 ± 0.08‰, no MIF, n = 7; ESM Table S1). Spikes of MeHg and MeHg/iHg into acidified, aqueous matrices that were subject only to resin separation (termed resin checks) had an average isotopic composition of δ202MeHg − 0.53 ± 0.10‰ and Δ199MeHg 0.04 ± 0.04‰ (n = 8; ESM Table S2, Fig. 1a, b). This isotopic composition was comparable to that of the MeHg stock standard, indicating that the resin separation step does not fractionate the MeHg spike. The distillation separation step was also tested independent of resin separation (termed distillation checks) and showed no fractionation of MeHg spikes (δ202MeHg − 0.53 ± 0.07‰, Δ199MeHg 0.02 ± 0.05‰, n = 15; ESM Table S2, Fig. 1a, b), as confirmed in previous studies [22, 26].
Isotopic compositions of (a) δ202MeHg (MDF) and (b) Δ199MeHg (MIF) in non-isotopic MeHg amendment experiments for Milli-Q lab water (teal), natural water (blue), anaerobic culture media (green), and natural sediment (orange). Resin check samples were only subject to resin separation, while distillation spikes were processed via distillation only and had no resin preconcentration. All other matrices were separated using distillation coupled to resin separation. Descriptions of natural matrices and processing steps can be found in ESM Tables S1 and S2, respectively. Dashed line represents (a) average δ202MeHg and (b) Δ199MeHg compositions of the MeHg stock standard analyzed directly with the solid box around average line showing 2 SDs. Boxplots represent the 25th–75th quartile for data with medians and means denoted by the center line and square symbol, respectively. Whiskers represent the 1.5 interquartile range of data, and outliers are denoted by filled symbols
Spikes of MeHg only and MeHg/iHg (MQ: MeHg and MQ: MeHg + iHg), subject to both distillation and resin separation steps, had isotopic compositions of δ202MeHg − 0.45 ± 0.12‰ and Δ199MeHg 0.07 ± 0.09‰ (n = 12; ESM Table S2, Fig. 1a, b) and δ202MeHg − 0.49 ± 0.11‰ and Δ199MeHg 0.05 ± 0.04‰ (n = 8; ESM Table S2, Fig. 1a, b), respectively. Across all filtered natural waters containing DOC (LMSW, PBSW, and FRSW), the isotopic composition of the MeHg spikes was also comparable to the stock (δ202MeHg − 0.46 ± 0.11‰, Δ199MeHg 0.07 ± 0.11‰, n = 13; ESM Table S2, Fig. 1a, b). Lastly, the processing of culture media also did not result in fractionation (average δ202MeHg − 0.49 ± 0.07‰, Δ199MeHg 0.09 ± 0.10‰, n = 3; ESM Table S2, Fig. 1a, b). These results show that the individual steps, resin separation and distillation, impart no fractionation. Additionally, no fractionation was observed when these two processing steps were combined for reagent water and complex natural aqueous matrix tests, indicating that the AG 1-X4 resin method is robust and well suited for the separation of MeHg using distillation pretreatment.
Isotopic separation of MeHg in sediments
Sediments provide an ideal environment for microorganisms that produce MeHg, which can subsequently enter the water column and food web [3]. However, the isotopic composition of MeHg in sediments has only been measured twice due to the labor-intensive method reliant on chemical ethylation to separate out the pool [22, 46], which can produce large fractionations if the reaction is incomplete. To improve upon measurements of MeHg isotopes in sediments, we coupled distillation to the successful AG 1-X4 resin separation, which avoids the complexities of ethylation, and provides a lower detection limit of ambient MeHg pools in sediment matrices.
Given that sediment matrices are more complex in comparison to method development tests performed in natural and reagent waters, sediment spike experiments were performed. The percent recovery of MeHg for amendments in two natural sediment matrices, with naturally low MeHg content, was within acceptable ranges for BRI-1 (100 ± 2%, n = 6; ESM Table S2) and SCNY (99 ± 4%, n = 6; ESM Table S2). The isotopic composition of the isolated MeHg spikes were in line with the isotopic composition of the MeHg stock standard for BRI-1 (δ202MeHg − 0.45 ± 0.08‰, Δ199MeHg 0.04 ± 0.03‰, n = 6; ESM Table S2, Fig. 1a, b) and SCNY (δ202MeHg − 0.55 ± 0.09‰, Δ199MeHg 0.05 ± 0.05‰, n = 6; ESM Table S2, Fig. 1a, b). These results indicate that the distillation and resin separation approach successfully removed potential matrix interferences and allowed for the quantitative recovery of MeHg from sediment.
In addition to spiked sediment experiments, three natural surface sediments were tested from a contaminated freshwater system (Fox River, Wisconsin). These sediments spanned the lotic to lentic transition zone of the lower river (20 km) and ranged in HgT concentration from 2210 to 6960 ng g−1 (dry wt.) and MeHg concentration from 9.1 to 15.8 ng g−1 (dry wt.) (ESM Table S1). Percent recovery was calculated based on the previously-measured MeHg concentration and the mass of the sample distilled. Using these values, these sediments produced recoveries 95 ± 5% (n = 6; ESM Table S2). These recoveries were slightly lower, but still acceptable (± 10%), compared to recoveries from spiked sediment experiments, highlighting the challenges of separating MeHg from complex natural matrices with low MeHg. Isotopically isolated MeHg pools were reproducible for the three natural sediments with δ202Hg 2 SDs ranging from 0.01 to 0.04 (n = 2 for each sediment, Fig. 2a) and Δ199Hg 2 SDs ranging from 0.01 to 0.13 (n = 2 for each sediment, Fig. 2b). There were distinct differences between the average HgT isotopic compositions of the three Fox River surface sediments (δ202HgT − 0.55 ± 0.09‰, n = 3; ESM Table S1, Fig. 2a) and in the isotopic composition of the MeHg (δ202MeHg 0.10 ± 0.15‰, n = 6; ESM Table S2, Fig. 2a). The isotopic enrichment of MeHg in comparison to HgT in these sediments supports previous work suggesting that the MeHg bioavailable pool of Hg to biota is isotopically heavier in δ202Hg [47] or that microbial demethylation plays a dominant role in the isotopic signature of the resultant MeHg pool [48, 49]. The ambient MeHg isotopic composition measured from this site have a smaller range than the previous measurements performed in estuary sediments [22], indicating that the composition of MeHg is highly dynamic and may be site specific.
Ambient isotopic data (a) δ202HgT and δ202MeHg (MDF) and (b) Δ199HgT and Δ199MeHg (MIF) for three surface sediments from the Fox River (Green Bay, Wisconsin) and two size-fractioned (> 500 and > 243 μm) plankton samples from Lake Mendota (Madison, Wisconsin). Estimation of the bioaccumulated MeHg pool prior to photodemethylation are denoted as δ202Hgcor in the main text and represented by the gray bars in panel (a). Distinct shifts in the δ202Hg isotopic composition are observed between the MeHg and iHg pools for the surface sediments and plankton. Error bars represent 2 SDs
Application of resin separation for biological matrices
While measurements of total isotopic composition are commonly performed in biota, the assumption that fish are > 90% MeHg [8] can be incorrect when examining prey fish species, as well as other organisms such as benthic invertebrates and plankton [9, 50]. Most of the currently developed species-specific methods have focused on examining biological pools of MeHg that are < 90% MeHg using offline (HPLC, direct ethylation, toluene extraction) and online (GC/MC-ICP-MS) approaches [21, 22, 27, 29]. While this biological tissue has been the target of many separation methods, there are still many downfalls related to poor reproducibility or the lack of vetting across different natural, non-CRM, samples which require a more robust method.
Biological CRMs were processed using distillation pretreatment coupled to resin separation and showed acceptable recoveries for TORT-2 (90 ± 3%; n = 4; ESM Table S3) and DOLT-2 (95 ± 3%; n = 6; ESM Table S3). Isotopic results for DOLT-2 (average δ202MeHg 0.15 ± 0.09‰, Δ199MeHg 1.05 ± 0.06‰, n = 6; ESM Table S3, Fig. 3) revealed MeHg isotopic compositions that were slightly more enriched in δ202MeHg, compared to previously published data for DOLT-4 [21, 27], potentially due to the different iterations (− 2 and − 4) of the DOLT source. Toluene extraction application, in addition to published results for DOLT-4 [21], confirmed this hypothesis, as shown in Fig. 3. Other attempts to isolate the MeHg from the DOLT-4 CRM using GC/MC-ICP-MS techniques were isotopically similar to our results but showed large variability (δ202MeHg 0.08 ± 0.34‰, Δ199MeHg 1.21 ± 0.22‰, n = 6; ESM Table S3, Fig. 3) [27]. Application of distillation/resin separation for TORT-2 resulted in δ202MeHg that was nearly identical to published values from toluene extraction [21] with similar precision (δ202MeHg 0.46 ± 0.15‰, Δ199MeHg 0.99 ± 0.14‰, n = 4; ESM Table S3, Fig. 3). Online separation for MeHg, using GC/MC-ICP-MS, differed from other published results and were more enriched in δ202MeHg than the resin separation results (δ202MeHg 0.96 ± 0.54‰, Δ199MeHg 0.93 ± 0.29‰, n = 6; ESM Table S3, Fig. 3) [27]. Overall, our comparison tests show good reproducibility and values in accordance with those measured using toluene extraction. Resin separation was also shown to have greater precision and accuracy in comparison to online GC methods for biological matrices.
Comparison of resin preconcentration and previous established methods for MeHg of CRM biological matrices for isotopic composition of δ202MeHg and Δ199MeHg for DOLT-2/DOLT-4 and TORT-2, respectively. The methods are labeled as follows: Distillation, distillation coupled to resin separation; OE, organic toluene extraction; and Online, analysis via online ethylation/propylation with a GC/MC-ICP-MS. The gray circle defines the isotopic variability of all DOLT data produced using different methods, while the blue circle defines the isotopic variability of all TORT-2 data. Error bars reflect 2 SDs. Data from Bouchet et al. [27] reported isotopic data with 2 standard error (SEs), and these values have been converted to 2 SDs to allow for comparison of method precision
Distillation and resin separation were also used to test more challenging natural matrices, specifically plankton. The MeHg in natural plankton samples (Lake Mendota > 500-μm and > 243-μm size fractions) was quantitatively recovered, with average % MeHg recoveries of 104 ± 2% (n = 4; ESM Table S2) and 104 ± 4% (n = 2; ESM Table S2) for the > 500-μm and > 243-μm size fractions, respectively. The isotopic composition of MeHg from natural plankton samples was precise for both size fractions: > 500 μm (δ202MeHg 0.63 ± 0.12‰, Δ199MeHg 1.62 ± 0.13‰, n = 4; ESM Table S2, Fig. 2a, b) and > 243 μm (δ202MeHg 0.76 ± 0.01‰, Δ199MeHg 1.68 ± 0.01‰, n = 2; ESM Table S2, Fig. 2a, b). These isotopic values for MeHg were significantly enriched in δ202MeHg relative to the δ202HgT isotopic composition for each size fraction: > 500 μm (δ202HgT 0.28 ± 0.15‰, Δ199HgT 1.33 ± 0.15‰, n = 3; ESM Table S1, Fig. 2a, b) and > 243 μm (δ202HgT 0.17 ± 0.08‰, Δ199HgT 1.24 ± 0.10‰, n = 3; ESM Table S1, Fig. 2a, b). It is noted that the published toluene extraction approach [21] did not produce viable MeHg separations for this matrix, despite being successful for CRM comparisons. Poor recoveries of the MeHg fraction for natural planktonic matrices were obtained (% MeHg recovery 65 ± 1%, n = 6; ESM Table S3), and the method could not be scaled up to acquire enough MeHg for isotope analysis. While organic extraction has been shown to be suitable for lipid-washed CRMs, the formation of a white emulsion (ESM Fig. S2) from the higher mass and lipid content in the plankton matrix resulted in the inability to isolate the organic phase. This further indicates that current available methods for the separation of MeHg in biota are limited by sample type and Hg concentrations, whereas the anion exchange had reproducible results for both processed CRMs and natural matrices.
Comparison to estimation approaches and future applications
Due to the lack of environmentally relevant MeHg isotope results, other than biota with > 90% HgT as MeHg, the isotopic assessment of MeHg cycling and bioaccumulation has predominantly relied on estimation approaches. These methods have commonly used photochemical corrections or mixing models based on % MeHg [20, 48, 49]. While more convenient than making direct MeHg isotope measurements, there is no independent confirmation that these approaches correctly predict the source of Hg to the food web. The photochemical estimation approach was applied to fish from the Fox River [51], to correspond with direct measurements of MeHg in the sediments, and to the Lake Mendota plankton. The δ202Hg signatures of the bioaccumulated MeHg pool prior to photochemical fractionation were estimated based on the predicted fraction of Δ199Hg and δ202Hg during photodemethylation (SI methods); the resultant value was termed δ202Hgcor.
Fish from the Fox River, which were co-located to bed sediments, had an estimated δ202Hgcor of − 0.20 ± 0.18‰ (Fig. 2a). These values were highly depleted in comparison to the direct measurement of sediment MeHg in the system (δ202MeHg 0.10 ± 0.15‰, n = 6; ESM Table S2, Fig. 2a). There are numerous factors that can cause a disconnection between the estimated fish value and sediment δ202MeHg, including factors related to foraging habitat, trophic position, and diet. However, previous isotope studies in contaminated regions have drawn the direct comparison from fish to bed sediments [48, 49], and if the sediments are a likely source of MeHg to the food web, the estimation approach may not capture the actual isotope composition.
For plankton, the direct comparison between the estimated δ202Hgcor of bioaccumulated Hg and the direct δ202MeHg is clearer. Plankton photochemical correction was based on the total isotopic values and resulted in a δ202Hgcor ranging from − 0.14 to 0.003‰, which was significantly depleted in comparison to direct measurements of δ202MeHg of the plankton (0.63‰ to 0.76‰) (Fig. 2a). It is even noted that if the photochemical correction is applied to the measured plankton MeHg pool to make a more direct comparison, values are still enriched in comparison (δ202MeHgcor = 0.29‰ to 0.41‰) to estimates. Another interesting aspect that the photochemical corrections do not account for the presence of higher MIF in the MeHg pool (Fig. 2b), which can add to the disconnection between the measured values and estimates. While photochemical estimations have been used for intercomparing fish populations with different extents of photochemistry, this approach is not suitable to estimate the MeHg pool or the sources of MeHg to the food web. Overall, the direct measurements of MeHg in sediments and plankton from two different systems were vastly different than the most common estimation approach for calculating bioaccumulated Hg prior to photochemical fractionation.
The sediments and plankton tested for method validation are not from the same system but do provide intriguing information about the potential shifts that can exist between these two matrices. Sediments from this study and another contaminated site [22] showed no odd MIF, despite shifts in MDF between the systems. The lack of odd MIF of MeHg in sediments, relative to aquatic biota that tend to have elevated MIF, specifically in the MeHg pool (Fig. 2b), raises further concerns about intercomparing these matrices and applying photochemical corrections. This difference in MIF possibly suggests two major scenarios: (1) there are early steps during the bioaccumulation of Hg, near the closest measurable point of uptake, that have the potential to enhance photochemical signatures, or (2) the sediments do not represent the pool of Hg that is bioavailable to the food web in these systems. Recent investigation of MIF during microalgal uptake and degradation of MeHg have shown large MIF shifts that may explain the higher MIF in the MeHg pool of biota as well as the lack of MIF thus far in sediment measurements [52]. Additionally, the current limited information we have about the sediment MeHg pool may not reflect the actual source entering the food web, and these direct measurements could be expanded into regions and matrices (e.g., invertebrates) directly linked to fish foraging. The further application of MeHg isotopes to the food web can provide more insight to how MeHg is created and transferred to biota in the environment.
Conclusion
This new application of distillation coupled with ion-exchange separation produced quantitative concentration and isotopic recovery of MeHg across matrices. Currently, this method has the potential to separate and isotopically analyze as little as 5 ng of MeHg in sediment and biota, greatly improving previous detection limits of species-specific measurements and making it more applicable to naturally occurring concentrations. Additionally, it is a low-cost alternative to online species-specific measurements and shows a wider range of application than organic phase separation (e.g., sediments and low concentration biota). It is further observed that the separated MeHg isotope pool in natural matrices is distinct from the bulk total isotopic compositions currently measured in the field and differs dramatically from estimation approaches for determining the bioaccumulated pool of MeHg in biota. This method allows us to increase the number and precision of measurements for MeHg isotopes in the environment, which is a vital step in better understanding the creation of MeHg within different environmental compartments and assessing different sources of MeHg to the food web.
References
Ullrich SM, Tanton TW, Abdrashitova SA. Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol. 2001;31:241–93.
Compeau GC, Bartha R. Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Appl Environ Microbiol. 1985;50:498.
Gilmour CC, Podar M, Bullock AL, Graham AM, Brown SD, Somenahally AC, et al. Mercury methylation by novel microorganisms from new environments. Environ Sci Technol. 2013;47:11810–20.
Hsu-Kim H, Kucharzyk KH, Zhang T, Deshusses MA. Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: a critical review. Environ Sci Technol. 2013;47:2441–56.
Munson KM, Lamborg CH, Boiteau RM, Saito MA. Dynamic mercury methylation and demethylation in oligotrophic marine water. Biogeosciences. 2018;15:6451–60.
Lehnherr I, St. Louis VL, Hintelmann H, Kirk JL. Methylation of inorganic mercury in polar marine waters. Nat Geosci. 2011;4:298–302.
Eckley CS, Hintelmann H. Determination of mercury methylation potentials in the water column of lakes across Canada. Sci Total Environ. 2006;368:111–25.
Bloom NS. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci. 1992;49:1010–7.
Lescord GL, Johnston TA, Branfireun BA, Gunn JM. Percentage of methylmercury in the muscle tissue of freshwater fish varies with body size and age and among species. Environ Toxicol Chem. 2018;37:2682–91.
UN Environment Economy Division, Report by UN Environment, The World Health Organization and Italian National Research Council - Institute of Atmospheric Pollution Research Regarding the Activities of the Global Environment Facility Project: development of a plan for global monitoring of human exposure to and environmental concentrations of mercury
Blum JD, Sherman LS, Johnson MW. Mercury isotopes in earth and environmental sciences. Annu Rev Earth Planet Sci. 2014;42:249–69.
Bergquist BA, Blum JD. Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science. 2007;318:417–20.
Schauble EA. Applying stable isotope fractionation theory to new systems. Rev Mineral Geochem. 2004;55:65–111.
Wiederhold JG. Metal stable isotope signatures as tracers in environmental geochemistry. Environ Sci Technol. 2015;49:2606–24.
Young ED, Galy A, Nagahara H. Kinetic and equilibrium mass-dependent isotope fractionation laws in nature and their geochemical and cosmochemical significance. Geochim Cosmochim Acta. 2002;66:1095–104.
Schauble EA. Role of nuclear volume in driving equilibrium stable isotope fractionation of mercury, thallium, and other very heavy elements. Geochim Cosmochim Acta. 2007;71:2170.
Buchachenko AL, Kouznetsov DA, Shishkov AV. Spin biochemistry: magnetic isotope effect in the reaction of creatine kinase with CH3HgCl. J Phys Chem A. 2004;108:707–10.
Rodriguez-Gonzalez P, Epov VN, Bridou R, Tessier E, Guyoneaud R, Monperrus M, et al. Species-specific stable isotope fractionation of mercury during Hg(II) methylation by an anaerobic bacteria (Desulfobulbus propionicus) under dark conditions. Environ Sci Technol. 2009;43:9183.
Kritee K, Barkay T, Blum JD. Mass dependent stable isotope fractionation of mercury during mer mediated microbial degradation of monomethylmercury. Geochim Cosmochim Acta. 2009;73(5):1285.
Tsui MTK, Blum JD, Kwon SY, Finlay JC, Balogh SJ, Nollet YH. Sources and transfers of methylmercury in adjacent river and forest food webs. Environ Sci Technol. 2012;46:10957–64.
Masbou J, Point D, Sonke JE. Application of a selective extraction method for methylmercury compound specific stable isotope analysis (MeHg-CSIA) in biological materials. J Anal Atom Spectrom. 2013;28:1620–8.
Janssen SE, Johnson MW, Blum JD, Barkay T, Reinfelder JR. Separation of monomethylmercury from estuarine sediments for mercury isotope analysis. Chem Geol. 2015;411:19–25.
Yang L, Sturgeon RE. Isotopic fractionation of mercury induced by reduction and ethylation. Anal Bioanal Chem. 2009;393:377–85.
Epov VN, Rodriguez-Gonzalez P, Sonke JE, Tessier E, Amouroux D, Bourgoin LM, et al. Simultaneous determination of species-specific isotopic composition of Hg by gas chromatography coupled to multicollector ICPMS. Anal Chem. 2008;80:3530–8.
Epov VN, Berail S, Jimenez-Moreno M, Perrot V, Pecheyran C, Amouroux D, et al. Approach to measure isotopic ratios in species using multicollector-ICPMS coupled with chromatography. Anal Chem. 2010;82:5652–62.
Dzurko M, Foucher D, Hintelmann H. Determination of compound-specific Hg isotope ratios from transient signals using gas chromatography coupled to multicollector inductively coupled plasma mass spectrometry (MC-ICP/MS). Anal Bioanal Chem. 2008;393:345.
Bouchet S, Bérail S, Amouroux D. Hg Compound specific isotope analysis at ultratrace levels using an on line gas chromatographic preconcentration and separation strategy coupled to multicollector-inductively coupled plasma mass spectrometry. Anal Chem. 2018;90:7809–16.
Rodríguez-González P, Epov VN, Pecheyran C, Amouroux D, Donard OFX. Species-specific stable isotope analysis by the hyphenation of chromatographic techniques with MC-ICPMS. Mass Spectrom Rev. 2011;31:504–21.
Entwisle J, Malinovsky D, Dunn PJH, Goenaga-Infante H. Hg isotope ratio measurements of methylmercury in fish tissues using HPLC with off line cold vapour generation MC-ICPMS. J Anal Atom Spectrom. 2018;33:1645–54.
Chen J, Hintelmann H, Dimock B. Chromatographic pre-concentration of Hg from dilute aqueous solutions for isotopic measurement by MC-ICP-MS. J Anal Atom Spectrom. 2010;25:1402–9.
Štrok M, Hintelmann H, Dimock B. Development of pre-concentration procedure for the determination of Hg isotope ratios in seawater samples. Anal Chim Acta. 2014;851:57–63.
Alderighi L, Gans P, Midollini S, Vacca A. Co-ordination chemistry of the methylmercury(II) ion in aqueous solution: a thermodynamic investigation. Inorg Chim Acta. 2003;356:8–18.
Korkisch J. Handbook of ion exchange resins: their application in inorganic analytical chemistry. Boca Raton: CRC; 1989.
Malinovsky D, Latruwe K, Moens L, Vanhaecke F. Experimental study of mass-independence of Hg isotope fractionation during photodecomposition of dissolved methylmercury. J Anal Atom Spectrom. 2010;25:950.
U.S. EPA. Method 1630 methyl mercury in water by distillation, aqueous ethylation, purge and trap, and cold vapor atomic fluorescence spectrometry. 1998.
U.S. EPA. Method 1631 revision E: mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry. EPA-821-R-02-019. 2002.
Delgado A, Prieto A, Zuloaga O, Diego A, Madariaga JM. Production of artifact methylmercury during the analysis of certified reference sediments: use of ionic exchange in the sample treatment step to minimise the problem. Anal Chim Acta. 2007;582:109–15.
Sanz J, Raposo JC, Larreta J, Martinez-Arkarazo I, Diego A, Madariaga JM. On-line separation for the speciation of mercury in natural waters by flow injection-cold vapour-atomic absorption spectrometry. J Sep Sci. 2004;27:1202–10.
Shetty P, Moosavi-Movahedi AA, Rengan K. Determination of trace level mercury in biological and environmental samples by neutron activation analysis. J Radioanal Nucl Chem. 1994;182:205–11.
Parker JL, Bloom NS. Preservation and storage techniques for low-level aqueous mercury speciation. Sci Total Environ. 2005;337:253–63.
Janssen SE, Lepak RF, Tate MT, Ogorek JM, DeWild JF, Babiarz CL, et al. Rapid pre-concentration of mercury in solids and water for isotopic analysis. Anal Chim Acta. 2018;1054:95–103.
Yin R, Krabbenhoft DP, Bergquist BA, Zheng W, Lepak RF, Hurley JP. Effects of mercury and thallium concentrations on high precision determination of mercury isotopic composition by Neptune Plus multiple collector inductively coupled plasma mass spectrometry. J Anal Atom Spectrom. 2016;31:2060–8.
Blum JD, Bergquist B. Reporting of variations in the natural isotopic composition of mercury. Anal Bioanal Chem. 2007;388:353.
National Institute of Standards and Technology. Report of investigation: RM8610—mercury isotopes in UM Almadén mono-elemental secondary standard. 2017. https://www-s.nist.gov/srmors/certificates/8610.pdf.
Rosera TJ, Janssen SE, Tate MT, Lepak RF, Ogorek JM, DeWild JF, et al. Isolation of methylmercury using distillation and anion-exchange chromatography for isotopic analyses in natural matrices data release: U.S. Geol Surv. 2019. https://doi.org/10.5066/P9LRHNL5.
Qin C, Chen M, Yan H, Shang L, Yao H, Li P, et al. Compound specific stable isotope determination of methylmercury in contaminated soil. Sci Total Environ. 2018;644:406–12.
Janssen SE, Schaefer JK, Barkay T, Reinfelder JR. Fractionation of mercury stable isotopes during microbial methylmercury production by iron- and sulfate-reducing bacteria. Environ Sci Technol. 2016;50:8077–83.
Kwon SY, Blum JD, Chen CY, Meattey DE, Mason RP. Mercury isotope study of sources and exposure pathways of methylmercury in estuarine food webs in the Northeastern U.S. Environ Sci Technol. 2014;48:10089–97.
Gehrke GE, Blum JD, Slotton DG, Greenfield BK. Mercury isotopes link mercury in San Francisco bay forage fish to surface sediments. Environ Sci Technol. 2011;45:1264.
Chasar LC, Scudder BC, Stewart AR, Bell AH, Aiken GR. Mercury cycling in stream ecosystems. 3. Trophic dynamics and methylmercury bioaccumulation. Environ Sci Technol. 2009;43:2733–9.
Madenjian CP, Janssen SE, Lepak RF, Ogorek JM, Rosera TJ, DeWild JF, et al. Mercury isotopes reveal an ontogenetic shift in habitat use by walleye in lower Green Bay of Lake Michigan. Environ Sci Technol Lett. 2019;6:8–13.
Kritee K, Motta LC, Blum JD, Tsui MT, Reinfelder JR. Photochemical visible light-induced magnetic mass independent fractionation of mercury in a marine microalga. Earth Space Chem. 2017;2(5):432–40.
Funding
This work was supported by the USGS Toxic Substances Hydrology Program. Support for TJR was provided by the Wisconsin Alumni Research Foundation through the University of Wisconsin Graduate School (award no. MSN194130) and through a U.S. Geological Survey - National Institutes for Water Resources internship under Cooperative Agreement G18ACOO3S4. Any use of trade, firm, or product names in this publication is for descriptive purposes only and does not imply the endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 857 kb)
Rights and permissions
About this article
Cite this article
Rosera, T.J., Janssen, S.E., Tate, M.T. et al. Isolation of methylmercury using distillation and anion-exchange chromatography for isotopic analyses in natural matrices. Anal Bioanal Chem 412, 681–690 (2020). https://doi.org/10.1007/s00216-019-02277-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02277-0