Abstract
The increasing number of children suffering from developmental disorders has raised questions regarding their association with the presence of environmental contaminants in mothers and children. We therefore developed a new method for the determination of 78 proven and potential developmental neurotoxicants, including polychlorinated biphenyls, legacy pesticides, pyrethroids, and old and new halogenated flame retardants in breast milk. The essential part of sample preparation was dialysis as a non-destructive clean-up step which was newly used at 10 °C and showed more efficient lipid removal (up to 96%) than the conventional methods such as gel permeation chromatography or freezing-lipid filtration and thus ensured low limits of detection (LOD) by reducing the sample volume prior to injection. Next advantages were significant solvent reduction and no risk of sample cross-contamination. Gas chromatography coupled with high resolution mass spectrometry (GC-HRMS) was subsequently used for the separation and compound quantification. The method was validated using breast milk samples fortified with the analyzed compounds. Recoveries for most of the compounds ranged from 63 to 121% with a relative standard deviation of 2–25%, and LODs ranged between 0.001 and 0.87 ng g−1 lipid weight. The method was applied to breast milk samples from a Dutch birth cohort where 35 out of the 78 compounds were quantified in more than 60% of the samples. For novel flame retardants, the method provides unique results regarding their occurrence in human matrices in Europe. Overall, the analysis of a complex mixture of developmental neurotoxicants could be useful for the assessment of the influence of the studied compounds to child health and development.

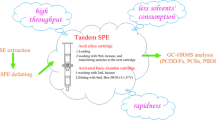
Flow diagram of the method and levels of the developmental neurotoxicants in Dutch human milk samples
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Worldwide, up to 15% of all children are affected by developmental disorders such as attention deficit hyperactivity disorder (ADHD), autism spectrum disorders (ASD), or learning problems [1]. A possible link has been revealed between these disorders and early life exposure to several environmental pollutants, which include polychlorinated biphenyls (PCBs), selected organochlorine pesticides (OCPs), pyrethroids, organophosphate pesticides, polybrominated diphenyl ethers (PBDEs), and novel flame retardants (NFRs) [2–6]. The toxic effects of NFRs are still mostly unknown, but some NFRs can due to structural similarities also act as developmental neurotoxicants, such as decabromodiphenyl ethane (DBDPE) and BDE-209 [7]. PCBs, OCPs, and PBDEs are persistent compounds, which are still present in both the environment (including the food chain) and humans despite bans and restrictions; pyrethroids and NFRs are currently used as alternatives to these legacy pesticides and flame retardants. While research has focused on testing toxic effects of various classes of environmental contaminants, there is still a lack of data regarding the levels of some of these compounds in the human body. The monitoring of compounds, such as NFRs, in human matrices would thus contribute to a better understanding of the impact of these chemicals on human health and fill one of the gaps identified in recent research [8].
Newborns are a susceptible group in terms of exposure to DNTs, and breast milk constitutes a significant exposure pathway for them. Due to the limited amount of breast milk samples usually obtained from cohort studies, multi-class analyses are a desirable approach. Two different approaches to milk sample preparation/extraction are currently being used. When solid-phase extraction (SPE), Quick, Easy, Cheap, Effective, Rugged and Safe (QuEChERS) method or ultrasonication of freeze-dried milk are applied, part of the lipids are removed, which prevents us from determining lipid content (i.e., lipid content must be determined separately) [9–11]. In contrast, use of liquid-liquid-extraction (LLE) or pressurized liquid extraction (PLE) allows us determining lipids gravimetrically as they are co-extracted with the target compounds [12–14].
Following extraction, the clean-up method must be applied due to the removal of lipids interfering with the instrumental analysis. In the case of determination of persistent compounds such as PCBs, OCPs, PBDEs, or their combinations, all of which are frequently analyzed in breast milk, a destructive clean-up method using concentrated sulfuric acid (mostly fixed on silica particles) is often applied [15–19]. The inclusion of non-persistent compounds, which would be broken down by the acid, calls for the use of different and often multiple clean-up methods. The number of methods suitable for the non-destructive multi-class analysis of DNTs in human breast milk is also currently limited [20]. A frequently reported non-destructive clean-up method for organic compounds in breast milk is gel permeation chromatography (GPC), which shows good separation of milk lipids from persistent pesticides and/or PBDEs with average recoveries between 66 and 84% [21, 22]. Freezing-lipid filtration (FLF) is an alternative method, often used for fish tissue samples when determining endocrine disrupting phenols or OCPs with sufficient recoveries (70–120 or 80–115%, respectively) [23, 24]. The lipid removal efficiency of FLF may reach 88.7–93.7% [20, 23, 24]. Chen et al. applied FLF to milk samples in order to analyze persistent as well as non-persistent pesticides with recoveries ranging from 34 to 102% [20]. Dialysis, based on the use of a semipermeable membrane (SPM), is another potential lipid removal method for the analysis of persistent and non-persistent organic compounds in breast milk. SPM has been used for fatty foods such as butter, egg yolk, chocolate, fish oil, or seal blubber, providing high recoveries of PCBs, PBDEs, polychlorinated dibenzodioxins/furans (PCDD/Fs), ranging from 50 to 97%, and a very high lipid removal efficiency (97–99) [25–29]. The applicability of dialysis to milk extracts was shown with DDT where the high recovery of this compound was reported (87 and 96% after 24 and 72 h, respectively). However, lipid removal (86 and 78% after 24 and 72 h, respectively) [30] was lower than for FLF.
The aims of this study were to develop a robust method for the determination of selected groups of 78 environmental contaminants such as indicator PCBs, OCPs, PBDEs, pyrethroids, and NFRs with proven or potential developmental neurotoxicity in human milk; to determine the method performance characteristics; and to apply it to breast milk samples from the Netherlands. In order to reach the lowest achievable limits of detection (LODs) and simultaneously suppress gas chromatography–high resolution mass spectrometry (GC-HRMS) analysis interferences, the proposed method should (a) use the maximum available volume of samples provided by cohort studies, (b) incorporate lipid determination into sample preparation, (c) select and optimize an efficient clean-up method that guarantees maximum fat removal, and (d) minimize the final sample volume, e.g., lower than 30 μL.
Materials and methods
Chemicals and reagents
Native indicator PCBs (PCB 28, 52, 101, 138, 153, 180), dioxin-like PCB 118, and selected OCPs (aldrin, α-, γ-chlordane, α-endosulfan, chlordecone, dieldrin) were purchased from LGC Standards (Lomianki, Poland). Other OCPs (β-endosulfan, endosulfan-sulfate, endrin, endrin aldehyde, endrin ketone, pentachlorobenzene, hexachlorbenzene (HCB), α-, β-, γ-, and δ-hexachlorocyclohexane (HCH), heptachlor, heptachlor epoxide, methoxychlor, mirex, o,p′- and p,p′-DDE, o,p′- and p,p′-DDT, o,p′- and p,p′-DDD) were obtained from Supelco (Bellefonte, PA, USA). Native PBDEs (BDE 28, 47, 66, 85, 99, 100, 153, 154, 183) were purchased from AccuStandard, Inc. (New Haven, CA, USA) and native pyrethroids from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA). NFRs included allyl 2,4,6-tribromophenyl ether (ATE), 2-bromoallyl-2,4,6-tribromophenyl ether (BATE), 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane (α- and β-TBECH), 2,3,5,6-tetrabromo-p-xylene (p-TBX), 1,2,5,6-tetrabromocyclooctane (α- and β-TBCO), pentabromobenzene (PBBZ), tetrabromo-o-chlorotoluene (TBCT), 1,2,3,4,5-pentabromo-6-methylbenzene (PBT), pentabromoethylbenzene (PBEB), 2,3-dibromopropyl 2,4,6-tribromophenyl ether (DPTE), hexabromobenzene (HBB), hexachlorocyclopentenyl-dibromocyclooctane (HCDBCO), 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EHTBB), 1,2-bis(2,4,6-tribromophenoxy)ethane (BTBPE), decabromodiphenylethane (DBDPE), bis(hexachlorocyclopentadieno)cyclooctane (syn- and anti-DP), and dechlorane Plus® Mono adduct (DPMA). NFRs and isotope-labeled internal standards of PBDEs were purchased from Wellington laboratories (Ontario, Canada). Labeled standard of PCB-7 (indicator PCBs and PCB 118), OCPs, and pyrethroids were purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA).
Solvents and reagents used in extraction and clean-up were dichloromethane (DCM) and n-hexane (Pestiscan grade) obtained from Lab-Scan (Gliwice, Poland), acetonitrile (ACN, LC-MS grade) from Biosolve BV (Valkenswaard, Netherlands), and n-nonane picograde from Promochem LGC Standards (Wesel, Germany). Methanol Chromasolv gradient grade was purchased from Sigma-Aldrich (Steinheim, Germany) and sodium sulfate, anhydrous (analytical-reagent grade) from Lach-Ner (Neratovice, Czech Republic).
Samples
A total of 120 breast milk samples from the mother-child cohort LInking endocrine disruptive compounds in maternal Nutrition to Child health (LINC) were used [31]. Samples were collected between 2011 and 2015 in the area of Zwolle and den Helder in the Netherlands. Women were recruited during their first antenatal visit to the midwife and written informed consent was obtained. Breast milk samples were collected by mothers during weeks 4 to 8 after delivery in pre-treated (prewashed and solvent rinsed) bottles and stored at −20 °C until analysis.
Extraction
Prior to extraction, 8–10 mL of liquid milk were freeze dried for 36 h using ScanVac CoolSafe Pro freeze-dryer (Copenhagen, Denmark). Approximately 1.2 g of obtained freeze-dried milk were ground with 15 g of sodium sulfate in a mortar and quantitatively transferred into a 40-mL PLE cell between two layers of cleaned Ottawa sand. Known amounts of labeled internal standards were added. PLE was performed on a Speed Extractor E-914 from Büchi (Switzerland). A three-cycle extraction programme with n-hexane:DCM:methanol (5:2:1; v:v:v) at 65 °C and 100 bar was used. Following solvent reduction using a Syncore Analyst concentrator Büchi (Switzerland), the lipid extract was placed into an oven at 60 °C for 1 h to evaporate the residual solvent. After the PLE step, lipids were determined gravimetrically.
Gel permeation chromatography
GPC was conducted on an automated system Gilson (Middleton USA) equipped with a 402 syringe pump, 231 XL Sampling Injector, 307 binary pump, and was coupled to a variable wavelength detector (UVD 200, Deltachrom, Watrex, Czech Republic). Two styrene-divinylbenzene GPC columns (19 × 150 mm and 19 × 300 mm, 15 μm particles, Envirogel, Waters) were connected in series. DCM was used as a mobile phase with the flow rate of 5 mL min−1. Milk fat was dissolved in DCM to obtain a total volume of 2 mL which was then injected into the GPC system.
Freezing-lipid filtration
Forty milliliters of ACN were added to milk fat and sonicated for 15 min. The extract was placed into a freezer (−24 °C) for 2 h. Suspended frozen lipids were removed via filtration performed inside the freezer through folded cellulose filter (Munktell, Germany). The process was repeated twice.
Dialysis
A portion of low-density polyethylene tubing (5 cm × 10 cm, nominal thickness of 85 μm, Brentwood Plastics, Missouri, USA) was macerated in n-hexane for 48 h prior to use. The tubing was heat-sealed on both ends and PLE extracted milk fat dissolved in 400 μL of n-hexane was carefully injected into the tubing at the upper sealed end using a syringe and a needle. The vial previously containing the milk fat was then rinsed with another 300 μL of n-hexane and the rinsate was added to the sample. Once the needle was removed, excess air was mechanically squeezed out from the tubing and the puncture was carefully heat-sealed again. The tubing was subsequently rolled into an amber vial containing 20 mL of n-hexane. After 24 h, n-hexane was poured into a 40-mL vial and 20 mL of fresh n-hexane were added to the original vial containing the membrane. Total dialysis time was 48 h. After dialysis, n-hexane was evaporated under a stream of nitrogen and the solvent was exchanged to ACN.
Column chromatography
Column chromatography was necessary in order to remove remaining lipids following each of the GPC/FLF/dialysis methods outlined above. Into a commercially available 6-mL column with 500 mg of C18 sorbent (Agilent Technologies, Lake Forest, CA, USA), 3 g of 90 active basic alumina (Merck, Darmstadt, Germany) deactivated by 5% of water were added for capturing mainly free fatty acids [32]. After loading the sample in 4 mL of ACN to the column, the original vial containing the sample was rinsed twice with 3 mL and 2.5 mL of ACN and the rinsate was added to the column. The ACN volume was optimized to elute the target compounds before lipids start eluting. The column eluent was collected and after reducing ACN to approximately 200 μL under the stream of nitrogen, it was quantitatively transferred to a conical 1.2-mL vial and evaporated nearly to dryness. Twenty-five microliters of 13C12 PCB 162 (20 ng mL−1) recovery standard in n-nonane were added to the sample to be analyzed for OCPs + PCBs-7 and pyrethroids. Subsequently, 10 μL of 13C12 BDE 77 and 13C12 BDE 138 [32] (100 ng mL−1) were added for analysis of PBDEs and NFRs.
Instrumental analysis
Four instrumental GC-HRMS methods were used to measure five groups of compounds. Two GC-HRMS instruments were used for the measurement. The instrumental analyses of OCPs, PCBs-7, and pyrethroids were carried out using a Trace 1310 GC (Thermo Scientific, USA) coupled to a double-focusing magnetic sector HRMS DFS (Thermo Scientific). The analyses of PBDEs and NFRs were performed using a 7890A (Agilent, USA) GC coupled to a double-focusing magnetic sector HRMS AutoSpec Premier (Waters, UK). The GC conditions for the methods are summarized in Table 1. In both instruments, 0.6-m × 0.53-μm Restek deactivated Rxi®-guard column was used. Helium was used as the carrier gas with a constant flow rate of 1 mL min−1. Splitless injection of 2 μL was used in all methods.
In both instruments, electron impact ionization in the positive mode (EI+) was used with electron energy of 35 eV (AutoSpec) and 48 eV (DFS). The MS resolution mode in both mass spectrometers was set at ≥10,000 (10% valley). The GC-MS transfer line temperature was 280 °C for all analyses. Quantification was based on the isotope dilution method if an analyte surrogate was available, or on the use of the labeled internal standard method or on external calibration, when no proper internal standard was available (selected NFRs). Detailed information regarding individual compounds, their masses, and used internal standards are provided in the Table S1 in Electronic Supplementary Material (ESM). PBDE and NFR chromatograms were processed using Waters Target Lynx software. OCP + PCBs-7 and pyrethroids chromatograms were processed using Thermo Scientific TargetQuan 3.2.
Method performance characteristics
Analytical performance characteristics of the method, based on validation guidelines included in the EU Commission Decision 2002/657/EC [33], were evaluated. The validation parameters included linearity, accuracy (comprising both trueness and precision), and LOD, and they were assessed using fortified breast milk obtained from a volunteer mother at two known concentration levels. Linearity in a calibration range was expressed as the relative standard deviation (RSD) of response factors calculated from the GC-HRMS responses of native and labeled analytes in calibration standards. Acceptable RSD values were below 20%. Trueness, evaluated as average recoveries, was calculated as the ratio of determined concentrations in spiked samples to their target level*100%. The range between 70 and 110% was considered satisfactory [33]. Precision was expressed as intra-day RSD for six measurements of fortified breast milk samples and between-day RSD from an analysis of the fortified samples analyzed weekly. Expanded uncertainties (U) were calculated from the combined standard uncertainties (u c) including the uncertainty from derived from precision (u p) and from trueness (u b). The details of the calculation are provided in ESM (p. S4). The limits of detection were calculated as 3*standard deviations of the levels found in procedural blanks (n = 18) divided by the median lipid content of the analyzed milk samples (0.038 g) to adjust the LOD to the lipid weight. LODs of compounds which were not present in the blanks were calculated as concentrations corresponding to S/N ratios of 3. In order to lower the background levels of the compounds in procedural blanks, all detergent-washed glassware was baked at 400 °C for 5 h and rinsed with organic solvents prior to analysis. The use of plasticware was avoided as much as possible in order to reduce the background levels of PBDEs/NFRs.
Results and discussion
Extraction
A pressurized solvent extraction was used due to the lower solvent consumption and shorter extraction time in comparison with the traditionally used liquid-liquid extraction. The choice of solvent mixture, temperature, and pressure used here (n-hexane:DCM:MeOH (5:2:1; v/v/v) 65 °C, 100 bar [34]) allowed us to quantitatively co-extract all target lipophilic compounds together with milk lipids. The lipid content constitutes important information in the analysis of non-polar organic compounds owing since their levels are of adjusted to lipid weight. The fat content obtained after PLE in this study (3.40 ± 0.15 % of fat in whole cow’s milk, n = 4) was in a good agreement with the LLE Röse-Gottlieb reference method [35] for fat determination in milk (3.38 ± 0.18% of fat in whole cow’s cow milk, n = 4).
Dutch breast milk samples analyzed in this study contained a varying amount of fat (from 0.06 to 1.2 g in 10 mL of milk). A robust clean-up technique had to be optimized for sufficient lipid removal. The results from the three different clean-up methods utilized in the present study are discussed in section below.
Clean-up
Three clean-up techniques, i.e., FLF, GPC, and dialysis, were compared in order to obtain the lowest lipid carryover and highest analytes recoveries. Lipid removal was tested using 350 mg of milk fat, which corresponds to 10 mL of milk sample with average fat content of 3.5%. In order to compare the recoveries of individual clean-up techniques, standard solutions were used because the lipid presence would require additional clean-up step.
Gel permeation chromatography
A GPC column system consisting of two Envirogel columns connected in series were tested in order to establish the most efficient separation of lipids and analytes. The elution profile of fat was visualized by measuring absorbance using a UV detector (254 nm) and analyte content was determined by GC-HRMS in the individual fractions collected every 30 s (Fig. 1).
Since pyrethroids (mainly cyhalothrin, permethrins, cyfluthrin, tetramethrin) elute early, in order to obtain recoveries higher than 70%, the fraction between minute 13.8 and 25 for the assessment of the total fat removal was collected. The lipid carryover from 350 mg of the milk fat in this fraction was 15%, i.e., approximately 50 mg of fat. The recoveries of all groups of analytes are provided in Fig. 2. Additionally, Envirogel column capacity was tested. Taking into account varying fat amount in breast milk samples, we tested the lipid elution profile with 650 mg of milk lipids. Significant broadening of the lipid peak was observed, suggesting that the GPC column capacity was exceeded. Lipid carryover increased to 30–35%. However, when the lipid amount was lowered to approximately 130 mg, the lipid peak was sharp and showed a good separation from pyrethroids. The optimum milk fat amount for the satisfactory separation of fat from early eluting pyrethroids was thus determined as approximately 250 mg. More fatty milk samples would require repeated GPC runs, which would constitute a time- and solvent-consuming process.
Freezing-lipid filtration
After two freezing-lipid filtration (FLF) cycles, mean lipid carryover was 19% (n = 4), i.e., approximately 66 mg out of 350 mg fat remained in the extract. This amount of lipids was still too high to be removed by the C18/b-Al2O3 column, which is able to retain max. 20–40 mg of fat. An additional clean-up step such as GPC would be therefore necessary for total fat removal. The recoveries of most of the analytes were higher than 75% (Fig. 2), but for some compounds (BDE 153, BDE 154, BDE 183, EHTBB, BTBPE, DBDPE, s-DP, and a-DP) the recoveries were 23.5–54.6%. This could be explained by their higher partitioning lipid–acetonitrile coefficients or by an occlusion of analytes in the precipitated matrix and by adsorption in the filtration process [36].
Dialysis
In the case of dialysis, several basic parameters affect compound recovery and lipid carryover, including LDPE membrane length, dialytic solvent selection and its volume, and dialytic time and temperature. Cyclopentane, n-hexane, or an n-hexane:DCM mixture were recommended previously [29]. Since cyclopentane has limited availability and high cost and the presence of DCM in n-hexane increases lipid carryover, we chose n-hexane, which showed recoveries of over 80% for labeled PCB 52 when the solvent volume was 40 times higher than the volume inside the membrane [25]. Thus, for approximately 400 μL of the milk fat dissolved in 700 μL of n-hexane (equal to the approximate total inside volume 1 ml), we used twice 20 mL of n-hexane with one solvent exchange after 24 h. A 48-h dialysis period was shown to be sufficient for recoveries higher than 80% for all the analytes. The effect of dialysis temperature on both lipid carryover and analyte recoveries was also studied. There is clear evidence of the decrease of lipid carryover with decreasing temperature [25, 29], but information regarding recoveries under different temperatures is inconsistent. While Roszko et al. showed a significant decrease of the average recovery of indicator PCBs in the range of 20–40 °C [29], Meadows et al. reported that the changes in temperature within the range of 15–30 °C do not affect the recovery of the labeled PCB 52 [25]. Exploring recoveries of other DNTs from this study at different temperatures and taking into account a lower transfer of lipids at lower temperatures, we selected two different temperatures: 10 and 25 °C. The lipid carryover was 4–6 and 8–10% at 10 °C (n = 4) and 25 °C (n = 4), respectively. Analyte recoveries provided in Fig. 2 indicate that the majority of the compounds fall within the 70–110% interval with the use of 10 or 25 °C which confirmed that there were no significant differences in analyte recoveries, but the use of lower temperature provided lower lipid carryover compared to 25 °C.
The comparison of dialysis with GPC and FLF showed that dialysis at 10 °C provided the lowest lipid carryover and high recoveries for all the compounds within the range of 70–120%. Due to the low lipid carryover (approximately 14 mg from 10 mL of 3.5% milk up to 40 mg from 10% milk), no other clean-up step had to be used prior to column chromatography. While the duration of the dialysis (48 h) may be viewed as a disadvantage, many parallel samples can be processed at the same time. Moreover, the procedure presents no risk of cross-contamination and constitutes a cost-effective solution. An additional advantage of dialysis is the significant reduction of matrix effects during instrumental analysis compared to GPC or FLF clean-up methods. The summary of three used clean-up methods discussed here is shown in Table 2.
Instrumental method
Several GC-MS instruments with electron ionization available in our center were tested (GC-MS/MS, GC-HRMS) for the five analyzed groups of compounds. GC-HRMS showed the highest sensitivity (optimization not shown here). Since the injection volume was 2 μL in each of four instrumental methods, the final sample volume prior to instrumental analysis was 25 μL. The accuracy of quantification was sporadically influenced by increased levels of interfering compounds eluting in the analyte retention time which caused suppressions of the MS signal of pyrethroids, especially when their lower mass fragments were monitored. That could be observed as a decrease of otherwise stable lock and calibration mass signals. However, after the use of dialysis clean-up, most of the matrix interferents were suppressed, and no significant decreases of MS responses during elution of analyte peaks were recorded. The final method scheme including the clean-up steps is shown in Fig. 3.
Method performance characteristics
As a part of a validation procedure, the following parameters were evaluated. Linearity expressed as RSD of the response factors for all compounds ranged from 1 to 20%. Analysis of breast milk samples spiked at two different levels (n = 6) provided recoveries ranging between 81 and 121% for OCPs, PCBs-7, and PBDEs while recoveries of pyrethroids and NFRs were lower, 58–120 and 40–121%, respectively, which could be caused by the deficiency of the isotope-labeled analogues for some compounds. For several NFRs (p-TBX, TBECH, α-TBCO, DPMA, DPTE, HCDBCO, EHTBP), no proper internal standard used within the method was found; therefore, their results were not corrected to the losses within the sample preparation. Labeled standards for analyzed compounds are shown in Table S1 in ESM. Intra-day precisions expressed as RSDs ranged from 2 to 22% for all compounds, but for β-cyfluthrin-3, the RSD was 28% and for endosulfan-sulfate intra-day precision was 36% what can be caused by the deficiency of the isotope-labeled standard. Between-day precisions ranged from 5 to 29% for all compounds. LODs ranged from 0.001 to 0.731 ng g−1 lw for all analytes which allowed us to determine compounds with trace levels in the milk samples such as BDE 66 or BDE 154 or selected NFRs such as BATE or ATE. The expanded uncertainties for all compounds ranged from 5 to 57% for the spiking level 1 and from 11 to 48% for the spiking level 2, respectively. The highest uncertainties were for pyrethroids and NFRs, for many of which no labeled standard were available. Detailed information regarding all validation parameters can be found in Table S2 in ESM. Blank samples contained PeCB, HCB, γ-HCH, p,p′-DDE, endosulfan-sulfate, PCB 28, BDE 47, and BDE 99, but their levels were low in comparison with levels present in milk samples. Qiu et al. also observed the presence of BDE 47 and BDE 99 in the blanks and reported their contribution to 2 and 9% of the sample levels, respectively [37]. In this study, their contribution was below 1.5% of the median levels in the samples for both congeners.
Application to Dutch breast milk samples
In order to demonstrate the applicability of the method, 120 breast milk samples with various fat contents were analyzed (Table 3). p,p′-DDE was the neurotoxicant present at the highest concentrations in Dutch samples (median 49 ng g−1 lw) followed by PCB 153 (median 16.0 ng g−1 lw), PCB 180, and PCB 138 (10.7 ng g−1 lw for both congeners). These compounds were found in 100% of all samples. Levels of persistent pesticides and PCBs as well as PCB congener profile were comparable to other European countries, such as Sweden, Belgium, or Croatia with the sample collection between 2009 and 2012 [38–40]. However, the PCB levels in Dutch milk samples were approximately six to eight times lower than those collected in 2009 in the Czech Republic which belongs to one of the most PCB-polluted countries in the world [41, 42]. Similarly, the levels of HCB and ΣDDX were approximately seven and five times lower, respectively, than those in the Czech Republic with high former use of persistent pesticides [42]. Median levels of PBDEs were below 0.5 ng g−1 lw. The highest levels were observed for BDE 153 (median 0.48 ng g−1 lw) followed by BDE 47 (median 0.197 ng g−1 lw). The PBDE 47, 99, 100, and 153 congeners were detected in all samples. A similar congener profile and comparable levels were found in Belgian breast milk samples from 2006 (median 0.29 ng g−1 lw for BDE 153, 0.16 ng g−1 lw for BDE 47) [39], whereas PBDE levels were approximately six times lower in the Netherlands compared to Great Britain with high PBDE concentrations in Europe [43].
Median levels of non-persistent neurotoxicants measured in this study were lower than 0.43 ng g−1 lw for pyrethroids and below 0.13 ng g−1 lw for NFRs. The detection frequency of pyrethroids in Dutch breast milk samples was very low. Generally, only cis- and trans-permethrins (median 0.127 ng g−1 lw) and pyrethroid synergist piperonyl butoxide (0.26 ng g−1 lw), which suggests previous exposure to pyrethroids, were found in over 80% of the samples. Unlike in the Netherlands, levels of all determined pyrethroids in human milk from Spain (tetramethrin, bifenthrin, cyhalothrin, deltamethrin, tralomethrin, fenvalerate, permethrin, and cypermethrin) were detected in more than 67% of all samples [11]. The median level of Σpermethrins which were present at highest concentrations in Spain was approximately 20 times lower than in our study. Other pyrethroid levels in Spain and the Netherlands were comparable. From among NFRs, p-TBX, PBBZ, ATE, EHTBB, and TBECH were detected in over 50% of the Dutch samples. To the best of our knowledge, no reference data regarding the levels of these compounds in breast milk in Europe has been published to date.
Conclusions
A novel method for the determination of 78 organic pollutants such as organochlorine pesticides, polychlorinated biphenyls, polybrominated diphenyl ethers, pyrethroids, and novel flame retardants with proven or potential developmental neurotoxicity in human milk (8–10 ml) was developed and validated. The advantage of the method is the determination of a whole mixture of persistent and non-persistent compounds together including fat determination. Two clean-up steps comprising dialysis and column chromatography with C18/basic-Al2O3 were used. Dialysis, newly used at 10 °C, showed the removal of more than 94–96% of interfering milk lipids and high analyte recoveries (65–121% for most of the analytes). The thorough lipid removal from milk samples with varying fat content (from 0.6 to 12%) allowed us to minimize the final sample volume to 25 μL and thus lower the LODs to sub-ng g−1 lipid weight and detect the compounds with trace levels in human milk such as pyrethroids or novel flame retardants. The dialysis procedure used in the current study was applied for the first time to an analysis of a complex mixture of the DNT compounds in a milk extract and showed several advantages over the traditionally used GPC method such as cost-effectiveness, low amount of (non-chlorinated) solvent, and a significant reduction of interferences influencing on GC-HRMS measurement.
The applicability of the method was verified using 120 Dutch milk samples with various lipid contents. Of the 78 analytes, 35 compounds were present in over 60% of the samples. The results from this and following biomonitoring studies using samples obtained from other European countries will provide useful data regarding levels of selected current use pesticides or flame retardants in human matrices which are still scarce in the literature and will be additionally used for an epidemiological evaluation of the influence of presence of these compounds and their mixtures in mother’s body to child health.
References
Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2016;13:330–8. doi:10.1016/S1474-4422(13)70278-3.
Schantz SL. Developmental neurotoxicity of PCBs in humans: what do we know and where do we go from here? Neurotoxicol Teratol. 1996;18:217–27. doi:10.1016/S0892-0362(96)90001-X.
Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol. 2010;171:593–601. doi:10.1093/aje/kwp427.
Viel J-F, Warembourg C, Le Maner-Idrissi G, Lacroix A, Limon G, Rouget F, et al. Pyrethroid insecticide exposure and cognitive developmental disabilities in children: the PELAGIE mother–child cohort. Environ Int. 2015;82:69–75. doi:10.1016/j.envint.2015.05.009.
Lee I, Eriksson P, Fredriksson A, Buratovic S, Viberg H. Developmental neurotoxic effects of two pesticides: behavior and biomolecular studies on chlorpyrifos and carbaryl. Toxicol Appl Pharmacol. 2015;288:429–38. doi:10.1016/j.taap.2015.08.014.
Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–67. doi:10.1016/j.neuro.2007.08.007.
EPA U. An alternatives assessment for the flame retardant decabromodiphenyl ether (decaBDE). 2014.
Covaci A, Harrad S, Abdallah MA-E, Ali N, Law RJ, Herzke D, et al. Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Environ Int. 2011;37:532–56. doi:10.1016/j.envint.2010.11.007.
Jeong I-S, Kwak B-M, Ahn J-H, Jeong S-H. Determination of pesticide residues in milk using a QuEChERS-based method developed by response surface methodology. Food Chem. 2012;133:473–81. doi:10.1016/j.foodchem.2012.01.004.
Luzardo Ruiz-Suárez N, Almeida-González M, Henríquez-Hernández LA, Zumbado M, Boada LDOP. Multi-residue method for the determination of 57 persistent organic pollutants in human milk and colostrum using a QuEChERS-based extraction procedure. Anal Bioanal Chem. 2013;405:9523–36. doi:10.1007/s00216-013-7377-0.
Corcellas C, Feo ML, Torres JP, Malm O, Ocampo-Duque W, Eljarrat EBD. Pyrethroids in human breast milk: occurrence and nursing daily intake estimation. Environ Int. 2012;47:17–22.
Sørensen LK. Determination of phthalates in milk and milk products by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:1135–43.
Saito K, Sjödin A, Sandau CD, Davis MD, Nakazawa H, Matsuki Y, et al. Development of a accelerated solvent extraction and gel permeation chromatography analytical method for measuring persistent organohalogen compounds in adipose and organ tissue analysis. Chemosphere. 2004;57:373–81. doi:10.1016/j.chemosphere.2004.04.050.
Morlock G, Schwack W. Determination of isopropylthioxanthone (ITX) in milk, yoghurt and fat by HPTLC-FLD, HPTLC-ESI/MS and HPTLC-DART/MS. J Anal Bioanal Chem. 2006;385:1618–2642.
Focant J-F, Sjödin A, Turner WE, Patterson DG. Measurement of selected polybrominated diphenyl ethers, polybrominated and polychlorinated biphenyls, and organochlorine pesticides in human serum and milk using comprehensive two-dimensional gas chromatography isotope dilution time-of-flight mass spectro. Anal Chem. 2004;76:6313–20. doi:10.1021/ac048959i.
Jaraczewska K, Lulek J, Covaci A, Voorspoels S, Kaluba-Skotarczak A, Drews K, et al. Distribution of polychlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers in human umbilical cord serum, maternal serum and milk from Wielkopolska region, Poland. Sci Total Environ. 2006;372:20–31. doi:10.1016/j.scitotenv.2006.03.030.
Tsydenova OV, Sudaryanto A, Kajiwara N, Kunisue T, Batoev VB, Tanabe S. Organohalogen compounds in human breast milk from Republic of Buryatia, Russia. Environ Pollut. 2007;146:225–32. doi:10.1016/j.envpol.2006.04.036.
Liu X, Zhao A, Zhang A, Liu H, Xiao W, Wang C, et al. Dispersive liquid-liquid microextraction and gas chromatography-mass spectrometry determination of polychlorinated biphenyls and polybrominated diphenyl ethers in milk. J Sep Sci. 2011;34:1084–90. doi:10.1002/jssc.201000767.
Inoue K, Harada K, Takenaka K, Inoue K, Harada K, Takenaka K, Uehara S, Kono M, Shimizu T, Koizumi A. Levels and concentration ratios of polychlorinated biphenyls and polybrominated diphenyl ethers in serum and breast milk in Japanese mothers. 2006;114(8). doi:10.1289/ehp.9032. Environ Heal Perspect 114:1179–1185.
Chen X, Panuwet P, Hunter RE, Riederer AM, Bernoudy GC, Barr DB, et al. Method for the quantification of current use and persistent pesticides in cow milk, human milk and baby formula using gas chromatography tandem mass spectrometry. J Chromatogr B. 2014;970:121–30. doi:10.1016/j.jchromb.2014.08.018.
Hayward DG, Pisano TS, Wong JW, Scudder RJ. Multiresidue method for pesticides and Persistent Organic Pollutants (POPs) in milk and cream using comprehensive two-dimensional capillary gas chromatography−time-of-flight mass spectrometry. J Agric Food Chem. 2010;58:5248–56. doi:10.1021/jf100021p.
Zheng G, Han C, Liu Y, Wang J, Zhu M, Wang C, et al. Multiresidue analysis of 30 organochlorine pesticides in milk and milk powder by gel permeation chromatography-solid phase extraction-gas chromatography-tandem mass spectrometry. J Dairy Sci. 2014;97:6016–26. doi:10.3168/jds.2014-8192.
Ahn YG, Shin JH, Kim H-Y, Khim J, Lee M-K, Hong J. Application of solid-phase extraction coupled with freezing-lipid filtration clean-up for the determination of endocrine-disrupting phenols in fish. Anal Chim Acta. 2007;603:67–75. doi:10.1016/j.aca.2007.09.045.
Hong J, Kim H-Y, Kim D-G, Seo J, Kim K-J. Rapid determination of chlorinated pesticides in fish by freezing-lipid filtration, solid-phase extraction and gas chromatography–mass spectrometry. J Chromatogr A. 2004;1038:27–35. doi:10.1016/j.chroma.2004.03.003.
Meadows J, Tillitt D, Huckins J, Schroeder D. Large-scale dialysis of sample lipids using a semipermeable membrane device. Chemosphere. 1993;26:1993–2006. doi:10.1016/0045-6535(93)90026-2.
Strandberg B, Bergqvist PA, Rappe C. Dialysis with semipermeable membranes as an efficient lipid removal method in the analysis of bioaccumulative chemicals. Anal Chem. 1998;70:526–33.
Rantalainen A-L, Crewe NF, Ikonomou AL. Comparison of three techniques for lipid removal from seal blubber: gel permeation, acid treatment, and dialysis with semipermeable membrane. Int J Environ Anal Chem. 2000;76:31–47.
Surma-Zadora M, Grochowalski A. Using a membrane technique (SPM) for high fat food sample preparation in the determination of chlorinated persistent organic pollutants by a GC/ECD method. Food Chem. 2008;111:230–5. doi:10.1016/j.foodchem.2008.03.053.
Roszko M, Rzepkowska M, Szterk A, Szymczyk K, Jędrzejczak R, Bryła M. Application of semi-permeable membrane dialysis/ion trap mass spectrometry technique to determine polybrominated diphenyl ethers and polychlorinated biphenyls in milk fat. Anal Chim Acta. 2012;748:9–19. doi:10.1016/j.aca.2012.08.037.
Bjerregaard-Olesen C, Hjelmborg PS, Bonefeld-Jørgensen EC. Isolation of lipophilic persistent organic pollutants from human breast milk. Anal Lett. 2012;45:1412–25. doi:10.1080/00032719.2012.675488.
de Cock M, Quaak I, Sugeng EJ, Legler J, van de Bor M. LInking EDCs in maternal Nutrition to Child health (LINC study)—protocol for prospective cohort to study early life exposure to environmental chemicals and child health. BMC Public Health. 2016;16:147. doi:10.1186/s12889-016-2820-8.
Esteve-Turrillas FA, Pastor A, de la Guardia M. Determination of pyrethroid insecticide residues in vegetable oils by using combined solid-phases extraction and tandem mass spectrometry detection. Anal Chim Acta. 2005;553:50–7. doi:10.1016/j.aca.2005.08.004.
European Commission L 221 (17. 08. 02). Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Commun. 2002.
Richardson RK, Colin GH. Determination of fat in dried milk products using accelerated solvent extraction (ASE). Dionex Appl Note. 1998;340:1–5.
Standard I. Milk-determination of fat content (reference method) Brussel: International Dairy Federation. 1996.
Walters SM. Clean-up techniques for pesticides in fatty foods. Anal Chim Acta. 1990;236:77–82. doi:10.1016/S0003-2670(00)83301-2.
Qiu X, Bigsby RM, Hites RA. Hydroxylated metabolites of polybrominated diphenyl ethers in human blood samples from the United States. Environ Health Perspect. 2009;117:93–8. doi:10.1289/ehp.11660.
Klinčić D, Herceg Romanić S, Matek Sarić M, Grzunov J, Dukić B. Polychlorinated biphenyls and organochlorine pesticides in human milk samples from two regions in Croatia. Environ Toxicol Pharmacol. 2014;37:543–52. doi:10.1016/j.etap.2014.01.009.
Croes K, Colles A, Koppen G, Govarts E, Bruckers L, Van de Mieroop E, et al. Persistent organic pollutants (POPs) in human milk: a biomonitoring study in rural areas of Flanders (Belgium). Chemosphere. 2012;89:988–94. doi:10.1016/j.chemosphere.2012.06.058.
Lignell S, Aune MAG, et al. Levels of persistent halogenated organic pollutants (POP) in mother’s milk from first-time mothers in Uppsala, Sweden: results from year 2012 and temporal trends for the time period 1996–2012. 2014.
WHO/UNEP human milk survey. Human exposure to POPs across the Globe: POPs levels and human health implications. 2013.
Mikeš O, Čupr P, Kohút L, Krsková A, Černá M. Fifteen years of monitoring of POPs in the breast milk, Czech Republic, 1994–2009: trends and factors. Environ Sci Pollut Res. 2012;19:1936–43. doi:10.1007/s11356-012-0798-z.
Bramwell L, Fernandes A, Rose M, Harrad S, Pless-Mulloli T. PBDEs and PBBs in human serum and breast milk from cohabiting UK couples. Chemosphere. 2014;116:67–74. doi:10.1016/j.chemosphere.2014.03.060.
Acknowledgments
Jakub Martiník and Marcela Kadlecová are acknowledged for their help during sample preparation. The research was supported by the Seventh Framework Programme of EU, Grant Agreement No. 282957: Developmental Neurotoxicity Assessment of Mixtures in Children (DENAMIC), by the “Employment of Best Young Scientists for International Cooperation Empowerment” (CZ.1.07/2.3.00/30.0037), co-financed by the European Social Fund, the state budget of the Czech Republic, and by the Czech Ministry of Education, Youth and Sports (LO1214 and LM 2015051).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics committee of the Vrije Universiteit Medical Center Amsterdam and has been performed in accordance with the ethical standards. Written informed consent was obtained from each individual participant.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 544 kb)
Rights and permissions
About this article
Cite this article
Čechová, E., Seifertová, M., Kukučka, P. et al. An effective clean-up technique for GC/EI-HRMS determination of developmental neurotoxicants in human breast milk. Anal Bioanal Chem 409, 1311–1322 (2017). https://doi.org/10.1007/s00216-016-0059-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-0059-y