Abstract
Aquatic toxins are a group of toxic compounds produced by several types of freshwater and marine algae and cyanobacteria and transported through the food chains of water bodies. Potential contamination of aquaculture products (raw and processed fish and seafood) with aquatic toxins requires the use of efficient screening methods for their control. In this study, a multiplex immunochromatographic test system for the simultaneous detection of three aquatic toxins—phycotoxins domoic acid (DA) and okadaic acid (OA), and cyanotoxin microcystin-LR (MC-LR)—is for the first time developed. For this, a competitive indirect immunochromatographic analysis (ICA) based on gold-labeled secondary antibodies was carried out. The LODs/cutoffs/working ranges of the ICA were 0.05/0.3/0.07–0.29, 1.3/100/3.2–58.2, and 0.1/2.0/0.2–1.1 ng/mL for MC-LR, DA, and OA, respectively. The assay duration was 18 min. The developed test system was used to analyze water samples from natural sources (salt and fresh water) and fish samples. For sample preparation of water, simple dilution with a buffer was proposed; for fish samples, methanol–water extraction was utilized. It was demonstrated that the triple LFIA specifically detected target aquatic toxins with recoveries of 85.0–121.5%. The developed multiplex LFIA can be considered a promising analytical solution for the rapid, easy, and sensitive control of water and food safety.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human impact on the environment is growing year by year. The active supply of nitrogen and phosphorus compounds with untreated wastewater leads to eutrophication of water bodies and the rapid development of algae and cyanobacteria. Aquatic toxins as products of their metabolism are very dangerous to human health. The ongoing planet climate change increases the urgency of this problem. A reduction in the ice cover of the world ocean leads to changes in water resources’ characteristics, which results in the appearance of aquatic toxins in those areas where they did not exist before [1, 2].
Aquatic toxins are produced by some species of algae and cyanobacteria [3, 4]. Upon entry to the human body, they can cause acute poisoning accompanied by characteristic symptoms (diarrheal, paralytic, amnesic, neurotropic, etc.) and have long-term effects (carcinogenesis, teratogenicity, hepatotoxicity, etc.) [5, 6]. The most toxic and widespread aquatic toxins include paralytic toxins (saxitoxin and its derivatives) [7], amnestic toxins (domoic acid, DA) [8, 9], diarrheal toxins (okadaic acid, OA, and dinophysistoxins) [10], and hepatotoxins (microcystin-LR (MC-LR) and its isoforms) [11, 12]. Given the extremely high harmfulness of aquatic toxins, the maximum residue levels (MRLs) were established by the European Union for the control of MC-LR, DA, and OA in water and seafood [13,14,15]. According to them, the MRL of total MC-LR (free plus bound to cells) was set at 1 µg per 1 L of drinking water with a permissible daily intake of 0.04 mg/kg of body weight. The content of OA and DA in mollusks should not exceed 0.16 mg/kg and 20 mg/kg, respectively. In this regard, the need to control aquatic toxins in water, fish, and seafood is undoubtedly urgent.
The most common method for the determination of aquatic toxins is liquid chromatography (LC) combined with various types of detectors. The LC mode is usually determined by the toxin structure. Thus, hydrophilic interaction chromatography is commonly used for DA measurement. The lipophilic toxins such as OA are separated by a reversed-phase LC [16]. The LC allows highly sensitive analysis and separation of even structurally similar analytes; however, this method requires complex, expensive equipment and can be implemented only in specialized laboratories by qualified personnel [17, 18]. For mass screening of samples for contamination by aquatic toxins, immunoanalytical methods, especially the enzyme-linked immunosorbent assay (ELISA) and the immunochromatographic analysis (ICA), seem optimal [3, 19]. Immunochromatographic test strips with preliminary immobilized specific immunoreagents are ready for use and do not require additional instrumentation or reagents. This allows for their effective application in out-of-laboratory conditions [20, 21]. The risks associated with water and fish contamination explain the interest of researchers in the development of the ICA of different aquatic toxins as a rapid and simple analytical approach [22,23,24,25,26]. Several studies present individual immunochromatographic test systems for the detection of MC-LR, OA, and DA in water and seafood [27,28,29,30,31,32,33,34].
Other direction in the development of portable devices for immunoassays is the use of microfluidic systems, allowing to manage multi-stage processes of analyses and synchronize them for simultaneous testing [35]. Thus, Guan T. et al. [36] developed a droplet microfluidic immunosensor for microcystin-LR that reach the detection limit of 0.6 ng/L.
One of the ways to increase the output of immunoassays is multiparametric testing, which implies the simultaneous detection of two and more analytes. Multiplex test strips are characterized by several analytical zones formed on the working membrane. Only two developments in the multiple detections of aquatic toxins are reported in the literature. Ling et al. (2019) developed the double ICA of OA and the neurotoxin tetrodotoxin with gold nanoparticles (AuNPs) as a marker [37]. The threshold values for OA and tetrodotoxin were 0.75 and 15 ng/mL, respectively, and the duration of the assay was 10 min. Zhang et al. (2019) presented a test system for the simultaneous ICA of OA and MC-LR based on the use of fluorescent microspheres as a label [38]. The assay was performed in 20 min and characterized by limits of detection (LOD) in fish samples of 0.074 and 2.42 µg/kg for MC-LR and OA, respectively. It should be noted that the transition from individual to multiparametric testing does not come down to a simple combination of optimized individual assays on one test strip. It is expected to solve several additional problems associated with choosing the conditions for immunochromatography, preventing nonspecific interactions of immunoreagents, and determining the position of binding zones on the membrane, which does not impair the parameters of the ICA immunoassay for each analyte [39, 40].
In the current study, the immunochromatographic test system for the multiparametric detection of lipophilic toxin OA, hydrophilic toxin DA, and cyanotoxin MC-LR as aquatic toxins the most common for inland and coastal waters was for the first time developed. The research describes the development of a test system based on the competitive indirect ICA using AuNPs as a marker and its approbation for toxins detection in samples of fresh water, seawater, and fish.
Experimental
Reagents and materials
Chloroauric acid (HAuCl4 × H2O), sodium azide, DA, OA, methanol, sucrose, dimethyl sulfoxide (DMSO), N-hydroxysuccinimide (NHS), Triton X-100, N-(3-dimethylaminopropyl)-N'-ethyl-carbodiimide hydrochloride (EDC), soybean trypsin inhibitor (STI), and bovine serum albumin (BSA) (Sigma-Aldrich, Saint Louis, MO, USA) were used. MC-LR was from Enzo Life Science (Lausen, Switzerland). Goat antibodies against mouse immunoglobulins (GAMI) and donkey antibodies against goat immunoglobulins (DAGI) were obtained from Arista Biologicals (Allentown, PA, USA). Peroxidase-labeled anti-mouse immunoglobulins (GAMI–HRP) were purchased from Jackson Immuno Research Labs (West Grove, PA, USA). Monoclonal antibodies (MAbs) to MC-LR were from Eximio Biotec (Hangzhou, China) and MC-LR–BSA conjugate was from Unibiotest (Wuhan, China). MAbs to OA (clone 7E1) were from Santa Cruz Biotechnology (Dallas, TX, USA). A one-component substrate solution of 3,3′,5,5′-tetramethylbenzidine (TMB) was from HBO Immunotech (Moscow, Russia). The purity of all other reagents was of analytical grade or higher. Female BALB/c mice (1.5–2 months of age) were purchased from the Scientific Center for Biomedical Technologies of the Federal Medical and Biological Agency (Stolbovaya branch, Moscow region, Russia).
Preparation of OA–protein and DA–protein conjugates
OA and DA were covalently attached to BSA and STI as described in [30, 41] with modifications. To synthesize DA–protein conjugates, 1 mg of DA was diluted in 50 µL DMSO before the addition of 20 µL of EDC in DMSO (25 mg/mL) and 30 µL of NHS in DMSO (30 mg/mL). The reaction mixture was shaken for 90 min at room temperature. Then, STI or BSA (both 5 mg in 250 µL of 85 mM borate buffer, pH 9.0) were added and stirred for 1.5 h.
To obtain OA–protein conjugates, 1 mg of OA was diluted in 200 µL of DMSO before the addition of 100 µL EDC in DMSO (5 mg/mL) and 100 μL of NHS in DMSO (9 mg/mL) and vortexed for 0.5 h at room temperature. Then, 400 μL of STI (5 mg/mL) or BSA (2.5 mg/mL) in 50 mM sodium carbonate buffer, pH 9.5, was added dropwise and stirred for 2 h at room temperature. The resultant conjugates were dialyzed against 50 mM potassium phosphate buffer with 0.1 M NaCl, pH 7.4 (PBS) for 16 h at 4 °C.
Production of MAbs
Anti-DA MAbs were obtained in mice by standard hybridoma technology using DA–STI as an antigen [42].
ELISAs of MC-LR, OA, and DA
MC-LR–BSA or DA–BSA, or OA–BSA (1 μg/mL in PBS, 100 μL per well) were added to the wells of the microplate (Costar 9018, Corning Costar, Tewksbury, MA, USA) and incubated overnight at 4 °C. Every step of the ELISA was followed by fourfold washing of the microplate with PBS with 0.05% Triton X-100 (PBST). For MC-LR detection, MC-LR solutions (2.5 pg/mL–50 ng/mL in PBST, 50 μL) and anti-MC-LR MAbs (50 ng/mL in PBST, 50 μL) were added and incubated for 1 h at 37 °C. For DA detection, DA solutions (556 ng/mL–17 µg/mL, 50 μL) and anti-DA MAbs (10 ng/mL in PBST, 50 μL) were added to the wells and incubated for 1 h at 37 °C. For OA detection, OA solutions (9 pg/mL–0.9 ng/mL, 50 μL) and anti-OA MAbs (50 ng/mL in PBST, 50 μL) were poured into the wells and incubated for 1 h at 37 °C. After washing, the GAMI–HRP conjugate (dilution in PBST was 1:5,000, 100 μL) was added to the wells and incubated for 1 h at 37 °C. To detect the formed immune complexes, 100 μL of TMB was added to the wells. After 15-min incubation at room temperature, the reaction was stopped using 50 μL of 1 M H2SO4, and the optical density (OD) at 450 nm was measured using a Zenyth 3100 microplate reader (Anthos Labtec Instruments, Wals, Austria).
Preparation and characterization of AuNPs
AuNPs were obtained according to the protocol described in [43]. HAuCl4 × H2O (1% water solution, 1.0 mL) was added to 97.5 mL of distilled water and heated to reach boiling. After that, sodium citrate dihydrate (1% solution, 1.5 mL) was added. After boiling for 25 min, the mixture was cooled and stored at 4 °C. The colloidal gold preparation was characterized by the transmission electron microscopy (TEM) using a CX-100 microscope (Jeol, Japan) as reported in [44].
Conjugation of AuNPs with GAMI
GAMI were dialyzed against 10 mM Tris–HCl buffer, pH 8.5, and added to AuNPs (OD520 = 1, pH 9.0) in the concentration of 6 μg/mL [44]. The mixture was incubated for 45 min with stirring at room temperature. BSA solution (10%) was added to a final concentration of 0.25%, and vigorous stirring was carried out for 15 min. Then, the GAMI–AuNPs conjugate was separated by centrifugation at 13,400 g for 20 min at 4 °C. The pellet was resuspended to an OD520 = 15 in 10 mM Tris–HCl buffer, pH 8.5, containing 1% BSA, 1% sucrose, and 0.05% sodium azide and stored at 4 °C.
Preparation of immunochromatographic test strips
Plastic support with CNPC-SS15 nitrocellulose working membrane, a GFB-R4 sample pad, and an APO45 adsorption pad (Advanced Microdevices, Ambala Cantt, India) were used. Reagents were immobilized using an Iso-Flow dispenser (Imagene Technology, Hanover, NH, USA) at a rate of 0.1 μL per mm of the working membrane. For the individual ICAs, MC-LR or DA–BSA, or OA–BSA conjugates (all in the concentration of 0.5 mg/mL in PBS) were applied to make a test (T) zone. For the multiparametric test system, these conjugates were applied in the same concentrations with the formation of three independent T zones. The control zone (C) was formed by DAGI (0.15 mg/mL in PBS). After drying overnight at room temperature and for 1.5 h at 37 °C, the membrane components were assembled and cut into strips of a 3.0 mm width using an Automatic Cutter (KinBio, Shanghai, China). Test strips were stored at room temperature in a sealed package containing silica gel.
Preparation of spiked water and fish samples
Natural water samples were taken from the Aegean Sea (Turkey), the Sea of Okhotsk (Kurile Islands, Russia), and the Volkhov River (Velikiy Novgorod, Russia) in autumn, 2021 and stored at 4 °C. Fish (trout) were purchased from a local food store in January 2022 and stored at − 20 °C until further use.
Before analysis, Triton X-100 was added (0.05%) to all water samples. Then, the obtained mixtures were spiked with known concentrations of MC-LR, DA, and OA. Spiked water samples were diluted 10 times by PBST and used for the ICA. Fish samples were minced into a homogeneous mass using a blender. To a 0.5 g of the sample, MC-LR (40 μL, 1 μg/mL, which corresponds to 80 ng/g), DA (10 μL, 1 mg/mL, which corresponds to 20 μg/g), OA (200 μL, 1 μg/mL, which corresponds to 400 ng/g), and 5 mL of the methanol–water mixture (1:1) were added. The mixtures were stirred for 5 min and centrifuged at 1,500 g for 10 min. The supernatants were collected, diluted 10 times by PBST, and used for the ICA.
ICAs
Single ICAs of MC-LR, OA, and DA
For the ICA of MC-LR, its solutions (0.0012–3.3 ng/mL in PBST, 50 μL) were mixed with anti-MC-LR MAbs (200 ng/mL in PBST, 50 μL) and GAMI–AuNPs (2 μL, OD520 = 15) and incubated for 3 min at room temperature. For the ICA of DA, its solutions (0.3–667 ng/mL in PBST, 50 μL) were mixed with anti-DA MAbs (100 ng/mL in PBST, 50 μL) and GAMI–AuNPs (2 μL, OD520 = 15) and incubated in the same manner. For the ICA of OA, its solutions (0.004–25 ng/mL in PBST, 50 μL) were mixed with MAbs (100 ng/mL in PBST, 50 μL) and GAMI–AuNPs (2 μL, OD520 = 15) and incubated as described above. Then, the test strips were immersed in the tested samples. After 15-min incubation, test strips were blotted and scanned with a CanoScan LiDE 90 scanner (Canon, Tokyo, Japan). The obtained images were transformed to a gray-scale format and then processed with TotalLab software (TotalLab, Newcastle upon Tyne, UK). The image processing algorithm [45] is based on the same principle as for 1D electrophoretic images. The software determines the boundaries of the colored zones, subtracts the background level and sums up the coloring intensity for all points of the selected zone. This ensures a good correlation of the recorded signal with the number of formed immune complexes containing colored gold nanoparticles.
Triple ICA of MC-LR, OA, and DA
The following solutions in PBST were mixed: MC-LR (0.5 pg/mL–10 ng/mL, 25 μL), DA (0.1 ng/mL–2 μg/mL, 25 μL), OA (1 pg/mL–25 ng/mL, 25 μL), and 25 µL of antibody mixture containing anti-MC-LR MAbs (1.17 μg/mL, 8.3 μL), anti-DA MAbs (0.67 μg/mL, 8.3 μL), and anti-OA MAbs (0.47 μg/mL, 8.3 μL). Then, GAMI–AuNPs (5 μL, OD520 = 15) were added, and the resultant mixture was incubated for 3 min at room temperature. After that, the test strips were immersed in the solutions for 15 min, and the detection was performed as described above.
Triple ICA of spiked seawater or fish extracts
25 μL of seawater or fish extracts were mixed with 50 μL of PBST and 25 µL of antibody mixture containing anti-MC-LR MAbs (1.17 μg/mL, 8.3 μL), anti-DA MAbs (0.67 μg/mL, 8.3 μL), and anti-OA MAbs (0.47 μg/mL, 8.3 μL). Then, GAMI–AuNPs (5 μL, OD520 = 15) were added and incubated for 3 min at room temperature. The test strips were immersed in the mixed solutions for 15 min, and detection was performed as described above.
Evaluation of the ICA and ELISA results
The calibration curves were plotted as dependences of the color intensity of the T zone or OD (y) versus the analyte concentrations (x) and approximated by a four-parameter sigmoid function y = (A – D)/(1 + (x/C)B) + D using the Origin 7.5 software (OriginLab, USA). The LODs, cutoffs, and working ranges were evaluated as described in [46].
Results and Discussion
Obtainment of the immunoreagents
When developing the immunochromatographic test system, commercial monoclonal antibodies to MC-LR and OA were used. Anti-DA antibodies were produced by immunizing mice with DA–STI conjugate as an immunogen. Protein conjugates of OA and DA were obtained by the carbodiimide method. Spectra of the synthesized DA–protein and OA–protein conjugates showed the presence of peaks characteristic of haptens and proteins, which confirmed complex formation (Figure S1). Specific MAbs to MC-LR, DA, and OA were characterized by the indirect competitive ELISAs. Anti-MC-LR and anti-OA MAbs and the corresponding protein conjugates allowed for the detection with LODs of 0.01 and 0.5 ng/mL for MC-LR and OA, respectively. For DA detection, among 9 obtained clones of MAbs, the Dom D3 clone was chosen, which provided the minimum LOD of DA (180 ng/mL).
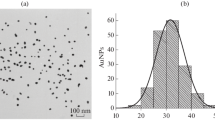
AuNPs obtained by the reduction of HAuCl4 with sodium citrate were used as a label [43]. TEM studies revealed homogeneous nanoparticles with an average diameter of 31.2 ± 2.9 nm and ellipticity of 1.3 ± 0.2 (169 nanoparticles were processed; their minimum and the maximum diameters were 19.1 and 38.2 nm) (Fig. 1). It should be noted that AuNPs with a diameter of about 30 nm are considered optimal in the ICA [47].
For the indirect competitive ICA of aquatic toxins, conjugates of AuNPs with GAMI were obtained. The choice of GAMI concentration for conjugation was carried out using a flocculation curve—a dependence of OD of the gold solution on the concentration of antibodies immobilized on the surface of AuNPs in the presence of a coagulant (10% sodium chloride) [44]. We chose a GAMI concentration of 6 μg/mL, which corresponds to the lower plateau of the flocculation curve indicating the stabilization of the AuNPs surface by adsorbed immunoglobulins [48].
Individual ICAs of MC-LR, DA, and OA
First of all, test systems for the analysis of individual aquatic toxins were optimized. To increase the sensitivity of the ICA, we used indirect labeling, when the detected immune complexes included specific MAbs and AuNPs-labeled anti-species antibodies [44, 49]. Such a format, on the one hand, enables the reduction of the concentration of specific MAbs and, consequently, increases the assay sensitivity. On the other hand, increasing the concentration of the labeled anti-species antibodies allows for the significant growth of the analytical signal intensity without loss in sensitivity. T and C zones were formed on the working membrane by immobilization of the MC-LR–BSA or DA–BSA, or OA–BSA conjugates and anti-species antibodies (DAGI) having specificity to GAMI in the labeled conjugate, respectively. The ICA included the incubation of a sample containing aquatic toxin with specific MAbs and the GAMI–AuNPs conjugate (1st step) and then incubation of the test strips with this mixture (2nd step). If there is no toxin in the sample, specific MAbs bind to gold-labeled anti-species antibodies. Then, this complex moves to the T zone where it is concentrated to form the first red-colored band. In the presence of the toxin in the sample, it interacts with MAbs, blocking their active centers and preventing binding to the toxin–protein conjugate immobilized in the T zone. Therefore, the coloration in the T zone decreases inversely to the toxin concentration.
The ICA was optimized to reach maximum assay sensitivity. Thus, in the case of MC-LR, we varied the concentration of the MC-LR–BSA conjugate in the T zone (0.75–0.5 mg/mL), the concentration of specific MAbs (100–300 ng/mL), the volume of GAMI–AuNPs conjugate (1–4 µL), and the duration of the assay stages. As an example of such optimization experiments, Fig. S2 shows the dependence of the color intensity on the concentration of MC-LR, obtained at different concentrations of the MC-LR–BSA conjugate immobilized in the T zone. It was shown that a decrease in the concentration of the MC-LR–BSA conjugate from 0.75 to 0.5 mg/mL allowed for the twofold reduction of the visual LOD (cutoff). A further decrease in the conjugate concentration led to a significant decrease in the amplitude of the ICA calibration curve. Therefore, the concentration of the MC-LR–BSA conjugate of 0.5 mg/mL was chosen for absorption, which provided a sufficient coloration intensity in the T zone. Immobilization of DAGI at a concentration of 0.15 mg/mL in the C zone and addition of the GAMI–AuNPs conjugate in the volume of 2 µL enabled to obtain the optimal combination of coloration brightness and low reagent consumption.
The calibration curve for the immunochromatographic determination of MC-LR obtained under optimized conditions was characterized by a working range of detectable concentrations of 0.07–0.29 ng/mL, an instrumental LOD of 0.05 ng/mL, and a cutoff of 0.3 ng/mL (Fig. 2a). The duration of the analysis was 18 min and included 3-min 1st step and 15-min 2nd step.
Calibration curve of MC-LR (a), DA (b), and OA (c) in the corresponding ICAs (n = 3) and the appearance of the test strips in the absence of the analyte (–), at a concentration of MC-LR of 0.1 ng/mL, DA of 10 ng/mL, and OA of 0.25 ng/mL (+ /–), and at the analyte concentration greater or equal to cutoff ( +). The error bars mean relative standard deviation
When optimizing the ICA of DA, the concentration of the immobilized DA–BSA in the T zone of 0.5 mg/mL (the tested range was 0.2–1.0 mg/mL), DAGI in the C zone of 0.5 mg/mL (the tested range was 0.1–0.5 mg/mL), and the volume of the GAMI–AuNPs conjugate of 2 µL (the tested range was range 1–4 µL) were selected. The duration of the pre-incubation of the analyzed sample with MAbs and labeled antibodies was 3 min (the tested interval was 2–5 min), and the incubation time of the test strip with the reaction mixture was 15 min (the tested interval was 10–20 min). The concentration of MAbs was 100 ng/mL. According to the DA calibration curve obtained under the selected conditions, the DA LOD was 1.3 ng/mL, the cutoff was 100 ng/mL, and the working range was 3.2–58.2 ng/mL (Fig. 2b).
The ICA of OA was also optimized to achieve a minimum LOD and high colorimetric signal amplitude. These requirements were met under the following conditions: OA–BSA concentration of 0.5 mg/mL, DAGI concentration of 0.5 mg/mL, MAbs concentration of 100 ng/mL, and labeled conjugate volume of 3 μL. The LOD of OA was 0.1 ng/mL, the cutoff was 2.0 ng/mL, and the working range was 0.2–1.1 ng/mL (Fig. 2c). Both for OA and DA determinations, the assay duration was 18 min.
Triple ICA of MC-LR, DA, and OA
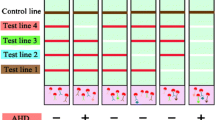
Based on the results obtained, a test system was developed for the simultaneous determination of three aquatic toxins: MC-LR, DA, and OA. The test strip included three T zones with immobilized MC-LR–BSA, OA–BSA, and DA–BSA conjugates and a C zone with DAGI (Fig. 3). During the multianalysis, all components of the test system were mixed, namely, solutions of aquatic toxins (25 mL of each analyte), a mixture of three MAbs specific to individual toxins (total volume of 25 µL), and GAMI–AuNPs conjugate (5 µL).
When implementing a triple test system for the simultaneous determination of MC-LR, DA, and OA, the concentrations of immunoreagents selected during the optimization of individual tests were initially used. However, the concentration of the GAMI–AuNPs conjugate was increased from 2 to 5 µL, as the number of binding zones (and hence, the conjugate consumption) increased from two to four. It was shown that the order of the T zones did not affect the assay sensitivity for all three analytes but determined the intensity of T zones’ coloration. Uniform intensities were obtained with the following location of the analytical zones (according to the flow direction): T1 zone (OA–BSA) ⇒ T2 zone (DA–BSA) ⇒ T3 zone (MC-LR–BSA) ⇒ C zone (DAGI). To determine the mutual influence of immunoreagents, all analytes were tested in the absence of two others. Non-specific binding was not registered: in the absence of any toxin in the sample, a uniform colored band is observed in the corresponding T zone. Its intensity did not differ from what appeared in the case of the negative sample (Fig. 4a).
a Images of test strips after the triple ICA in the absence of two or three analytes. b Images of test strips after the triple ICA of MC-LR, DA, and OA. MC-LR/DA/OA concentrations are 10/2000/25 (1), 3.3/667/8.3 (2), 1.1/222/2.8 (3), 0.4/74/0.9 (4), 0.1/25/0.3 (5), 0.04/8.2/0.1 (6), 0.01/2.7/0.03 (7), 5 × 10−3/0.91/0.01 (8), 2 × 10–3/0.3/3.8 × 10−3 (9), 5 × 10−4/0.1/1.3 × 10−3 (10), and 0/0/0 (11) ng/mL
The concentration of the immobilized DAGI in the C zone was reduced from 0.5 to 0.15 mg/mL, which enabled to equalization of the coloration intensity of all bands. Taking into account additional dilution in the reaction mixture, the concentration of MAbs was increased in comparison to individual tests (1.17 µg/mL for anti-MC-LR MAbs, 0.47 µg/mL for anti-OA MAbs, and 0.67 µg/mL for anti-DA MAbs).
The chosen 15-min time for immersion of the test strips in the sample solution was found to be optimal. With a longer time, the distribution of the coloration over the membrane no changes. On the other hand, with a decrease in this time, the coloration intensities of the binding zones first become less reproducible and then decrease.
Under optimized conditions, the LODs in the triple test did not differ from those in the individual tests for MC-LR, DA, and OA (Fig. 4b).
The registered coloration of all binding zones is quite stable when storing the prepared tests packed in sealed bags at room temperature. For a storage time of 5 months, the coloration intensities decreased no more than 10% and did not affect any of the detection limits.
Triple ICA of aquatic toxin seawater
To approbate the triple ICA, natural water samples were taken from the Aegean Sea, the Sea of Okhotsk, and the Volkhov River. In the study of Hendrickson et al. (2022), it was shown that for successful testing of water samples, a preliminary addition of 0.05% Triton X-100 detergent to the water sample followed by a tenfold dilution with PBST was required [50]. In this case, the matrix effect was eliminated and uniform movement of the GAMI–AuNPs conjugate along the membrane was ensured. Under the selected sample preparation conditions, MC-LR, DA, and OA in spiked water samples were determined. As can be seen from Table 1, the developed test system provides recoveries of aquatic toxins in the range of 85.0–121.5%.
Triple ICA of aquatic toxins in fish
The developed test system was used to determine aquatic toxins in trout extracts. The obtained results indicate that the developed test system provides recoveries in the range of 95.0–101.5% (Table 2). The accuracy of the developed test was validated using the DA and OA ELISA kits (EuroProxima, Arnhem, The Netherlands). The correlation coefficient between the amounts of aquatic toxins determined by ICA and ELISA was 0.978 (n = 10).
Overall estimation of the developed test system
The proposed assay format allows using the conjugate of gold nanoparticles with anti-species antibodies as a universal reagent that provides coloration in all binding zones by the same manipulations and thus excludes necessity in more complicated tools for multiplexing.
To evaluate the possibility of on-site application of the developed tests, we checked available tolls for data registration and processing. It was found that smartphone photos of test strips (obtained through a hole in a closed chamber to prevent backlighting) provide sufficient resolution for further processing of test strip images, including using open tools such as Appuente (https://www.appuente.com/) or LFA App (https://lfapp.shinyapps.io/mobile_app/).
Considering completion of the proposed multiplex test system and its future competitive potential as a commercial device, the following two specific features of the chosen design should be taken into account. Firstly, reagents for the determination of all three compounds are applied to the membranes of the same standard test strip, thus excluding additional consumption of membranes. Secondly, as our previous studies have demonstrated [51, 52], indirect labeling of specific antibodies allows reduce the consumption of these expensive reagents an order of magnitude or more by their application in native form in combination with much cheaper anti-species antibodies.
Conclusions
An immunochromatographic test system has been for the first time developed for the simultaneous detection of the three relevant aquatic toxins—MC-LR, DA, and OA. For this, a competitive indirect ICA format based on gold-labeled anti-species antibodies was performed. The LODs/cutoffs/working ranges of the ICA were 0.05/0.3/0.07–0.29, 1.3/100/3.2–58.2, and 0.1/2.0/0.2–1.1 ng/mL for MC-LR, DA, and OA, respectively, with a testing time of 18 min. It was shown that the developed test system is suitable for the determination of MC-LR, DA, and OA in fresh and seawater, as well as in fish. The ability of simultaneous control of three toxins significantly increases the assay performance, which enables the recommendation of this approach for the rapid and easy detection of aquatic toxins in various objects.
References
Louzao MC, Vilariño N, Vale C, Costas C, Cao A, Raposo-Garcia S, Vieytes MR, Botana L. Current trends and new challenges in marine phycotoxins. Mar Drugs. 2022;20198. https://doi.org/10.3390/md20030198.
Botana LM. Toxicological perspective on climate change: aquatic toxins. Chem Res Toxicol. 2016;29:619–25. https://doi.org/10.1021/acs.chemrestox.6b00020.
Daguer H, Hoff RB, Molognoni L, Kleemann CR, Felizardo LV. Outbreaks, toxicology, and analytical methods of marine toxins in seafood. Curr Opin Food Sci. 2018;24:43–55. https://doi.org/10.1016/j.cofs.2018.10.006.
Vilarino N, Louzao MC, Abal P, Cagide E, Carrera C, Vieytes MR, Botana LM. Human poisoning from marine toxins: unknowns for optimal consumer protection. Toxins. 2018;10(8):324. https://doi.org/10.3390/toxins10080324.
Murk AJ, Nicolas J, Smulders FJM, Burk C, Gerssen A. Marine biotoxins: types of poisoning, underlying mechanisms of action and risk management programmes Food safety assurance and veterinary public health. Chem Haz Foods Animal Origin. 2019;7:207–39. https://doi.org/10.3920/978-90-8686-877-3_09.
Farabegoli F, Blanco L, Rodriguez LP, Vieites JM, Cabado AG. Phycotoxins in marine shellfish: origin, occurrence and effects on humans. Mar Drugs. 2018;16(6):188. https://doi.org/10.3390/md16060188.
Christensen VG, Khan E. Freshwater neurotoxins and concerns for human, animal, and ecosystem health: a review of anatoxin-a and saxitoxin. Sci Total Environ. 2020;736: 139515. https://doi.org/10.1016/j.scitotenv.2020.139515.
Petroff R, Hendrix A, Shum S, Grant KS, Lefebvre KA, Burbacher TM. Public health risks associated with chronic, low-level domoic acid exposure: a review of the evidence. Pharmacol Ther. 2021;227: 107865. https://doi.org/10.1016/j.pharmthera.2021.107865.
Lefebvre KA, Robertson A. Domoic acid and human exposure risks: a review. Toxicon. 2010;56(2):218–30. https://doi.org/10.1016/j.toxicon.2009.05.034.
Fu LL, Zhao XY, Ji LD, Xu J. Okadaic acid (OA): Toxicity, detection and detoxification. Toxicon. 2019;160:1–7. https://doi.org/10.1016/j.toxicon.2018.12.007.
Arman T, Clarke JD. Microcystin toxicokinetics, molecular toxicology, and pathophysiology in preclinical rodent models and humans. Toxins. 2021;13(8):537. https://doi.org/10.3390/toxins13080537.
Fessard V, Le Hegarat L. A strategy to study genotoxicity: application to aquatic toxins, limits and solutions. Anal Bioanal Chem. 2010;397(5):1715–22. https://doi.org/10.1007/s00216-010-3699-3.
EFSA. Marine biotoxins in shellfish – summary on regulated marine biotoxins Scientific opinion of the panel on contaminants in the food chain in feed and food. EFSA J. 2009;1306:1–23. https://doi.org/10.2903/j.efsa.2009.1306.
Guidelines for drinking-water quality. fourth edition incorporating the first and second addenda. Geneva: World Health Organization; 2022.
World Health Organization. Cyanobacterial toxins: microcystins. Background document for development of WHO Guidelines for drinking-water quality and Guidelines for safe recreational water environments. Geneva: World Health Organization; 2020 (WHO/HEP/ECH/WSH/2020.6). https://apps.who.int/iris/bitstream/handle/10665/338066/WHO-HEP-ECH-WSH-2020.6-eng.pdf.
Rasmussen SA, Andersen AJC, Andersen NG, Nielsen KF, Hansen PJ, Larsen TO. Chemical diversity, origin, and analysis of phycotoxins. J Natur Prod. 2016;79(3):662–73. https://doi.org/10.1021/acs.jnatprod.5b01066.
Liang Y, Li A, Chen J, Tan Z, Tong M, Liu Z, Qiu J, Yu R. Progress on the investigation and monitoring of marine phycotoxins in China. Harmful Algae. 2022;111: 102152. https://doi.org/10.1016/j.hal.2021.102152.
D’Amore T, Lo Magro S, Vita V, Di Taranto A. Optimization and validation of a high throughput UHPLC-MS/MS method for determination of the EU regulated lipophilic marine toxins and occurrence in fresh and processed shellfish. Mar Drugs. 2022;20(3):173. https://doi.org/10.3390/md20030173.
Dubois M, Demoulin L, Charlier C, Singh G, Godefroy SB, Campbell K. Development of ELISAs for detecting domoic acid, okadaic acid, and saxitoxin and their applicability for the detection of marine toxins in samples collected in Belgium. Food Additiv Contam: Part A. 2010;27:859–68. https://doi.org/10.1080/19440041003662881.
Dzantiev BB, Byzova NA, Urusov AE, Zherdev AV. Immunochromatographic methods in food analysis. TrAC Trends Anal Chem. 2014;5:81–93. https://doi.org/10.1016/j.trac.2013.11.007.
Mahmoudi T, de la Guardia M, Shirdel B, Mokhtarzadeh A, Baradarn B. Recent advancements in structural improvements of lateral flow assays towards point-of-care testing TrAC Trends Anal Chem. 2019;116:13–30. https://doi.org/10.1016/j.trac.2019.04.016.
Dillon M, Zaczek-Moczydlowska MA, Edwards C, Turner AD, Miller PI, Moore H, McKinney A, Lawton L, Campbell K. Current trends and challenges for rapid smart diagnostics at point-of-site testing for marine toxins. Sensors. 2021;21(7):2499. https://doi.org/10.3390/s21072499.
Zhang K, Wu J, Li Y, Wu Y, Huang T, Tang D. Hollow nanogold microsphere-signalized lateral flow immunodipstick for the sensitive determination of the neurotoxin brevetoxin B. Microchim Acta. 2014;181(11–12):1447–54. https://doi.org/10.1007/s00604-014-1291-9.
Hendrickson OD, Zvereva EA, Zherdev AV, Dzantiev BB. Ultrasensitive lateral flow immunoassay of phycotoxin microcystin-LR in seafood based on magnetic particles and peroxidase signal amplification. Food Control. 2022;133: 108655. https://doi.org/10.1016/j.foodcont.2021.108655.
Li Y, Xu X, Liu L, Kuang H, Xu L, Xu C. A gold nanoparticle-based lateral flow immunosensor for ultrasensitive detection of tetrodotoxin. Analyst. 2020;145:2143–51. https://doi.org/10.1039/d0an00170h.
Akter S, Vehniäinen M, Kankaanpää HT, Lamminmäki U. Rapid and highly sensitive non-competitive immunoassay for specific detection of nodularin. Microorganisms. 2017;5(3):58. https://doi.org/10.3390/microorganisms5030058.
Liu B-H, Hung C-T, Lu C-C, Chou H-N, Yu F-Y. Production of monoclonal antibody for okadaic acid and its utilization in an ultrasensitive enzyme-linked immunosorbent assay and one-step immunochromatographic strip. J Agric Food Chem. 2014;62:1254–60. https://doi.org/10.1021/jf404827s.
Hu L, Liu J, Wang Q, Zhang Y, Jia R, Cai C, Wu W, Chen S-F. Development of an immunochromatographic strip test for the rapid detection of okadaic acid in shellfish sample. J Appl Phycol. 2013;25:1091–9. https://doi.org/10.1007/s10811-012-9949-3.
Lu S-Y, Lin C, Li Y-S, Zhou Y, Meng X-M, Yu S-Y, Li Z-H, Li L, Ren H-L, Liu Z-S. A screening lateral flow immunochromatographic assay for on-site detection of okadaic acid in shellfish products. Anal Biochem. 2012;422:59–65. https://doi.org/10.1016/j.ab.2011.12.039.
Wang R, Zeng L, Yang H, Zhong Y, Wang J, Ling S, Farhan Saeed A, Yuan J, Wang S. Detection of okadaic acid (OA) using ELISA and colloidal gold immunoassay based on monoclonal antibody. J Hazard Mater. 2017;339:154–60. https://doi.org/10.1016/j.jhazmat.2017.06.030.
Tsao Z-J, Liao Y-C, Liu B-H, Su C-C, Yu F-Y. Development of a monoclonal antibody against domoic acid and its application in enzyme-linked immunosorbent assay and colloidal gold immunostrip. J Agric Food Chem. 2007;55(13):4921–7. https://doi.org/10.1021/jf0708140.
Jawaid W, Meneely J, Campbell K, Hooper M, Melville K, Holmes S, Rice J, Elliott C. Development and validation of the first high performance-lateral flow immunoassay (HP-LFIA) for the rapid screening of domoic acid from shellfish extracts. Talanta. 2013;116:663–9. https://doi.org/10.1016/j.talanta.2013.07.027.
Xing C, Liu L, Song S, Feng M, Kuang H, Xu C. Ultrasensitive immunochromatographic assay for the simultaneous detection of five chemicals in drinking water. Biosens Bioelectron. 2015;66:445–53. https://doi.org/10.1016/j.bios.2014.12.004.
Melnik S, Neumann AC, Karongo R, Dirndorfer S, Stubler M, Ibl V, Niessner R, Knopp D, Stoger E. Cloning and plant-based production of antibody MC10E7 for a lateral flow immunoassay to detect [4-arginine]microcystin in freshwater. Plant Biotechnol J. 2018;16:27–38. https://doi.org/10.1111/pbi.12746.
Boobphahom S, Ly MN, Soum V, Pyun N, Kwon OS, Rodthongkum N, Shin K. Recent advances in microfluidic paper-based analytical devices toward high-throughput screening. Molecules. 2020;25(13):2970. https://doi.org/10.3390/molecules25132970.
Guan T, Liu D, Li X, Shu B, Li M, Liu Y, Xu Z, Shen Y, Sun Y, Lei H, Shen X. Magnet-actuated droplet microfluidic immunosensor coupled with gel imager for detection of microcystin-LR in aquatic products. Talanta. 2020;219: 121329. https://doi.org/10.1016/j.talanta.2020.121329.
Ling S, Li X, Zhang D, Wang K, Zhao W, Zhao Q, Wang R, Yuan J, Xin S, Wang S. Detection of okadaic acid (OA) and tetrodotoxin (TTX) simultaneously in seafood samples using colloidal gold immunoassay. Toxicon. 2019;165:103–9. https://doi.org/10.1016/j.toxicon.2019.04.011.
Zhang H, Luo J, Beloglazova N, Yang S, De Saeger S, Mujtaba Mari G, Zhang S, Shen J, Wang Z, Yu X. Portable multiplex immunochromatographic assay for quantitation of two typical algae toxins based on dual-color fluorescence microspheres. J Agric Food Chem. 2019;67:6041–7. https://doi.org/10.1021/acs.jafc.9b00011.
Zvereva EA, Byzova NA, Sveshnikov PG, Zherdev AV, Dzantiev BB. Cut-off on demand: adjustment of the threshold level of an immunochromatographic assay for chloramphenicol. Anal Methods. 2015;7:6378–84. https://doi.org/10.1039/C5AY00835B.
Bartosh AV, Sotnikov DV, Hendrickson OD, Zherdev AV, Dzantiev BB. Design of multiplex lateral flow tests: a case study for simultaneous detection of three antibiotics. Biosensors. 2020;10:17. https://doi.org/10.3390/bios10030017.
Finlay WJJ, Shaw I, Reilly JP, Kane M. Generation of high-affinity chicken single-chain Fv antibody fragments for measurement of the Pseudonitzschia pungens toxin domoic acid. Appl Environ Microbiol. 2006;72(5):3343–9. https://doi.org/10.1128/AEM.72.5.3343-3349.2006.
Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7. https://doi.org/10.1038/256495a0.
Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci. 1973;241:20–2. https://doi.org/10.1038/physci241020a0.
Hendrickson OD, Zvereva EA, Shanin IA, Zherdev AV, Tarannum N, Dzantiev BB. Highly sensitive immunochromatographic detection of antibiotic ciprofloxacin in milk. Appl Biochem Microbiol. 2018;54(6):670–6. https://doi.org/10.1134/S000368381806008X.
TotalLab 1D v14.1 manual. TotalLab Ltd. 2015. https://irp-cdn.multiscreensite.com/20593b6f/files/uploaded/userguide.pdf. Accessed 9 Sept 2022.
Uhrovcik J. Strategy for determination of LOD and LOQ values–some basic aspects. Talanta. 2014;119:178–80. https://doi.org/10.1016/j.talanta.2013.10.061.
Huang X, Aguilar ZP, Xu H, Lai W, Xiong Y. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: a review. Biosens Bioelectron. 2016;75:166–80. https://doi.org/10.1016/j.bios.2015.08.032.
Hermanson GT. Bioconjugate Techniques. 3rd ed. Pierce Biotechnology, Thermo Fisher Scientific: Rockford, IL, USA; 2013. https://doi.org/10.1016/C2009-0-64240-9.
Urusov AE, Petrakova AV, Zherdev AV, Dzantiev BB. “Multistage in one touch” design with a universal labelling conjugate for high-sensitive lateral flow immunoassays. Biosens Bioelectron. 2016;86(57):5–579. https://doi.org/10.1016/j.bios.2016.07.027.
Hendrickson OD, Zvereva EA, Zherdev AV, Dzantiev BB. Cascade-enhanced lateral flow immunoassay for sensitive detection of okadaic acid in seawater, fish, and seafood. Foods. 2022;11:1691. https://doi.org/10.3390/foods11121691.
Urusov AE, Zherdev AV, Dzantiev BB. Use of gold nanoparticle-labeled secondary antibodies to improve the sensitivity of an immunochromatographic assay for aflatoxin B1. Microchim Acta. 2014;181(15–16):1939–46. https://doi.org/10.1007/s00604-014-1288-4.
Byzova NA, Urusov AE, Zherdev AV, Dzantiev BB. Multiplex highly sensitive immunochromatographic assay based on the use of nonprocessed antisera. Anal Bioanal Chem. 2018;410(7):1903–10. https://doi.org/10.1007/s00216-018-0853-9.
Acknowledgements
The authors are grateful to S.M. Pridvorova for TEM studies and D.S. Popravko for the design of Fig. 3.
Funding
This research was funded by the Russian Science Foundation (grant number 20–43-07001; development and validation of the test system) and by the Ministry of Science and Higher Education of the Russian Federation (consideration of registration and processing tools for the tests).
Author information
Authors and Affiliations
Contributions
Elena A. Zvereva: Methodology, investigation, formal analysis, writing—original draft. Olga D. Hendrickson: Investigation; data curation; writing, original draft; writing, review and editing. Olga N. Solopova: Investigation, data curation. Anatoly V. Zherdev: Conceptualization, writing—review and editing. Peter G. Sveshnikov: Conceptualization, supervision. Boris B. Dzantiev: Supervision, project administration.
Corresponding author
Ethics declarations
Ethics approval
Studies in animals were performed following the EU Directive 2010/63/EU for animal experiments and authorized by the Institutional Ethics Committee of the Research Center of Biotechnology (protocol code N22-D February 12, 2020).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zvereva, E.A., Hendrickson, O.D., Solopova, O.N. et al. Triple immunochromatographic test system for detection of priority aquatic toxins in water and fish. Anal Bioanal Chem 414, 7553–7563 (2022). https://doi.org/10.1007/s00216-022-04298-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04298-8