Abstract
Interference-free conditions, allowing straightforward As and Se determination, can be obtained by using CH3F (a mixture of 10 % CH3F and 90 % He) as a reaction gas in tandem ICP–mass spectrometry (ICP–MS/MS). Both target elements react via CH3F addition and subsequent HF elimination, rendering AsCH2 + and SeCH2 + the respective favored reaction product ions. Instrumental limits of detection were 0.2 ng L−1 for As and below 10 ng L−1 for Se, using either 77Se, 78Se, or 80Se. Neither addition of carbon to the solutions, nor admixing of additional He into the octopole reaction cell resulted in a further improvement of the LoDs, despite the increase in analyte signal intensity. By using synthetic matrices, containing elements giving rise to ions interfering at either the original mass-to-charge ratios or those of the reaction products, absence of spectral overlap could be demonstrated. This conclusion was corroborated by successful As and Se determination in a collection of reference materials from plant, animal, or environmental origin, displaying a considerable range of As and Se contents. These accurate results were obtained via external calibration using Te as an internal standard. The high efficiency reaction between As and CH3F and the possibility to use the major isotope of Se provides enhanced detection power versus other techniques, such as sector-field ICP–mass spectrometry, while the possibility to monitor at least three Se isotopes interference-free also enables isotopic analysis.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The determination of ultra-trace concentrations of As and Se in a large variety of samples has been the objective of numerous studies for many years. Arsenic is a toxic element that has been classified in category 1 by the International Agency for Research on Cancer (IARC), which means that it is carcinogenic for humans. [1] Selenium is one of the minor elements that, due to its presence in several proteins and enzymes, is essential for biological processes. However, already at concentrations slightly higher than optimum, it becomes toxic to humans. [2] In addition, the antagonistic metabolic effect between As and Se [3] has been typically used to reduce As poisoning effects, and different As-related diseases are linked with Se deficiency [4–6]. For this reason, and although the toxicity and/or beneficial effects of these elements strongly depend(s) on the chemical form [7–10], the determination of the total concentration of As and Se gives invaluable and fast information concerning the associated risks. [11] As a result, analysis of biological and environmental samples for their As and/or Se content(s) is of interest from a biomedical point of view. However, the determination of these metalloids at ultra-trace levels is not simple by means of any atomic spectroscopy technique [12–16]. Inductively coupled plasma–mass spectrometry (ICP–MS) can be considered as the technique of choice for the monitoring of these elements. However, despite its detection power, the determination of As and Se by ICP–MS is seriously hampered by the occurrence of both spectral and nonspectral interferences [17].
Matrix effects (nonspectral interferences [18]) are more pronounced for As and Se than for many other elements, due to their high ionization energy (9.81 and 9.75 eV, respectively), and the so-called “carbon effect,” which means that a high amount of carbon, dissolved in the sample or admixed into the ICP, leads to a signal enhancement for both As and Se. Although this effect has been widely described in the literature [19–21], there is no clear consensus concerning the mechanism responsible for it. The carbon effect is hypothesized to result from the charge transfer between C+ and CH+ (ionization energy of 11.26 and 10.64 eV, respectively) [22, 23] to atoms of elements with a high ionization energy. However, other characteristics need to be involved, because not for all elements with a high ionization energy such an enhancement is observed. In addition, extremely high amounts of carbon could also result in a signal intensity decrease due to a reduction of the plasma temperature.
Nonspectral interferences [24, 25] can be tackled in different ways (e.g., use of a suitable internal standard [26], or quantification by means of standard addition [27], or by means of isotope dilution [28–31]), but the issue of spectral interferences remains a serious one due to the formation of Ar-based polyatomic ions [32, 33] that affect the determination of 75As+ (40Ar35Cl+ and 38Ar37Cl+) and 77,78,80Se+ (40Ar36Ar1H+, 40Ar37Cl+, 40Ar38Ar+, and 40Ar40Ar+). While in the case of Se, 82Se can often be monitored interference-free, no such possibility exists for the monoisotopic As.
Different options have been explored to overcome the influence of these interferences, such as the use of analyte/matrix separation (which obviously represents additional work and degrades sample throughput) [34], of mathematical equations (which may not work efficiently if the ratio between the signal of the interferent and that of the analyte is too high) [35], of cool plasma conditions (which results in a significant drop in sensitivity for these elements, owing to their poor ionization efficiency under such working conditions) [36] and of vapor generation systems (the use of which also involves some additional sample pretreatment) [37].
Additionally, more advanced ICP–MS devices permit these spectral interferences to be overcome in a more straightforward way. The use of a sector-field ICP–MS (SF–ICP–MS) instrument, operated at higher mass resolution, is an elegant choice to resolve spectral interferences [38]. However, the maximum resolution setting (m/Δm~10,000) should be used in the case of As and Se, which involves a reduction in sensitivity of about 2 orders of magnitude, which is sometimes too much for the targeted concentrations. Also, and even using the maximum resolution setting, some polyatomic interferences still cannot be completely resolved (e.g., interference-free measurement of 80Se+ is hard to accomplish due to the very high intensity of the 40Ar2 + dimer).
The most widespread approach to deal with the spectral overlap affecting the determination of As and Se nowadays is probably the use of a quadrupole-based ICP–MS (Q–ICP–MS) instrument equipped with a collision–reaction cell [39]. Different gasses can be used to pressurize the cell and ameliorate the conditions for As and/or Se determination. For instance, the combination of a collision gas [40] (typically He) and kinetic energy discrimination can be used to reduce the interferences as, due to their larger size, polyatomic ions lose a larger fraction of their kinetic energy than do mono-atomic ions. However, this strategy is incapable of removing doubly charged interferences and is accompanied by a substantial reduction in sensitivity. Also, reactive gasses (e.g., NH3, H2, CH4, O2) can be used to selectively react with the interfering ions [41–43], aiming at the monitoring of the analyte ions at their original mass-to-charge ratio. Alternately, also a selective reaction between the analyte ions and the gas molecules [44, 45], resulting in the formation of a reaction product ion that can be measured interference-free at another m/z ratio, is sometimes viable. The efficacy of the latter approach strongly depends on the efficiency and rate of the reaction involved.
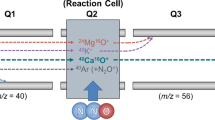
Recently, a new configuration of Q–ICP–MS instrumentation was introduced, the so-called triple quadrupole ICP–MS/MS [46–49] setup, which consists of a tandem mass spectrometer with an octopole reaction cell located in-between two quadrupole mass analyzers. This new configuration allows removing all the ions with an m/z ratio different from that of the target nuclide with the first quadrupole, which results in a full control over the reactions that are subsequently taking place in the octopole cell. The second quadrupole then selects one of the possibly many reaction products formed for monitoring and quantification of the target element. This improved control enables the application of nontypical, but highly reactive gasses, offering new possibilities via the conversion of the target elements in reaction product ions, without sacrificing sensitivity by using low efficiency reactions.
Methyl fluoride is a reaction gas that has only been used scarcely in ICP–MS before [50, 51]. However, in a previous work [49], we have demonstrated the possibilities of this reaction gas to resolve spectral overlap in the determination of light metals (Al, Co, Cr, Mn, Ni, Ti, and V) in clinical samples using ICP–MS/MS. The main goal of the current project was to investigate the potential of methyl fluoride as a reaction gas in ICP–MS/MS for dealing with the interferences affecting ultra-trace determination of both As and Se in diverse sample types.
Experimental
Instrumentation
All measurements were carried out using an Agilent 8800 triple quadrupole ICP–MS/MS instrument (Agilent Technologies, Japan). The sample introduction system comprises a MicroMist nebulizer (400 μL/min) and a Peltier-cooled Scott-type spray chamber (2 °C). The instrument is equipped with two quadrupole mass analyzers (Q1 and Q2) and an octopole collision–reaction cell (ORS3) mounted in-between the two quadrupole units (Q1–ORS3–Q2). The instrument can be used in different operation modes, (i) with the first quadrupole fully open (single quadrupole mode, SQ) or (ii) using both quadrupoles as mass filters (MS/MS mode). This MS/MS mode allows for a better control over the reactions proceeding in the cell and provides the user with the possibility of product ion and precursor ion scanning as a powerful tool for evaluating the reaction product ions formed and selecting the optimum one for quantification processes, and for identifying the parent ion at the origin of polyatomic ions formed through cell reaction, respectively. The cell can be pressurized with different inert or reactive gasses, typically He, H2, O2, and NH3, but in this work, the merits of CH3F (a mixture of 10 % CH3F and 90 % He) were evaluated. The CH3F/He mixture was introduced via the fourth line (typically used for O2) and with the flow controller calibrated for this gas. As a result, the flow rates given below are expressed in O2-equivalent milliliters per minute.
A Thermo Element XR sector-field ICP–MS instrument (ThermoScientific, Germany) was used with the aim of comparing the capabilities of both techniques for ultra-trace determination of As and Se.
Reagents and standards
Only high-purity reagents were used during all of the experiments. Water was purified using a Milli-Q Element water purification system (Millipore, France). Pro-analysis 14 M HNO3 (ChemLab, Belgium) was further purified via sub-boiling distillation. Ultrapure 28 M HF (Fisher Chemicals, Great Britain) and 9.8 M H2O2 (Fluka, Belgium) were chosen for sample digestion. Trace select 25 M MeOH for studying the carbon effect was acquired from Sigma-Aldrich, Germany. Throughout the work, appropriate dilutions of 1 g L−1 single-element standard solutions (Instrument Solutions, The Netherlands) were carried out for optimization (As and Se), for internal standardization (Te), for external calibration (standard solutions with concentrations ranging between 0 and 5 μg L−1 of As and Se), and for matrix-matching (Ca, Cl, Gd, Mo, Nb, Nd, Ru, Sm, Y, Zr).
Samples
Reference materials were analyzed for method validation purposes. The list comprises NIST SRM 1515 (Apple leaves), NBS SRM 1575 (Pine needles), NBS SRM 1573 (Tomato leaves), NIST SRM 1568a (Rice flour), BCR CRM 526 (Tuna fish tissue), NCR-CNRC DORM-4 (Fish protein), BCR CRM 414 (Plankton), NBS SRM 1646 (Estuarine sediment), NIST SRM 1566a (Oyster tissue), and NRC-CNRC TORT-3 (Lobster hepatopancreas).
Sample preparation
To avoid contamination, only metal-free tubes were used for standard and sample preparation (15 or 50 mL polypropylene centrifuge tubes, VWR, Belgium). The samples were digested via acid digestion in Teflon Savillex beakers, which had been pre-cleaned with HNO3 and HCl and subsequently rinsed with Milli-Q water. To complete the mineralization, between 0.1 and 0.2 g of the respective reference materials (except for the apple leaves reference material, for which 0.5 g was digested due to the low concentration of both As and Se) were digested with 4 mL of 14 M HNO3 and 1 mL of 9.8 M H2O2 and, only in the case of NBS SRM 1646 (Estuarine Sediment), an additional 1 mL of 28 M HF. The procedure was completed after heating at 110 °C on a hot plate overnight.
Prior to ICP–MS/MS analysis, the digested materials were diluted 40- and 20-fold for As and Se, respectively, with Milli-Q water.
The final results were calculated on a dry mass basis, taking into account the water content of the reference materials, previously calculated after drying until constant weight.
Results and discussion
As discussed in the introduction, the determination of ultra-trace levels of As and Se using ICP–MS is still a challenge. Different problems are encountered when addressing determination of ultra-trace concentrations of these elements, and there is no straightforward method to deal with all the problems encountered without sacrificing some analytical properties. The objective of this work was to investigate the capabilities of ICP–MS/MS with CH3F/He as a reaction gas in this context.
Selection of the main product ions formed upon reaction between As and Se and CH3F/He
In order to study the reactions between CH3F/He and As and Se, product ion scanning was used to identify the main reaction products. Standard solutions of 5 μg L−1 of As and of Se were measured using different CH3F/He flow rates to find the most suitable reaction product ions, in terms of sensitivity and limits of detection (LoDs). Due to the monoisotopic character of As, the mass-to-charge ratio of the first quadrupole was fixed at 75, while for Se, an m/z setting of 80 was selected because this is the most abundant nuclide. The results are summarized in Fig. 1. In both cases, it is clear that a single product ion shows the highest sensitivity; for both As and Se, there is a selective and efficient reaction with CH3F/He, consisting of CH3F addition and subsequent HF elimination, resulting in the formation of AsCH2 + and SeCH2 +. This reaction was studied in a previous work using ICP–SIFT–MS[52], where the same behavior was found for As, but not for Se. With ICP–SIFT–MS, no reaction was seen to take place between Se and CH3F, which indicates that some differences in reactivity can occur when other types of devices are used. Figure 1 also provides valuable information concerning the optimum CH3F/He flow rate settings. For As, the AsCH2 + signal intensity reaches a maximum at approximately 0.75 mL min−1 and then decreases, while the maximum sensitivity for Se is obtained at the highest flow rate setting attainable. It can be assumed that the use of higher flow rates could increase the SeCH2 + signal sensitivity even further. In any case, these reaction product ions seem promising to develop interference-free methods for As and Se determination in complex matrices using the MS/MS mode, and they were selected for further work. A schematic representation of the ICP–MS/MS operation using CH3F/He for As and Se is shown in Fig. 2.
Method development and optimum instrumental parameters for AsCH2 + and SeCH2 + monitoring
Once the most suited reaction product ions were identified, the corresponding analytical methods were developed. To further optimize the CH3F/He flow rate settings, the signal intensity of a standard solution containing 5 μg L−1 of As and of Se and a blank solution (0.14 M HNO3) were measured at different flow rates, adjusting the first quadrupole for transition of the mass of the target nuclide (75 and 80 for 75As and 80Se, respectively) and the second for transmission of the mass of the selected reaction product ion (89 and 94 for 75AsCH2 + and 80SeCH2 +, respectively). The results obtained are shown in Fig. 3, indicating an optimum CH3F/He flow rate setting of 0.72 (range between 0.60 and 0.80 mL min−1) and 1.00 mL min−1 for 75AsCH2 + and 80SeCH2 +, respectively. As previously said, it can be hypothesized that at an even higher CH3F/He flow rate, the results for Se could even be better. Despite this limitation, the two methods were fully optimized, resulting in the instrument parameters summarized in Table 1. Under the optimum conditions for each of the target elements, a set of standard solutions (concentration between 0 and 5 μg L−1) were measured 10 consecutive times in order to obtain the calibration data and the instrumental limits of detection and of quantification (LoDs and LoQs were calculated as 3 and 10 times sblank/slope, respectively). The results are given for 75As and for three different nuclides of Se (77Se, 78Se, and 80Se) in Table 2. These nuclides were selected in view of the strong Ar-based polyatomic interferences they suffer from and thus, to further prove the robustness of the methods developed.
From the data in Table 2, it is possible to appreciate the difference in sensitivity between As and Se, which cannot be due to the monoisotopic character of As only, but probably also to the higher efficiency of the reaction between CH3F/He and As. However, excellent instrumental LoDs that are suitable for analysis of real samples were obtained in all cases: 0.2 ng L−1 for As as 75AsCH2 + and below 10 ng L−1 for Se as 77,78,80SeCH2 +. It needs to be stressed that these are instrumental LoDs, so procedural blanks and sample dilution need to be taken into account for the final LoDs.
Signal intensity enhancement: addition of C and surplus addition of He
Different approaches known to enhance the signal intensity for a variety of analytes were tested in this work. Due to their high ionization energy, As and Se are poorly ionized in the plasma. As described in the introduction, high amounts of carbon can give rise to a substantial increase in the As and Se signal intensities because of the carbon effect. While on the one hand, the situation can complicate quantification and necessitates matrix-matching of the external standards or the method of standard additions, it is also frequently used to improve the sensitivity. With the aim to evaluate this possibility, different amounts of MeOH (between 0 and 10 %) were added to both the As and Se standards (5 μg L−1). The results are shown in Fig. 4. A signal enhancement is observed in both cases with increasing MeOH-concentration, reaching a maximum when between 4 and 8 % of MeOH is added. This leads to, approximately, a 2- and 2.5-fold improvement for As and Se, respectively. A further increase of the MeOH-concentration in the samples leads to a decrease in the signal intensity for both elements. However, from Fig. 4, it can also be seen that the signal-to-background ratios and thus, also the LoDs, were actually deteriorated by the use of MeOH, probably because of the impurities present in the solvent. For this reason, admixing MeOH was not further considered.
Another experiment was conducted to investigate whether the addition of a supplemental He flow in the octopole collision/reaction cell (introduced via another line) could affect the reaction between CH3F/He and the target analytes. This turned out to be the case for As. The results of this experiment are presented in Fig. 5, which shows the signal intensity for 5 μg L−1 of As at different CH3F/He flow rate settings (range of 0.10–1.00 mL min−1), while the cell was also pressurized with surplus He (range of 0–5 mL min−1).
It can be clearly seen that a higher supplemental He flow shifts the maximum signal intensity for 75AsCH2 + toward lower CH3F/He flow rates. Moreover, the net sensitivity is increased up to 3-fold, if the CH3F/He flow rate is reduced from 0.72 to 0.20 mL min−1 and the He flow rate is increased from 0 to 5 mL min−1. These results demonstrate that He plays an important role in the reaction between As and CH3F. It can be hypothesized that the presence of more He slows down the As+ ions, resulting in collisional stabilization, which improves the efficiency of the reaction between As and CH3F. This behavior has already been mentioned in the literature for reactions between other elements and CH3F [52]. The same behavior was not observed in the case of Se, for which the addition of a supplemental He flow resulted in a decrease of the signal intensity that could not be compensated with a reduction in the CH3F/He flow rate, as was the case for As. It seems that for Se, the CH3F/He flow rate was not high enough to observe the same phenomenon, perhaps due to the instrumental restriction in the CH3F/He flow rate to 1.00 mL min−1 and the differences in reaction efficiency, as discussed before.
However, it was observed that the signal-to-background ratio also deteriorated when He was added, which in turn led to degraded LoDs. Therefore, use of an additional He flow was disregarded for further work.
Comparison with other cell gasses
In order to better assess the potential of CH3F/He as a reaction gas for As and Se determination, alternative cell conditions were evaluated (“vented mode,” addition of He only, and addition of O2), using both the single quadrupole mode (SQ) and the MS/MS mode. Moreover, an SF–ICP–MS device operated in high-resolution mode was used to compare the capabilities of both techniques. The instrument settings for all the methods are provided in the Electronic Supplementary Material (ESM, Table S1 and Table S2 for ICP–MS/MS and SF–ICP–MS, respectively).
To summarize all the data obtained, the instrumental LoDs observed under different conditions are shown in Table 3. Additionally, all the calibration data are presented in Table 4. Attention should be paid to the fact that this information is partial because the instrumental LoDs reported are not affected by spectral interference due to the absence of the parent elements at the origin of interfering species (basically, Cl) for 75As and 77Se. For 78Se and 80Se, the overlap with 38Ar40Ar+ and 40Ar2 +, respectively, is always affecting the signals irrespective of the matrix considered. Despite this, Table 3 provides valuable information concerning the instrumental possibilities of the various methods.
Generally speaking, the use of MS/MS involves a reduction in sensitivity, which means that, for samples without spectral interferences, the SQ mode should provide a higher signal intensity. The differences in overall ion transmission efficiency between both modes could be most easily observed when the cell was used in “vented mode” or pressurized with He because in these cases, Q1 and Q2 were set at the same mass (see Table 4). This was not the case with O2 and CH3F because in these cases, Q1 is set to the mass of the target nuclide itself and Q2 to that of the corresponding reaction product ion. Under these conditions, both the reaction efficiency and the overall ion transmission efficiency govern the signal intensity. However, the LoD is also determined by the blank level at the mass of the reaction product ion, such that the LoDs when O2 or CH3F was used to avoid spectral overlap turned out to be better with double mass selection (MS/MS mode).
When O2 was selected, the LoD for 80Se was higher than that for 78Se, despite the higher abundance of the former nuclide. The efficiency of the reaction of As and Se with O2 is lower than that with CH3F/He, as can be seen from the sensitivity of both methods, presented in Table 4. Additionally, the signal stability for Se was substantially deteriorated (RSD > 10 %) when using O2. A final comparison between the three pressurized cell options (He, O2, and CH3F/He) used to enable interference-free determinations of As and Se indicates that the use of the novel method, using CH3F/He and the MS/MS mode, provides improved LoDs for both As and Se in all cases.
When comparing the figures of merit thus obtained with those obtained using SF–ICP–MS, the ICP–MS/MS approach with CH3F/He as a reaction gas shows an improvement in the LoDs by a factor of 50, 5, and 100 for 75As and 77,78Se, respectively, while the accurate monitoring of 80Se is not feasible using SF–ICP–MS.
In addition, the LoDs obtained in this work using CH3F/He in ICP–MS/MS were compared with those obtained using different setups as reported in the literature. This information is shown in Table 5, from which it can be seen that the ICP–MS/MS LoDs are superior to those obtained using SF–ICP–MS or other types of Q–ICP–MS instrumentation equipped with a collision–reaction cell.
The true potential and robustness of the methods developed were subsequently assessed through the use of matrix-matched standard solutions containing elements that can give rise to interfering ions. Solutions containing 5 μg L−1 of As or of Se plus 100 μg L−1 of Ca; 500 mg L−1 of Cl, 100 μg L−1 of Gd, of Nd, and of Sm; and 1 μg L−1 of Mo, of Nb, of Ru, of Y, and of Zr were analyzed. While the use of He allows the polyatomic interferences (except for 40Ar40Ar+) [40] to be resolved, doubly charged interferences (e.g., 150Nd2+, 150,154Sm2+, 154,156,160Gd2+) cannot be dealt with. When using O2 or CH3F/He, in the SQ mode, isobaric ions having the same m/z ratio as the reaction product ion (e.g., 89Y+, 91,94,96Zr+, 93Nb+, 94,96Mo+, and 96Ru+), as well as all polyatomic interfering ions, formed either in the plasma or in the cell, still present a problem. However, in MS/MS mode, both reaction gasses were able to resolve all the spectral overlaps. Moreover, CH3F/He shows a better performance than O2 because of the higher reaction efficiency with both As and Se, thus leading to better LoDs.
Results obtained for the determination of As and Se in reference materials
A number of reference materials were selected to further validate the methods relying to the use of ICP–MS/MS with CH3F/He. The materials were of plant, animal, or environmental origin, and a wide range of concentrations was covered.
External calibration was used for quantification with Te as an internal standard (selected on the basis of the closeness of its ionization energy and chemical behavior to those of the target elements) to correct for matrix effects, instrument instability, and signal drift. For each reference material, several aliquots from four separate digestions were diluted and analyzed.
The results are presented in Table 6, which shows the average of 20 measurements, obtained from four different digestions, each one measured five consecutive times. At the 95 % level of significance, all the results obtained are in good agreement with the corresponding certified value (t < t critical), which demonstrates that the methods developed enable the straightforward determination of As and Se at different concentration levels and in various matrix types, whereby the problem of both spectral and nonspectral interferences is avoided.
Additionally, a good agreement was found among the three selected Se nuclides, thus enabling the monitoring of the most abundant isotope (80Se), which is often prohibited when using other approaches (e.g., SF–ICP–MS), and also permitting isotopic analysis when required in the context of elemental assay via isotope dilution or tracer experiments.
The accuracy of the determination was proven for all the reference materials, including NIST SRM 1515 (Apple Leaves), despite its low As and Se concentrations and the presence of considerable amounts of Nd and Sm (leading to doubly charged interferences that cannot be overcome by using a collision gas (e.g., He), as discussed in the previous section).
The precision attainable was checked via the RSD (%), which includes the measurement precision, as well as the contribution from the sample preparation. Still, RSD values lower than 5 % were found for all reference materials in the case of As. These RSD values improve down to 1–2 % for reference materials with concentrations exceeding 1 μg g−1. In the case of Se, RSD values better than 3 % were found for 80Se, better than 5 % for 78Se and better than 10 % for 77Se, except for NIST SRM 1515 (Apple leaves), for which, due to the low concentration, the RSD values found were 13, 6, and 8 % for 77,78,80Se, respectively. Except in this case, the RSDs found are in good agreement with the trend expected on the basis of the isotopic abundance of the nuclides monitored (RSD via 77Se > 78Se > 80Se).
Conclusion
In this work, the capabilities of CH3F/He as a reaction gas in ICP–MS/MS for the ultra-trace determination of As and Se in diverse sample types have been demonstrated. This approach provides interference-free conditions, permitting As and Se determination via AsCH2 + and SeCH2 +, respectively, with high accuracy and precision. The instrumental LoD for As was calculated to be 0.2 ng L−1, while those for all of the Se nuclides studied were below 10 ng L−1. For Se, the approach developed is suited for interference-free element determination via all of the isotopes investigated. As a result, also the utility in speciation and isotopic analysis (e.g., tracer studies and elemental assay via isotope dilution) can be assumed feasible.
References
Roy P, Saha A (2002) Metabolism and toxicity of arsenic: a human carcinogen. Curr Sci 82:38–45
Tinggi U (2003) Essentiality and toxicity of selenium and its status in Australia: a review. Toxicol Lett 137:103–110
Sun H-J, Rathinasabapathi B, Wu B, Luo J, Pu L-P, Ma LQ (2014) Arsenic and selenium toxicity and their interactive effects in humans. Environ Int 69:148–158
Huang Z, Pei Q, Sun G, Zhang S, Liang J, Gao Y, Zhang X (2008) Low selenium status affects arsenic metabolites in an arsenic exposed population with skin lesions. Clin Chim Acta 387:139–144
Kolachi NF, Kazi TG, Wadhwa SK, Afridi HI, Baig JA, Khan S, Shah F (2011) Evaluation of selenium in biological sample of arsenic exposed female skin lesions and skin cancer patients with related to non-exposed skin cancer patients. Sci Total Environ 409:3092–3097
Sah S, Vandenberg A, Smits J (2013) Treating chronic arsenic toxicity withhigh selenium lentil diets. Toxicol Appl Pharmacol 272:256–262
Vassileva E, Becker A, Broekaert JAC (2001) Determination of arsenic and selenium species in groundwater and soil extracts by ion chromatography coupled to inductively coupled plasma mass spectrometry. Anal Chim Acta 441:135–146
Iserte MO, Roig-Navarro AF, Hernádez F (2004) Simultaneous determination of arsenic and selenium species in phosphoric acid extracts of sediment samples by HPLC-ICP-MS. Anal Chim Acta 527:97–104
Wang R-Y, Hsu Y-L, Chang L-F, Jiang S-J (2007) Speciation analysis of arsenic and selenium compounds in environmental and biological samples by ion chromatography-inductively coupled plasma dynamic reaction cell mass spectrometer. Anal Chim Acta 590:239–244
Tonietto GB, Godoy JM, Oliveira AC, de Souza MV (2010) Simultaneous speciation of arsenic (As(III), MMA, DMA, and As(V)) and selenium (Se(IV), Se(VI), and SeCN−) in petroleum refinery aqueous streams. Anal Bioanal Chem 397:1755–1761
Balaba RS, Smart RB (2012) Total arsenic and selenium analysis in Marcellus shale, high-salinity water, and hydrofracture flowback wastewater. Chemosphere 89:1437–1442
Chan CCY, Sadana RS (1992) Determination of arsenic and selenium in environmental samples by flow-injection hydride generation atomic absorption spectrometry. Anal Chim Acta 270:213–238
Ding WW, Sturgeon RE (1996) Evaluation of electrochemical hydride generation for the determination of arsenic and selenium in sea water by graphite furnace atomic absorption with in situ concentration. Spectrochim Acta B 51:1325–1334
Cai Y (2000) Speciation and analysis of mercury, arsenic, and selenium by atomic fluorescence spectrometry. TrAC Trends Anal Chem 19:62–66
Beccaloni E, Musmeci L, Stacul E (2002) Determination of As in environmental solid matrix. Anal Bioanal Chem 374:1230–1236
Resano M, Flórez MR, García-Ruiz E (2014) Progress in the determination of metalloids and non-metals by means of high-resolution continuum source atomic or molecular absorption spectrometry. A critical review. Anal Bioanal Chem 406:2239–2259
Goossens J, Vanhaecke F, Moens L, Dams R (1993) Elimination of interferences in the determination of arsenic and selenium in biological samples by inductively coupled plasma mass spectrometry. Anal Chim Acta 280:137–143
Vanhaecke F, Riondato J, Moens L, Dams R (1996) Non-spectral interferences encountered with a commercially available high resolution ICP-mass spectrometer. Fresenius J Anal Chem 355:397–400
Allain P, Jaunault L, Mauras Y, Mermet J-M, Delaporte T (1991) Signal enhancement of elements due to the presence of carbon-containing compounds in inductively coupled plasma mass spectrometry. Anal Chem 63:1497–1498
Larsen EH, Stürup S (1994) Carbon-enhanced inductively coupled plasma mass spectrometric detection of arsenic and selenium and its application to arsenic speciation. J Anal At Spectrom 9:1099–1105
Kovačevič M, Goessler W, Mikac N, Veber M (2005) Matrix effects during phosphorus determination with quadrupole inductively coupled plasma mass spectrometry. Anal Bioanal Chem 383:145–151
Grindlay G, Mora J, Md L-V, Vanhaecke F (2013) A systematic study on the influence of carbon on the behavior of hard-to-ionize elements in inductively coupled plasma-mass spectrometry. Spectrochim Acta B 86:42–49
Nakazawa T, Suzuki D, Sakuma H, Furuta N (2014) Comparison of signal enhancement by co-existing carbon and by co-existing bromine in inductively coupled plasma mass spectrometry. J Anal At Spectrom 29:1299–1305
Kim Y-S, Kawaguchi H, Tanaka T, Mizuike A (1990) Non-spectroscopic matrix interferences in inductively coupled plasma-mass spectrometry. Spectrochim Acta B 45(3):333–339
Vanhaecke F, Dams R, Vandecasteele C (1993) ‘Zone Model’ as an explanation for signal behaviour and non-spectral interferences in inductively coupled plasma mass spectrometry. J Anal At Spectrom 8:433–438
Al-Ammar AS, Gupta RK, Barnes RM (1999) Correction for non-spectroscopic matrix effects in inductively coupled plasma-mass spectrometry by common analyte internal standardization. Spectrochim Acta B 54:1849–1860
Salin ED, Antler M, Bort G (2004) Evaluation of the simultaneous use of standard additions and internal standards calibration techniques for inductively coupled plasma mass spectrometry. J Anal At Spectrom 19:1498–1500
Jr RLW, Eberhardt KR, Beary ES, Fassett JD (1997) Protocol for isotope dilution using inductively coupled plasma-mass spectrometry (ICP-MS) for the determination of inorganic elements. Metrologia 34:87–96
Reyes LH, Gayón JMM, Alonso JIG, Sanz-Medel A (2003) Determination of selenium in biological materials by isotope dilution analysis with an octapole reaction system ICP-MS. J Anal At Spectrom 18:11–16
Park CJ, Song H (2005) Determination of arsenic in biological samples by inductively coupled plasma mass spectrometry with selenium as an internal standard. J Anal At Spectrom 20:436–440
Castillo A, Boix C, Fabregat N, Roig-Navarro AF, Rodríguez-Castrillón LA (2012) Rapid screening of arsenic species in urine from exposed human by inductively coupled plasma mass spectrometry with germanium as internal standard. J Anal At Spectrom 27:354–358
Boulyga SF, Becker JS (2001) ICP-MS with hexapole collision cell for isotope ratio measurements of Ca, Fe, and Se. Fresenius J Anal Chem 370:618–623
Darrouzès J, Bueno M, Lespès G, Holeman M, Potin-Gautier M (2007) Optimisation of ICPMS collision/reaction cell conditions for the simultaneous removal of argon based interferences of arsenic and selenium in water samples. Talanta 71:2080–2084
Zhang Z, Chen S, Yu H, Sun M, Liu W (2004) Simultaneous determination of arsenic, selenium, and mercury by Ion exchange-vapor generation-inductively coupled plasma-mass spectrometry. Anal Chim Acta 513:417–423
Colon M, Hidalgo M, Iglesias M (2009) Correction strategies over spectral interferences for arsenic determination in aqueous samples with complex matrices by quadrupole ICP-MS. J Anal At Spectrom 24:518–521
Tanner SD (1995) Characterization of ionization and matrix suppression in inductively coupled ‘cold’ plasma mass spectrometry. J Anal At Spectrom 10:905–921
Musil S, Pétursdóttir AH, Raab A, Gunnlaugsdóttir H, Krupp E, Jr F (2014) Speciation without chromatography using selective hydride generation: inorganic arsenic in rice and samples of marine origin. Anal Chem 86:993–999
Townsend AT (1999) The determination of arsenic and selenium in standard reference materials using sector field ICP-MS in high resolution mode. Fresenius J Anal Chem 364:521–526
D'Ilio S, Violante N, Majorani C, Petrucci F (2011) Dynamic reaction cell ICP-MS for determination of total As, Cr, Se and V in complex matrices: still a challenge? A review. Anal Chim Acta 698:6–13
Niemelä M, Perämäki P, Kola H, Piispanen J (2003) Determination of arsenic, iron and selenium in moss samples using hexapole collision cell, inductively coupled plasma-mass spectrometry. Anal Chim Acta 493:3–12
Resano M, Ruiz EG, Mihuez VG, Móriez ÁM, Záray G, Vanhaecke F (2007) Rapid screening method for arsenic speciation by combining thin layer chromatography and laser ablation-inductively coupled plasma-dynamic reaction cell-mass spectrometry. J Anal At Spectrom 22:1158–1162
Colon M, Hidalgo M, Iglesias M (2011) Arsenic determination by ICP-QMS with octapole collision/reaction cell. Overcome of matrix effects under vented and pressurized cell conditions. Talanta 85:1941–1947
Guo W, Hu S, Wang Y, Zhang L, Hu Z, Zhang J (2013) Trace determination of selenium in biological samples by CH4-Ar mixed gas plasma DRC-ICP-MS. Microchem J 108:106–112
Guo W, Hu S, Li X, Zhao J, Jin S, Liu W, Zhang H (2001) Use of ion–molecule reactions and methanol addition to improve arsenic determination in high chlorine food samples by DRC-ICP-MS. Talanta 84:887–894
Grotti M, Frache R (2007) Direct determination of arsenic in sea-water by reaction cell inductively coupled plasma mass spectrometry. J Anal At Spectrom 22:1481–1487
Fernández SD, Sugishama N, Encinar JR, Sanz-Medel A (2012) Triple Quad ICPMS (ICPQQQ) as a new tool for absolute quantitative proteomics and phosphoproteomics. Anal Chem 84:5851–5857
Balcaen L, Woods G, Resano M, Vanhaecke F (2013) Accurate determination of S in organic matrices using isotope dilution ICP-MS/MS. J Anal At Spectrom 28:33–39
Balcaen L, Bolea-Fernandez E, Resano M, Vanhaecke F (2014) Accurate determination of ultra-trace levels of Ti in blood serum using ICP-MS/MS. Anal Chim Acta 809:1–8
Bolea-Fernandez E, Balcaen L, Resano M, Vanhaecke F. Potential of methyl fluoride as a universal reaction gas to overcome spectral interference in the determination of ultra-trace concentrations of metals in biofluids using ICP-MS/MS. Anal Chem 86:7969–7977
Moens LJ, Vanhaecke F, Bandura DR, Baranov VI, Tanner SD (2001) Elimination of isobaric interferences in ICP-MS, using ion-molecule reaction chemistry: Rb/Sr age determination of magmatic rocks, a case study. J Anal At Spectrom 16:991–994
Nonose N, Ohata M, Narukawa T, Hioki A, Chiba K (2009) Removal of isobaric interferences in isotope dilution analysis of vanadium in silicon nitride fine ceramics powder by DRC-ICP-MS. J Anal At Spectrom 24:310–319
Zhao X, Koyanagi GK, Bohme DK (2006) Reactions of methyl fluoride with atomic transition-metal and main-group cations: gas-phase room-temperature kinetics and periodicities in reactivity. J Phys Chem A 110:10607–10618
Sloth JJ, Larsen EH (2000) The application of inductively coupled plasma dynamic reaction cell mass spectrometry for measurement of selenium isotopes, isotope ratios and chromatographic detection of selenoamino acids. J Anal At Spectrom 15:669–672
Chéry CC, Günther D, Cornelis R, Vanhaecke F, Moens L (2003) Detection of metals in proteins by means of polyacrylamide gel electrophoresis and laser ablation-inductively coupled plasma-mass spectrometry: application to selenium. Electrophoresis 24:3305–3313
Frank J, Krachler M, Shotyk W (2005) Direct determination of arsenic in acid digests of plant and peat samples using HG-AAS and ICP-SF-MS. Anl Chim Acta 530:307–316
Pick D, Leiterer M, Einax JW (2010) Reduction of polyatomic interferences in biological material using dynamic reaction cell ICP-MS. Microchem J 95:315–319
Li X, Dai S, Zhang W, Li T, Zheng X, Chen W (2014) Determination of As and Se in coal and coal combustion products using closed vessel microwave digestion and collision/reaction cell technology (CCT) of inductively coupled plasma mass spectrometry (ICP-MS). Int J Coal Geol 124:1–4
Acknowledgments
The authors acknowledge Agilent Technologies for the ACT-UR research project grant as well as the Spanish Ministry of Economy and Competitiveness (Project CTQ2012-33494) and the Aragón Government (Fondo Social Europeo). Elisabeth Nissen is acknowledged for her support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection celebrating ABCs 13th Anniversary.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 23 kb)
Rights and permissions
About this article
Cite this article
Bolea-Fernandez, E., Balcaen, L., Resano, M. et al. Interference-free determination of ultra-trace concentrations of arsenic and selenium using methyl fluoride as a reaction gas in ICP–MS/MS. Anal Bioanal Chem 407, 919–929 (2015). https://doi.org/10.1007/s00216-014-8195-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8195-8