Abstract
Nanoparticles are increasingly used in medical products and devices. Their properties are critical for such applications, as particle characteristics determine their interaction with the biological system, and, therefore, the performance and safety of the final product. Among the most important nanoparticle characteristics and parameters are particle mass distribution, composition, total particle mass, and number concentration. In this study, we utilize single-particle inductively coupled plasma time-of-flight mass spectrometry (spICP-TOFMS) for the characterization of inorganic nanoparticles in complex biological fluids. We report online microdroplet calibration for reference-nanomaterial-free and matrix-matched calibration of carbon-coated iron carbide nanoparticles (C/Fe3C NPs). As a case study, we analyze C/Fe3C NPs designed for targeted blood purification. Through the analysis of NP mass distributions, we study the effect of the NP surface modification on aggregation of C/Fe3C NPs in whole blood. We also demonstrate the efficiency of removal of coated C/Fe3C NP from saline by magnetically enhanced filters. Magnetic filtering is shown to reduce the mass concentration of detectable C/Fe3C NPs by 99.99 ± 0.01% in water.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of nanomaterials, including metal-containing nanoparticles (NPs), is rapidly growing for many pharmaceutical and medical applications [1, 2]. Nanoparticles are of interest due to their advanced properties in terms of, e.g., optical and magnetic tunability, cell membrane penetration, and controlled drug release [3,4,5]. Defining critical quality attributes (CQAs) is essential for the development of NPs for use in biomedical applications. However, many initially approved nanomedicines have been withdrawn due to insufficient clinical performance and/or toxic effects due to poor understanding of the mechanisms of action at cellular and sub-cellular levels [6]. Medical devices that contain nanomaterials are also increasingly regulated, and so biomedical researchers and device developers must conduct detailed risk assessments for these devices [7]. This gap between nanomaterial development and accurate assessment of safety and efficacy of the technology is partly due to a lack of standardized characterization methods of the nanomaterials.
In the past, drug regulatory agencies faced the difficulty that nanomaterial characterization results had to be evaluated using a suite of techniques with limited prior knowledge and available scientific literature [8]. However, in recent years, a variety of technologies have emerged to better characterize engineered nanoparticles (ENPs) for use in biosystems [9,10,11,12,13,14]. Average (bulk) size measurements are possible with techniques like dynamic light scattering (DLS), ultraviolet–visible (UV–Vis) spectrophotometry, field-flow fractionation separations, and nanoparticle tracking analysis (NTA). The size and morphology of individual particles can be obtained with scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Particle number concentrations can be determined with techniques such as nanoparticle tracking analysis (NTA) and UV–Vis [8]. Bulk or size-fractionated element compositions of NPs and mass concentrations can be determined by inductively coupled plasma mass spectrometry (ICP-MS) [10]. Inductively coupled plasma optical emission spectroscopy (ICP-OES) and ICP-MS are considered “gold standard” techniques to determine the biodistribution and fate of inorganic NPs in organs and tissues due to their low detection limits [15,16,17,18] [19, 20].
Despite guideline-defined methods, there are also emerging techniques that are of interest in nanomaterial research and development, including single-particle techniques. Such methods directly analyze individual particle events instead of averaging the properties over a large quantity of particles. This enables one to not only analyze the average behavior but also to look at sub-populations and clusters of particles present in the sample. Single-particle inductively coupled plasma mass spectrometry (spICP-MS) is one of the technologies providing more accurate characterization of metal-containing nanomedicines [21,22,23,24]. Its advantages are the low limits of detection for particle number concentrations and the ability to analyze samples directly in suspension without sample preparation necessary for other techniques such as electron microscopy [25]. Among different types of mass spectrometers, TOFMS with its quasi-simultaneous measurement of all elements is very attractive for nanoparticle analysis [26]. Important characteristics are high-throughput and multiplex analysis, which enables the determination of the compositions of the associated particles [27]. One of the limitations of the spICP-MS is the need of reference nanoparticles for calibration. Particles, including Fe-containing ones, are among those for which there is no standard reference material, and so there is need for indirect particle mass calibration [28]. However, online microdroplet calibration combined with ICP-TOFMS makes reference-free nanoparticle characterization possible [29,30,31].
Iron oxide (FeO/Fe2O3) NPs have been approved for different medical and pharmaceutical applications [2]. Initially, these NPs were approved to treat iron deficiency. However, the particles are popular in the medical field because of their magnetic properties and nontoxic body response [32]. Applications of FeO/Fe2O3 NPs range from food supplements to contrast agents for magnetic resonance imaging (MRI) to cancer tumor hyperthermia agents [33]. A particularly promising application of magnetic nanoparticles is their use in extracorporeal blood purification systems [34, 35]. NPs used for such an application must have high magnetic saturation in order to be efficiently removed from whole blood by applying a magnetic field. It has been previously shown that non-oxidic carbon-coated iron carbide nanoparticles (C/Fe3C NPs) can be used for blood purification [34]. In these systems, C/Fe3C NPs functionalized with antibodies or other pathogen-binding molecular structures are injected into extracorporeally diverted blood. Targeted molecules or microbes are captured on the surface of the C/Fe3C NPs, the particles are subsequently removed by a magnet, and the blood is recirculated to the patient [34].
Measuring the removal efficiency of magnetic iron NPs from whole blood following magnetic separation is critical and a current analytical challenge. Nanoanalytical techniques are needed to determine and validate the removal efficiency of these NPs. Recently, Bougas et al. [36] reported a method that employs a high-performance atomic magnetometer for the real-time non-destructive detection of ferromagnetic NPs in flowing water and whole blood. The limit of detection (LOD) of this method was approximately 2 µg L−1 for C/Fe3C NPs of a particular size in whole blood. While this method is useful for confirming the online separation of magnetic particles through change in measured magnetic fields, the approach does not provide direct quantification of particles or information about their particle mass distributions and agglomeration states. Multiple studies focus on the characterization of Fe NPs in blood, both in vivo and in vitro [36,37,38,39]. A challenge of directly quantifying C/Fe3C NPs in whole blood is that the blood matrix contains significantly more iron than that injected as NPs, which renders direct bulk elemental analysis approaches impossible [40, 41]. Herrmann et al. [32] injected Pt-labeled Fe NPs into a mouse followed by the digestion of the tissue. They measured platinum to quantify the remaining Fe-Pt particles in the tissue. Nwoko et al. [38] used asymmetric flow field-flow fractionation (AF4) to separate Fe NPs from rat blood cells and rat blood plasma, then measured the particulate fraction by the spICP-MS. Kruszewska et al. [42] simulated the physiological conditions of blood by adjusting their synthetic matrix pH to mimic that of blood and measured Fe-NPs in this synthetic, and Fe-free, blood.
In the current work, we demonstrate the use of spICP-TOFMS for direct quantification of diluted whole blood and discuss the advantages and limitations of this approach. To reduce the dissolved Fe background to a level above which single-particle signals could be recorded, we diluted blood 106 times. These extremely dilute samples were analyzed by spICP-TOFMS combined with online microdroplet calibration to quantify the mass and concentration of Fe NPs directly. We applied our measurement system for the characterization of C/Fe3C NPs used in a blood purification system and for the quantification of particle-removal efficiency in a magnetic filtration medical device.
Materials and methods
Iron nanoparticle and functionalization
Carbon-coated iron carbide NPs (C/Fe3C NPs) with saturation magnetization of ~ 93 emu/g were used in this study. The particles were coated with a hydrophilic polymer and streptavidin to reduce surface fouling and agglomeration, which permits the preparation of stable NP dispersions in water and in biological fluids. A more detailed description of these particles can be found elsewhere [34]. The size of the nanoparticle iron carbide core (Table 2, average of at least 100 particles) was analyzed by a scanning electron microscope in transmission mode (Nova NanoSEM 450, Thermo Fisher Scientific, FEI, accelerating voltage 20 kV). The TEM images shown in Fig. 2a were recorded by a transmission electron microscope (HT7700, Hitachi High-Technologies Corporation, accelerating voltage 100 kV).
Single-particle ICP-TOFMS analysis
We used an icpTOF-2R (TOFWERK AG, Thun, Switzerland) instrument, which has a mass resolving power (m/Δm at FWHM) of ∼6000. Details about instrument settings are provided in Table 1. TOFMS allows quasi-simultaneous measurement of most naturally occurring elements. This multi-element and multi-isotope detection allows for single-particle measurements and calibration at the same time, as well as the detection of multi-metal NPs. As shown in Fig. 1, for online calibration, we used dual sample introduction system consisting of a cyclonic spray chamber, nebulizer (MicroFlow, Element Scientific Inc., USA), and falling tube with Microdroplets generator (Microdrop Technologies GmbH, Germany) equipped with a 50-µm diameter Autodrop Pipette (AD-KH-501-L6).
a Schematic of the dual sample introduction system combined to a spICP-TOFMS. The sample is transformed into a fine aerosol via a pneumatic nebulizer. Calibration droplets are introduced into the falling tube and partially dried before being connected to the nebulized sample through the T-piece and injected into the plasma. b Time trace (only 250 ms shown) of NP measurement in diluted blood for three different elements in the sample. Note the calibration droplet signals are only at the beginning of each measurement run
Particle digestion and measurement
Particles were microwave digested in 2 replicates [39]. For each digestion, 0.5 mL of the suspension containing C/Fe3C nanoparticles was mixed with 0.25 mL each of HNO3 (mass fraction of 65%, DuoPUR sub-boiling distillation system, Milestone GmbH, Germany) and HCl (mass fraction of 37%, TraceSelect, Fluka Analytical, Switzerland), and then 0.1 mL of H2O2 (mass fraction of 35%, Sigma-Aldrich, USA) was added. A Rh-spike (Inorganic Ventures, USA) of 1 mg L−1 was added, which served as a recovery standard for digestion. Samples were treated at 200 °C and 40 bar in a microwave-based digestion system (ultraWAVE, Milestone Inc., Germany). After digestion, the samples were further diluted 1:100 and spiked with Co as an internal standard (10 µg L−1). Subsequently, the samples were measured by ICP-TOFMS.
Online microdroplet calibration
Element-mass and particle-mass concentration determinations were performed according to our previously published online microdroplet calibration method [31]. A scheme for this calibration setup is provided in Fig. 1. In this system, monodisperse droplets with known size are generated, transported quantitatively into the plasma, and used to determine the absolute sensitivity (counts/g) of each element standard in the microdroplets. Here, microdroplets were composed of aqueous dilute nitric acid (mass fraction of 1%) with 50 µg L−1 of each Cr, Fe, and Cs. The absolute sensitivities from the microdroplets are used as single-point calibration factors to convert measured NP signals to mass amounts of each element. The determined iron masses were converted to particle masses assuming a density of 4.93 g cm−3 for Fe3C. The carbon of the C/Fe3C particles is not measured, instead, the known stoichiometry of the Fe3C particles was used to convert the determined Fe masses to particle masses. Particle number concentration (PNC) was determined as the number of particle events registered divided by the volume of sample introduced into the plasma (Vplasma) during the measurement time (tmeas). To determine Vplasma, online microdroplet calibration with a plasma uptake standard of Cs was used as described previously to measure the flow rate into to the plasma (\({q}_{\mathrm{plasma}}\)) [31, 43]. The PNC of iron-containing NPs is calculated as shown in Eq. 1, where NFe-NP is the number of detected iron-containing particles. Particle mass concentration is the PNC multiplied by average particle mass.
Absolute sensitivity and presence of background signal—either from noise or dissolved analyte—determine the mass of NPs that can be measured. In the ICP-TOFMS time trace, signals above a critical value (LC,sp) with a defined false-positive rate are registered as NP events. When this critical value is reported in terms of mass, it is called the critical mass. Critical value thresholds were determined according to previously published work [44].
At low particle detection frequencies, counting statistics limit the precision of the PNC that can be determined, and thus for a small number of measurements also limit the accuracy of determined particle mass concentrations. Here, we define particle mass concentration and number concentration limits of detection in terms of Poisson statistics according to the IUPAC definition, as shown in Eq. 2 [45, 46] where LOD is the limit of detection in particle mass concentration, \({\sigma }_{\mathrm{Blank}}\) is the standard deviation of the number of detected NPs in the blank and \(\mathrm{sensitivity}\) is the slope of the calibration curve in units of particle events per particle mass concentration. Likewise, Eq. 2 can be used to determine the LOD in terms of PNC by measuring sensitivity as particle events per particle number concentration.
Nanoparticle separation study
We used a setup designed to mimic an extracorporeal blood circuit. Saline solution or blood (bovine whole blood, heparin anticoagulated, Fiebig Nährstofftechnik, Germany) was pumped with a Miniflex pump (Masterflex, USA) at 100 mL min−1 through medical-grade tubing lines (TYGON ND-100–65, ID: 3.2 mm, Saint-Gobain, USA). C/Fe3C NPs were continuously dispersed in an ultrasonic bath (GT SONIC-L10, GuangDong GT Ultrasonic Co., Ltd, China) and injected with a 4-channel peristaltic pump (Ismatec Reglo, Germany) at 1 mL min−1 into the solution stream resulting in a C/Fe3C NP concentration of 100 mg L−1. Subsequently, the C/Fe3C NPs were removed from the solution stream using high-gradient magnetic filters. Three different magnetic filters were employed: Filter 1 contained a material made of low alloy steel, filter 2 a material made of high alloy steel, and filter 3 a material made of polymer-coated high-alloy steel (all provided by hemotune AG, Switzerland). Over the course of 2 h, blood samples were taken before and after the magnetic filter and nanoparticle concentration was determined by spICP-TOFMS.
Results and discussion
Effect of functionalization on particle characteristics
Several steps of surface treatment are needed to prepare C/Fe3C NPs for blood purification. Monitoring the characteristics of the particles during these processes is important. In Fig. 2a, we provide TEM images taken after two main steps during the preparation of these particles, including the polymer coating (S1) and streptavidin functionalization (S2). TEM images show that there is no significant change in the particles’ primary shape and size (see also Table 2). However, TEM images of the particles only show primary size and shape of the particles and not the actual status of them when dispersed in media. Further analyses of these particles by spICP-TOFMS (see Fig. 2b) give insights into the transitions that the particles made during the modification steps. Comparing the mass distribution of detected particles in S1 and S2 in water, median NP mass increased from 0.61 fg for S1 to 2.0 fg for S2. Polymer coating on S1 particles prevents them from aggregation because the polymer creates a physical barrier between particles [47]. The median determined mass of the streptavidin functionalized particles S2 is significantly greater than that of the S1 particles. This increased mass is most likely due to aggregation of the protein-coated S2 particles. The median mass of detected S2 is 3.3 times higher than that of particles S1. This indicates that the formed aggregates are in the order of few particles, though some large outlier particles are also apparent.
Effect of functionalization on C/Fe3C. a TEM image of S1 (polymer coated) and S2 (streptavidin functionalized) C/Fe3C particles. Scale bars are 100 nm. b Boxplot of mass of measured C/Fe3C NPs and single-particle mass concentration recovery for C/Fe3C. Mass concentrations for the C/Fe3C NPs are calculated based on measured mass of Fe in NPs. Mass concentration error bars represent the standard deviation of measurements (N = 3) c Serial dilution of C/Fe3C (S2) in water and in blood diluted a million fold
In Fig. 2b, we also plot the recorded particle mass distributions and mass concentrations as boxplots for S1 and S2 in water and in bovine blood. The boxplots reveal a positively skewed mass distribution of particles. This distribution skew could, in part, be the result of the spICP-TOFMS measurement in which particles below the critical mass are not detectable. In all analyses, particle suspensions with a concentration of 100 ± 1 mg L−1 (which corresponds to 64 ± 3 mg L−1 iron) were analyzed. In all cases, particle mass concentration recoveries were less than 50%. Based on ICP-TOFMS quantification of a particle digest, the C/Fe3C NPs are composed of roughly mass fraction of 63% iron, with the rest likely attributed to structural carbon (mass fraction of 7% in Fe3C) and the coated shell of carbon plus polymer and streptavidin (mass fraction of ca. 30%). Considering this mass fraction of iron, quantification by spICP-TOFMS events still underestimates the true particle mass concentration. This underestimation occurs because particles below the method’s critical mass are not detectable. Critical mass is the function of instrument sensitivity and dissolved (i.e., background) iron level of the sample [44]. When the targeted particle mass range is near the critical mass of the given measurement, aggregation becomes an important factor in how efficiently particle events are recorded. Aggregation causes an increase in the mass of Fe in individual events, which improves the chances of detection above the critical mass. On the other hand, dispersion of particles will potentially result in more undetected particles below the critical mass. As seen in Fig. 2b, S1 has a slightly lower determined particle mass concentration, which could be because these particles have polymer branches that act as active anti-aggregation mechanisms on their surface. If the dispersed particles were, on average, smaller than the critical mass, they would not be detected quantitatively, and so recovery would be poor. On the other hand, particles from S2 are slightly more aggregated, and are larger in size (i.e., they have more mass) which allows a larger fraction of them to be detected by spICP-TOFMS, and results in an improved particle mass concentration recovery. For the rest of the study, we use S2 and for simplicity just call it C/Fe3C.
Fe-NP calibration in blood
In order to detect the presence of nanoparticles in water and blood (bovine whole blood, heparin anticoagulated), it is essential to know the LOD in terms of mass concentration. Therefore, we analyzed C/Fe3C NPs at several concentrations and determined the Poisson-based LOD using Eq. 2. This LOD represents the minimum particle mass concentration above which we can record Fe-NPs above the background. In addition to detection in water, we studied 106-times diluted whole blood. In all cases, C/Fe3C NPs were added at variable concentrations to the matrix before dilution. Mass quantification and calibration was constructed for several Fe-NP concentrations recorded in each matrix and are provided in Fig. 2c. Summarized calibration results are presented in Table 3. The LOD in DI water is lower compared to that of the diluted blood because the lower background of iron in water leads to a lower critical mass and a higher sensitivity (Fe-NP events/(ng L−1)).
In Fig. 2b, we provide mass distributions and particle mass concentration for C/Fe3C NPs measured in water and diluted blood. In our study, we tried several dilution factors for whole blood and found that the best particle mass concentration recovery was obtained for 106-times diluted blood and that the average mass of Fe per particle increases in blood compared to that in water which is likely due to increased formation of C/Fe3C aggregates.
In spICP-MS, particles with analyte mass below the critical mass (which depends on sensitivity and background signal levels) are not recorded, and so spICP-MS methods are sometimes unable to detect the full mass distribution of particles. However, even with this limitation, spICP-MS is beneficial for detection of low concentrations of particles compared to digestion methods. For example, the LODs for ICP-TOFMS analysis of the dissolved iron detection in water and undiluted blood respectively are 300 ng L−1 and 11.3 mg L−1. On the other hand, we demonstrate that spICP-TOFMS provides an LOD improvement that is 300 times lower in the case of water and 3.8 times lower in the case of blood. The detection of particles in whole blood is worse than in water because high dilutions of whole blood are necessary to reduce the iron background and the background noise from the blood samples is high.
Magnetic purification case study
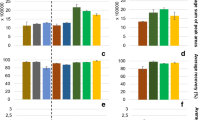
Magnetic blood purification is a new and advanced method for the removal of targeted molecules or microbes directly from a biological sample using surface-modified C/Fe3C NPs, followed by subsequent removal of C/Fe3C NPs from the bloodstream using a magnetic separation unit. Characterization of the magnetic purification system in terms of PNCs and particle-mass concentrations released provides diagnostic information to guide develop and characterize performance of these devices and offers data unavailable via bulk analysis approaches. Here, we performed spICP-TOFMS to assess the magnetic separation unit in terms of both the removal efficiency of spiked particles in saline and the potential for inorganic nanoparticle impurities or contaminations. Figure 3a shows a schematic of the C/Fe3C NP removal test setup. The filter and tubing were washed before injection of C/Fe3C NPs with 9 g L−1 sodium chloride blank. This sample is called “pre-wash.” In the pre-wash step, the goal was to investigate the possible release of the NPs and dissolved impurities from the filter material itself. After this step, a known concentration of C/Fe3C NPs in saline was injected; this sample is called the “injected NP.” Following, injected particles were removed online in the magnetic filter and the remaining sample is called the “post-separation.” spICP-MS analysis results from these three steps are provided in Fig. 3b. In this plot, we show results from three types of magnetic filters, F1-3 (see the “Materials and methods” section). Filter 1 shows substantially more particles released in the pre-wash steps. Filter 3 has the lowest pre-wash particle concentrations.
Magnetic separation of C/Fe3C NPs in saline study. a Schematic of the experiment setup. b Quantification of particle mass concentration at different steps of separation study. c Removal efficiency of particles, for different filter types. d Fe–Cr particulate release from the three filters in the pre-wash step
With spICP-TOFMS, complete elemental spectra are recorded, which also enables screening for possible metal contaminations. In our analysis, we found that Cr contaminations were the most common impurity. Fe–Cr was detected as an impurity in the pre-washed samples of filter 1 and filter 2. These particles could be potentially released from the filter itself since after coating the filters, the Fe–Cr contamination fell below the detection level. The efficiency of removing the injected C/Fe3C NPs by the magnetic filters was determined based on comparing the mass concentration of detected C/Fe3C NPs before and after separation by spICP-TOFMS in saline solution. According to the results in Fig. 3c, filter 1 has the lowest particle removal efficiency, while filter 2 and filter 3 showed similar C/Fe3C NP removal efficiencies. All filters showed C/Fe3C NP removal efficiencies greater than 99.8%; however, these removal efficiencies are calculated based on the detectable particles, i.e., particles above the critical mass. Single-particle ICP-TOFMS cannot detect particles below the critical mass, and so the removal efficiency of smaller particles cannot be determined, which is a limitation of this method. For future removal efficiency studies in whole blood, blood samples must be diluted 106 times, at which the LOD is 3.0 mg L−1 of Fe in whole blood. Because C/Fe3C particles are spiked at a maximum concentration of 100 mg L−1 (64 ± 3 mg L−1 of Fe) prior to magnetic separation of the particles, spICP-TOFMS may be used to quantify removal efficiency up to 97 ± 1% removal in whole blood and up to 99.99 ± 0.01% in water. The C/Fe3C NP particle removal efficiency as determined in the saline solution demonstrates that the magnetic purification unit can efficiently remove C/Fe3C particles. Future spICP-TOFMS studies on whole blood with a next-generation magnetic particle separation unit could be used to confirm at least a lower floor for particle-removal efficiency.
Conclusion
We have demonstrated a multi-element, matrix-matched quantification technique for the analysis of magnetic iron nanoparticles based on single-particle ICP-TOFMS, which could be used with minimum sample preparation in solution mode. The average Fe content of whole blood is around 450 mg L−1; here, we demonstrated Fe-NP detection at mass concentrations down to 3 mg L−1 in whole blood. Consequently, with spICP-TOFMS, Fe-NPs can be determined down to a Fe-NP mass concentration 1/150th of the mass concentration of the blood matrix. This mass detection limit depends on Fe-NP mass distributions, blank particle concentrations, background Fe concentrations, and measurement time. Future studies focusing on improving this mass detection limit of Fe-NPs should emphasize reducing the dissolved background through further dilution or online size fractionation coupled to spICP-TOFMS. We showed the utility of our spICP-TOFMS method for studying the presence and release of inorganic nanoparticles from a medical device and emphasize the potential of this approach for measuring CQAs in the nanomedicine field.
References
D’Mello SR, Cruz CN, Chen M-L, Kapoor M, Lee SL, Tyner KM. The evolving landscape of drug products containing nanomaterials in the United States. Nat Nanotechnol. 2017;12(6):523–9.
Weissig V, Elbayoumi T, Flühmann B, Barton A. The growing field of nanomedicine and its relevance to pharmacy curricula. Am J Pharm Educ. 2021;85(8):8331.
Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–31.
Murthy SK. Nanoparticles in modern medicine: state of the art and future challenges. Int J Nanomed. 2007;2(2):129.
Verma A, Uzun O, Hu Y, Hu Y, Han H-S, Watson N, et al. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat Mater. 2008;7(7):588–95.
Frtús A, Smolková B, Uzhytchak M, Lunova M, Jirsa M, Kubinová Š, et al. Analyzing the mechanisms of iron oxide nanoparticles interactions with cells: a road from failure to success in clinical applications. J Controlled Release. 2020;328:59–77.
European Commission. Medical Device Regulation (MDR) . Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. Official Journal of the European Union. 2017.
McNeil SE. Challenges for nanoparticle characterization. In: McNeil SE, editor. Characterization of nanoparticles intended for drug delivery. Totowa, NJ: Humana Press; 2011. p. 9–15.
Svendsen C, Walker LA, Matzke M, Lahive E, Harrison S, Crossley A, et al. Key principles and operational practices for improved nanotechnology environmental exposure assessment. Nat Nanotechnol. 2020;15(9):731–42.
Laborda F, Bolea E, Cepria G, Gomez MT, Jimenez MS, Perez-Arantegui J, et al. Detection, characterization and quantification of inorganic engineered nanomaterials: a review of techniques and methodological approaches for the analysis of complex samples. Anal Chim Acta. 2016;904:10–32.
Shang J, Gao XH. Nanoparticle counting: towards accurate determination of the molar concentration. Chem Soc Rev. 2014;43(21):7267–78.
Geertsen V, Barruet E, Gobeaux F, Lacour JL, Tache O. Contribution to accurate spherical gold nanoparticle size determination by single-particle inductively coupled mass spectrometry: a comparison with small-angle X-ray scattering. Anal Chem. 2018;90(16):9742–50.
Tuoriniemi J, Cornelis G, Hassellov M. Improving the accuracy of single particle ICPMS for measurement of size distributions and number concentrations of nanoparticles by determining analyte partitioning during nebulisation. J Anal At Spectrom. 2014;29(4):743–52.
Laramy CR, Brown KA, O’Brien MN, Mirkin CA. High-throughput, algorithmic determination of nanoparticle structure from electron microscopy images. ACS Nano. 2015;9(12):12488–95.
Feng Q, Liu Y, Huang J, Chen K, Huang J, Xiao K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci Rep. 2018;8(1):2082.
Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm. 2008;5(2):316–27.
Álvarez-FernándezGarcía R, Fernández-Iglesias N, López-Chaves C, Sánchez-González C, Llopis J, Montes-Bayón M, et al. Complementary techniques (spICP-MS, TEM, and HPLC-ICP-MS) reveal the degradation of 40 nm citrate-stabilized Au nanoparticles in rat liver after intraperitoneal injection. J Trace Elem Med Biol. 2019;55:1–5.
Kuznetsova OV, Mokhodoeva OB, Maksimova VV, Dzhenloda RK, Jarosz M, Shkinev VM, et al. High-resolution ICP-MS approach for characterization of magnetic nanoparticles for biomedical applications. J Pharm Biomed Anal. 2020;189: 113479.
Patil US, Adireddy S, Jaiswal A, Mandava S, Lee BR, Chrisey DB. In vitro/in vivo toxicity evaluation and quantification of iron oxide nanoparticles. Int J Mol Sci. 2015;16(10):24417–50.
Chemnitzer R. Strategies for achieving the lowest possible detection limits in ICP-MS. Spectroscopy. 2019;34(10):12–6.
Montano MD, Olesik JW, Barber AG, Challis K, Ranville JF. Single Particle ICP-MS: advances toward routine analysis of nanomaterials. Anal Bioanal Chem. 2016;408(19):5053–74.
Kocic J, Günther D, Hattendorf B. Improving detection capability for single particle inductively coupled plasma mass spectrometry with microdroplet sample introduction. J Anal At Spectrom. 2021;36(1):233–42.
Mozhayeva D, Engelhard C. A critical review of single particle inductively coupled plasma mass spectrometry – a step towards an ideal method for nanomaterial characterization. J Anal At Spectrom. 2020;35(9):1740–83.
Rua-Ibarz A, Bolea-Fernandez E, Pozo G, Dominguez-Benetton X, Vanhaecke F, Tirez K. Characterization of iron oxide nanoparticles by means of single-particle ICP-mass spectrometry (SP-ICP-MS) – chemical versus physical resolution to overcome spectral overlap. J Anal At Spectrom. 2020;35(9):2023–32.
Uusimaeki T, Wagner T, Lipinski HG, Kaegi R. AutoEM: a software for automated acquisition and analysis of nanoparticles. J Nanopart Res. 2019;21(6):122.
Hendriks L, Gundlach-Graham A, Hattendorf B, Guther D. Characterization of a new ICP-TOFMS instrument with continuous and discrete introduction of solutions. J Anal At Spectrom. 2017;32(3):548–61.
Mehrabi K, Kaegi R, Gunther D, Gundlach-Graham A. Emerging investigator series: automated single-nanoparticle quantification and classification: a holistic study of particles into and out of wastewater treatment plants in Switzerland. Environ Sci Nano. 2021;8(5):1211–25.
Pace HE, Rogers NJ, Jarolimek C, Coleman VA, Higgins CP, Ranville JF. Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal Chem. 2011;83(24):9361–9.
Ramkorun-Schmidt B, Pergantis SA, Esteban-Fernandez D, Jakubowski N, Gunther D. Investigation of a combined microdroplet generator and pneumatic nebulization system for quantitative determination of metal-containing nanoparticles using ICPMS. Anal Chem. 2015;87(17):8687–94.
Hendriks L, Ramkorun-Schmidt B, Gundlach-Graham A, Koch J, Grass RN, Jakubowski N, et al. Single-particle ICP-MS with online microdroplet calibration: toward matrix independent nanoparticle sizing. J Anal At Spectrom. 2019;34(4):716–28.
Mehrabi K, Gunther D, Gundlach-Graham A. Single-particle ICP-TOFMS with online microdroplet calibration for the simultaneous quantification of diverse nanoparticles in complex matrices. Environ Sci-Nano. 2019;6(11):3349–58.
Herrmann IK, Beck-Schimmer B, Schumacher CM, Gschwind S, Kaech A, Ziegler U, et al. In vivo risk evaluation of carbon-coated iron carbide nanoparticles based on short- and long-term exposure scenarios. Nanomedicine (Lond). 2016;11(7):783–96.
Viet Long N, Minh Thi C, Yong Y, Cao Y, Wu H, Nogami M. Synthesis and characterization of Fe-based metal and oxide based nanoparticles: discoveries and research highlights of potential applications in biology and medicine. Recent Pat Nanotechnol. 2014;8(1):52–61.
Herrmann IK, Urner M, Koehler FM, Hasler M, Roth-Z’Graggen B, Grass RN, et al. Blood purification using functionalized core/shell nanomagnets. Small. 2010;6(13):1388–92.
Doswald S, Stark WJ, Beck-Schimmer B. Biochemical functionality of magnetic particles as nanosensors: how far away are we to implement them into clinical practice? Journal of Nanobiotechnology. 2019;17(1):73.
Bougas L, Langenegger LD, Mora CA, Zeltner M, Stark WJ, Wickenbrock A, et al. Nondestructive in-line sub-picomolar detection of magnetic nanoparticles in flowing complex fluids. Sci Rep. 2018;8(1):3491.
Costo R, Heinke D, Grüttner C, Westphal F, Morales MP, Veintemillas-Verdaguer S, et al. Improving the reliability of the iron concentration quantification for iron oxide nanoparticle suspensions: a two-institutions study. Anal Bioanal Chem. 2019;411(9):1895–903.
Nwoko KC, Raab A, Cheyne L, Dawson D, Krupp E, Feldmann J. Matrix-dependent size modifications of iron oxide nanoparticles (ferumoxytol) spiked into rat blood cells and plasma: characterisation with TEM, AF4-UV-MALS-ICP-MS/MS and spICP-MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1124:356–65.
Schumacher CM, Herrmann IK, Bubenhofer SB, Gschwind S, Hirt A-M, Beck-Schimmer B, et al. Quantitative recovery of magnetic nanoparticles from flowing blood: trace analysis and the role of magnetization. Adv Funct Mater. 2013;23(39):4888–96.
Bellusci M, La Barbera A, Padella F, Mancuso M, Pasquo A, Grollino MG, et al. Biodistribution and acute toxicity of a nanofluid containing manganese iron oxide nanoparticles produced by a mechanochemical process. Int J Nanomed. 2014;9:1919.
Kolosnjaj-Tabi J, Javed Y, Lartigue L, Volatron J, Elgrabli D, Marangon I, et al. The one year fate of iron oxide coated gold nanoparticles in mice. ACS Nano. 2015;9(8):7925–39.
Kruszewska J, Sikorski J, Samsonowicz-Górski J, Matczuk M. A CE-ICP-MS/MS method for the determination of superparamagnetic iron oxide nanoparticles under simulated physiological conditions. Anal Bioanal Chem. 2020;412(29):8145–53.
Gundlach-Graham A, Mehrabi K. Monodisperse microdroplets: a tool that advances single-particle ICP-MS measurements. J Anal At Spectrom. 2020;35(9):1727–39.
Gundlach-Graham A, Hendriks L, Mehrabi K, Gunther D. Monte Carlo simulation of low-count signals in time-of-flight mass spectrometry and its application to single-particle detection. Anal Chem. 2018;90(20):11847–55.
Currie LA. Nomenclature in evaluation of analytical methods including detection and quantification capabilities (IUPAC Recommendations 1995). Pure Appl Chem. 1995;67(10):1699–723.
Tanner M. Shorter signals for improved signal to noise ratio, the influence of Poisson distribution. J Anal At Spectrom. 2010;25(3):405–7.
Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51.
Acknowledgements
The authors thank Roland Mäder from the ETH mechanical workshop for manufacturing components of the dual sample introduction setup. A. Gundlach-Graham and K. Mehrabi acknowledge funding through an Ambizione grant of the SNSF, project no. PZ00P2_174061. A. Gundlach-Graham also acknowledges Iowa State University for faculty start-up funds. M. Dengler, I. Nilsson, M. Baumgartner, and C. A. Mora acknowledge funding through a grant of Wyss Zurich Translational Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Dengler, I. Nilsson, M. Baumgartner, and C.A. Mora are employed by hemotune AG but declare no financial or non-financial interest in the results of this publication. K. Mehrabi, D. Günther, and A. Gundlach-Graham have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehrabi, K., Dengler, M., Nilsson, I. et al. Detection of magnetic iron nanoparticles by single-particle ICP-TOFMS: case study for a magnetic filtration medical device. Anal Bioanal Chem 414, 6743–6751 (2022). https://doi.org/10.1007/s00216-022-04234-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04234-w