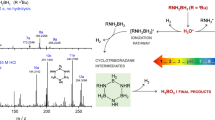
Abstract
The aqueous-phase reaction of dimethylarsinc acid (DMAs(V)) with NaBH4 (THB) was studied under non-analytical conditions (1000 μg/mL As, 0.1 M HCl, 1% NaBH4) with the aim of identifying intermediates and reaction products. The use of direct analysis in real time (DART) with high-resolution mass spectrometry (HRMS), in combination with two different chemical vapor generation systems, allowed the identification of some species not detected by GC-MS such as Me2As–AsMe–AsMe2 and the arsonium species [Me3As–AsMe2]+ and [Me2As–AsMe2–AsMe2]+. Many other methylated species of arsenic containing up to four arsenic atoms have been observed. Unfortunately, the oxidation mechanism that took place in the DART source interfered with the identification of some of those species formed in solution following THB reduction. The species identified by DART-HRMS, together with those previously identified by GC-MS (Me2AsH, Me2AsOH, Me3As, Me3AsO, Me2AsAsMeH, Me2AsAsMe2, and Me2As–O–AsMe2)‚ enabled the formulation of hypotheses on the possible reaction pathways and revealed an aqueous-phase reactivity of DMAs(V) which could not be explained on the basis of current knowledge.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemical vapor generation (CVG) of volatile arsanes by reaction of inorganic As(III, V) and methylated As(V) acids with aqueous boranes (MBH4, M = Na, K; R3NBH3, R = H, alkyl), besides its analytical relevance for trace arsenic determination and speciation [1,2,3,4,5,6,7,8,9,10,11,12], represents an useful tool for the investigation of the mechanisms controlling aqueous-phase CVG [13,14,15,16]. The good stability of arsane and methylated arsanes, combined with the use of mass spectrometry and deuterated reagents, allowed the identification of the mechanism of hydrogen transfer [13, 15, 17, 18] and reaction intermediates [13, 16, 17], taking place in CVG under various reaction conditions.
The present state of knowledge can be outlined with the aids of Schemes 1 and 2. In general, an As(V) substrate, R2AsO(OH) (R = OH, Me), is converted to the corresponding arsanes, R2AsH (R = H, Me), by the stepwise, direct transfer of hydride from borane to arsenic substrate (Scheme 1). The hydride transfer takes place through the formation of analyte-borane complexes, ABC, between the analytical substrate and the borane or a hydridoboron species, B–H. Reaction 1 (Scheme 1) represents the reduction step, which is common for all pentavalent arsenic species.
In the case of trivalent arsenic species, the derivatization reaction is described by reactions 2–4, where reaction 4 represents the classical arsane formation from arsenous acid. The stepwise replacement of hydroxyls by the hydride leads to formation of hydrido-metal(oid) complexes (HMC) intermediates I–VI, which contain both As–H and As–OH moieties.
The HMC can react among them, with the final arsane and with the analytical substrate through condensation reactions leading to formation of oligomers containing two or more arsenic atoms [14, 16, 17](Scheme 2).
The formation of arsane would be better described by a reaction scheme that takes into account the competition between the hydride formation pathway (Scheme 1) and the condensation pathway (Scheme 2) [16].
Under analytical conditions, where total concentration of THB is several orders of magnitude higher than the analytical substrates, the hydride derivatization pathway prevails. Under these conditions, all possible mutual interaction among reaction intermediates I–VI, analytical substrate, and final arsanes are negligible. All arsenic species react with the large excess of THB, and/or its hydrolysis products (B-H), and they are quickly converted to final arsanes, which are the desired derivatization products.
When the arsenic concentration increases and/or the THB/As ratio decreases (non-analytical conditions), the occurrence of condensation reactions became increasingly important. In this case, the formation of arsane oligomers has been detected by various mass spectrometric techniques [13, 14, 17].
Under non-analytical conditions (up to 10 mM As), the volatilization efficiency of arsenic species dramatically decreased [17]. For inorganic As(III), about 65% of arsenic remains in the condensed phase (45% in solution and 20% as solid precipitate). For both MeAsO(OH)2 and Me2AsO(OH), the amount fraction of the total As remaining in solution as non-volatile compounds is > 98%. The identification of arsenic compounds which are present in the gas phase [13, 16, 17] and liquid phase [14], together with the evidence obtained by deuterium-labelled experiments [13, 15, 16], serves as a support of the mechanism of CVG depicted in Schemes 1 and 2.
In the case of reaction between Me2AsO(OH) (DMAs(V)) and NaBH4 (THB) under non-analytical conditions, the current reaction model based on the competition between reduction (Scheme 1) and condensation (Scheme 2), which is followed by As(III) [16] and MMAs(V) [14] substrates, would have suggested that the major product was Me2AsAsMe2, i.e., the condensation product of Me2AsOH with Me2AsH. Experimental evidences indeed indicate that most of arsenic remained in solution (> 98%) at the end of reaction. Many transient volatile species were detected by GC-MS such as Me2AsH (the desired analytical derivatives), Me2AsX (X = OH, Cl), Me2AsAsMe2, and Me2AsOAsMe2 during reaction and the only volatile species detected in the headspace at the end of reaction was Me3As (< 2%) [17]. In spite of the high amount fraction of arsenic remaining in solution, very little precipitate was observed [17], indicating formation of soluble arsenic species which remained unidentified.
This paper reports a study performed by direct analysis in real time (DART) with high-resolution mass spectrometry (HRMS), devoted to the identification of volatile and non-volatile arsenic species formed by reaction of DMAs(V) with THB under non-analytical conditions. The experimental evidences allowed clarifying the mechanism governing the reactivity of arsenic-THB reaction systems, to explain the anomalous behavior of DMAs(V) with respect to inorganic As and MMAs(V) and discloses a reactivity which is not reported previously in the literature.
Experimental
Reagents and materials
An aqueous standard solution of dimethylarsinic acid was prepared from cacodylic acid (Me2As(O)OH, 99.3% purity, Sigma-Aldrich) in 0.1 M HCl at a mass fraction of 1000 μg/g of As. A reducing solution of sodium tetrahydroborate (1% w/v) was prepared by dissolving the solid salt (NaBH4, 98% purity, Alfa Aesar) in aqueous 0.1 M NaOH.
Instrumentation and apparatus
Direct analysis in real time (DART; IonSense, Saugus MA, USA) with a LTQ linear ion trap-Orbitrap mass spectrometer (Thermo Fisher Scientific Inc., Bremen, Germany) was used for identification of arsenic intermediates. The DART source was placed 17 mm away from the orifice of the Orbitrap. The mass spectrometer was tuned in positive mode and calibrated according to the manufacturer’s operating instructions. The DART was operated with a helium flow of 3.5 L/min at 100 °C. All other parameters were standard settings: needle current = 9990 mA; needle voltage = 3500 V; discharge electrode current = 7 mA; discharge electrode voltage = 150 V; grid electrode current = 10 mA; grid electrode voltage = 250 V.
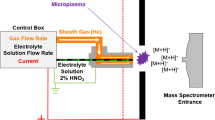
The reaction between DMAs(V) and THB was carried out in a 4-mL borosilicate vial equipped with a screw cap and a silicone/teflon septum. The gaseous phase (Fig. 1a) or the liquid phase (Fig. 1b) was injected in a glass vessel (30 mm O.D., 90 mm length) containing a porous glass frit. The apparatus was equipped with a screw cap silicone/teflon septum for injection, and an outlet line for delivery of the sample to the DART source. The outlet line was connected to the DART source with a short Teflon tube (1.5 mm I.D., 25 cm length). A glass capillary was adapted at the end of the Teflon tube (a DIP-it™ glass stick, IonSense Inc., USA) and positioned into the DART source. The apparatus was flushed with argon (150 mL/min) and the injected sample was swept to the DART source.
Schematic representation of the experimental apparatus employed for the investigation of DMAs(V)-THB reaction (see the “Instrumentation and apparatus” section for details). (A) the headspace of reaction vial was introduced in the glass vial; (B) the liquid phase of reaction vial was introduced in the gas vial
Procedures
Reactions were carried out in batch mode (Fig. 1) by mixing 1 mL volume of 1000 μg/g As as DMAs(V) in 0.1 M HCl with 200 μL of borohydride solution (1% w/w NaBH4 in 0.1 M NaOH).
The reaction resulted in formation of dense white fumes. While such fumes formed, the headspace was sampled several times in the time interval from 1 to 12 min and injected into the apparatus (1-mL injection; Fig. 1a). After about 20 min, the white fumes’ evolution ended. The vial hosting the reaction was opened and the liquid phase sampled and injected into the apparatus (0.5-mL injection; Fig. 1b).
DART-HRMS spectra were collected at 30,000 resolution mode during the reaction between DMAs(V) and THB. The spectra of both headspace and liquid sample were background corrected. The spectrum of the sample is the result of averaging 13 spectra around peak apex for headspace (narrow peak) and 26 spectra for liquid phase (broad peak). Signal acquisition was performed with Xcalibur Thermo Tune Plus software (Thermo Fisher Scientific).
Safety considerations
Arsenic intermediates generated by reduction with THB must be considered highly toxic and volatile. All experiments were conducted in a vented lab with adequate PPEs.
Results and discussion
DART-HRMS spectra and identification of arsenic species
DART-HRMS spectra collected during the reaction between DMAs(V) and THB are reported in Fig. 2. The spectrum in Fig. 2a corresponds to the headspace after 1-min reaction time; headspace spectra recorded at higher times (i.e., 8, 10, 12 min) did not show remarkable differences. We only observed some ionic species disappearing over time (i.e., [C2H8AsO2]+ at m/z = 138.9722, and [C4H13As2O]+ at m/z = 226.9375), and a moderate change of relative signal intensity. Also the spectrum of the liquid phase, recorded after 20-min reaction (Fig. 2b), is quite similar to the headspace one, with the only exception that the species below m/z = 160 are absent or reduced in intensity.
DART-HRMS spectra of reaction products detected during the DMAs(V)-THB reaction under the conditions reported in the “Procedures” section. Resolution 30,000. The signals with relative abundance < 1% were filtered out. a Headspace. b Liquid phase
The list of all arsenic species detected by DART-HRMS with relative intensities > 0.1% is reported in Table 1. DART-HRMS confirmed some species that were already identified by GC-MS in previous works, such as Me2AsH, Me2AsOH, Me3As, Me3AsO, Me2AsAsMeH, Me2AsOAsMe2, and Me2AsAsMe2 [13, 17]. In addition, the data reported in Table 1 indicates a variety of intermediates containing two, three, and also four arsenic atoms. Not all signals detected by HRMS corresponded to species generated in solution, but some could be formed within the DART ion source following one or more reactions comprising protonation, hydride abstraction, and oxidation [14, 19].
Species with one arsenic atom
The dimethylarsenic species identified by DART-HRMS (Table 1) could be originated, in principle, by Me2AsH through reactions taking place into the DART source through the pathway of hydride abstraction:
and by oxidation reactions:
The ions 1h can be also generated by the oxidation of Me2As(O)OH, the DMAs(V) analytical substrate, and Me2AsOH. Together with Me2AsH, they represent the expected reduction pathways of DMAs(V) with THB (Scheme 1).
The presence of trimethylarsenic species is confirmed by the signals 1c, 1e, and 1g. Similarly, the ions 1i can be formed from both Me3As and Me3AsO. In a previous study, Me2AsH, Me2AsOH, Me3As, and Me3AsO were identified by GC-MS in a similar reaction system [17].
Methylated polyarsanes
Tetramethyl diarsane (2f) is one of the most abundant species present in the spectra of both liquid and gaseous phases (Table 1 and Fig. 2). Pentamethyl triarsane (3b) is also present but with low abundance.
These species can generate many other signals by methyl loss:
The species 2e can be formed also by hydride abstraction from trimethyldiarsane, Me2AsAsMeH, a species which is present in the reaction environment as confirmed by GC-MS [17].
Oxidation products of polyarsanes
In the DART ion source, polyarsanes containing As–H bonds can undergo both oxidation and hydride abstraction reactions, [(M+mO)-H]+ [14, 19]. For example, starting from Me2AsAsMeH (2e), this ionization pathway can generate ions 2g (m = 1) and 2i (m = 2). For fully methylated polyarsane, hydride abstraction is prevented, but these molecules can be oxidized and protonated in the ion source, [(M+mO)+H]+. For example, molecules like Me4As2 (2f) can be converted to ions 2j (m = 1), 2m (m = 2), and 2p (m = 3).
Due to the gas-phase reactivity taking place in the ion source, a single molecular structure can produce several m/z signals, and these effects need to be considered when identifying arsenic intermediates by DART-HRMS. In this regard, certain ion signals could arise from gas-phase reaction of different precursors: as reported in Table 1, the ions 2j and 2m could have been obtained by hydride abstraction from other species, such as H(OH)(Me2)AsAsMe2, that could be condensation production of intermediates I or II (Scheme 1) with Me2AsH or Me2AsOH.
Arsonium species
Fully methylated arsonium ions, 2h and 3c, are among the most abundant species detected. The structure [Me3AsAsMe2]+ can be assigned to the species 2h, while two isomeric structures—[Me3AsAs(Me)AsMe2]+ and [Me2AsAs(Me)2AsMe2]+—can be assigned to species 3c. It seems unlikely that these arsonium species are formed into the DART source, but they rather seem to be ions generated by aqueous reaction of DMA and THB. Being permanently charged cationic species, these arsonium ions do not require further ionization. Similar behavior was observed in the hydrolysis products of dimethylamine borane, where the boronium species [(Me2N)2BH2]+ were detected by DART-HRMS [20].
Many other oxidized arsonium species were detected (2l, 2o, 2r, 2s, 3e, 3f, 3m), some of them with relatively high abundance. There are some doubts that these species were formed in the reaction media and not in the DART ion source by oxidation of [Me5As2]+ (2h) and [Me6As3]+ (3c):
It is reasonable that the oxidized cationic species do not undergo further process, as protonation or hydride abstraction, which would lead to the formation of double charge ionic species.
Arsenic species containing oxygen
As in the previous work [14], uncertainty remains whether the detected arsenic species containing oxygen are species actually formed in solutions from chemical reactions, or they are generated by oxidation in the DART source or the MS interface region. In this case, however, we know from independent GC-MS experiment that Me4As2O (2j; Table 1) is formed during reaction and in this case is detected as a protonated species [Me4As2OH]+ according to mechanism (c). For most polyarsanes containing two or more oxygen atoms, the reducing environment of the reaction suggests that it is unlikely that the arsenic species remained in the higher oxidation state. Therefore, the arsenic species containing high number of oxygen atoms are probably formed in DART source. For the above reason, the species containing oxygen atoms must be considered with cautions in the individuation of reaction pathways leading to the formation of non-volatile or less volatile arsenic species.
Reaction of DMAs(V) with THB
In order to explain the reactivity of DMAs(V) with THB under non-analytical conditions, the starting point is represented by the evidence of the species that have been identified with reliability in this work by DART-HRMS, and previously by GC-MS (Table 2) [17]. With the exception of Me2AsOAsMe2, oxidized polyarsanes were not included because they could also be formed in the DART source as discussed in the “Arsenic species containing oxygen” section.
Apart from condensation reactions, which can explain the formation of some polyarsanes:
the formation of trimethylarsanes (Me3As and Me3AsO), arsonium species ([Me3AsAsMe2]+ and [Me6As3]+), and trimethyldiarsane (Me2AsAsMeH) cannot be explained on the base of current knowledge of the reactivity of methylarsanes and polymethyl arsanes.
Previous investigations performed in organic media (benzene, toluene, and diethylether) [21,22,23] indicated the lability of As–As and As–H bonds, and a non-ionic, four-centered redistribution mechanism was considered the most likely to explain, for example, the D–H exchange in dialkylarsanes [23]:
and the reaction of MeAsH2 with Me2AsAsMe2 [22]:
According to the redistribution mechanism proposed for the above reaction,

there is no methyl transfer and there is no breakage of As–C bond involved in the formation of Me2AsH from MeAsH2. The redistribution mechanism observed in organic media [21,22,23] cannot therefore explain the formation of Me2As–AsMeH following reaction of Me2AsH with Me2AsAsMe2, as well as it cannot explain the formation of species containing the trimethylarsenic group such as Me3As, Me3AsO, [Me3As–AsMe2]+, [Me3As–AsMe–AsMe2]+, and [MeAs2–AsMe2–AsMe2]+.
The investigated reaction system took place in aqueous solvent, and it is composed by many different arsenic species (Table 2) which can undergo mutual interaction among them and with intermediates I and II of the reduction step (Scheme 1). The aqueous solvent at different acidities can favor reaction pathways forming ionic intermediates and reaction products.
We believe that a primary role in the investigated DMAs(V)-THB reaction system is played by reaction intermediates I and II and Me2AsOH with the other species which are present in the reaction environment, in particular Me2AsAsMe2. Hypothesis on some of the possible reaction pathways bringing to the formation of trimethylarsanes and arsonium species can be drawn as reported below.
Considering the evidence on the methylating properties of the reaction solution [17], trimethylarsane could be formed by methyl transfer from Me2AsAsMe2:
and Me2AsAsMeOH undergoes both reduction with hydridoboron species (B–H) and condensation with dimethylarsane [16]:
Arsonium species 2h could be formed by reaction:
The two possible isomers of the arsonium species [Me6As3]+, 3c, termed here 3c′ and 3c″, could be formed by two different reactions. The first reaction is similar to reaction 18:
In the second reaction, the formation of the other isomer 3c″ could be based on condensation among one of the possible specie 2j (see Table 1) and Me2AsOH,
where 2j corresponds to the structure (Table 1):

That can be formed by condensation reaction between Me2AsOH and intermediate II (Scheme 1):
The discussion reported above, although it has a certain speculative aspect, is based on hypotheses of reactions involving species for which experimental evidence exists (Tables 1 and 2). It is possible that other species containing oxygen may also be involved in other reaction pathways, but at the moment, there is no reliable evidence they are actually real species formed in the reacting solution.
A comparison of the behavior of DMAs(V) with respect to As(III) and MMAs(V)
From the results shown in the present and previous studies [13,14,15,16,17], it is possible to draw some conclusions about the behavior of the different arsenic species. In all cases, under analytical conditions, As(III) and As(V), MMAs(V) and DMAs(V) are converted to arsanes by direct transfer of hydrogen from THB to arsenic. The hydrogen transfer takes place through analyte-borane complex intermediates (ABC) and it is also confirmed recently in the reaction of As-sugar with THB [8]. Arsanes are formed stepwise, passing through the formation of intermediate hydrido-metal complexes (HMC) (Scheme 1). A more recent work of Marschner et al. [9] reported that at trace level, the derivatization of methylated arsenic species can proceed with some degree of demethylation. The extent of demethylation depends on solution composition and seems to decrease in the order Me3AsO, Me2AsO(OH), MeAsO(OH)2.
Increasing the analyte concentration slows down the final hydride formation and the HMC intermediates can live for a longer time with respect to analytical conditions. Under these conditions, we observed the formation of new arsenic species due to reactions involving HMC intermediates. Condensation reactions among HMC intermediates, final arsanes, and analytical substrates are predominant for inorganic arsenic and MMAs; they led to formation of dimeric, trimeric, and oligomeric arsanes, depending on THB/As ratio [13, 17] (Scheme 2). When the As concentration increases, some reactions of demethylation and methylation occur, but they are of little relevance and the main product remains MeAsH2. Increasing the As concentration increases the extent of condensation reactions, whereas the generation efficiency of arsane and methylarsane drops down due to formation of high molecular weight oligomeric arsanes. The dimeric, trimeric, and oligomeric arsanes formed from MMAS(V) reaction have been characterized by DART-HRMS [14], and monomeric, dimeric, and trimeric arsanes also by GC-MS [13, 17]. In the case of inorganic As, monomeric, dimeric, and trimeric arsanes have been identified by GC-MS [13, 17] whereas no arsenic species could be detected by DART-HRMS at higher As concentration. According to literature, the nature of the solid phase which is formed at higher As concentration is not elemental As but rather a solid arsenic hydrides [24].
For DMAs(V), high analyte concentration should bring to formation of the dimeric species Me2AsAsMe2, the terminus of condensation reaction. On the opposite, the dimers of inorganic arsenic (H2AsAsH2) and MMAs (HMeAsAsMeH) are open to further condensation. Notably, the highly reactive tetramethyldiarsane further interacts with the other As intermediate species, also through gaseous-phase reactions giving rise to methyl transfer reactions and formation of water-soluble arsonium species. The complex of transient arsenic species found both in the gas and liquid phases—such as Me2AsH, Me2AsOH, Me2AsAsMe2, and Me2AsOAsMe2—constitutes a reactive mixture that can evolve into stable, water-soluble arsonium species.
Previous studies demonstrated that the generation pathways of different arsanes (reactions 2, 3, 4; Scheme 1) can interact among them and with the formation pathways of other hydrides [17]. In the reduction of Sb(III) in the presence of As(III) or DMAs(V), H2As–SbH2 and MeSbH2 were identified, respectively, indicating that these condensation and methyl transfer reactions can be source of interference in the speciation of As and other hydride-forming elements.
Conclusion
The use of DART-HRMS in the study of the aqueous-phase reaction of DMAs(V) with THB, in non-analytical conditions, led to the detection of some arsenic species which have not been previously identified by GC-MS [13, 16] and allowed to clarify the anomalous behavior of DMAs(V) with respect to inorganic As and MMAs(V). The main limitation of the DART source is the potential formation of oxidized species which could complicate the interpretation of arsenic species formation. The formation of some arsonium species, not containing oxygen, could be confirmed with certainty. Their formation is in agreement with the evidence that in this reaction system, a fraction ≥ 98% of total arsenic remains in solution as soluble species [16]. The observed reactivity cannot be explained in the light of previous studies based on organic-phase reactions of methylated arsanes and polyarsanes [21,22,23, 25], where the lability of As–As and As–H bonds, and a non-ionic redistribution mechanism were considered to control the observed reactivity. It is reasonable that in aqueous-phase reactions, pathways passing through ionic intermediates are most favored and contribute to a reactivity that is not entirely known.
The hypotheses of possible reaction pathways, based on the reactions of identified species and reaction products, constitute a working hypothesis for further investigations aimed to clarify the mechanisms that govern the aqueous-phase reaction of methylarsenate-THB. It remains confirmed the conclusion of previous studies [17] about possible interference behavior of DMAs(V) in the speciation of arsenic and other hydride forming elements.
References
Dědina J, Tsalev DL. Hydride generation atomic absorption spectrometry. Chichester: Wiley; 1995.
Musil S, Matoušek T, Currier JM, Stýblo M, Dědina J. Speciation analysis of arsenic by selective hydride generation-cryotrapping-atomic fluorescence spectrometry with flame-in-gas-shield atomizer: achieving extremely low detection limits with inexpensive instrumentation. Anal Chem. 2014;86:10422–8. https://doi.org/10.1021/ac502931k.
Proch J, Niedzielski P. In–spray chamber hydride generation by multi–mode sample introduction system (MSIS) as an interface in the hyphenated system of high performance liquid chromatography and inductivity coupled plasma optical emission spectrometry (HPLC/HG–ICP–OES) in arsenic species determination. Talanta. 2020;208:120395. https://doi.org/10.1016/j.talanta.2019.120395.
Marschner K, Pétursdóttir ÁH, Bücker P, Raab A, Feldmann J, Mester Z, et al. Validation and inter-laboratory study of selective hydride generation for fast screening of inorganic arsenic in seafood. Anal Chim Acta. 2019;1049:20–8. https://doi.org/10.1016/j.aca.2018.11.036.
Douillet C, Matoušek T, Stýblo M, Wang Z, Musil S. Direct speciation analysis of arsenic in whole blood and blood plasma at low exposure levels by hydride generation-cryotrapping-inductively coupled plasma mass spectrometry. Anal Chem. 2017;89:9633–7. https://doi.org/10.1021/acs.analchem.7b01868.
Pitzalis E, Ajala D, Onor M, Zamboni R, D’Ulivo A. Chemical vapor generation of arsane in the presence of L-cysteine. Mechanistic studies and their analytical feedback. Anal Chem. 2007;79:6324–33. https://doi.org/10.1021/ac070513p.
Pitzalis E, Onor M, Mascherpa MC, Pacchi G, Mester Z, D’Ulivo A. Chemical generation of arsane and methylarsanes with amine boranes. Potentialities for nonchromatographic speciation of arsenic. Anal Chem. 2014;86:1599–607. https://doi.org/10.1021/ac4032466.
Marschner K, Musil S, Mikšík I, Dědina J. Investigation of hydride generation from arsenosugars - is it feasible for speciation analysis? Anal Chim Acta. 2018;1008:8–17. https://doi.org/10.1016/j.aca.2018.01.009.
Marschner K, Musil S, Dědina J. Demethylation of methylated arsenic species during generation of arsanes with tetrahydridoborate(1-) in acidic media. Anal Chem. 2016;88:6366–73. https://doi.org/10.1021/acs.analchem.6b00735.
Liu M, Liu T, Mao X, Liu J, Na X, Ding L, et al. A novel gas liquid separator for direct sampling analysis of ultratrace arsenic in blood sample by hydride generation in-situ dielectric barrier discharge atomic fluorescence spectrometry. Talanta. 2019;202:178–85. https://doi.org/10.1016/j.talanta.2019.04.041.
Liu M, Liu T, Liu J, Mao X, Na X, Ding L, et al. Determination of arsenic in biological samples by slurry sampling hydriDe generation atomic fluorescence spectrometry using: in situ dielectric barrier discharge trap. J Anal At Spectrom. 2019;34:526–34. https://doi.org/10.1039/c8ja00374b.
dos Santos QO, Silva Junior MM, Lemos VA, Ferreira SLC, de Andrade JB. An online preconcentration system for speciation analysis of arsenic in seawater by hydride generation flame atomic absorption spectrometry. Microchem J. 2018;143:175–80. https://doi.org/10.1016/j.microc.2018.08.004.
D’Ulivo A, Mester Z, Meija J, Sturgeon RE. Mechanism of generation of volatile hydrides of trace elements by aqueous tetrahydroborate(III). Mass spectrometric studies on reaction products and intermediates. Anal Chem. 2007;79:3008–15. https://doi.org/10.1021/ac061962c.
Pagliano E, Onor M, McCooeye M, D’Ulivo A, Sturgeon RE, Mester Z. Application of direct analysis in real time to a multiphase chemical system: identification of polymeric arsanes generated by reduction of monomethylarsenate with sodium tetrahydroborate. Int J Mass Spectrom. 2014;371:42–6. https://doi.org/10.1016/j.ijms.2014.07.048.
D’Ulivo A, Mester Z, Sturgeon RE. The mechanism of formation of volatile hydrides by tetrahydroborate(III) derivatization: a mass spectrometric study performed with deuterium labeled reagents. Spectrochimica Acta B. 2005;60:423–38. https://doi.org/10.1016/j.sab.2005.03.015.
Pagliano E, D’Ulivo A, Mester Z, Sturgeon RE, Meija J. The binomial distribution of hydrogen and deuterium in arsanes, diarsanes, and triarsanes generated from As(III)/[BH n D4-n ] - and the effect of trace amounts of Rh(III) ions. J Am Soc Mass Spectrom. 2012;23:2178–86. https://doi.org/10.1007/s13361-012-0489-5.
D’Ulivo A, Meija J, Mester Z, Pagliano E, Sturgeon RE. Condensation cascades and methylgroup transfer reactions during the formation of arsane, methyl- and dimethylarsane by aqueous borohydride and (methyl) arsenates. Anal Bioanal Chem. 2012;402:921–33. https://doi.org/10.1007/s00216-011-5503-4.
Pagliano E, Onor M, Meija J, Mester Z, Sturgeon RE, D’Ulivo A. Mechanism of hydrogen transfer in arsane generation by aqueous tetrahydridoborate: interference effects of Au(III) and other noble metals. Spectrochim Acta B. 2011;66:740–7. https://doi.org/10.1016/j.sab.2011.09.009.
Cody RB. Observation of molecular ions and analysis of nonpolar compounds with the direct analysis in real time ion source. Anal Chem. 2009;81:1101–7. https://doi.org/10.1021/ac8022108.
D’Ulivo L, Pagliano E, Onor M, Mester Z, D’Ulivo A. Application of direct analysis in real time to the study of chemical vapor generation mechanisms: identification of intermediate hydrolysis products of amine-boranes. Anal Bioanal Chem. 2019;411:1569–78. https://doi.org/10.1007/s00216-019-01598-4.
Rheingold AL, Lewis JE, Bellama JM. Homoatomic organoarsine ladder polymers. Synthesis and physical properties. Inorg Chem. 1973;12:2845–50. https://doi.org/10.1021/ic50130a021.
Gupta VK, Krannich LK, Watkins CL. NMR studies of the reaction of MeAsH2 with Me2AsAsMe2. Inorg Chim Acta. 1987;132:163–4. https://doi.org/10.1016/S0020-1693(00)81736-3.
Cullen WR, Leeder WR. Kinetics of the exchange reaction of dimethylarsenic deuteride with diethylarsine. Can J Chem. 1970;48:3757–60. https://doi.org/10.1139/v70-631.
Jolly WL, Anderson LB, Beltrami RT. Solid arsenic hydrides. J Am Chem Soc. 1957;79:2443–7. https://doi.org/10.1021/ja01567a024.
Rheingold AL, Pleau EJ, Ferrar WT. Dynamic NMR study of dimethylarsenic condensation reactions. Inorg Chim Acta. 1977;22:215–8. https://doi.org/10.1016/S0020-1693(00)90921-6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pagliano, E., Onor, M., Mester, Z. et al. Application of direct analysis in real time to study chemical vapor generation mechanisms: reduction of dimethylarsinic(V) acid with aqueous NaBH4 under non-analytical conditions. Anal Bioanal Chem 412, 7603–7613 (2020). https://doi.org/10.1007/s00216-020-02896-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02896-y