Abstract
Identification of bioactive compounds directly from complex herbal extracts is a key issue in the study of Chinese herbs. The present study describes the establishment and application of a sensitive, efficient, and convenient method based on surface plasmon resonance (SPR) biosensors for screening active ingredients targeting tumor necrosis factor receptor type 1 (TNF-R1) from Chinese herbs. Concentration-adjusted herbal extracts were subjected to SPR binding assay, and a remarkable response signal was observed in Rheum officinale extract. Then, the TNF-R1-bound ingredients were recovered, enriched, and analyzed by UPLC-QTOF/MS. As a result, physcion-8-O-β-d-monoglucoside (PMG) was identified as a bioactive compound, and the affinity constant of PMG to TNF-R1 was determined by SPR affinity analysis (K D = 376 nM). Pharmacological assays revealed that PMG inhibited TNF-α-induced cytotoxicity and apoptosis in L929 cells via TNF-R1. Although PMG was a trace component in the chemical constituents of the R. officinale extract, it had considerable anti-inflammatory activities. It was found for the first time that PMG was a ligand for TNF receptor from herbal medicines. The proposed SPR-based screening method may prove to be an effective solution to analyzing bioactive components of Chinese herbs and other complex drug systems.

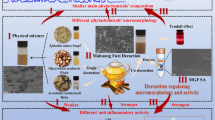
Scheme of the method based on SPR biosensor for screening and recovering active ingredients from complex herbal extracts and UPLC-MS for identifying them. Scheme of the method based on SPR biosensor for screening and recovering active ingredients from complex herbal extracts and UPLC-MS for identifying them
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chinese herbs play an important role in the treatment of various diseases in China, and some natural products from Chinese herbal medicine have shown significant pharmacological activities. However, only a small number of the whole chemical constituents of herbs have been clarified for bioactive ingredients responsible for their pharmacological activities [1, 2]. Therefore, identification of medicinal compounds from a complex matrix that may contain hundreds or even thousands of components with different structural types and concentrations is a key issue in the study of Chinese herbs. Traditional methods of screening active components depend on the isolation and purification of natural products, structural determination, and bioassays, which are usually laborious and expensive [3]. Some methods that directly screen compound mixtures from herbal extracts have aroused increasingly more attention [4]. Cell membrane chromatography has been developed to screen active compounds that could act on membrane proteins from herbs [5–7]. Pharmacokinetic studies have been performed to screen active compounds present in blood and urine with appropriate blood concentrations and urinary excretion rates after the administration of herbal extracts [8]. Besides, computational approaches such as data-mining [9] and network pharmacology [10] could also be used for the prediction of active ingredients of herbal medicines.
The surface plasmon resonance (SPR) biosensor is a refractometer that measures changes in the optical reflectivity of a thin metal film when species adsorb or bind to its surface or to any material coated onto its surface [11, 12]. Due to its high sensitivity, resolution, and specificity [13], SPR has been applied in numerous fields including medical diagnostics, environmental monitoring, food safety and security, and pharmaceutical analysis [14]. Recently, it has been used to capture bioactive components from compound mixtures for the purpose of high-throughput screening [15, 16]. The major advantage of SPR-based screening is that it can give a real-time indication of the dynamic process of ligand-receptor interaction by using compound mixtures without any prior separation. But as SPR cannot provide information for structural identification of captured components, some hyphenated systems that combine the biosensor with liquid chromatography and mass spectrometry have been established [17, 18]. However, these methods were confined to complicated interfaces and therefore it is difficult to use them conveniently and extensively. In addition, previous practices of SPR-based screening usually obtained dozens of bioactive compounds, and did not focus on the trace but strong affinity components, therefore, not profiting from the highly sensitive SPR biosensor.
In the present study, we chose five Chinese herbs including Rheum officinale (RO), Salviae miltiorrhizae (SM), Folium isatidis (FI), Radix glycyrrhizae (RG), and Astragalus membranaceus (AM), which are known to have potential anti-inflammatory activities [19–23], for screening active ingredients targeting tumor necrosis factor receptor type 1 (TNF-R1) by using a sensitive and specific SPR-based method. The method consists of SPR-based screening, chip-bound ingredients recovery, and ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS) identification. Physcion-8-O-β-d-monoglucoside (PMG) was identified as a bioactive compound. Although PMG was a trace component in the chemical constituents of the R. officinale extract, it had considerable anti-inflammatory activities. These results proved that the proposed SPR-based bioactive component screening method was sensitive, efficient, and convenient, suggesting that the method may prove to be an effective solution to analyzing bioactive components of Chinese herbs and other complex drug systems.
Materials and methods
Reagents and materials
R. officinale, S. miltiorrhizae, F. isatidis, R. glycyrrhizae, and A. membranaceus were purchased from Leiyunshang Pharmacy (Shanghai, China). Physcion-8-O-β-d-monoglucoside was purchased from Shanghai Liding Biotech (Shanghai, China). Matrine, schizandrin, chrysophanol, tanshinone IIA, indirubin, liquiritin, and calycosin-7-glucoside were purchased from the National Institute for the Pharmaceutical and Biological Products of China (Beijing, China). The purity of all the standard compounds was greater than 99.9 %. HPLC-grade acetonitrile was purchased from Merck Chemicals (Darmstadt, Germany). Actinomycin D was purchased from Sigma-Aldrich (St. Louis, MO, USA). TNF-α and TNF-R1 were purchased from PeproTech (Rocky Hill, NJ, USA).
Preparation of herbal extracts and standard solutions
Dried herbs were homogenized with a mill and passed through a 40-mesh sieve. Powder samples in 1 g were extracted by ultrasonic extracting method with 10 mL 80 % methanol for 30 min. Then, the extract was centrifuged and the supernatant was filtered through a 0.22-μm nylon filter. Physcion-8-O-β-d-monoglucoside, chrysophanol, tanshinone IIA, indirubin, liquiritin, and calycosin-7-glucoside stock solutions were prepared in DMSO for a final concentration of 20 mM and filtered through a 0.22-μm nylon filter.
Quantitative determination of representative component from each herb
A relative concentration of each herbal extract was estimated by HPLC analysis performed on an Agilent 1100 series HPLC system (Agilent Corp., Germany) consisting of a quaternary solvent delivery system, a thermostatically controlled column apartment, and a diode array detector. Chromatographic separation was carried out on a C18 column (Hypersil ODS2, 4.6 mm × 200 mm, 5 μm, Elite, Dalian, China) at 37 °C. The injection volume is 10 μL. For A. membranaceus extract and calycosin-7-glucoside standard solution, the mobile phase consisted of methanol (A) and water (B), using a gradient elution of 10–100 % A at 0–60 min. The flow rate was 1.0 mL/min. The detection wavelength was 260 nm. The chromatographic conditions of other herbal extracts and standard solutions were set according to Chinese Pharmacopoeia [24]. Then, the concentration of representative component in herbal extract (C r ) was calculated by an external standard method. The equation is: C r = C s × A r /A s , where C r is the concentration of standard solution, A r is the peak area of compound in herbal extract, and A s is the peak area of external standard.
Immobilization of TNF-R1 on sensor surface and SPR screening
SPR analysis was carried out on a Biacore T200 system (GE Healthcare, Sweden). TNF-R1 were diluted in 10 mM sodium acetate pH 5.0 and immobilized by the amine coupling method on a CM5 sensor chip according to the manufacturer’s protocol. The target immobilization level of ligand was 5,000 RU. The specificity for TNF-R1 binding was characterized by TNF-α which was used as positive control and matrine and schizandrin which were used as negative controls. The running buffer is phosphate buffer saline (PBS). Samples were then injected to the TNF-R1 sensor surface for 60 s at a flow rate of 30 μL/min. Sensorgrams of five herbs were recorded and analyzed.
Recovery of TNF-R1-bound ingredients
Ingredients that were bound to TNF-R1 on the sensor surface were recovered by an injection and recovery program using Biacore T200 system. Briefly, R. officinale extract was injected over the sensor surface for 180 s at 5 μL/min. The flow system was washed with distilled water. A small volume of 2 μL recovery solution was injected into the flow cells and incubated for 20 s to allow the bound ingredients to dissociate into the recovery solution. Then, the flow direction over the sensor surface was reversed and the recovery solution containing TNF-R1-bound ingredients was deposited in 10 μL ammonium bicarbonate (50 mM).
UPLC-QTOF/MS analysis
The analyses of herbal extracts and SPR-recovered samples were performed on an Agilent 1290 series UPLC system (Agilent Corp., USA). Chromatographic separation was carried out on a Waters XSelect® HSS T3 2.5 μM column (2.1 × 100 mm) at 25 °C. The injection volume is 5 μL. The mobile phase consisted of 0.1 % aqueous formic acid (v/v) (A) and acetonitrile (B), using a gradient elution. Elution procedure is 10 % B at 0–0.5 min, 10–90 % B at 0.5–4.5 min, and 90 % B at 4.5–5 min for herbal extracts, and 10 % B at 0–1 min, 10–90 % B at 1–7 min, and 90 % B at 7–8 min for SPR-recovered samples. The flow rate was kept at 3.5 mL/min, and a post-column split was used to maintain a flow rate of 0.4 mL/min into the mass spectrometer source to obtain good nebulization efficiency. Detection was performed by an Agilent 6538 UHD Accurate-Mass QTOF/MS (Agilent Corp., USA). The analysis was performed using full-scan mode and mass range was set at m/z 100–1000 in positive ion mode. The conditions of ESI source were as follows: drying gas (N2) flow rate, 10 L/min; drying gas temperature, 350 °C; nebulizer, 35 psig; capillary voltage, 4000 V; fragmentor voltage, 120 V; skimmer voltage, 60 V; octopole RF, 250 V. All the data were processed by Agilent MassHunter Software ver. B.02.00 (Agilent Technologies). Tuning mix (G1969-85000, Agilent Corp., USA) was used for lock mass calibration in the assay.
SPR affinity analysis
PMG was diluted in running buffer PBS containing 5 % DMSO at concentrations ranging from about 40 nM to 4 μM. Analytes were injected through reference and active channels at a flow rate of 30 μL/min. The association and dissociation times were 100 and 300 s. The affinity fitting was carried out with Biacore T200 evaluation software by global fitting using a steady-state affinity model to obtain the affinity constant K D .
Cell culture
L929 cells were obtained from the Department of Biochemical Pharmacy, Second Military Medical University. L929 cells were cultured in DMEM supplemented with 10 % FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C with 5 % CO2 atm.
Cytotoxicity assay
Exponentially growing L929 cells were harvested and plated at a density of 2 × 104 cells/well in 96-well plates per 100 μL complete medium. After 24 h incubation at 37 °C, cells were pretreated for 3 h with SPD304 (20 μM) and physcion-8-O-β-d-monoglucoside (20 μM) respectively. Then, TNF-α (10 ng/mL) and actinomycin D (1 μg/mL) were added. After 48 h incubation at 37 °C, 10 μL of CCK-8 solution was added to each well and incubated at 37 °C for 1 h. The resulting color was assayed at 450 nM using a Biotek Synergy 4 microplate reader (Hopkinton, MA, USA).
Apoptosis assay
L929 cells were plated at a density of 1 × 106 cells/well in 12-well plates per 1 mL complete medium. After 24 h incubation at 37 °C, cells were pretreated for 3 h with 20 μM physcion-8-O-β-d-monoglucoside. Then, TNF-α (10 ng/mL) and actinomycin D (1 μg/mL) were added. After 24 h incubation at 37 °C, cells were washed with PBS and collected by trypsinization. After centrifugation at 400×g for 5 min at 4 °C, cells were washed twice with cold PBS and resuspended in PBS to which were added 5 μL of fluorescein isothiocyanate (FITC)-labeled annexin V (annexin V-FITC) and 5 μL of propidium iodide (PI) solution. The cells were gently vortexed and incubated for 15 min at 4 °C in the dark. Cell fluorescence was measured on a FACSCalibur flow cytometer (BD Biosciences, Mountain View, CA, USA). The percentages of apoptotic cells were determined as the sum of annexin V-FITC+ and annexin V-FITC+/PI+ cells.
Results and discussion
Adjustment of concentrations of herbal extracts
An equal amount of each herb was used to prepare their extracts. But as the content level and extraction efficiency were different from each other, the final concentration of each extract was different. In order to adjust them to a similar concentration and to get a nondiscriminatory response value in SPR screening, the relative concentration of each extract was estimated by HPLC analysis (Fig. 1). Chrysophanol, tanshinone IIA, indirubin, liquiritin, and calycosin-7-glucoside were selected as the representative components of the five herbs respectively according to Chinese Pharmacopoeia [24]. The concentration of each herbal extract was evaluated and compared by the quantitative determination of its representative component. The quantification results are shown in Table 1. Then, each herbal extract prepared for SPR screening was diluted by PBS to a definite concentration that contain 1 μM representative component.
Chromatograms obtained by HPLC analysis. The relative concentration of each extract was estimated by quantitative determination of representative component from each herb. In each panel, the upper is herbal extract and the lower is standard solution. a Rheum officinale. b Salviae miltiorrhizae. c Folium isatidis. d Radix glycyrrhizae. e Astragalus membranaceus. (1) Chrysophanol. (2) Tanshinone IIA. (3) Indirubin. (4) Liquiritin. (5) Calycosin-7-glucoside
Recognition and recovery of TNF-R1-bound ingredients by SPR biosensor
TNF-R1 protein was immobilized by amine method on a CM5 sensor chip. The specificity for TNF-R1 binding was first characterized. TNF-α is a known ligand for TNF-R1 [25], so different concentrations of TNF-α were used as positive controls. As a result, TNF-α could bind to the sensor surface, and the response unit varied in a concentration-dependent manner. These sensorgrams are shown in Fig. 2a. Meanwhile, matrine and schizandrin, which are unrelated to TNF-R1, were used as negative controls. The response unit of these compounds was the same as running buffer PBS, and the sensorgrams are shown in Fig. 2b. These results indicate that the TNF-R1 sensor chip could recognize its ligands specifically.
Recognition and recovery of TNF-R1-bound ingredients by SPR biosensor. The specificity for TNF-R1 binding was characterized by positive control a TNF-α and negative controls b matrine and schizandrin. c Herb samples were subjected to SPR analysis to screen TNF-R1-bound components. Of the five herbs, the sensorgram of R. officinale showed a significant increase, and no significant increase was observed on the other sensorgrams. d Schematic diagram of the injection and recovery procedure in our proposed screening method
Then, the diluted herb samples were subjected to SPR analysis to screen TNF-R1-bound components. The sensorgrams are shown in Fig. 2c. Of the five herbs, the sensorgram of R. officinale showed a significant increase when it flowed through the TNF-R1 sensor surface, while no significant increase was observed in the other sensorgrams, indicating that one or more ingredients in R. officinale could bind to TNF-R1, inducing the signal rise. But as SPR analysis could not provide structural information of the active ingredients, further identification was needed.
TNF-R1-bound ingredients from R. officinale were recovered by an injection and recovery program using Biacore T200 system. As illustrated in Fig. 2d, (1) the compound mixture firstly passed through the sensor surface, during which the active components associated with TNF-R1. After that, (2) the system (including the injection needle and flow path) was washed with distilled water to thoroughly remove the remaining sample solution without interfering with the chip-bound ingredients in the recovery step. Then, (3) the recovery solution was injected and incubated to allow the TNF-R1-bound ingredients to dissociate. Finally, (4) the recovery buffer containing recovered compounds was drawn back and mixed with the deposition solution. These steps were repeated for 5 cycles to collect 20 μL of recovered sample in a vial.
Identification of active ingredients
As the amount of chip-bound ingredients was quite low in one single injection and recovery procedure, it was insufficient to meet the detection limit of QTOF/MS. The injection and recovery procedures were repeated to obtain a series of sample vials. Different numbers (1–5) of the vials were merged, dried under nitrogen, redissolved by mobile phase, and analyzed by UPLC-QTOF/MS system. Consequently, the credible identification result was obtained from at least four merged vials of the recovered sample. According to the accurate mass data obtained by QTOF/MS and searching from the chemical compound database of R. officinale containing 146 compounds built by our group, only physcion-8-O-β-d-monoglucoside (PMG) was identified as a recovered ingredient (Fig. 3a, b and Table 2). This result was further confirmed by PMG standard (Fig. 3c). The major components of R. officinale have been elucidated by previous studies [26, 27]; however, PMG have not been mentioned. So PMG may be an attractive ligand for TNF-R1 with low content in RO. The goal of such identification is to find out the trace TNF-R1-bound component that may possess the important pharmacological effect by using the least amount of the sample.
Identification of TNF-R1-bound ingredients from R. officinale. a Total ion chromatography of the recovered sample. b Mass spectrometry and chemical structure of PMG. c Total ion chromatography of the PMG standard. d Investigation of three recovery solutions (0.5 % TFA, 0.5 % FA, and 0.5 % AA) and different numbers (1–5) of vials merged required for each recovery solution to accurately identify PMG
Additionally, three MS compatible recovery solutions containing mild organic acids, 0.5 % (v/v) trifluoro acid (TFA), formic acid (FA), or acetic acid (AA), were investigated. At least four merged vials of the recovered sample were required for each recovery solution to identify PMG. However, the peak areas of PMG in different conditions were different (Fig. 3d). The peak area of TFA and FA was similar, and larger than that of AA in both cases, indicating that TFA and FA could not only help the dissociation of TNF-R1-bound ingredients but enhance the sensitivity of MS detection, and therefore may prove to be ideal recovery solutions for screening active components from a complex mixture by combined use of SPR and MS.
Validation of PMG binding to TNF-R1 by SPR analysis
Direct binding of PMG to TNF-R1 was determined by SPR affinity analysis. As shown in Fig. 4a, serial concentrations of PMG ranging from about 40 nM to 4 μM were tested. The dissociation constant (K D ) for PMG was calculated as 376 nM (Fig. 4b). The nanomolar K D indicated that PMG was a potent compound binding to TNF-R1, and PMG was a potential anti-inflammatory ingredient from Chinese herb R. officinale.
Inhibition of TNF-α-mediated cytotoxicity in L929 cells
The inhibitory effects on TNF-α-mediated L929 cytotoxicity of positive control SPD304 and PMG were tested by CCK-8 assay. As shown in Fig. 5a–b, TNF-α and actinomycin D changed the cell morphology and caused cell damage, and the cell viability was only 32.20 ± 0.74 %. SPD304 inhibited the killing effect and the cell viability increased to 66.10 ± 2.51 %. PMG also showed an inhibitory effect on cytotoxicity with a cell viability percentage of 52.54 ± 5.49 %. These results indicate that PMG inhibited the TNF-α-mediated cytotoxicity in L929 cells via TNF-R1, and suggest that the SPR screening method was accurate and specific in recognizing of PMG as TNF-R1-binding ingredient.
Blocking effects of PMG on TNF receptor. a The inhibitory effect of PMG on TNF-α-mediated L929 cytotoxicity by CCK-8 assay. b The percentage of cell viability was calculated. c The inhibitory effect of PMG on TNF-α-induced L929 cell apoptosis by FACS analysis. d The percentage of apoptotic cells was determined
Inhibition of TNF-α-induced apoptosis in L929 cells
Next, we tested the inhibitory effect of PMG on TNF-α-induced L929 cell apoptosis using annexin V-FITC/PI double staining method. As shown in Fig. 5c–d, only 2.02 ± 0.03 % apoptotic cells were found in the control group. TNF-α sensitized by actinomycin D significantly enhanced the apoptosis with an apoptosis rate of 56.05 ± 3.17 %. Interestingly, the apoptosis rate decreased to 22.89 ± 1.62 % after 24-h PMG treatment. These results imply that PMG inhibited TNF-α-induced apoptosis in L929 cells via TNF-R1, indicating that PMG is a new ligand of TNF-R1 and a regulator of inflammation. These blocking effects of PMG on TNF-R1 collectively demonstrate that the active ingredients screening method based on SPR and MS had given us credible results. Taken together with other advantages of the screening system, such as simply using of compound matrix and high sensitivity, it could be employed to discover trace but strong bioactivity components from complex compound mixtures of Chinese herbs.
Conclusions
In SPR analysis, a response signal is generated by interactions between the bioactive compound and the immobilized target protein, and the signal intensity is closely related to the concentration of the compound. However, an inactive compound cannot bind to the protein so as not to cause a response signal regardless of concentration [28]. Therefore, existence of a large amount of inactive ingredients in a complex compound mixture does not interfere with the detection of trace bioactive components. This is a distinct advantage of activity-based SPR analysis compared to other chemistry-based detection methods, such as ultraviolet spectroscopy and mass spectrometry. Nevertheless, SPR does not provide information for structural identification of protein-bound compounds in an unknown mixture. The active ingredients need to be recovered, enriched, and analyzed by high-resolution MS.
In the present study, we described a method for screening active components from Chinese herbs by using SPR biosensor and MS in combination, used this method to screen five Chinese herbs for potential anti-inflammatory ingredients targeting TNF-R1, and found that PMG was a bioactive compound. Pharmacological experiments revealed that the binding of PMG to TNF-R1 resulted in an inhibitory effect on TNF-α-mediated cytotoxicity and apoptosis in L929 cells. To the best of our knowledge, this is the first study reporting PMG as a ligand for TNF-R1. The active compound identified by this screening method was not only in a low amount of herbal extract but in strong affinity compared to most other natural products, indicating that the method is highly sensitive and specific. Our study showed the feasibility of applying SPR-based screening, chip-bound ingredients recovery, and UPLC-MS identification method for screening active ingredients from herbal extracts. This method may prove to be an effective solution to analyzing active components of Chinese herbs and other complex drug systems.
Abbreviations
- AA:
-
Acetic acid
- FA:
-
Formic acid
- PMG:
-
Physcion-8-O-β-d-monoglucoside
- SPR:
-
Surface plasmon resonance
- TFA:
-
Trifluoro acid
- TNF:
-
Tumor necrosis factor
References
Liu S, Yi L-Z, Liang Y-Z. Traditional Chinese medicine and separation science. J Sep Sci. 2008;31(11):2113–37.
Chen X, Cao Y, Lv D, Zhu Z, Zhang J, Chai Y. Comprehensive two-dimensional HepG2/cell membrane chromatography/monolithic column/time-of-flight mass spectrometry system for screening anti-tumor components from herbal medicines. J Chromatogr A. 2012;1242:67–74.
Harvey AL. Natural products in drug discovery. Drug Discov Today. 2008;13(19–20):894–901.
Zhu Y, Zhang Z, Zhang M, Mais DE, Wang M-W. High throughput screening for bioactive components from traditional Chinese medicine. Comb Chem High Throughput Screen. 2010;13(10):837–48.
Wang S, Sun M, Zhang Y, Du H, He L. A new A 431/cell membrane chromatography and online high performance liquid chromatography/mass spectrometry method for screening epidermal growth factor receptor antagonists from Radix sophorae flavescentis. J Chromatogr A. 2010;1217(32):5246–52.
Chen X, Cao Y, Zhang H, Zhu Z, Liu M, Liu H, et al. Comparative normal/failing rat myocardium cell membrane chromatographic analysis system for screening specific components that counteract doxorubicin-induced heart failure from Acontium carmichaeli. Anal Chem. 2014;86(10):4748–57.
Cao Y, Chen X-F, Lü D-Y, Dong X, Zhang G-Q, Chai Y-F. Using cell membrane chromatography and HPLC-TOF/MS method for in vivo study of active components from roots of Aconitum carmichaeli. J Pharm Anal. 2011;1(2):125–34.
Wang X, Sun H, Zhang A, Jiao G, Sun W, Yuan Y. Pharmacokinetics screening for multi-components absorbed in the rat plasma after oral administration traditional Chinese medicine formula Yin-Chen-Hao-Tang by ultra performance liquid chromatography-electrospray ionization/quadrupole-time-of-flight mass spectrometry combined with pattern recognition methods. Analyst. 2011;136(23):5068–76.
Yang H, Chen J, Tang S, Li Z, Zhen Y, Huang L, et al. New drug R&D of traditional Chinese medicine: role of data mining approaches. J Biol Syst. 2009;17(03):329–47.
Liang X, Li H, Li S. A novel network pharmacology approach to analyse traditional herbal formulae: the Liu-Wei-Di-Huang pill as a case study. Mol BioSyst. 2014;10(5):1014–22.
Campbell CT, Kim G. SPR microscopy and its applications to high-throughput analyses of biomolecular binding events and their kinetics. Biomaterials. 2007;28(15):2380–92.
Homola J. Present and future of surface plasmon resonance biosensors. Anal Bioanal Chem. 2003;377(3):528–39.
Phillips KS, Cheng Q. Recent advances in surface plasmon resonance based techniques for bioanalysis. Anal Bioanal Chem. 2007;387(5):1831–40.
Homola J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem Rev. 2008;108(2):462–93.
Peng M, Zhang Y, Shi S, Peng S. Simultaneous ligand fishing and identification of human serum albumin binders from Eucommia ulmoides bark using surface plasmon resonance-high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2013;940:86–93.
Zhang Y, Shi S, Guo J, You Q, Feng D. On-line surface plasmon resonance-high performance liquid chromatography-tandem mass spectrometry for analysis of human serum albumin binders from Radix Astragali. J Chromatogr A. 2013;1293:92–9.
Marchesini GR, Buijs J, Haasnoot W, Hooijerink D, Jansson O, Nielen MWF. Nanoscale affinity chip interface for coupling inhibition SPR immunosensor screening with nano-LC TOF MS. Anal Chem. 2008;80(4):1159–68.
Stigter ECA, de Jong GJ, van Bennekom WP. Development of an on-line SPR-digestion-nanoLC-MS/MS system for the quantification and identification of interferon-γ in plasma. Biosens Bioelectron. 2009;24(7):2184–90.
Chu X, Wei M, Yang X, Cao Q, Xie X, Guan M, et al. Effects of an anthraquinone derivative from Rheum officinale Baill, emodin, on airway responses in a murine model of asthma. Food Chem Toxicol. 2012;50(7):2368–75.
Wang X, Jia W, Zhao A, Wang X. Anti-influenza agents from plants and traditional Chinese medicine. Phytother Res. 2006;20(5):335–41.
Shin EM, Zhou HY, Guo LY, Kim JA, Lee SH, Merfort I, et al. Anti-inflammatory effects of glycyrol isolated from Glycyrrhiza uralensis in LPS-stimulated RAW264.7 macrophages. Int Immunopharmacol. 2008;8(11):1524–32.
Lee D-Y, Noh H-J, Choi J, Lee K-H, Lee M-H, Lee J-H, et al. Anti-inflammatory cycloartane-type saponins of Astragalus membranaceus. Molecules. 2013;18(4):3725–32.
Wang B-Q. Salvia miltiorrhiza: chemical and pharmacological review of a medicinal plant. J Med Plants Res. 2010;4(25):2813–20.
Committee CP. Pharmacopoeia of the People’s Republic of China. Beijing: Chinese Medical Science and Technology Press; 2015.
Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214(2):149–60.
Shang X, Yuan Z. Determination of six components in Rhubarb by cyclodextrin-modified micellar electrokinetic chromatography using a mixed micellar system of sodium cholate and sodium taurocholate. Anal Chim Acta. 2002;456(2):183–8.
Gong YX, Li SP, Wang YT, Li P, Yang FQ. Simultaneous determination of anthraquinones in Rhubarb by pressurized liquid extraction and capillary zone electrophoresis. Electrophoresis. 2005;26(9):1778–82.
Guo X. Surface plasmon resonance based biosensor technique: a review. J Biophotonics. 2012;5(7):483–501.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81202496 and 81273472) and grants from the Second Military Medical University (No. 2011QN04).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Yan Cao, Ying-hua Li and Di-ya Lv contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cao, Y., Li, Yh., Lv, Dy. et al. Identification of a ligand for tumor necrosis factor receptor from Chinese herbs by combination of surface plasmon resonance biosensor and UPLC-MS. Anal Bioanal Chem 408, 5359–5367 (2016). https://doi.org/10.1007/s00216-016-9633-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9633-6