Abstract
Massive utilization of bisphenol A (BPA) in the industrial production of polycarbonate plastics has led to the occurrence of this compound (at μg/L to ng/L level) in the water treatment plant. Nowadays, the presence of BPA in drinking water sources is a major concern among society because BPA is one of the endocrine disruption compounds (EDCs) that can cause hazard to human health even at extremely low concentration level. Parallel to these issues, membrane technology has emerged as the most feasible treatment process to eliminate this recalcitrant contaminant via physical separation mechanism. This paper reviews the occurrences and effects of BPA toward living organisms as well as the application of membrane technology for their removal in water treatment plant. The potential applications of using polymeric membranes for BPA removal are also discussed. Literature revealed that modifying membrane surface using blending approach is the simple yet effective method to improve membrane properties with respect to BPA removal without compromising water permeability. The regeneration process helps in maintaining the performances of membrane at desired level. The application of large-scale membrane process in treatment plant shows the feasibility of the technology for removing BPA and possible future prospect in water treatment process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is one of the most frequently detected emerging pollutants in the environment. It is originated as a monomer in the production of epoxy resins and polycarbonate plastics (Pereira et al. 2015). BPA can be found in the form of white, crystalline solid powder or granule with chlorophenol-like odour (Tsai 2006). In terms of organic structure, BPA is constituted of phenolic group with hydroxyl group bounded to aromatic ring (Michałowicz 2014). As a single hydrocarbon molecule, BPA binds with other molecule to form polymers throughout manufacturing process (Erler and Novak 2010). Table 1 presents the physico-chemical properties of BPA. The molecular weight of BPA is 228 g/mol with relatively lower water solubility (120–300 mg/L) than phenol (8.20 × 104 mg/L) at 25 °C. However, its solubility tends to increase when dissolving in organic polar solvents or aqueous solution of alkaline conditions. This phenomenon is due to its dissociation constants (pKa) that range from 9.6 to 10.2 (Tsai 2006). The octanol–water partition coefficient (Kow) of BPA is the logarithm ratio of BPA concentration in n-octanol to water at equilibrium and specified temperature that varies between 2.2 and 3.4. Low value of the Kow indicates that BPA is strongly hydrophobic and has low potential for bioaccumulation (Thomas and Visakh 2011).
Although BPA was first synthesized in 1891, it only became a famous compound in food cans and beverage containers manufacturing in 1950s (Fu and Kawamura 2010). In 2011, it was reported that the annual production of BPA was more than 8 billion pounds. Of the total production, 100 tons might have been released into the atmosphere (Rubin 2011). Recent statistics revealed that approximately 95 % of BPA was used in the industrial production of polycarbonates and epoxy resins (Careghini et al. 2015).
Figure 1 shows the global consumption of polycarbonate that had been significantly increased from 1679 million tonnes in 2000 to 3442 million tonnes in 2010. Owing to the high demand of BPA, the trend for polycarbonate consumption was expected to go up to 4560 million tonnes by 2015 (Dutia 2012). These synthetic polymers are broadly utilized in many products such as plastic bottles, food packaging, toys, medical devices, tableware, disc manufacturing, etc. (Fig. 2) (Huang et al. 2012). Although polycarbonates are high in demand and very valuable for industrial productions, the utilization of polycarbonates is associated with BPA issue that is linked to human health (Erler and Novak 2010).
It is generally believed that BPA can impose health hazard through estrogenic activity when leaching from polycarbonate flasks during autoclaving process (Zhang et al. 2006). Even though these compounds are less persistent in the environment, their continuous introduction might lead to adverse effects (Houtman 2010). This is because BPA is an EDC that can interrupt the endocrine system by mimicking, blocking or disrupting functions of hormones in living organisms (Yüksel et al. 2013). Because of this, the migration of BPA to aquatic environment is a major concern among society over the last decade.
The conventional water treatment plant (WTP) has been known to be effective in treating surface water by removing majority of chemical and microbial contaminants. However, its application is limited for EDCs removal (Stackelberg et al. 2007; Sodré et al. 2010; Kleywegt et al. 2011; Chen et al. 2013). Furthermore, the demand for better water quality has required BPA to be removed from water sources, even though it only exists at extremely low concentration level (Zhang et al. 2006).
The US Environmental Protection Agency (EPA) has established the tolerable daily intake (TDI) for BPA at 50 μg/kg (body weight)/day (Rubin 2011) while the oral reference dose (RfD) for BPA is set at 100 μg/L as a total allowable concentration (TAC) in drinking water (Willhite et al. 2008). Nevertheless, there are no established standards or guidelines for the limit of BPA in drinking water sources at the moment. Therefore, the risks related to drinking water consumption have not yet been assessed. Poor quality of drinking water will definitely affect public health, but little is known on the chronic effects of daily exposure to low level BPA in drinking water (Sodré et al. 2010). The epidemiological and animal studies in laboratory revealed the adverse effects of BPA on human health. These include reproduction and developmental effects, metabolic disease, thyroid hormone function, albuminuria, oxidative stress, inflammation, epigenetics and gene expression (Rochester 2013). These negative impacts on human health can be possibly prevented if appropriate, reliable and safe water treatment process is implemented to eliminate BPA from water sources.
Back in the 1960s, a significant leap forward in the industrial applications of synthetic membranes has promoted the application of membrane technology in water treatment processes. Today, physical separation process of contaminants using membrane technology in water treatment plant has been getting a lot of attention. The progress of membrane science and technology leads to the invention of novel and improved membrane process with lower capital and operation costs (Fane et al. 2011). Intensive efforts on advanced treatment processes using membrane system have been made to determine the ability of the treatment to remove BPA (Zhang et al. 2006; Kim et al. 2008; Bing-zhi et al. 2008; Bing-zhi et al. 2010; Su-Hua et al. 2010; Yüksel et al. 2013). Types of membrane separation processes that are commonly used in water treatment are reverse osmosis (RO), nanofiltration (NF), ultrafiltration (UF) and microfiltration (MF) with ascending order of pore sizes (Gupta and Ali 2013). It was previously reported that about 60 million m3 of water was treated by membrane processes daily in which RO being the most widely applied membrane technology for water treatment. MF and UF on the other hand show the overall plant capacity of around 20 million m3/day, accounting 60 % of total drinking water production (Schrotter and Bozkaya-schrotter 2010). Membrane separation process is suitable for drinking water treatment as the process does not utilize chemicals, unless during cleaning process (Yang and Chen 2013). Unlike biodegradation and chemical oxidation process which tend to create by-products or new metabolites, membrane filtration is very environmentally friendly (Liu et al. 2009). The main objective of this article is to provide a review on the major issues of BPA occurrence and its effects toward living organisms. It is also the objective of this article to discuss the potential of membrane technology and its current development in removing BPA from water sources.
Occurrences and sources of BPA
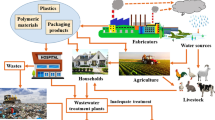
Santhi et al. (2012) have reported that BPA occurrences in the nation’s water supplies did not exceed the TDI set by US EPA, but many WTPs around the world have been found to contain BPA in the concentration ranging from μg/L (equivalent to ppb) to ng/L (equivalent to ppt) level. Table 2 shows the range of BPA concentrations that have been found in WTP worldwide. Direct discharge of BPA-based products to river can lead to severe accumulation as they can easily leach out or migrate from the polycarbonates via diffusion and hydrolysis of polymers (Michałowicz 2014). Figure 3 shows how the BPA can be found in drinking water source through point and non-point sources.
Point source contributes majority of BPA contamination either by direct discharge to the river or via sewage treatment plant (STP). Lee et al. (2013) reported that BPA concentration in the range of 0.01–44.65 μg/L could be detected in the river near highly industrialized and urbanized areas. In China, the concentrations of BPA detected in river waters and coastal waters were lower than 1 μg/L, except for several river waters which are located at highly developed industrial and commercial regions (Huang et al. 2012). BPA has also been found in drinking water supply with concentration (0.16 ± 0.03 μg/L) similar to median values detected in STP effluents and raw water samples collected in particular locations. This is due to the receiving of wastewater inputs to the river from the urban area and municipalities located nearby the city (Sodré et al. 2010).
The contamination of BPA from non-point sources is due to the utilization of BPA-based products by domestic that are disposed to the landfills. The presence of BPA in groundwater sources is related to the infiltration of leachates containing BPA from landfills (Canedo et al. 2013). The occurrence of BPA in groundwater at Mexico shows the frequency detection of 63 % with lowest concentration in the range of 1–10 ng/L. A review by Jurado et al. (2012) revealed that the highest concentration for BPA detected in groundwater by the European survey was 2299 ng/L.
Relatively high BPA quantification of 11.4 ng/L detected in the Cotia River, Brazil, presented the worst Public Water Supply Quality Index (IAP) according to Jardim et al. (2012). The polluted river is known to receive raw and treated discharges from the neighbouring urban region that is used to partially supply the city of Barueri. The concentrations of BPA in surface water (sea and river) and near-bottom water (sea) from the coastal zone of the Gulf of Gdansk ranged from <5.0 to 277.9 ng/L. The variability of BPA concentration could be attributed to increased tourism in the coastal region, water temperature and dissolved oxygen concentration (Staniszewska et al. 2015).
In wastewater effluents, BPA of 890 ng/L was detected in one of the wastewater treatment plants (WWTPs) located in Saudi Arabia. The high concentration of BPA might be due to the non-nitrifying biological treatment process (Alidina et al. 2014). Similarly, high concentration of BPA (∼336 ng/L) was also reported at the downstream of the largest WWTP in South Korea. This WWTP was known to receive wastewaters from industrial, municipal and manure sector (Ra et al. 2011). BPA concentrations in the range of 8.24–263 ng/L were reported in Songhua River of northeast China where the concentrations of this compound were typically higher in downstream locations compared to upstream locations of the city. The occurrence is due to the discharge of wastewater from the city that located near the downstream of the river (Zhang et al. 2014). In East Lake of China, the maximum BPA concentration of 37.1 ng/L was detected during the spring season. This is caused by the discharge of municipal wastewater into the lake (Wu et al. 2015).
In Malaysia, 98 % of potable water to households is supplied by the rivers that receive treated effluents, municipal and industrial wastewater prior to the conventional water treatment processes (Fulazzaky et al. 2009). Santhi et al. (2012) revealed the variation of BPA level, ranging from below quantitation limit to 215 ng/L in Langat River, Malaysia. This river is used as a source of potable water supply where the sampling site receives effluents from numerous sewage treatment plants, industries, housing estates and towns located upstream. It is showed that BPA is ubiquitous contaminant that can be found in surface, tap and bottled mineral water. However, the exposure of BPA from drinking water is very low and less than 0.01 % of the TDI.
The health effects of BPA toward living organisms
Based on the laboratory results, BPA has been suspected to cause adverse health effects as an endocrine disruptor that can bind to estrogen receptors in living organisms (Rochester 2013). BPA can act as agonists or antagonists toward the endocrine system and is capable of altering the activity of response elements of genes, block natural hormones from binding to their receptors, or act as a hormone mimic to its receptor (Rogers et al. 2013). Development of chronicle diseases such as prostate and breast cancer, Type 2 diabetes, obesity as well as impaired brain development may occur due to early exposure of BPA (Anderson et al. 2012). In vitro studies suggested that BPA exposures in prostate cancer might lead to tumour cell proliferation and activate mutant androgen receptors that are frequently selected during androgen-deprivation therapy (Wetherill et al. 2007). Maternal exposure to this compound is likely to result in postnatal changes in DNA methylation status, altering expression of specific genes in offspring and disrupting epigenetic programming of gene expression during child development (Kundakovic and Champagne 2011). BPA was also found to be carcinogenic in which the potential modes of action include estrogenic endocrine disruption, promotion of tumorigenic progression, genotoxicity and developmental reprogramming that increases susceptibility to other carcinogenic events (Keri et al. 2007). Table 3 summarizes the negative effects of BPA exposure on human health (Rochester 2013).
In a study of low doses of BPA ranging from 5 to 40 μg/kg/day during the prenatal and postnatal day in pregnant mice, an inducement of behavioural alterations was reported in adulthood. While in males, alterations of sexual behaviour were observed with decrease of anogenital and body sniffing as well as allo-grooming behaviours (Frye et al. 2012). A review of in vivo effects of BPA toward rodent showed BPA oral doses of 30 μg/kg/day and above in drinking water could reverse normal sex differences in brain structure and eliminate sex differences in behaviour. Lower BPA exposure (20 μg/kg/day) in drinking water had also caused an increased estrogen production in hippocampal neurons in male offspring of pregnant and lactating rat, owing to an increase in aromatase activity (Richter et al. 2007). The effects of BPA concentrations on living organisms are shown in Table 4.
Removal of BPA by membrane
Removal mechanisms
In membrane separation process, there are several mechanisms that are responsible for the removal of micropollutants. These include sieving, adsorption and electrostatic interaction as presented in Fig. 4. Generally, the removal mechanisms of BPA in NF and RO membrane are based on sieving and electrostatic interaction (Zhang et al. 2006; Bolong et al. 2010; Yüksel et al. 2013; Khazaali et al. 2013) while removal mechanism in microporous MF and UF is governed by adsorption (Bing-zhi et al. 2010; Bing-zhi et al. 2008). However, combination of removal mechanisms between sieving, adsorption and electrostatic interaction sometimes is also reported, depending on the intrinsic properties of membrane used.
Membrane removal of BPA via a sieving, b electrostatic interaction, c adsorption mechanism. Adapted from (Chattopadhyay et al. 2014)
Sieving mechanism (also known as size exclusion or steric hindrance) is the removal of solutes that is larger than the pore size of the membrane by preventing solutes from passing through membrane structure (Rana et al. 2014). The steric hindrance effect usually occurs in NF and RO membrane due to their (sub)nm-scale pore size (Verliefde et al. 2008). For MF and UF membrane, their pore sizes are usually much larger than the size of BPA, thus sieving mechanism does not contribute to the BPA removal. Nonetheless, removal of BPA by size exclusion of UF membrane is likely to occur in fouled membrane following the cake layer formation which creates additional barrier to retain BPA. Hu et al. (2014) showed that clean membrane could only remove BPA by adsorption while fouled membrane was capable of removing BPA by combination of adsorption and size exclusion. Results revealed that 64–76 % of BPA could be removed by the fouled membrane in comparison to only 34 % found in the clean membrane.
Electrostatic interaction between charged organic solutes and the charged membrane surface is also reported as one of the factors affecting removal efficiency of membrane against micropollutants. The electrostatic repulsion between negatively charged membrane surface and charged organic solutes can prevent the solutes from approaching to the membrane surface, increasing solute removal rate and improving permeate quality (Verliefde et al. 2008). The separation process of contaminant can be governed by either weak electrostatic (van der Waals) interactions such as dipole–dipole, ion–dipole and hydrogen bonding or strong electrostatic interactions (ion exchange) at the membrane surface (Basile et al. 2011). The separation of charged solutes by electrostatic (Donnan) exclusion is directly related to the density of surface charges for “loose” NF and UF membranes (Tiraferri and Elimelech 2012). Shao et al. (2011) showed that the appropriate charge modification on the neutral UF membrane could improve natural organic matter (NOM) removal and antifouling properties in comparison to unmodified UF membrane. The occurrence of NOM removal can be due to electrostatic repulsion between the negatively charged humic acid and the negatively charged membrane at pH 7.
Adsorptive removal takes place when contaminants are adsorbed onto the membrane (Rana et al. 2014). This mechanism can be considered by one or combination of these three steps: (i) mass transfer from liquid phase to the particles surface across the boundary layer, (ii) adsorption onto the membrane surface in which the energy will depend on the binding process (physical or chemical) and (iii) diffusion of BPA to an adsorption site in membrane either by a pore diffusion process through the liquid filled pores or by a solid surface diffusion mechanism (Cheung et al. 2007). Adsorption mechanism of micropollutants to the membrane surface can occur by both chemical (hydrogen bonding, ionic or covalent interaction) and physical (hydrophobic interactions) process as illustrated in Fig. 5. This is the main mechanism in MF and UF membrane for removing micropollutants as there are many adsorption sites available in microporous membranes (Muhamad et al. 2016). Comerton et al. (2007) reported that the highest adsorption of BPA could be achieved by the UF membrane followed by the NF and RO membrane. This is because solute adsorption not only occurs to the membrane surface but also to the membrane pores. Hence, the membrane with larger pore size such as UF will have higher adsorption site for the micropollutants compared to the smaller pore size of the NF and RO membrane. The initial removal of micropollutants by adsorption is usually higher until it reaches equilibrium state in which desorption of the compound may occur. The adsorbed compound can dissolve into the membrane top layer before moving across the membrane via convection or diffusion. The compound will eventually desorb into the permeate side of the membrane and affects the removal rate. This is likely to occur when the concentration of the feed compound is lower than the equilibrium concentration (Su-Hua et al. 2010).
Factors affecting the removal performance
It has been previously reported that as high as 90 % BPA removal could be achieved using membrane technology (Bing-zhi et al. 2008; Bolong et al. 2010; Yüksel et al. 2013). In order to achieve high BPA removal rate, one needs to understand the factors that can influence the removal of BPA during membrane process. These include the characteristics of BPA, feed water, membrane properties and operating conditions as presented in Fig. 6.
Hydrophobicity of a compound plays an important role in adsorption mechanism of membrane. Higher octanol–water partitioning coefficient (log Kow) indicates higher hydrophobicity of the compound. Hydrophobic interactions between the compound and membrane lead to the adsorption of the compound toward membrane surface and pores. This as a consequence will cause high removal rate of the compound at initial stage followed by decreasing removal rate before it reaches equilibrium state (Verliefde et al. 2007).
The adsorption of BPA onto the membrane surface is pH dependent. For instance, the removal of BPA decreases as the pH value closes to the acid dissociation constant pKa of BPA, i.e. 9.6–11.3. BPA exists in its neutral form (HO–C15H14–OH) at low pH and neutral environment. However, at high pH environments, BPA tends to dissociate and become negatively charged specie (HO–C15H14–O−) as the molecule loses its proton. Deprotonation of BPA results in charge repulsion force between BPA and negatively charged membrane surface, hindering the adsorption of BPA to the membrane surface and further decrease the removal rate (Bing-zhi et al. 2010; Comerton et al. 2007; Schäfer et al. 2006).
The presence of NOM in the water sample can slightly affect the removal rate of BPA as it will compete for the limited available adsorption sites with BPA. As a result, minor decrease of BPA removal could be experienced (Su-Hua et al. 2010; Bing-zhi et al. 2008). Heo et al. (2012) observed that BPA adsorption decreased linearly with increasing retained of dissolved organic compound (DOC). This is because high retention of DOC tends to have greater pore blockage in the membrane that creates more competition of adsorption sites for BPA. On contrary, NOM can also be an important factor for BPA removal by steric exclusion of UF membrane. The retention of NOM on membrane can lead to the formation of fouling layer that enhances BPA adsorption due to the NOM partitioning of BPA (Schäfer et al. 2006).
The molecular size of BPA (228 Da) that is far lower than that of typical UF membrane (2–500 kDa) has made it unable to be removed based on size exclusion. However, due to adsorption mechanism, UF membranes with higher molecular weight cutoff (MWCO) are found to have capability to remove BPA to certain extent. Bing-zhi et al. (2008) showed that the UF membrane with MWCO of 10 kDa could remove BPA in the range of 92–98 % while 2 kDa UF membrane removed BPA in the range of 95–98 %. These results suggested that the different membrane MWCO did not significantly affect the BPA removal rate as both membranes could achieve similar removal rate by adsorption mechanism. In addition, the authors also found that increasing the feed BPA concentration from 30 to 550 μg/L only slightly affected the permeate concentration, decreasing its removal from 96 to 92 %. It was suggested that initial concentration has no significant influence on BPA retention, owing to the constant partition coefficient for BPA between membrane and bulk solution.
Zhao et al. (2015) recently investigated the removal mechanism of BPA via size exclusion using low MWCO UF membrane (1 kDa). The possibility of hydrophobic BPA removal by membrane adsorption was excluded as hydrophilic membrane was used in this study. Without the adsorption mechanism, this membrane only exhibited <30 % BPA removal. It is also reported that the presence of ions such as Na+, Li+, Mg2+, H+ and OH− tended to decrease the removal rate of BPA. The Stokes radius of the hydrated BPA suggested that the ions may compete with BPA hydration molecules. Ions tend to attract water molecules due to stronger attractive forces, causing partial dehydration of the BPA and decrease of its effective size. The reduced size of BPA makes it easier to pass through the membrane pore, affecting the rejection efficiency.
Han et al. (2013) studied the effect of permeation rate toward the sorption of BPA by conducting cross-flow filtration using MF membrane at the flow rates of 10, 20 and 40 mL min−1. The 4-fold increase in membrane flux showed minimal effects of sorption in membranes due to rapid hydrogen bonding interactions between the membrane and BPA. Khazaali et al. (2013) showed that BPA rejection using RO membrane was higher with increased pressure until critical pressure was reached, then the rejection was decreased. Increasing pressure had improved membrane water flux, but led to the accumulation of BPA molecules on the membrane surface. It was pointed out that higher operating pressure than that of critical pressure had reduced the effective pressure driving force for water molecule to transport but increased solute passage through membrane.
Performances of membrane in BPA removal
Membrane technologies have been studied for BPA removal for many years (Schäfer et al. 2006; Su-Hua et al. 2010; Bing-zhi et al. 2010; Heo et al. 2012; Han et al. 2013; Khazaali et al. 2013). There are many advantages of using membrane in water treatment process. These include the ease of operational control, low-energy costs, compact in structure and potential material recovery compared to the conventional treatment process (Mehwish et al. 2014). The studies of membrane for BPA removal performances are commonly performed using commercially available membranes manufactured by several well-known companies such as Millipore Corporation, Toray Corporation, Koch Membrane Systems Incorporation and Dow Filmtec.
These commercial membranes are mainly made of polymeric or ceramic materials. Table 5 shows the performances of commercial membranes in removing BPA. The removal performance varies, depending on the types of membrane used and the component of feed water spiked with BPA. The “tight” membranes such as NF and RO are able to remove BPA effectively by size exclusion. However, these membranes are associated with higher operating cost due to higher operating pressure required and relatively low production of permeate even in long operation time (Schrotter and Bozkaya-schrotter 2010).
As opposed to that, “loose” MF and UF membranes have been considered as a promising alternative to reduce cost of operation as they can be operated at lower pressure (1–3 bar) and produces more permeate within shorter time (Igunnu and Chen 2012). However, it must be pointed out that microporous membranes do not able to remove BPA by size exclusion as effective as dense membrane. Thus, other mechanisms such as adsorption and electrostatic interaction should be taken into consideration.
The efficiency of UF and MF membrane in eliminating BPA can be as high as RO and NF membrane provided that the adsorption mechanism of the membrane plays a significant role in retaining BPA by adsorbing into the surface and internal pore walls of the membrane (Su-Hua et al. 2010). Nevertheless, the adsorption by membrane should be carefully monitored because BPA can also be easily desorbed from the membrane when the membrane is in saturation condition. Bing-zhi et al. (2010) found that the efficiency of a commercial MF membrane for BPA removal was reduced by 79 % after the membrane was saturated with the compound.
Modification of membranes for BPA removal
The use of modified membranes in treating BPA solution has not been widely studied as commercial membranes are generally reported to have capability of removing BPA for up to 90 %. Nonetheless, it should be pointed out that by performing surface modification on the membrane, an improved membrane surface characteristic can be tailored to enhance BPA removal rate. Table 6 summarizes the development of modified membranes for BPA removal process. A comprehensive review by Zhao et al. (2013) highlighted that membrane surface can be modified through various methods such as surface coating, grafting and blending. Kango et al. (2013) on the other hand reported that the surface of a membrane could be improved via sol–gel processing, in situ polymerization or in situ growth of nanoparticles in a polymer matrix. It was explained that the modification techniques of membrane could improve the interfacial interactions between inorganic nanoparticles and polymer matrices, resulting in unique properties in membrane such as improved anti-fouling resistance, in addition to filtration performance.
Surface coating process is carried out by coating the membrane surface using a thin film layer of additives (Zhao et al. 2013). Example of surface coating is illustrated in Fig. 7 where the membrane top layer is coated with nanoscopic dendrimer that could effectively enhance membrane surface hydrophilicity. Hou et al. (2014) conducted surface coating of polyvinylidene fluoride (PVDF) membrane using titanium dioxide (TiO2) nanoparticles at a low temperature of hydrothermal sol–gel process, prior to immobilization of laccase on the membranes by chemical coupling. As a result, the modified membrane was reported to offer significant improvement of BPA removal with minimum fouling tendency.
Surface coating of membrane with dendrimer. Adapted from (Sarkar et al. 2010)
The sol–gel process is by adding nanoparticles into the polymeric solution that forming interpenetrating networks between the nanoparticles and polymers under mild conditions as illustrated in Fig. 8. The process is initiated by dispersing nanoparticles into polymeric solution with subsequent gel formation. This method can increase the membrane hydrophilicity, chemical, mechanical and thermal stability as strong compatibility and interfacial interaction is built between organic and inorganic phases (Kango et al. 2013; Yu et al. 2009).
In situ polymerization is done by bulk or solution polymerization of dispersed nanoparticles into monomer as shown in Fig. 9 (Kango et al. 2013). This method is easy to handle with quicker process and results in better membrane performance (Zou et al. 2008). In situ growth of nanoparticles in a polymer matrix on the other hand is done by incorporating nanoparticles into bulk polymeric network. The inorganic precursor will diffuse into the bulk polymer network and convert into inorganic nanoparticles by the templating and catalysis of the inorganic-precipitating polymer (Pan et al. 2010).
Surface grafting is by grafting synthetic polymers to the substrate surface to enhance the chemical functionality and surface topology of the inherent inorganic and organic materials (Kango et al. 2013). The polymer chains can be grafted to the surface of nanoparticles either by “grafting-to” method that covalently attach the end-functionalized polymers to the surface or by “grafting-from” method, which is an in situ monomer polymerization where polymer chains are grown from immobilized initiators (Zou et al. 2008) as shown in Fig. 10. Ben-David et al. (2010) tailored the selectivity of commercial membranes toward BPA by surface grafting of commercial NF membrane using two monomers of 3-sulfopropyl methacrylate (SPM) and 2-hydroxyethyl methacrylate (HEMA). They found that the interactions of BPA with SPM that contains strong negatively charged sulfonic groups might attribute to the increase of BPA rejection by steric hindrance. Kim et al. (2008) modified thin film composite membrane by sequence which involved graft polymerization, cross-linking of grafted polymer chains and substitution of functional groups. The study showed that BPA rejection by the unmodified membrane was improved from 74 % to ≥95 % upon modifications.
Surface grafting of polymer chains by “grafting-to” and “grafting-from” method. Adapted from (Li et al. 2014)
Among the listed methods, blending is the most conventional and simple methods to modify membranes by direct-mixing of inorganic additives into the polymeric solution. It is somehow difficult to obtain uniform and well-dispersed nanoparticles in the polymer matrix, surface modification of nanoparticles prior to blending is highly recommended to reduce nanoparticle agglomeration and enhance its dispersibility in dope solution (Kango et al. 2013). Bolong et al. (2010) fabricated hollow fibre membrane by blending polyethersulfone (PES) with negatively charged surface modifying macromolecule (cSMM). The result showed that BPA removal of >90 % could be achieved owing to improved surface charge of the membrane prepared. In another study by Rana et al. (2014), it is reported that the cSMM-modified PES membrane could only achieve partial removal of BPA in the initial stage. No removal was observed in later stages. They concluded that adsorption was the main mechanism governing the BPA removal during filtration process.
The regeneration technologies of membrane
In water treatment process, the ability of membrane to recover high BPA removal after series of filtration is the key factor for economical and feasible application of the membrane. The reusability of membrane depends on the ease of regeneration, especially for the membrane that functions based on adsorption mechanism.
As mentioned earlier, the adsorption of BPA to the membrane tends to decrease as a function of filtration time. Therefore, regeneration of the BPA-saturated membrane by physical or chemical forces is required to disrupt the hydrogen bonds between BPA and membrane surface, leading to desorption of BPA from the membrane. Membrane regeneration is a process in which BPA is detached from the binding sites of used membrane. It is necessary in order to maintain and restore the membrane performances for the next filtration cycle (Son and Takaomi 2011).
Han et al. (2013) studied the regeneration of polyamide membrane using caustic solution (NaOH). The result showed that almost 100 % of BPA could be desorbed at pH 11.6 in 2-h contact time. Deprotonation of BPA occurs at alkali environment, disrupting the hydrogen bonds between the compound and membrane and resulting in BPA desorption. The membrane was found to have good reusability with consistent sorption capacities for BPA after three cycles of reuse. Although alkaline solution can be one of the effective ways to desorb BPA, long-term exposure of membrane to high pH condition can be detrimental to the membrane properties and needs further study (Muralidhara 2010).
As a comparison, other regeneration technique such as membrane backwashing using water is more preferable. Not only this method is much safer for the membrane process but also economical. A study by Bing-zhi et al. (2010) showed the recovery of a PVDF hollow fibre membrane by water backwashing method at 0.1 MPa. It is found that the membrane was able to restore up to 70 % BPA removal capability after two consecutive cycles of backwash, indicating the possible of using water to regenerate membrane properties.
Other study by Son and Takaomi (2011) demonstrated the use of solvent to weaken the attraction between BPA and the binding sites of membrane. The regeneration of BPA-saturated membrane was performed using ethanol for 10 min at volume flux of 116 L/m2 h. The membrane was then flushed with distilled water to remove remaining ethanol from its surface. Results revealed that the membrane showed fairly well separation of BPA even after three times of recycling.
Another alternative of regenerating membrane is reported in the work of Liu et al. (2010). In this study, the regeneration of the BPA-saturated membrane was performed via UV/Fenton treatment for 2 h. The rate of desorption and oxidation was increased with supersonic treatment. It was found that the adsorption capacity of the membrane immobilized with activated carbon fibre was recovered after the UV/Fenton treatment, indicating the possible use of UV/Fenton treatment to regenerate membrane properties.
The membrane applications in water treatment and future prospect
Membrane for BPA removal has been intensively studied in laboratory for the possible implementation in water treatment system. Most of the cases that relate to BPA removal in treatment plants have been reported for wastewater application in which membranes were purposely used to reduce the concentration of organic compounds present in wastewater. The integration of membrane unit as an advanced treatment is one of the ways to reduce BPA concentration in water.
The application of UF membrane as an advanced treatment in drinking water production has been previously reported in a hybrid pilot scale water treatment process that combines coagulation, ozonation, membrane filtration and granular activated carbon filtration. Over 50 % BPA removal efficiency was achieved using the membrane via adsorption mechanism, i.e. BPA was adsorbed to the fouling layer on membrane. Size exclusion mechanism was ruled out in this case as the membrane exhibited much larger pore size compared to the size of the micropollutants. Further treatment using the hybrid process resulted in high BPA removal of 98 %, owing to the integration of membrane and ozonation that play crucial role in enhancing the removal efficiency. This study showed that the integration of membrane technology is promising way to improve the efficiency of conventional water treatment plant for micropollutants removal (Fan et al. 2014).
A full-scale water recycling plant in Queensland, Australia, showed the potential of using membrane technology as advanced treatment process in treating the effluent discharged by WWTP, aiming to produce high quality recycled water. Complete elimination of BPA in the water source could be achieved by combining typical WWTP with membrane technology (Al-Rifai et al. 2011).
A study by Lee et al. (2008) showed the application of pilot scale membrane bioreactors (MBR) integrated with NF and RO membrane in removing various EDCs from sewage effluents in a treatment plant for water reuse purposes. The MBR system consisted of activated sludge process with MF membrane module, while the NF and RO membrane was applied separately as final stage treatment after the MBR system. The integration of NF and RO membranes to the subsequent MBR system had further improved BPA removal from 90 to >95 %. Macedonio et al. (2012) have predicted that in the future, more similar membrane treatment processes will be integrated into secondary treatment and substitute conventional secondary treatment.
Sahar et al. (2011) demonstrated the application of MBR and conventional activated sludge (CAS-UF) pilot plants that were integrated with RO system in treating the same raw sewage from WWTP located in Tel-Aviv, Israel. BPA removal in CAS/UF was higher (92 %) compared to that of MBR (70 %). However, the integration of RO membrane to each system could ensure 95 % BPA removal. These showed that the integration of membrane unit to the pilot plant is vital in order to achieve high BPA removal as membrane cannot serve as a stand-alone unit for the treatment of micropollutants.
The membrane innovation for future prospect in water treatment is the integration of membrane unit with advanced oxidation process (AOP) such as ozonation and photocatalytic. The application of photocatalytic reactor membrane pilot system for the treatment of river water can be found in the work of Benotti et al. (2009) in which >70 % of EDCs could be removed with this treatment method. Furthermore, the water treated by the photocatalytic reactor membrane contained no chemical residual, revealing the viable application of integrated AOP-membrane for large scale water treatment process.
Future applications of membrane technology also include the use of renewable energy powered membrane (RE-membrane) system in which small-scale membrane system was powered by renewable energy that could provide autonomous treatment option for rural areas and able to treat many water sources to meet drinking water standards. It is an attractive decentralized water treatment options in areas without infrastructure and experience high level of dissolved contaminants in water (Schäfer et al. 2014). The RE-membrane offers alternative solutions to reduce energy consumption and the dependency on fossil fuels. Of the renewable energy sources available, solar energy contributes about 70 % in the RE-membrane market (Macedonio et al. 2012).
Management issues related to membrane technology
Membrane technology is a sophisticated and versatile water treatment process that is able to attain high water quality standards (Macedonio et al. 2012). This technology has been applied in many countries in large scale as it offers effective barrier to remove unwanted contaminants and produce water of high quality. Nevertheless, there are several management issues related to this technology that need to be addressed. These include design of an effective membrane process (as an advanced treatment unit), membrane fouling, management of waste stream and costing.
It is no doubt that the membrane shows much better removal rate against micropollutants compared to the conventional treatment process. However, in order to reduce membrane fouling and extend its lifespan, membrane works best as an integration unit in the conventional treatment process. It has been reported that the integration process could offer synergistic effect to the overall treatment performance, achieving not only high removal rate of micropollutants but also minimizing membrane fouling propensity (Macedonio et al. 2012).
One of the most important issues when employing membrane technology is membrane fouling. In every membrane process, fouling is the shortcoming of filtration resulted from deposition of molecules or particulates onto the membrane surface or pore walls. The occurrences of fouling can be due to adsorption, chemical interactions, cake formation and pore blocking by particles (Cui et al. 2010). The negative effects of membrane fouling are the increasing of membrane resistance that reduces the water flux and alters membrane selectivity. Controlling the membrane fouling via periodic cleaning can maintain the performance of the membrane at desired level, but its effectiveness strongly depends on the factors such as temperature, pH and concentration of cleaning chemicals and contact time between the chemical solution and the membrane (Li and Chen 2010).
Typical clean-in-place (CIP) systems designed for large membrane systems involve the alternate use of water flush and chemical solutions (caustic or acid). Long-term exposure of chemical solutions can cause damage to membrane, leading to irreversible changes in membrane properties. The method also requires high usage of water, energy and chemicals. Other alternative cleaning method is the use of enzymatic cleaners that operates under mild alkaline conditions and thus less harmful to membrane. In addition, the optimization of cleaning protocols can reduce membrane fouling and the frequency of cleaning. The approach is to operate the cleaning at low transmembrane pressure (below critical flux) (Muralidhara 2010). Permeate flux is controlled based on the critical flux value which leads to minimum fouling (Cui et al. 2010). With this approach, the system can minimize the cost of operation by reducing chemicals usage and cleaning frequency.
In membrane processes, the management of waste streams leaving the membrane is an important issue for sustainable application of this technology. The waste streams refer to the concentrated stream of membrane filtration that comprises rejected compounds by the membrane and substances used to clean the membranes (Khan et al. 2009). The ability to recycle the concentrated stream such as for cooling water industry, fire water, aesthetic fountains, toilet flush, or grey water prove to be beneficial, economical and more environmental friendly toward sustainable livings. In addition, high membrane recovery (99.5 %) could be achieved with the introduction of backwash waste treatment facilities to the membrane plant that post-treated the waste stream before recycling back into the feed stream (Schrotter and Bozkaya-schrotter 2010).
Membranes are often considered as expensive, although the cost for membrane has been reducing over the years. The operation cost for membrane is quite expensive as membrane system required intensive energy to be operated (Muralidhara 2010). The advancement of research and technology in energy recovery and system design can help reduce energy consumption and cost of the system (Macedonio et al. 2012). Economical processes are also needed to fabricate large membrane modules with innovative ways to incorporate these modules for the operation of large scale system in water treatment plant (Fane et al. 2011).
In terms of design, the membrane module should be pH compatible, solvent resistant and eco-friendly. Standardization of the system components is crucial as it should be flexible for membranes from different manufacturers. The design and fabrication of module should be precise so that the module is fit to membrane housing and connectors. The by-pass line in membrane system design needs to be properly located or else will require additional pumping capacity. For the ease of operation and maintenance, the membrane control should be kept as simple as possible (Muralidhara 2010).
Conclusion
The abundance presence of BPA in water treatment plant is a major concern among public as conventional water treatment process is not designed to remove emerging micropollutants. In vivo and in vitro tests have shown the negative effects of BPA toward living organism even at extremely low concentration level exposure. Nevertheless, the absence of laws and guidelines of BPA limit in water sources indicated that the issue has not yet being profoundly deliberated by the authorities at the moment. The occurrences of BPA in the water treatment process are expected to become more serious in the future with consistent growing demand of industrial sector. With regard to this matter, the application of membrane technologies has been reported as a promising solution to eliminate BPA from water sources. The physical separation process using membrane offers huge potential to remove BPA effectively. The understandings of the mechanisms that involve in the removal process by membrane (i.e. sieving, adsorption and electrostatic interactions) are vital fundamental strategy to control and improve membrane performances. Literature revealed that the membranes with modified surface tended to have better BPA removal compared to the unmodified membranes. In light of this, further studies should focus on membrane surface modification that can be achieved by incorporation of inorganic additives. An ideal membrane property must be associated with efficient BPA removal without compromising water permeability. The regeneration of membrane is also important for maintaining efficient performance and prolonging the membrane lifespan. The successful application of membrane process (both pilot and large scale) as advanced treatment unit for removing BPA provides strong evidence of potential membrane technology to improve water quality. The management issues related to membrane technology had shed some light on the feasibility of using the technology for water treatment. In addition, the implementation of guidelines on BPA limit in the water can help in controlling the frequent discharge of this compound to the water sources and promote the adoption of membrane technology.
References
Alidina M, Hoppe-Jones C, Yoon M, Hamadeh AF, Li D, Drewes JE (2014) The occurrence of emerging trace organic chemicals in wastewater effluents in Saudi Arabia. Sci Total Environ 478:152–162
Al-Rifai JH, Khabbaz H, Schäfer AI (2011) Removal of pharmaceuticals and endocrine disrupting compounds in a water recycling process using reverse osmosis systems. Sep Purif Technol 77:60–67
Anderson OS, Nahar MS, Faulk C, Jones TR, Liao C, Kannan K, Weinhouse C, Rozek LS, Dolinoy DC (2012) Epigenetic responses following maternal dietary exposure to physiologically relevant levels of Bisphenol A. Environ Mol 53:334–342
Arnold SM, Clark KE, Staples CA, Klecka GM, Dimond SS, Caspers N, Hentges SG (2012) Relevance of drinking water as a source of human exposure to bisphenol A. J Expo Sci Environ Epidemiol 23:137–144
Basile T, Petrella A, Petrella M, Boghetich G, Petruzzelli V, Colasuonno S, Petruzzelli D (2011) Review of endocrine-disrupting-compound removal technologies in water and wastewater treatment plants: an EU Perspective. Ind Eng Chem Res 50:8389–8401
Bellona C, Marts M, Drewes JE (2010) The effect of organic membrane fouling on the properties and rejection characteristics of nanofiltration membranes. Sep Purif Technol 74:44–54
Ben-David A, Bernstein R, Oren Y, Belfer S, Dosoretz C, Freger V (2010) Facile surface modification of nanofiltration membranes to target the removal of endocrine-disrupting compounds. J Memb Sci 357:152–159
Benotti MJ, Stanford BD, Wert EC, Snyder SA (2009) Evaluation of a photocatalytic reactor membrane pilot system for the removal of pharmaceuticals and endocrine disrupting compounds from water. Water Res 43:1513–1522
Bing-Zhi D, Lin W, Nai-Yun G (2008) The removal of bisphenol A by ultrafiltration. Desalination 221:312–317
Bing-Zhi D, Hua-Qiang C, Lin W, Sheng-Ji X, Nai-Yun G (2010) The removal of bisphenol A by hollow fiber microfiltration membrane. Desalination 250:693–697
Bolong N, Ismail AF, Salim MR, Rana D, Matsuura T, Tabe-Mohammadi A (2010) Negatively charged polyethersulfone hollow fiber nanofiltration membrane for the removal of bisphenol A from wastewater. Sep Purif Technol 73:92–99
Canedo TEF, Álvarez JCD, Cisneros BJ (2013) The occurrence and distribution of a group of organic micropollutants in Mexico City’s water sources. Sci Total Environ 454–455:109–118
Careghini A, Mastorgio AF, Saponaro S, Sezenna E (2015) Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: a review. Environ Sci Pollut Res 22:5711–5741
Chattopadhyay S, Heine E, Keul H, Moeller M (2014) Azetidinium functionalized polytetrahydrofurans: antimicrobial properties in solution and application to prepare non leaching antimicrobial surfaces. Polymers 6:1618–1630
Chen HW, Liang CH, Wu ZM, Chang EE, Lin TF, Chiang PC, Wang GS (2013) Occurrence and assessment of treatment efficiency of nonylphenol, octylphenol and bisphenol-A in drinking water in Taiwan. Sci Total Environ 449:20–28
Cheung WH, Szeto YS, Mckay G (2007) Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour Technol 98:2897–2904
Colin A, Bach C, Rosin C, Munoz J-F, Dauchy X (2014) Is drinking water a major route of human exposure to alkylphenol and bisphenol contaminants in France? Arch Environ Contam Toxicol 66:86–99
Comerton AM, Andrews RC, Bagley DM, Yang P (2007) Membrane adsorption of endocrine disrupting compounds and pharmaceutically active compounds. J Memb Sci 303:267–277
Cui ZF, Jiang Y, Field RW (2010) Fundamentals of pressure-driven membrane separation processes. In: Chui ZF, Muralidhara HS (eds) Membr. Technol., 1st ed. Elsevier Ltd, Oxford, UK, pp 1–18
Dupuis A, Migeot V, Cariot A, Albouy-Llaty M, Legube B, Rabouan S (2012) Quantification of bisphenol A, 353-nonylphenol and their chlorinated derivatives in drinking water treatment plants. Env Sci Pollut Res 19:4193–4205
Dutia P (2012) Polycarbonate resins: A techno-commercial profile. Chemical Weekly. http://www.chemicalweekly.com/Profiles/Polycarbonate_resins.pdf. Accessed 2 March 2016
Erler C, Novak J (2010) Bisphenol A exposure: human risk and health policy. J Pediatr Nurs 25:400–407
Escalona I, de Grooth J, Font J, Nijmeijer K (2014) Removal of BPA by enzyme polymerization using NF membranes. J Memb Sci 468:192–201
Fan X, Tao Y, Wang L, Zhang X, Lei Y, Wang Z, Noguchi H (2014) Performance of an integrated process combining ozonation with ceramic membrane ultra-filtration for advanced treatment of drinking water. Desalination 335:47–54
Fane AGT, Wang R, Jia Y (2011) Membrane technology: past, present and future. In: Wang LK, Chen JP, Hung YT, Shammas NK (eds) Membr. Desalin. Technol. Humana Press, Totowa, NJ, pp 1–45
Frye CA, Bo E, Calamandrei G, Calzà L, Dessì-Fulgheri F, Fernández M, Fusani L, Kah O, Kajta M, Le Page Y, Patisaul HB, Venerosi A, Wojtowicz AK, Panzica GC (2012) Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J Neuroendocrinol 24:144–159
Fu P, Kawamura K (2010) Ubiquity of bisphenol A in the atmosphere. Environ Pollut 158:3138–3143
Fulazzaky MA, Seong TW, Masirin MIM (2009) Assessment of water quality status for the Selangor River in Malaysia. Water Air Soil Pollut 205:63–77
Garoma T, Matsumoto S (2009) Ozonation of aqueous solution containing bisphenol A: effect of operational parameters. J Hazard Mater 167:1185–1191
Gonçalves CR, Cunha RW, Barros DM, Martínez PE (2010) Effects of prenatal and postnatal exposure to a low dose of bisphenol A on behavior and memory in rats. Environ Toxicol Pharmacol 30:195–201
Gupta VK, Ali I (2013) Water Treatment by Membrane Filtration Techniques. Environ. Water, 1st ed. Elsevier B.V., Oxford, UK, pp 135–154
Han J, Meng S, Dong Y, Hu J, Gao W (2013) Capturing hormones and bisphenol A from water via sustained hydrogen bond driven sorption in polyamide microfiltration membranes. Water Res 47:197–208
Hassan ZK, Elobeid MA, Virk P, Omer SA, ElAmin M, Daghestani MH, AlOlayan EM (2012) Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid Med Cell Longev 194829:1–6
Heo J, Flora JRV, Her N, Park Y-G, Cho J, Son A, Yoon Y (2012) Removal of bisphenol A and 17β-estradiol in single walled carbon nanotubes–ultrafiltration (SWNTs–UF) membrane systems. Sep Purif Technol 90:39–52
Hou J, Dong G, Ye Y, Chen V (2014) Enzymatic degradation of bisphenol-A with immobilized laccase on TiO2 sol–gel coated PVDF membrane. J Memb Sci 469:19–30
Houtman CJ (2010) Emerging contaminants in surface waters and their relevance for the production of drinking water in Europe. J Integr Environ Sci 7:271–295
Hu Z, Si X, Zhang Z, Wen X (2014) Enhanced EDCs removal by membrane fouling during the UF process. Desalination 336:18–23
Huang YQ, Wong CKC, Zheng JS, Bouwman H, Barra R, Wahlström B, Neretin L, Wong MH (2012) Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int 42:91–99
Igunnu ET, Chen GZ (2012) Produced water treatment technologies. Int J Low-Carbon Technol 9:157–177
Jardim WF, Montagner CC, Pescara IC, Umbuzeiro GA, Di Dea Bergamasco AM, Eldridge ML, Sodré FF (2012) An integrated approach to evaluate emerging contaminants in drinking water. Sep Purif Technol 84:3–8
Jones BA, Watson NV (2012) Hormones and Behavior Perinatal BPA exposure demasculinizes males in measures of affect but has no effect on water maze learning in adulthood. Horm Behav 61:605–610
Jurado A, Vàzquez-Suñé E, Carrera J, López de Alda M, Pujades E, Barceló D (2012) Emerging organic contaminants in groundwater in Spain: a review of sources, recent occurrence and fate in a European context. Sci Total Environ 440:82–94
Kango S, Kalia S, Celli A, Njuguna J, Habibi Y, Kumar R (2013) Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—a review. Prog Polym Sci 38:1232–1261
Keri RA, Ho S-M, Hunt PA, Knudsen KE, Soto AM, Prins GS (2007) An evaluation of evidence for the carcinogenic activity of bisphenol A. Reprod Toxicol 24:240–252
Khan SJ, Murchland D, Rhodes M, Waite TD (2009) Management of concentrated waste streams from high-pressure membrane water treatment systems. Crit Rev Environ Sci Technol 39:367–415
Khazaali F, Kargari A, Rokhsaran M (2013) Application of low-pressure reverse osmosis for effective recovery of bisphenol A from aqueous wastes. Desalin Water Treat 52:7543–7551
Kim J-H, Park P-K, Lee C-H, Kwon H-H (2008) Surface modification of nanofiltration membranes to improve the removal of organic micro-pollutants (EDCs and PhACs) in drinking water treatment: graft polymerization and cross-linking followed by functional group substitution. J Memb Sci 321:190–198
Kleywegt S, Pileggi V, Yang P, Hao C, Zhao X, Rocks C, Thach S, Cheung P, Whitehead B (2011) Pharmaceuticals, hormones and bisphenol A in untreated source and finished drinking water in Ontario, Canada-Occurrence and treatment efficiency. Sci Total Environ 409:1481–1488
Kundakovic M, Champagne FA (2011) Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun 25:1084–1093
Lee J, Lee BC, Ra JS, Cho J, Kim IS, Chang NI, Kim HK, Kim SD (2008) Comparison of the removal efficiency of endocrine disrupting compounds in pilot scale sewage treatment processes. Chemosphere 71:1582–1592
Lee C, Jiang L, Kuo Y, Hsieh C-Y, Chen CS, Tien C-J (2013) The potential role of water quality parameters on occurrence of nonylphenol and bisphenol A and identification of their discharge sources in the river ecosystems. Chemosphere 91:904–911
Li H, Chen V (2010) Membrane fouling and cleaning in food and bioprocessing. In: Chui ZF, Muralidhara HS (eds) Membr. Technol., 1st ed. Elsevier Ltd, Oxford, UK, pp 213–254
Li D, Zheng Q, Wang Y, Chen H (2014) Polymer Chemistry Combining surface topography with polymer chemistry: exploring new interfacial biological phenomena. Polym Chem 5:14–24
Liu Z-H, Kanjo Y, Mizutani S (2009) Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment-physical means, biodegradation, and chemical advanced oxidation: a review. Sci Total Environ 407:731–748
Liu L, Zheng G, Yang F (2010) Adsorptive removal and oxidation of organic pollutants from water using a novel membrane. Chem Eng J 156:553–556.
Macedonio F, Drioli E, Bardow A, Semiat R, Kurihara M, Gusev AA (2012) Efficient technologies for worldwide clean water supply. Chem Eng Process Process Intensif 51:2–17
Mehwish N, Kausar A, Siddiq M (2014) Advances in polymer-based nanostructured membranes for water treatment. Polym Plast Technol Eng 53:1290–1316
Michałowicz J (2014) Bisphenol A—sources, toxicity and biotransformation. Environ Toxicol Pharmacol 37:738–758
Moon MK, Kim MJ, Jung IK, Koo YD, Kim SH, Yoon YC, Jang HC, Park YJ (2012) Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J Korean Med Sci 27:644–652
Muhamad MS, Salim MR, Lau WJ, Hadibarata T, Yusop Z (2016) Removal of Bisphenol A by adsorption mechanism of PES-SiO2 composite membranes. Environ Technol 1–30. doi: 10.1080/09593330.2015.1137359
Muralidhara HS (2010) Challenges of membrane technology in the XXI Century. Membr. Technol., First Edit. Elsevier Ltd, pp 19–32.
Nam S, Jo B, Yoon Y, Zoh K (2014) Occurrence and removal of selected micropollutants in a water treatment plant. Chemosphere 95:156–165
Nghiem LD, Vogel D, Khan S (2008) Characterising humic acid fouling of nanofiltration membranes using bisphenol A as a molecular indicator. Water Res 42:4049–4058
Nie M, Yang Y, Liu M, Yan C, Shi H, Dong W, Zhou JL (2014) Environmental estrogens in a drinking water reservoir area in Shanghai: occurrence, colloidal contribution and risk assessment. Sci Total Environ 487:785–791
Ozaki H, Ikejima N, Shimizu Y, Fukami K, Taniguchi S, Takanami R, Giri RR, Matsui S (2008) Rejection of pharmaceuticals and personal care products (PPCPs) and endocrine disrupting chemicals (EDCs) by low pressure reverse osmosis membranes. Water Sci Technol 58:73–81
Padhye LP, Yao H, Kung’u FT, Huang C-H (2014) Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res 51:266–276
Pan F, Jia H, Cheng Q, Jiang Z (2010) Bio-inspired fabrication of composite membranes with ultrathin polymer–silica nanohybrid skin layer. J Memb Sci 362:119–126
Pereira LC, de Souza AO, Bernardes MFF, Pazin M, Tasso MJ, Pereira PH, Dorta DJ (2015) A perspective on the potential risks of emerging contaminants to human and environmental health. Environ Sci Pollut Res 22:13800–13823
Qiu L, Wang X, Zhang X, Zhang Z, Gu J, Liu L, Wang Y, Wang X, Wang S-L (2013) Decreased androgen receptor expression may contribute to spermatogenesis failure in rats exposed to low concentration of bisphenol A. Toxicol Lett 219:116–124
Ra J-S, Lee S-H, Lee J, Kim HY, Lim BJ, Kim SH, Kim SD (2011) Occurrence of estrogenic chemicals in South Korean surface waters and municipal wastewaters. J Environ Monit 13:101–109
Rana D, Narbaitz RM, Garand-Sheridan A-M, Westgate A, Matsuura T, Tabe S, Jasim SY (2014) Development of novel charged surface modifying macromolecule blended PES membranes to remove EDCs and PPCPs from drinking water sources. J Mater Chem A 2:10059–10072
Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, Frederick S (2007) In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 24:199–224
Rochester JR (2013) Bisphenol A and human health: a review of the literature. Reprod Toxicol 42:132–155
Rogers JA, Metz L, Yong VW (2013) Review: endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol Immunol 53:421–430
Rubin BS (2011) Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol 127:27–34
Ruíz FN, Arévalo AA, Álvarez JCD, Cisneros BJ (2012) Operating conditions and membrane selection for the removal of conventional and emerging pollutants from spring water using nanofiltration technology: the Tula Valley case. Desalin Water Treat 42:117–124
Sadmani AHMA, Andrews RC, Bagley DM (2014) Nanofiltration of pharmaceutically active and endocrine disrupting compounds as a function of compound interactions with DOM fractions and cations in natural water. Sep Purif Technol 122:462–471
Sahar E, David I, Gelman Y, Chikurel H, Aharoni A, Messalem R, Brenner A (2011) The use of RO to remove emerging micropollutants following CAS/UF or MBR treatment of municipal wastewater. Desalination 273:142–147
Santhi VA, Sakai N, Ahmad ED, Mustafa AM (2012) Occurrence of bisphenol A in surface water, drinking water and plasma from Malaysia with exposure assessment from consumption of drinking water. Sci Total Environ 427–428:332–338
Sarkar A, Carver PI, Zhang T, Merrington A, Bruza KJ, Rousseau JL, Keinath SE, Dvornic PR (2010) Dendrimer-based coatings for surface modification of polyamide reverse osmosis membranes. J Memb Sci 349:421–428
Schäfer AI, Nghiem LD, Oschmann N (2006) Bisphenol A retention in the direct ultrafiltration of greywater. J Memb Sci 283:233–243
Schäfer AI, Hughes G, Richards BS (2014) Renewable energy powered membrane technology: a leapfrog approach to rural water treatment in developing countries ? Renew Sustain Energy Rev 40:542–556
Schrotter J, Bozkaya Schrotter B (2010) Current and emerging membrane processes for water treatment. In: Peinemann KV, Nunes SP (eds) Membr. Technol. Membr. Water Treat, 4th edn. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, pp 53–91
Shao J, Hou J, Song H (2011) Comparison of humic acid rejection and flux decline during filtration with negatively charged and uncharged ultrafiltration membranes. Water Res 45:473–482
Sodré FF, Locatelli MAF, Jardim WF (2010) Occurrence of emerging contaminants in Brazilian drinking waters: a sewage-to-tap issue. Water Air Soil Pollut 206:57–67
Son LT, Takaomi K (2011) Hollow-fiber membrane absorbents embedded molecularly imprinted polymeric spheres for bisphenol A target. J Memb Sci 384:117–125
Stackelberg PE, Gibs J, Furlong ET, Meyer MT, Zaugg SD, Lippincott RL (2007) Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci Total Environ 377:255–272
Staniszewska M, Koniecko I, Falkowska L, Krzymyk E (2015) Occurrence and distribution of bisphenol A and alkylphenols in the water of the gulf of Gdansk (Southern Baltic). Mar Pollut Bull 91:372–379
Stavrakakis C, Colin R, Hequet V, Faur C, Cloirec PL (2008) Analysis of endocrine disrupting compounds in wastewater and drinking water treatment plants at the nanogram per litre level. Environ Technol 29:279–286
Su-Hua W, Bing-zhi D, Yu H (2010) Adsorption of bisphenol A by polysulphone membrane. Desalination 253:22–29
Thomas S, Visakh PM (2011) Handbook of Engineering and Speciality Thermoplastics. In: Polyethers and Polyesters, vol. 3. Wiley, vol. 63.
Tiraferri A, Elimelech M (2012) Direct quantification of negatively charged functional groups on membrane surfaces. J Memb Sci 389:499–508
Tiwari D, Kamble J, Chilgunde S, Patil P, Maru G, Kawle D, Bhartiya U, Joseph L, Vanage G (2012) Clastogenic and mutagenic effects of bisphenol A: An endocrine disruptor. Mutat Res - Genet Toxicol Environ Mutagen 743:83–90
Tsai W-T (2006) Human health risk on environmental exposure to Bisphenol-A: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 24:225–255
Verliefde A, Cornelissen E, Amy G, Van der Bruggen B, van Dijk H (2007) Priority organic micropollutants in water sources in Flanders and the Netherlands and assessment of removal possibilities with nanofiltration. Environ Pollut 146:281–289
Verliefde ARD, Cornelissen ER, Heijman SGJ, Verberk JQJC, Amy GL, Van der Bruggen B, van Dijk JC (2008) The role of electrostatic interactions on the rejection of organic solutes in aqueous solutions with nanofiltration. J Memb Sci 322:52–66
Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, Lu J, Chen Y, Wang W, Li X, Liu Y, Bi Y, Lai S, Ning G (2012) Urinary Bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab 97:E223–E227
Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM (2007) In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol 24:178–198
Willhite CC, Ball GL, McLellan CJ (2008) Derivation of a bisphenol A oral reference dose (RfD) and drinking-water equivalent concentration. J Toxicol Environ Health B Crit Rev 11:69–146
Wolkowicz IRH, Herkovits J, Coll CSP (2014) Stage-dependent toxicity of Bisphenol A on Rhinella arenarum (Anura, Bufonidae) embryos and larvae. Environ Toxicol 29:146–154
Wray HE, Andrews RC, Bérubé PR (2014) Surface shear stress and retention of emerging contaminants during ultrafiltration for drinking water treatment. Sep Purif Technol 122:183–191
Wu C, Huang X, Lin J, Liu J (2015) Occurrence and fate of selected endocrine-disrupting chemicals in water and sediment from an urban lake. Arch Env Contam Toxicol 68:225–236
Xie M, Nghiem LD, Price WE, Elimelech M (2012) Comparison of the removal of hydrophobic trace organic contaminants by forward osmosis and reverse osmosis. Water Res 46:2683–2692
Yang YX, Chen J (2013) Advanced treatment of drinking water by ultrafiltration membrane. Adv Mater Res 647:543–547
Yu S, Zuo X, Bao R, Xu X, Wang J, Xu J (2009) Effect of SiO2 nanoparticle addition on the characteristics of a new organic–inorganic hybrid membrane. Polymer 50:553–559
Yüksel S, Kabay N, Yüksel M (2013) Removal of bisphenol A (BPA) from water by various nanofiltration (NF) and reverse osmosis (RO) membranes. J Hazard Mater 263:307–310
Zhang Y, Causserand C, Aimar P, Cravedi JP (2006) Removal of bisphenol A by a nanofiltration membrane in view of drinking water production. Water Res 40:3793–3799
Zhang Z, Ren N, Kannan K, Nan J, Liu L, Ma W, Qi H, Li Y (2014) Occurrence of endocrine-disrupting phenols and estrogens in water and sediment of the Songhua River, Northeastern China. Arch Env Contam Toxicol 66:361–369
Zhao C, Xue J, Ran F, Sun S (2013) Modification of polyethersulfone membranes—a review of methods. Prog Mater Sci 58:76–150
Zhao F, Tang C, Liu X, Shi F-J, Song X-R, Yu T, Zhan-Shuang L (2015) Transportation characteristics of bisphenol A on ultrafiltration membrane with low molecule weight cut-off. Desalination 362:18–25
Zou H, Wu S, Shen J (2008) Polymer/silica nanocomposites : preparation, characterization, properties, and applications. Chem Rev 18:3893–3957
Acknowledgements
The authors wish to thank Universiti Teknologi Malaysia and Ministry of Higher Education Malaysia (MOHE) for providing LRGS grant (R.J30000.7809.4 L810) for Water Security entitled Protection of Drinking Water: Source Abstraction and Treatment (203/PKT/6720006) as financial support of this project. This support is greatly appreciated. M.S. Muhamad thanks the MOHE for the MyBrain15 (MyPhD) sponsorship received during her PhD studies.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Muhamad, M.S., Salim, M.R., Lau, W.J. et al. A review on bisphenol A occurrences, health effects and treatment process via membrane technology for drinking water. Environ Sci Pollut Res 23, 11549–11567 (2016). https://doi.org/10.1007/s11356-016-6357-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6357-2