Abstract
An improved method based on tandem solid phase extraction (SPE) cleanup and gas chromatography-high resolution mass spectrometry (GC-HRMS) has been validated for a rapid determination of dibenzo-p-dioxins/furans (PCDD/Fs), dioxin-like polychlorinated biphenyls (PCBs), marker polychlorinated biphenyls (M-PCBs), and polybrominated diphenyl ethers (PBDEs) using a large volume (50 mL) of human milk. This method was well validated for the measurement of these analytes in human milk from the general population with low limits of detection (LODs, 0.004–0.12 ng/g lipid), satisfactory accuracy (75–120 % of recoveries), and precision [less than 10 % of relative standard deviations (RSDs)]. To comprehensively evaluate the performance of this method, a good, presently validated and routinely used method based on an automated sample clean-up system (ASCS, based on the commercial acid multilayer silica, basic alumina, and carbon columns) was used in parallel for comparison. Compared with the ASCS method, this method presented comparable specificity. Additionally, this method, in contrast to ASCS method, highly reduced consumption of solvents (40 mL versus 500 mL), which results in much lower background in the procedural blank, reduced time, and enhanced sample pretreatment throughput. This method was also applied in a pilot study to measure a batch of human milk samples with satisfactory results.

Characteristics of the application of tandem SPE cleanup for determination of PCDD/Fs, DL-PCBs,M-PCBs and PBDEs in human milk
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human milk has been considered as an ideal matrix for generally measuring persistent organic pollutants (POPs) exposure because of its high lipid contents and advantage over other sampling matrices (e.g., tissue and blood) in allowing a larger volume of human milk to be sampled in a short period via a noninvasive way and in providing POPs exposure information of both humans and their human milk-fed infants [1–4]. These POPs, including polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (PCBs), marker PCBs (M-PCBs), and polybrominated diphenyl ethers (PBDEs) are generally accumulated in the human body of the general population at ultra-trace levels (pg/g) [4–9]. Therefore, in an attempt to achieve a high-sensitive measurement of these POPs for the accurate assessment of their internal exposure risk on the general population, several tens of milliliters (generally 40–50 mL) of human milk are normally needed to enrich the target POPs in a highly reduced volume (10–20 μL) [4, 8]. Simultaneously, this enrichment will undoubtedly result in large amounts of lipids and other interferences presented at the concentrations several orders of magnitude higher than the analytes [10]. Therefore, it is critically important to develop and validate not only a comprehensive pretreatment strategy for removing/eliminating these interferences during the process of POPs enrichment but also a highly sensitive and accurate instrumental analysis for their chromatographic separation and MS detection. Additionally, facing the current situation where large numbers of human milk samples collected are waiting for measurement, the method involving sample pretreatment and instrumental analysis should be characterized with rapidness, cheap, automation, and high sample throughput. Recently, to avoid the drawbacks of the generally used instrument gas chromatography high resolution mass spectrometry (HRMS) based on magnetic-electric sector instruments, predominantly involving a high cost in investment and maintenance and a high operation-skill requirement for analyst, an instrumental analysis based on gas chromatography triple quadrupole mass spectrometry (GC-QqQ MS) has been developed for the rapid determination of these POPs at relatively high concentration levels in samples [11–13]. However, fewer advances in recent studies were involved in the sample pretreatment for the rapid and high-throughput determination of POPs.
Regarding the entire analysis of POPs in human milk, sample pretreatment always plays an extremely important role as it was admittedly considered to be highly associated with much consumption of time, labor, and organic solvents as well as the error sources [10, 14–17]. The traditional sample pretreatment method, based on a Soxhlet extraction and a cleanup through multiple chromatographic columns using silica, acid silica, florisil, and alumina as adsorbents, has limited use because of its well-known disadvantages in manual operation, much time and organic solvent consuming, and large deviations (e.g., QC and measured results) frequently occurred from batch to batch or even from sample to sample in the same batch [10, 14–19]. An integrated method, based on accelerated solvent extraction (ASE) of lyophilized human milk, defatting based on the sulfuric acid silica gel, and cleaning up with a fluid management system (FMS, based on acid multilayer silica, basic alumina, and carbon columns) [4, 14, 18, 20, 21], or a FMS liked technique-automate sample clean-up system (ASCS, with the same columns as FMS) [8], has been widely adopted in sample pretreatment with the characters of high effectiveness and automation. However, the cleanup method via FMS or ASCS would use large amounts of solvents to quantitatively recover analytes and remove cross-contamination by washing with extra solvents [14, 18, 19]. Additionally, the method still lacks high throughput, and each instrument only treats a sample in each batch (in total at least 4 h needed for chromatographic column conditioning, sample loading, washing and eluting, and finally post-washing), which also generates high cost in the use of solvents and commerical chromatographic columns. A tandem solid phase extraction (SPE) based on sulfuric acid silica cartridge coupled with basic alumina cartridge was developed for the determination of PCBs and PBDEs in fishery and aquaculture products with many advantages over ASCS method [19]. The mean lipid content of human milk is about 5 % and comparable to that of the common fish. Almost no obvious matrix interferences in 150 human milk samples were observed in our previous PCDD/Fs and DL-PCBs study using ASCS method [8]. These results make it interesting for us to expand and validate the tandem SPE method covering not only PBDEs and PCBs but also PCDDs/Fs in the sample matrix of human milk.
The aim of this study is to validate a method for rapid determination of PCDD/Fs, DL-PCBs, M-PCBs, and PBDEs in human milk using tandem SPE method coupled with gas chromatography-high resolution mass spectrometry (GC-HRMS). To thoroughly assess the method performance, ASCS method, as a compared method, and certificated reference materials and real samples are employed in this study.
Experimental
Chemicals and materials
PCDDs/Fs, DL-PCBs, M-PCBs, and PBDEs standard solutions including calibration solutions (EDF-9999, EC-5380, EC-5385, and EO-5279), isotopic internal standards (EDF-8999, EC-5372, EC-5379, and EO-5277), and injection internal standards (EDF-5999, EC-5371, and EO-5275) were purchased from Cambridge Isotope Laboratories (Andover, MS, USA). Silica gel (0.063–0.100 mm, 100–200 mesh) was from Merck (Darmstadt, Germany). Nonane (anhydrous, ≥99.0 %), basic alumina (150 mesh), and anhydrous sodium sulphate (≥99.0 %) were from Sigma Aldrich (Steinheim, Germany). Hexane and dichloromethane were from Fisher Scientific (Fair Lawn, NJ, USA) with Optima grade purity. Three columns used in ASCS (Polytech Instrument Ltd, China), including acid multilayer silica (PCBS-ABN-STD), basic alumina (PCBA-BAS-011), and AX-21 carbon (PCBC-CCE-034) were from Fluid Management Systems Inc. (Waltham, MA, USA). Certified reference material (CRM) samples including SRM 1954 (NIST) and human milk sample of interlaboratory comparison on dioxins in food 2006 (2006 B in abbreviation, from Norwegian Institute of Public Health) were used for method development and validation.
Preparation of standard solutions
The calibration solution serials of PCDDs/Fs used were prepared by diluting EDF-9999 to 1/10 its original level in nonane, and this step was omitted for the calibration solution serials of DL-PCBs, M-PCBs, and PBDEs. A working internal standard mixture solution was prepared by diluting EDF-8999, EC-5372, EC-5379, and EO-5277 in nonane to a level of 10 ng/mL for PCDDs/Fs and DL-PCBs, and 100 ng/mL for M-PCBs and PBDEs. EDF-5999 and EC-5371 were individually diluted in nonane as working injection internal standard solution to 10 ng/mL.
Preparation of SPE cartridges
The lab-made SPE cartridges used were prepared according to our previous study [19]. Briefly, silica gel, basic alumina, and anhydrous sodium sulfate were rinsed with dichloromethane (20 mL per g) and then activated/baked under the condition of 180 °C for 1 h, 600 °C for 24 h, and 400 °C for 1 h, respectively. Forty-four percent acid silica gel was prepared by thoroughly mixing 100 g activated silica gel with 78.6 g concentrated sulfuric acid, which was applied as the sorbents both for D-SPE and acid silica SPE cartridge. Acid silica SPE cartridges were prepared by packing 5 g acid silica gel, a polypropylene frit, 1 g granular anhydrous sodium sulfate, and a polypropylene frit into a 12 mL empty SPE cartridge from bottom to top. Similarly, alumina SPE cartridges were prepared using 5 g activated basic alumina and 1 g anhydrous sodium sulfate. After preparation, all SPE cartridges were immediately vacuum-packaged for use within 3 mo.
Sample pretreatment
Lyophilization, ASE extraction, and defatting
The procedure of lyophilization, ASE extraction, and defatting was similar to our previous study [8]. Briefly, 40 g of human milk samples were lyophilized (–52 °C and 0.03 mbar for 48 h), and then extracted using ASE based on the following conditions: hexane and dichloromethane (1:1, v/v) as extraction solvents, 100 °C of extraction temperature and 1500 psi of extraction pressure. The extracts were concentrated to dryness and reconstituted in hexane (15 mL per g lipid) for dispersive solid phase extraction (D-SPE) defatting. The defatting was conducted by treating the extracts with acid silica (10 g per l g lipid). The extracted solution from D-SPE were concentrated to 0.2 mL for tandem SPE cleanup or to 2 mL for ASCS multi-column cleanup.
Tandem SPE cleanup
Tandem SPE cleanup was performed based on our previous work [19]. Acid silica SPE cartridge was conditioned with 9.0 mL hexane and activated basic alumina SPE cartridge was conditioned with a 9.0 mL mixture of hexane and dichloromethane (HEX-DCM, 1:1, v/v) and 9.0 mL hexane, successively. The acid silica SPE cartridge and the basic alumina SPE cartridge were tandem from top to bottom. After the sample was loaded on the conditioned acid silica SPE cartridge, 9 mL hexane was used to elute the target analytes from the acid silica SPE to the activated basic alumina SPE. Afterwards, the acid silica cartridge was removed and 2.0 mL hexane and 8.0 mL of HEX-DCM (1:1, v/v) were successively applied in the basic alumina cartridge. The HEX-DCM elution fraction (8.0 mL) was collected, concentrated to dryness, and reconstituted with the two working injection internal standard solutions (10 µL for each) for the GC-HRMS analysis.
ASCS multi-column cleanup
For comparison with tandem SPE, an ASCS, identical FMS cleanup (Fluid Management Systems, Waltham, MA, USA) [14], was used. Details were reported in our previous study [8]. Disposable multi-layer silica columns (4 g acid, 2 g basic, and 1.5 g neutral), basic alumina (8 g), and PX-21 (2 g) carbon columns were used, and two eluents including PCDDs/Fs fraction and PCBs + PBDEs fraction were collected. The two eluents were individually concentrated to near dryness and reconstituted with 10 μL of their corresponding working injection internal standard solution for GC-HRMS analysis.
GC-HRMS analysis
The analysis was carried out on a high resolution mass spectrometer (HRMS) (MAT95XP, ThermoFinnigan, Bremen, Germany) coupled with two gas chromatography (GC) instruments, each equipped with a CTC autosampler (CTC Analytics AG, Zwingen, Switzerland). Detailed operation parameters involving PCDD/Fs and PCBs were reported in our previous studies [8]. A DB-5MS-UI separation column (60 m × 0.25 mm i.d., 0.25 μm film thickness; Agilent, CA, USA) was used for the analysis of PCDD/Fs and PCBs, and DB-5HT separation column (15 m × 0.25 mm i.d., thickness 0.1 μm; Agilent) was for PBDEs. Ultrapure helium (99.999 %) was used as carrier gas with a constant flow of 0.8, 1.2, and 1.3 mL/min for PCDDs/Fs, PBDE, and PCBs, respectively. Surge splitless and splitless injection modes with a constant injection temperature of 260 °C were used for PCDDs/Fs and PCBs, respectively. PTV splitless injection mode was used for PBDEs with the following temperature program: initial temperature was 90 °C (hold 0.8 min), and ramped to 330 °C (hold 10 min) at the rate of 600 °C/min. The transfer line temperature was set at 310 °C for these three families of chemicals, and the oven temperature program was varied between them. For PCDD/Fs: hold at 120 °C for 1 min after injection, and ramped to 220 °C at the rate of 43 °C/min and held for 5 min at this temperature, and ramped to 250 °C at 2.3 °C/min, and ramped to 260 °C at 0.9 °C/min, and ramped to 310 °C at 20 °C/min and held for 5 min at this temperature; for PCBs: initial temperature was 80 °C (hold 2 min), and ramped to 150 °C at the rate of 15 °C /min, and ramped to 270 °C (hold 3 min) at the rate of 2.5 °C/min, and ramped to 315 °C (1 min) at the rate of 15 °C/min; for PBDEs: initial temperature was 80 °C (hold 1.6 min), and ramped to 140 °C at the rate of 4.6 °C/min, and ramped to 310 °C (hold 5 min) at the rate of 4 °C/min.
The HRMS was operated in EI mode, with 0.55 A of emission current, 42 eV of electron energy and 260 °C of ion source temperature. Multiple ions detection (MID) mode was performed with PFK as the reference gas and 10,000 MS resolution. Two isotopic ions of known relative abundance, representing a group of isomers, were monitored for each molecular ion cluster of native and 13C-labeled congeners. The detail MID parameters were summarized in our previous studies for PCDD/Fs and PCBs, and in Complementary Materials [see Electronic Supplementary Material (ESM) Table S1] for PBDEs. The injection volume was 2 μL for PCDDs/Fs and 1 μL for PCBs and PBDEs.
Method specificity and method validation and quality control
Two CRM samples including SRM 1954 and 2006 B were used for the studies of method specificity (mainly involving ion abundance ratio) and method accuracy. Ten samples similar to a batch of samples collected in our previous study [8] were used in a pilot study. These samples were transferred, each with an appropriate volume, to mix as a pooled sample for method development and validation (limit of detection (LOD), method accuracy and precision), as well as QC. After sampling and additional treatment, such as transferring, all the samples were immediately stored at –25 °C until chemical analysis. ASCS cleanup was used in parallel for assessment of the performance of tandem SPE. ASCS method is an on-going validated method in our laboratory. Based on ASCS method, satisfactory results (involving PCDDs/Fs, PBDEs, DL-PCBs, and M-PCBs) were obtained in our laboratory in Interlaboratory Comparison on POPs in Food (Lab nos. in 2010, 2011, 2012, 2014, and 2015 are 84, 87, 72, 75, and 35, respectively) and Biennial Interlaboratory Assessment on POPs (in 2013, Lab no. L137) organized by the Norwegian Institute of Public Health and United Nations Environment Programme (UNEP), respectively.
All integrated chromatograms were thoroughly examined based on the analytical standards recommended by the US EPA method 1613B [16], 1668B [10], and 1614 [17] and EN 1948-4 [22]. The peaks detected in samples were identified as the positive congeners according to the follow criteria: retention time within a 6 s window compared with the corresponding 13C-labeled isomer, signal-to-noise ratio (S/N) equal to or greater than 3 and ion abundance ratio of corresponding two monitored ions within ±15 % of its theoretical value. Lock-mass traces were examined for evidence of ionization suppression as well. A procedural blank and a quality control sample (SRM 1954) were analyzed in each sample batch. Linearity of the calibration curves was checked based on the variation of relative response for all analytes.
Results and discussion
Optimization of tandem SPE
Regarding PCBs and PBDEs, the optimized condition of tandem SPE was well investigated in our previous study [19]. Therefore, this study mainly focused on method optimization for PCDDs/Fs with some efforts made for the on-going validation for PCBs and PBDEs. Like PCBs and PBDEs, PCDDs/Fs were observed with quantitative recoveries in the step of eluting the target analytes from the acid silica cartridge to the basic alumina cartridge. PCDDs/Fs have equal or slightly lower polarity and higher polarity compared with PBDEs and PCBs, respectively. Therefore, PCDDs/Fs possess equal or slightly weaker retention capacity on basic alumina cartridge in contrast to PBDEs but stronger retention capacity in contrast to PCBs. Thus, the original mixture (Hex:DCM, 1:1, v/v) utilized for eluting PCBs and PBDEs [19] was used in our previous study. The experimental results showed that 8 mL Hex:DCM (1:1, v/v) could present 51–105 % of recoveries for PCDDs/Fs, PCBs, and PBDEs, and no more improvement was observed when more volume of eluting solvent was used. Therefore, 8 mL Hex:DCM (1:1, v/v) is set as the optimized condition for eluting the target analytes from the basic alumina cartridge.
Method specificity
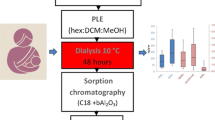
In this study, method specificity was studied by comparing ion abundance ratio of two diagnostic ions with its expected value for each native and isotopic congener, and by evaluating the procedural blank in an attempt to detect the presence of possible interferences of the samples treated by tandem SPE method and to assess the effects of these interferences on the highly sensitive and accurate identification and quantification of PCDDs/Fs, PCBs, and PBDEs. CRM 2006B was used for the study of ion abundance ratio assessment. For comparison, ASCS method was used in parallel. The relative deviations of ion abundance ratios of 2006B (subjected to tandem SPE and ASCS cleanup) to the expected are illustrated in Fig. 1. The experimental results showed that for tandem SPE, the deviations of these ratios to the theoretical ratios all fall within the limits (±15 %) set by EPA method 1613 [16], 1614B [17], and 1668B [10], with their values less than 14 % for PCDD/Fs and 8 % for M-PCBs, DL-PCBs, and PBDEs (except BDE-28 (13 %) and CB-77L (11 %) in ASCS, and CB-169L(14 %) in tandem SPE). Compared with ASCS method, tandem SPE method presented the deviations with no statistically significant difference for PCDD/Fs and DL-PCBs (p (2-tailed) = 0.21 and 0.056, respectively, paired samples t-test), and even better results for PBDEs and M-PCBs (p (2-tailed) = 0.04 and 0.006, respectively, paired samples t-test). Additionally, tandem SPE method exhibited the satisfactory peak shape and the unobvious shift of retention time for each target analyte in analysis of 2006B, indicating no obvious effect from interferences on chromatographic separation.
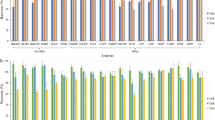
To evaluate the procedural blank of tandem SPE, procedural blank samples (from our daily routine projects from the year of 2010 to 2014) subjected to ASCS method was used for comparison. The compared data acquired based on both methods are displayed in Fig. 2. For PCDDs/Fs, tandem SPE method presented more than two times lower concentration levels than ASCS method for nearly half the number of congeners, including 2,3,7,8-TCDD, 1,2,3,7,8-PeCDD, 1,2,3,6,7,8-HxCDD, 2,3,7,8-TCDF, 2,3,4,7,8-PeCDF, 2,3,4,6,7,8-HxCDF, 1,2,3,4,6,7,8-HpCDF, and OCDF (Fig. 2), and comparable levels for the rest of congeners. For PCBs and PBDEs, tandem SPE method presented more than two times lower concentration levels for all target congeners, especially for those congeners (displayed in Fig. 2) with an order of magnitude lower levels compared with ASCS method. The profile and concentration levels of these target analytes in the procedural blank were observed to be surprisingly similar to that of the highly concentrated (10,000 times) organic solvent used in sample pretreatment of these POPs. Thus, the concentration levels of the procedural blank were believed to be mainly contributed by the consumption of organic solvent. Owing to less volume of organic solvent used in tandem SPE (40 mL) than ASCS (500 mL), tandem SPE presented lower concentration levels in the procedural blank. The reported concentrations of samples are generally obtained by subtraction of the measured concentration of samples from that of the corresponding procedural blank. However, for an accurate determination in our laboratory, the subtraction is performed based on a general criterion that the concentration of the procedural blank is not more than 20 % of concentration of the samples. Based on the criterion, tandem SPE presented the satisfactory procedure blank with all congeners presented at a concentration lower than 20 % of the measured mean concentration of the 10 samples used in this study. This result is much better than that of ASCS method (with five congeners beyond the criterion). Due to the low concentration levels of the procedural blank in tandem SPE, a specific treatment-organic solvent distillation-was avoided during the preparation of materials and solvents for POPs analysis.
Method validation
Commercial calibration standard solutions were used for the isotope dilution quantification. For each analyte, the relative response was observed with less than 20 % variation over the five-point concentration range. Therefore, an averaged relative response was used for the quantification. The LODs were estimated as the concentration of the pooled sample (5 % lipid content) extracts, in which concentration of each congener was adjusted by spiking standard solutions or diluting with nonane to a concentration where signal-to-noise ratio (s/n) was in a range of 3–5. LOD values were in a range of 0.004–0.017 ng/g lipid for PCDD/Fs, 0.05–0.07 ng/g lipid for DL-PCBs, 0.04–0.07 ng/g lipid for M-PCBs, and 0.032–0.12 ng/g lipid for PBDEs, among which the LODs of PCDD/Fs and DL-PCBs are comparable to the values obtained from ASCS method [8]. These LODs were low enough to allow quantification of these POPs in human milk samples from the general population . The pooled sample was spiked with various amounts of standards, depending on analytes (shown in Table 1), and aliquoted into three 50-mL subpools. These subpools were analyzed for a study of method accuracy and precision. The unspiked samples were also analyzed in duplicate for recovery correction. The intra-day (n = 3) recoveries were 75–120 % with relative standard deviations (RSDs) equal to or less than 10 %, indicating satisfactory method accuracy and precision that the tandem SPE presented.
Application in CRM samples
Two human milk CRM samples, 2006B and SRM 1954, were used to assess method performance. To thoroughly evaluate method performance presented by tandem SPE, 2006B was also treated using ASCS method in parallel for comparison. The measured results are summarized in Table 1. In the analysis of 2006B, tandem SPE presented satisfactory results with |Z| ≤ 2 for all congeners (Table 1, the Z values were not listed here for ASCS method) according to the consensus median concentrations assigned by the Norwegian Institute of Public Health. These results were comparable to ASCS method. For ASCS method, among a total of 43 congeners, seven congeners (including 2,3,7,8-TCDF, 1,2,3,7,8-PeCDF, 1,2,3,7,8,9-HxCDF, 1,2,3,4,7,8,9-HpCDF, OCDF, CB-52, and BDE-209) have |Z| ∈ [1, 2) (meaning 20 % < RSD ≤ 40 % compared with the consensus values); whereas for tandem SPE method, 10 congeners (including 1,2,3,7,8-PeCDD, 1,2,3,4,7,8-HxCDD, 2,3,4,7,8-PeCDF, 1,2,3,4,7,8-HxCDF, 1,2,3,6,7,8-HxCDF, 1,2,3,4,6,7,8-HpCDF, OCDF, CB-28, CB-101, and BDE-209) have |Z| | ∈ [1, 2]. In the analysis of 2006B, recoveries of isotopic internal standards in method of tandem SPE were in the range of 62-100 %, 51-69 %, and 46-72 %, respectively, for PCDD/Fs, DL-PCBs, and M-PCBs. Although these recoveries are not better compared with that of ASCS method, it is worth mentioning that these recovery values all fall into the normal range (17-197 % for PCDD/Fs and 25-150 % for PCBs) adopted by EPA Method 1613 [16] and 1668B [10].
For SRM1954 sample, tandem SPE also presented very satisfactory |Z| values all within a range of 0–1. These measured results indicate tandem SPE method is characterized with a satisfactory performance in method accuracy.
Application in real samples
The method has been applied to determine a batch of samples, including 10 human milk samples and procedural blank and QC (SRM 1954) samples, with the purpose of addressing the on-going method specificity and performance. These validation parameter values, including ion abundance ratios, concentration levels of the procedural blank, concentration deviations of QC to the assigned concentrations, and recoveries of internal standards of these 10 samples all fell within the ranges specified during the initial studies in method specificity and validation. Just like in the analysis of CRM 2006B, the method presented excellent chromatographic peaks without retention time shift and obvious interfering peaks (Fig. 3 for PCDDs/Fs and Figs. S1 and S2 in the ESM for PCBs and PBDEs) observed in the entire analysis. Ion abundance ratios for signal peaks were assessed for agreement with theoretical abundances, and the variation in response factors for reference standard solutions within a run was limited to 15 %. Each lock-mass of each sample was observed not to vary by more than 20 % throughout its respective retention time window and thus no failures in mass-drift correction of electric field using lock and calibration masses were found in the entire analysis. These satisfactory results indicate less or even no co-eluted ions associated with interference or suppression of the target analyte ions and lock and calibration masses because of the effective cleanup of tandem SPE. Owing to the good performance of tandem SPE, activated carbon, generally adopted to further treat these POPs by removing the residual lipids and other potential interferences from samples and separating the target coplanar congeners [including 17 PCDD/F congeners and four PCB congeners (CB-77, 81, 126, and 169)] from the interfered un-coplanar isomers, was ignored in this method. The procedural blank-corrected concentrations of samples (n = 10) are summarized in Table 1, and these values are generally in line with our reported results acquired based on the same batch of sampling as these 10 samples but with a different cleanup method (using ASCS). Due to the low LODs presented by this method, all congeners of DL-PCBs, M-PCBs, and PBDEs were detected in these 10 samples with detection rates of 100 %, and a majority of congeners of PCDDs/Fs with detection rates of over 70 %.
Conclusion
A tandem SPE cleanup based on sulfuric acid silica cartridge coupled with basic alumina cartridge was developed and validated for determination of PCDDs/Fs, PCBs, and PBDEs in human milk sample. The method was well validated with LODs low enough to allow low exposure level detection for the general population, and with satisfactory method accuracy and precision. To access the performance of this cleanup method, the traditional cleanup method-ASCS method-was performed in parallel and two CRM samples were used. This method could be very useful in reducing workload, time, and solvents consumption, and enhancing throughput of sample pretreatment compared with ASCS method. At least 10 samples in a batch of analysis can be allowed for simultaneous cleanup with less than 1 h and each sample with no more than 40 mL organic solvents consumed. Compared with ASCS method where each batch consumed about 4 h and each instrument treated only one sample in each batch, and each sample needed at least 500 mL organic solvent, tandem SPE cleanup has the overwhelming advantages. In summary, the use of tandem SPE has been shown with the potential to serve as an alternative method of ASCS to flawlessly meet the requirement of high throughput of sample pretreatment in POPs analysis of human milk.
References
US EPA. Exposure and human health reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds. Nat Acad Sci Review Draft. Washington, DC: National Center for Environmental Assessment, Office of Research and Development; 2004.
WHO. Fourth WHO-coordinated survey of human milk for persistent organic pollutants in cooperation with UNEP- guidelines for developing a national protocol. 2007.
LaKind JS, Brent RL, Dourson ML, Kacew S, Koren G, Sonawane B, et al. Human milk biomonitoring data: interpretation and risk assessment issues. J Toxicol Environ Health A. 2005;68(20):1713–69.
Li J, Zhang L, Wu Y, Liu Y, Zhou P, Wen S, et al. A national survey of polychlorinated dioxins, furans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (dl-PCBs) in human milk in China. Chemosphere. 2009;75(9):1236–42.
Zhang L, Li J, Zhao Y, Li X, Yang X, Wen S, et al. A national survey of polybrominated diphenyl ethers (PBDEs) and indicator polychlorinated biphenyls (PCBs) in Chinese mothers' milk. Chemosphere. 2011;84(5):625–33.
Ryan JJ, Rawn DFK. Polychlorinated dioxins, furans (PCDD/Fs), and polychlorinated biphenyls (PCBs) and their trends in Canadian human milk from 1992 to 2005. Chemosphere. 2014;102:76–86.
Wong TW, Wong AHS, Nelson EAS, Qiu H, Ku SYK. Levels of PCDDs, PCDFs, and dioxin-like PCBs in human milk among Hong Kong mothers. Sci Total Environ. 2013;463:1230–8.
Lu D, Lin Y, Feng C, Wang D, She J, Shen H, et al. Levels of polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (DL-PCBs) in breast milk in Shanghai, China: a temporal upward trend. Chemosphere. 2015;137:14–24.
Lu D, Wang D, Ni R, Lin Y, Feng C, Xu Q, et al. Organochlorine pesticides and their metabolites in human breast milk from Shanghai, China. Environ Sci Pollut Res Int. 2015. doi:10.1007/s11356-015-4072-z.
US EPA. Method 1668, revision B: chlorinated biphenyl congeners in water, soil, sediment, biosolids, and tissue by HRGC/HRMS. Washington DC: US Environmental Protection Agency; 2008.
Planche C, Ratel J, Mercier F, Blinet P, Debrauwer L, Engel E. Assessment of comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry based methods for investigating 206 dioxin-like micropollutants in animal-derived food matrices. J Chromatogr A. 2015;1392:74–81.
L’Homme B, Scholl G, Eppe G, Focant JF. Validation of a gas chromatography-triple quadrupole mass spectrometry method for confirmatory analysis of dioxins and dioxin-like polychlorobiphenyls in feed following new EU Regulation 709/2014. J Chromatogr A. 2015;1376:149–58.
García-Bermejo Á, Ábalos M, Sauló J, Abad E, González MJ, Gómara B. Triple quadrupole tandem mass spectrometry: a real alternative to high resolution magnetic sector instrument for the analysis of polychlorinated dibenzo-p-dioxins, furans, and dioxin-like polychlorinated biphenyls. Anal Chim Acta. 2015;889:156–65.
Pirard C, De Pauw E, Focant J-F. New strategy for comprehensive analysis of polybrominated diphenyl ethers, polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and polychlorinated biphenyls by gas chromatography coupled with mass spectrometry. J Chromatogr A. 2003;998(1/2):169–81.
Focant JF, Pirard C, De Pauw E. Automated sample preparation-fractionation for the measurement of dioxins and related compounds in biological matrices: a review. Talanta. 2004;63(5):1101–13.
US EPA. Method 1613, revision B tetra- through octa-chlorinated dioxins and furans by isotope dilution HRGC/HRMS. Washington DC: US Environmental Protection Agency; 1994.
US EPA. Method 1614 brominated diphenyl ethers in water, soil, sediment, and tissue by HRGC/HRMS. Washington DC: US Environmental Protection Agency; 2007.
Pirard C, Focant J-F, De Pauw E. An improved clean-up strategy for simultaneous analysis of polychlorinated dibenzo-p-dioxins (PCDD), polychlorinated dibenzofurans (PCDF), and polychlorinated biphenyls (PCB) in fatty food samples. Anal Bioanal Chem. 2002;372(2):373–81.
Lu D, Lin Y, Feng C, Wang D, Qiu X, Jin Y, et al. Determination of polybrominated diphenyl ethers and polychlorinated biphenyls in fishery and aquaculture products using sequential solid phase extraction and large volume injection gas chromatography/tandem mass spectrometry. J Chromatogr B. 2014;945(946):75–83.
Pirard C, De Pauw E, Focant JF. Suitability of tandem-in-time mass spectrometry for polybrominated diphenylether measurement in fish and shellfish samples: comparison with high resolution mass spectrometry. J Chromatogr A. 2006;1115(1/2):125–32.
Li JG, Yu HF, Zhao YF, Zhang G, Wu YN. Levels of polybrominated diphenyl ethers (PBDEs) in breast milk from Beijing, China. Chemosphere. 2008;73(2):182–6.
BSI. BS EN 1948-4 Stationary source emissions - determination of the mass concentration of PCDDs/PCDFs and dioxin-like PCBs Part 4: sampling and analysis of dioxin-like PCBs. British Standards. 2009.
Acknowledgments
This study was supported by the National Key Scientific Instrument and Equipment Development Projects (2013YQ150557), Shanghai Municipal Key Discipline of Public Health (no. 15GWZK0301), Shanghai Municipal Overseas High-End Talent Training (no. GWTD2015S03), the Research Grand Award (no. 201540210, Shanghai Municipal Commission of Health and Family Planning, China), and the Fourth 3-Year Action Plan of System Establishment of Shanghai Municipal Public Health (2016–2018).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Yuanjie Lin and Chao Feng contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 102 kb)
Rights and permissions
About this article
Cite this article
Lin, Y., Feng, C., Xu, Q. et al. A validated method for rapid determination of dibenzo-p-dioxins/furans (PCDD/Fs), polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in human milk: focus on utility of tandem solid phase extraction (SPE) cleanup. Anal Bioanal Chem 408, 4897–4906 (2016). https://doi.org/10.1007/s00216-016-9576-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9576-y