Abstract
A sensitive and selective method for separating fluoroquinolones (FQs) from bovine milk samples was successfully developed using montmorillonite magnetic molecularly imprinted polymers (MMMIPs) as adsorbents. MMMIPs were prepared using montmorillonite as carrier, fleroxacin (FLE) as template molecule, and Fe3O4 magnetite as magnetic component. MMMIPs possessed high adsorption capacity of 46.3 mg g−1 for FLE. A rapid and convenient magnetic solid-phase extraction procedure coupled with capillary electrophoresis was established with MMMIPs as adsorbents for simultaneous and selective extraction of four FQs in bovine milk samples. Limits of detection ranged between 12.9 and 18.8 μg L−1, and the RSDs were between 1.8 % and 8.6 %. The proposed method was successfully applied to spike bovine milk samples with recoveries of 92.7 %–108.6 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoroquinolones (FQs) are important antibiotics with broad-spectrum activities against pathogenic Gram-negative and Gram-positive bacteria by inhibiting DNA synthesis in bacteria [1, 2]. FQs are normally used in human and veterinary medicine to treat infectious diseases. However, the extensive use of FQs in food-producing animals has resulted in considerable concern because antibiotic residues in humans may exert possible toxic effects and allergic reactions [1]. Numerous countries have established tolerance levels and maximum residue limits of food products obtained from animals that are commonly treated with FQs to ensure safe human consumption [1, 3–5]. To protect consumer health, the maximum residue levels (MRLs) in milk have been strictly regulated by the European Union (EU) [3]. The MRLs for veterinary drug residues in milk were set at 30–100 μg kg−1 for four FQs regulated in bovine milk. These low values demand the development of analytical methods that are sensitive enough to monitor and determine these drugs in bovine milk. Thus, developing a sensitive and selective analytical method to monitor FQ residue levels and comply with legislative requirements is necessary.
The majority of widely applied analytical methods for monitoring FQ residues are based on high-performance liquid chromatography mainly coupled with different detection techniques, such as UV spectroscopy [6], fluorescence [7–9], diode array detection [10], and mass spectrometry (MS) [11–13]. MS is highly sensitive and selective, thereby ensuring proper identification of compounds in samples. However, the compatibility of MS should be considered when coupled with other separation techniques. In addition, not all laboratories can implement this technique because MS is fairly expensive to perform.
Capillary electrophoresis (CE) has been increasingly applied for analyzing FQ residues because it offers many advantages over other techniques, such as fast separation, high separation efficiency, and low sample and reagent consumption [14–17]. However, this method has limited sensitivity when UV detectors are used despite the feasibility of obtaining excellent separation efficiency. Thus, several in- or off-line preconcentration steps should be employed to assay trace-level FQs in complex samples. Liquid–liquid extraction [18, 19], solid-phase extraction (SPE) [9, 20–22], solid-phase micro-extraction [23–25], and matrix solid-phase dispersion extraction [26, 27] are commonly employed for sample pretreatment. However, most of these methods show low selectivity, so a more effective pretreatment method should be explored.
Molecularly imprinted polymers (MIPs) are artificial materials with highly selective binding capacity and molecular recognition ability toward the template. Magnetic MIPs (MMIPs), which are obtained by embedding ferroferric oxide nanoparticles inside MIPs, have recently attracted much interest because of their beneficial characteristics, such as excellent magnetic response, high dispersibility, relatively large surface area, fast and effective binding of targets, easy surface medication, and reversible and controllable flocculation. MMIPs have been widely used as SPE sorbents [magnetic SPE (MSPE)] in environmental and food sample pretreatment [28–32], which is performed in biological processes, such as bioseparation, drug delivery, and biomolecular sensing [33–37]. Contrary to traditional SPE, MSPE sorbent does not need to be packed into an SPE cartridge and is easily separated from the matrix when an external magnetic field is applied. Therefore, MSPE with magnetic separation provides a simple, convenient, and highly efficient sample pretreatment protocol.
Montmorillonite (MMT) is a natural layered silicate, which is formed by the intermolecular forces between each layer. It is composed of silicon-oxygen tetrahedron and alumina-oxygen octahedron according to the ratio of 2:1, and connected by a common oxygen atom. Al atom is located in the center of the aluminum oxygen octahedron. Silicon-oxygen tetrahedron is arranged in a hexagonal mesh and extends indefinitely, so that MMT is highly ordered [38]. The above-mentioned structural characteristics makes it possess unique physical and chemical properties, such as low cost, non-toxicity, lamellar structure, large surface area, high sorption capacity, high cation-exchange capacity, swelling ability, and so on. This has provided a broad space for the structural modification, functional design, and industrial utilization of MMT. MMT has been applied as an effective sorbent in medicinal and environmental applications [39–43].
However, MMT, as an SPE stationary phase, also has some drawbacks, such as long process of column packing, high back pressure, and low flow rate. Hence, MMT powder and magnetic nanoparticles can be used to prepare magnetic MMT nanoparticles (MMNPs). MMNPs, as a SPE sorbent, can easily extract and magnetically separate the target analyte [44–46].
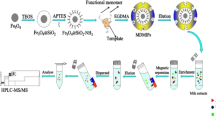
Various approaches have also been recently applied for preparing FQ-imprinted polymers [7, 47–50]. Sun et al. [7] prepared MIPs by adopting a dummy template for FQs to solve the leakage of the residual template. However, few studies have reported the determination of FQs in milk samples based on MMT MMIPs (MMMIPs) and CE. Thus, a rapid and selective method was developed in this study to improve and simplify the determination of FQs in milk samples. MMMIPs were synthesized using MMT as carrier, fleroxacin (FLE) as template molecule, and Fe3O4 magnetite as magnetic component. The characteristics and binding properties of the MMMIPs were investigated. A rapid and selective MSPE procedure with MMMIPs as adsorbents was established, and simultaneous detection of multiple FQs in bovine milk samples was performed.
Materials and methods
Chemicals and reagents
Fleroxacin (FLE), gatifloxacin (GAT), lomefloxacin (LOM), and norfloxacin (NOR) were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). 4-Vinylpyridine (4-VP), ethylene glycol dimethacrylate (EDMA), and azaodiisobutyronitrile (AIBN) were purchased from Aladdin Chemical (Shanghai, China). Ammonium iron sulfate hexahydrate, ammonium ferrous sulfate tetrahydrate, and MMT (ϕ5 μm) were supplied by Xilong Chemical (Shantou, China). Ultrapure water was used throughout the experiment. All solutions for CE analysis were passed through a 0.45 mm nylon filter before use, degassed by sonication, and freshly prepared each day.
Instrumentation
A P/ACE TM MDQ capillary electrophoresis system (Beckman Coulter, Brea, CA, USA) equipped with a UV detector was applied for CE experiments. The pH values were measured using a PHS-3CT pH meter (Dapu Instrumentation, Shanghai, China).
Preparation of MMMIPs
Preparation of MMNPs
Ferric ammonium sulfate hexahydrate (15.4 g), MMT (2.1 g), and ultrapure water (180 g) were placed in a three-necked flask, ultrasonicated for 15 min, stirred at 300 rpm, and stored at 30 °C for 3 h. A stable liquid suspension was subsequently obtained. Ammonium ferrous sulfate tetrahydrate (3.54 g) was dissolved into the liquid suspension by stirring at 600 rpm and 80 °C under a nitrogen atmosphere. Ammonium hydroxide was then added dropwise to the mixture with vigorous stirring to adjust the pH to 9. The reaction was allowed to proceed for 30 min, after which magnetic separation was performed. The MMNPs were washed with ultrapure water until they were neutralized and dried in an oven at 35 °C.
Preparation of MMMIPs
FLE (0.4 g), 4-VP (6 mmol), and EDMA (2.26 mL) were added to 30.0 mL of a mixed solution of methanol and chloroform (1:1, v/v) under ultrasonic stirring for 60 min. In a separate vessel, 10.0 mL of methanol was mixed with 1.5 g of MMNPs. The two solutions were then mixed, and 0.04 g of AIBN was added to the resultant solution under a nitrogen atmosphere over a period of 5 min. The mixture was then sealed and allowed to react at 60 °C for 12 h. The product was filtered, washed successively with distilled water and ethanol for several times, and dried in vacuum.
After polymerization, MMMIPs were acquired by magnetic separation and washed several times with methanol/acetic acid (8:2, v/v) under vigorous shaking to completely remove FLE. Finally, MMMIPs were rinsed with methanol to remove remaining acetic acid and then dried under vacuum at 40 °C for 12 h.
Magnetic MMT non-imprinted polymers (MMNIPs) were synthesized following the same procedures described above but without the template molecule FLE.
Rebinding experiment
The adsorption capacity of MMMIPs or MMNIPs was measured by equilibrating 20 mg of MMMIPs or MMNIPs with 2.0 mL of various concentrations of FLE standard solutions (0.1–4.0 mg/mL in CAN), respectively. The samples were oscillated in an incubator at room temperature for 24 h. The MMMIPs or MMNIPs were magnetically separated from the solution, and the remaining FLE in the solution was analyzed by CE. The binding test was performed in duplicate, and the mean value was used.
The adsorption capacity (Q, mol/L) was calculated as follows:
where C 0 and C denote the initial and equilibrium concentrations of FLE, respectively; V (L) is the volume of the solution; M is the molecular weight of FLE; and W is the mass of polymers (g).
Selective adsorption
Four FQs (FLE, GAT, NOR, and LOM) were selected to evaluate the selectivity of MMMIPs. MMMIPs (20 mg) were incubated with 1 mL of each analyte solution in ACN at 2 mg/mL at room temperature for 24 h. The supernatant was magnetically separated from polymers and quantified by CE analysis. Selective adsorption was executed in duplicate, and the mean value was obtained.
Electrophoretic procedure
Separation was performed in a bare fused-silica capillary of 70 cm effective length × 50 μm i.d. (375 μm o.d.) from Polymicro Technologies (Phoenix, AZ, USA). A negative voltage of 15 kV was applied at 25 °C. At the beginning of each day, the capillary was continuously rinsed with 0.1 M NaOH (10 min), ultrapure water (10 min), and running buffer solution (10 min). The capillary was flushed with buffer solution for 5 min between runs to maintain reproducibility. The UV detection wavelength was set to 280 nm. The hydrodynamic injection mode was applied at 0.8 psi pressure for 5 s, and the buffer solution was 50 mmol L−1. The mixture of STEDE and phosphoric acid yielded a pH of 9.0.
Sample preparation
The sample was measured (5.0 mL) and placed in a centrifuge tube with a plug. Acetone (5.0 mL) was added to the samples to precipitate proteins, and the mixture solution was centrifuged at 10,000 rpm for 10 min using a vortex mixer. The supernatants were collected, and the residues were centrifuged again. All extract solutions were merged, filtered through a 0.22 μm membrane filter, and stored in a refrigerator for further use.
Results and discussion
Preparation of MMMIPs
In this work, MMT was selected as the initial raw material for synthetic MMMIP. The preparation procedure of MMMIP is illustrated in Scheme 1.
Fe3O4@MMT was prepared via wet impregnation process followed by co-precipitation process [51]. At the beginning, Fe2+ and Fe3+ ions were introduced onto the surface of the MMT through complexation reactions. After that, Fe2+ and Fe3+ ions were transformed to Fe3O4 and immobilized on the surface of MMT under alkaline conditions.
MMMIPs were grafted onto the surface of Fe3O4@MMT by surface molecularly imprinted technology using FLE as the template molecule, 4-VP as the functional monomer, EDGMA as the cross-linker, and AIBN as the initiator. In order to obtain high adsorption capacity and selective recognition of MMMIPs, some acritical factors such as functional monomer (MAA and 4-VP), porogenic solvent (ethanol, methanol, and chloroform), and the molar ratio of template:functional monomer:cross-linker (1:6:10, 1:6:20, and 1:6:30) were chosen to optimize the condition during the preparation procedure.
The experiment results indicated that the highest adsorption capacity and molecular recognition ability of the polymer could be achieved under the following conditions: 4-VP as functional monomer, methanol-chloroform (1:1, v/v) as porogenic solvent, and the molar ratio of template:functional monomer:cross-linker at 1:6:20.
Characterization of MMMIPs
The micromorphologic structures and sizes of MMMIPs and MMNIPs were investigated by SEM as shown in Fig. 1. MMMIPs and MMNIPs exhibited uniform shapes with diameters in the range of 0.2–0.8 μm. The imprinted film was well distributed on the surface of Fe3O4 nanoparticles.
The FT-IR spectra of MMT, Fe3O4@MMT, MMMIP, and MMNIP are shown in Fig. 2. For MMT, the broad bands at 3633 and 3435 cm−1 were assigned to the O–H stretching vibration of Si–OH and H–OH vibration of the retained water molecules, respectively [52]. The peak at 1639 cm−1 was the bending vibration of O–H bonds of the retained water molecules. The strong absorption peak at 1035 cm−1 corresponded to the Si–O–Si groups. For Fe3O4@MMT, the peak at 3406 cm−1 was due to the O–H stretching vibration from Fe3O4 [53]. The strong absorption peak at 588 cm−1 derived from the Fe–O stretching vibration.
For MMMIP and MMNIP, the strong absorption peak at 588 cm−1 was characteristic of the Fe–O stretching vibration, and 1054 cm−1 was assigned to the stretching vibration of Si–O [54, 55]. The peak at 3425 cm−1 was the stretching vibration of O–H bonds, and the peak at 2950 cm−1 was the stretching vibration of C–H bonds. The strong absorption peak at 1730 cm−1 corresponded to the stretching vibration of C = O bonds. These peaks indicated that Fe3O4 successfully bonded to the polymer.
Adsorption property of MMMIPs
The adsorption abilities of MMMIPs and MMNIPs on FLE were investigated. The adsorption performance of MMMIPs and MMNIPs was investigated in standard FLE solutions of different concentrations. The resulting isothermal adsorption diagrams are plotted in Fig. 3a.
The adsorption capacities of MMMIPs and MMNIPs increased with increasing standard solution concentration. The adsorption capacity reached saturation at 2.0 mmol/L solution, at which the adsorption curves were mild. The adsorption capacity of MMMIPs in the substrate was far greater than that of MMNIPs. Specifically, the maximum adsorption capacity of MMMIPs was 43.1 mg/g, whereas that of MMNIPs was 22.6 mg/g. The high adsorption capacity is due to more active binding sites on the internal and external layers of MMMIPs being available [38, 45, 56, 57].
The resulting isothermal adsorption diagrams are plotted in Fig. 3b. The isothermal adsorption results indicated that the absorption capacities of MMMIPs and MMNIPs increased with increasing adsorption time at the beginning of adsorption, and the adsorption capacity of MMMIPs was much higher than that of MMNIPs. The adsorption capacities reached maximum values with an adsorption time of 30 min and then plateaued with increasing time. Therefore, the time for selective adsorption was set to 30 min in subsequent experiments.
Selective adsorption
Analogs of GAT, NOR, and LOM were selected to investigate the adsorption property of MMMIPs. The results of selective adsorption tests are listed in Table 1. MMMIPs exhibited high binding amounts with the four FQs. GAT, NOR, and LOM and matched the imprinting cavities very well because of their highly similar functional groups and molecular structure with FLE. The adsorption capacity of MMMIPs in the substrate was significantly greater than those of the analogs with selectivity coefficients (K) of 1.9, 1.8, and 1.5. The K values of MMNIPs in the template molecule were 1.1, 1.2, and 1.1, which were smaller than those of MMMIPs.
Optimization of MSPE conditions
The influencing factors of extraction efficiency of MSPE parameters, namely, sample pH, mass of adsorbent, extraction time, elution solvents, and desorption time, were investigated and optimized. When one influencing factor was changed, the other factors were fixed at optimized values.
Adsorbent mass
The mass of the adsorbent required to achieve high extraction efficiency was determined. Different masses of MMMIPs ranging from 10 mg to 50 mg were used to extract FQs from 2 mL of milk sample. The peak areas of four FQs increased rapidly with increasing MIP mass and then became almost invariant above 30 mg of MMMIPs (Fig. 4a). Thus, 30 mg of MMMIPs was employed in further studies.
Sample pH
The effect of pH was determined at different pH values (3.0–9.0) of 2 mL of milk samples. The results are presented in Fig. 4b, which showed that the extraction progress was obviously affected by pH. The highest peak areas of four FQs were obtained at pH 6.0. The pH values were between pK a1 and pK a2 of FQs, ranging from 5.5 to 6.3 and from 9.1 to 9.3, respectively [58]. FQs were in cationic forms at below pK a1, but they were in anionic form at above pK a2. FQs in intermediate forms could be extracted by MMMIPs. Thus, pH 6.0 was the optimal pH for further experiments.
Extraction time
The effect of extraction time on peak areas was investigated. Adsorption equilibrium was reached after 20 min (Fig. 4c). Therefore, 20 min was chosen as the suitable extraction time.
Elution solvents
The effect of the ratio of MeOH and 50 mM KH2PO4 (pH 6) as eluting solvent on the extraction efficiencies of FQs was evaluated. The highest peak areas of FQs were obtained at 80 % MeOH (Fig. 4d). Therefore, 80 % MeOH was selected as the eluting solvent in subsequent experiments.
Desorption time
To optimize the desorption time, different time intervals (5–50 min) were evaluated. The results revealed that 20 min was sufficient to accomplish desorption (Fig. 4e). Therefore, 20 min was set as the optimal desorption time in this work.
Optimization of CE conditions
The running buffer significantly affects the separation of FQs. The effect of pH ranging from 8.0 to 9.5 of 12 mM Na2B4O7–38 mM NaH2PO4 on the separation performance was investigated according to a previous study [59]. Enhanced baseline separation was observed at pH 9.0. Thus, 12 mM Na2B4O7–38 mM NaH2PO4 at pH 9.0 was selected as the running buffer for separation.
The effect of different applied voltages (10–20 kV) was compared. Higher values of separation voltage resulted in faster migration rate of ions, shorter separation time, and lower resolution. The optimal separation performance was obtained when the voltage was 15 kV. Therefore, a separation voltage of 15 kV was used in subsequent experiments.
Analytical performance
A series of concentrations of FQ standard solution was prepared and separated under optimal electrophoresis conditions in the section “Optimization of MSPE conditions,” and relevant peak areas were acquired. A linear relationship was determined using concentration and corresponding peak area as horizontal and vertical coordinates, respectively. The parameters of this relationship are provided in Table 2. The correlation coefficients (R 2) of all four FQs exceeded 0.9956, which suggested a favorable linear relationship between concentration and peak area. The limits of detection (LODs) were 12.9–18.8 μg/L (S/N = 3), and the limits of quantitation (LOQs) were 42.8–61.9 μg/L (S/N = 10). The four FQs exhibited stable migration times and peak areas, with RSD values ranging from 1.8 % to 8.6 % and recovery rates from 92.7 % to 108.6 %. These results reflected the favorable accuracy and high precision of the established method.
Real sample analysis
Commercially available bovine milk samples were subjected to FQ detection. The detection results are listed in Table 3. The proposed method was successfully applied to measure bovine milk samples under optimized conditions, with recoveries of 92.7 %–108.6 % and RSDs of 1.8 %–8.6 %. FQ residues were not detected in these samples.
Comparison of MMMIPS with other methods
The proposed technique and other related methods for measuring FQs in bovine milk samples were compared, and the results are presented in Table 4. The LODs of this technique were better than on-line column-switching HPLC coupled with an FLD detector. The results indicated that MMMIPs had highly selective enrichment capabilities for analyzing the four FQs in complex samples. Moreover, MMIP preparation is simple and easy to perform to avoid potential environmental threat.
Conclusion
A sensitive and selective method was successfully developed for extracting and determining four FQs from bovine milk samples using novel MMMIPs as adsorbents for MSPE. The MMMIPs were characterized in detail. The LODs were found to be 12.9–18.8 μg L−1, and the LOQs were 42.8–61.9 μg L−1 using MSPE coupled with CE analysis. Recoveries were 92.7 %–108.6 % with RSD values ranging from 1.8 % to 8.6 %. These results reflected the favorable accuracy and high precision of the established method. The proposed MSPE-HPCE method was successfully applied to detect FQs in bovine milk samples.
References
Andreu V, Blasco C, Picó Y (2007) Analytical strategies to determine quinolone residues in food and the environment. Trends Anal Chem 26:534–556
Brown SA (1996) Fluoroquinolones in animal health. J Vet Pharmacol Ther 19:1–14
Commission of the European Communities (2010) Off J Eur Commun L15:1–72
US Food and Drug Administration (FDA) (2006) Code of Federal regulation. Food and Drugs 6, Part 556
Pulgarín JAM, Molina AA, Muñoz SR (2011) Rapid chemiluminescent determination of enrofloxacin in eggs and veterinary drugs. Anal Lett 44:2194–2208
Chen B, Wang W, Huang Y (2012) Cigarette filters as adsorbents of solid-phase extraction for determination of fluoroquinolone antibiotics in environmental water samples coupled with high-performance liquid chromatography. Talanta 88:237–243
Sun X, Wang J, Li Y, Yang J, Jin J, Shah SM, Chen J (2014) Novel dummy molecularly imprinted polymers for matrix solid-phase dispersion extraction of eight fluoroquinolones from fish samples. J Chromatogr A 1359:1–7
Parrilla Vázquez MM, Parrilla Vázquez P, Martínez Galera M, Gil García MD (2012) Determination of eight fluoroquinolones in groundwater samples with ultrasound- assisted ionic liquid dispersive liquid–liquid microextraction prior to high-performance liquid chromatography and fluorescence detection. Anal Chim Acta 748:20–27
Rodríguez E, Navarro-Villoslada F, Benito-Peña E, Marazuela MD, Moreno-Bondi MC (2011) Multiresidue determination of ultratrace levels of fluoroquinolone antimicrobials in drinking and aquaculture water samples by automated online molecularly imprinted solid phase extraction and liquid chromatography. Anal Chem 83:2046–2055
Huang X, Wang Y, Liu Y, Yuan D (2013) Preparation of magnetic poly(vinylimidazole-co-divinylbenzene) nanoparticles and their application in the trace analysis of fluoroquinolones in environmental water samples. J Sep Sci 36:3210–3219
Aguilera-Luiz M, Vidal JLM, Romero-González R, Frenich AG (2008) Multi-residue determination of veterinary drugs in milk by ultra-high-pressure liquid chromatography-tandem mass spectrometry. J Chromatogr A 1205:10–16
Blasco C, Picó Y (2012) Development of an improved method for trace analysis of quinolones in eggs of laying hens and wildlife species using molecularly imprinted polymers. J Agr Food Chem 60:11005–11014
Bourdat-Deschamps M, Leang S, Bernet N, Daudin JJ, Nelieu S (2014) Multiresidue analysis of pharmaceuticals in aqueous environmental samples by online solid-phase extraction-ultra-high-performance liquid chromatography-tandem mass spectrometry: Optimization and matrix effects reduction by quick, easy, cheap, effective, rugged, and safe extraction. J Chromatogr A 1349:11–23
Alothman ZA, Dawod M, Kim J, Chung DS (2012) Single-drop microextraction as a powerful pretreatment tool for capillary electrophoresis: a review. Anal Chim Acta 739:14–24
Lara FJ, Garcia-Campana AM, Ales-Barrero F, Bosque-Sendra JM, Garcia-Ayuso LE (2006) Multiresidue method for the determination of quinolone antibiotics in bovine raw milk by capillary electrophoresis-tandem mass spectrometry. Anal Chem 78:7665–7673
Piñero MY, Garrido-Delgado R, Bauza R, Arce L, Valcarcel M (2012) Easy sample treatment for the determination of enrofloxacin and ciprofloxacin residues in raw bovine milk by capillary electrophoresis. Electrophoresis 33:2978–2986
Morales-Cid G, Cárdenas S, Simonet BM, Valcárcel M (2009) Fully automatic sample treatment by integration of microextraction by packed sorbents into commercial capillary electrophoresis-mass spectrometry equipment: application to the determination of fluoroquinolones in urine. Anal Chem 81:3188–3193
Tang C, Tan J, Wang C, Peng X (2014) Determination of perfluoro-octanoic acid and perfluoro-octane sulfonate in cooking oil and pig adipose tissue using reversed-phase liquid–liquid extraction followed by high performance liquid chromatography tandem mass spectrometry. J Chromatogr A 1341:50–56
Wägli P, Chang YC, Homsy A, Hvozdara L, Herzig HP, de Rooij NF (2013) Microfluidic droplet-based liquid–liquid extraction and on-chip IR spectroscopy detection of cocaine in human saliva. Anal Chem 85:7558–7565
Yan H, Qiao J, Wang H, Yang G, Row KH (2011) Molecularly imprinted solid-phase extraction combined with ultrasound-assisted dispersive liquid–liquid microextraction for the determination of four Sudan dyes in sausage samples. Analyst 136:2629–2634
Wierucka M, Biziuk M (2014) Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. Trends Anal Chem 59:50–58
González-Mariño I, Quintana JB, Rodríguez I, Gonzalez-Diez M, Cela R (2012) Screening and selective quantification of illicit drugs in wastewater by mixed-mode solid-phase extraction and quadrupole-time-of-flight liquid chromatography-mass spectrometry. Anal Chem 84:1708–1717
Mirnaghi F, Pawliszyn J (2012) Reusable solid-phase microextraction coating for direct immersion whole-blood analysis and extracted blood spot sampling coupled with liquid chromatography-tandem mass spectrometry and direct analysis in real time–tandem mass spectrometry. Anal Chem 84:8301–8309
Xu S, Zhang X, Sun Y, Yu D (2013) Microwave-assisted preparation of monolithic molecularly imprinted polymeric fibers for solid phase microextraction. Analyst 138:2982–2987
Cui X, Bao L, Gan J (2013) Solid-phase microextraction (SPME) with stable isotope calibration for measuring bioavailability of hydrophobic organic contaminants. Environ Sci Technol 47:9833–9840
Wang L, Duan C, Wu D, Guan Y (2014) Quantification of endogenous brass inosteroids in sub-gram plant tissues by in-line matrix solid-phase dispersion-tandem solid phase extraction coupled with high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1359:44–51
Vela-Soria F, Ballesteros O, Camino-Sánchez FJ, Zafra-Gómez A, Ballesteros L, Navalón A (2015) Matrix solid phase dispersion for the extraction of selected endocrine disrupting chemicals from human placental tissue prior to UHPLC-MS/MS analysis. Microchem J 118:32–39
Lan H, Gan N, Pan D, Hu F, Li T, Long N, Shen H, Feng Y (2014) Development of a novel magnetic molecularly imprinted polymer coating using porous zeolite imidazolate framework-8 coated magnetic iron oxide as carrier for automated solid phase microextraction of estrogens in fish and pork samples. J Chromatogr A 1365:35–44
Zheng H, Mo J, Zhang Y, Gao Q, Ding J, Yu Q, Feng Y (2014) Facile synthesis of magnetic molecularly imprinted polymers and its application in magnetic solid phase extraction for fluoroquinolones in milk samples. J Chromatogr A 1329:17–23
Yao G, Liang R, Huang C, Wang Y, Qiu J (2013) Surface plasmon resonance sensor based on magnetic molecularly imprinted polymers amplification for pesticide recognition. Anal Chem 85:11944–11951
Griffete N, Li H, Lamouri A, Redeuilh C, Chen K, Dong C, Nowak S, Ammar S, Mangeney C (2012) Magnetic nanocrystals coated by molecularly imprinted polymers for the recognition of bisphenol A. J Mater Chem 22:1807–1811
Zhang Z, Tan W, Hu Y, Li G, Zan S (2012) Microwave synthesis of gibberellin acid 3 magnetic molecularly imprinted polymer beads for the trace analysis of gibberellin acids in plant samples by liquid chromatography-mass spectrometry detection. Analyst 137:968–977
Kan X, Zhao Q, Shao D, Geng Z, Wang Z, Zhu J (2010) Preparation and recognition properties of bovine hemoglobin magnetic molecularly imprinted polymers. J Phys Chem B 114:3999–4004
Parisi OI, Morelli C, Puoci F, Saturnino C, Caruso A, Sisci D, Trombino GE, Picci N, Sinicropi MS (2014) Magnetic molecularly imprinted polymers (MMIPs) for carbazole derivative release in targeted cancer therapy. J Mater Chem B 2:6619–6625
Zhao M, Zhang C, Zhang Y, Guo X, Yan H, Zhang H (2014) Efficient synthesis of narrowly dispersed hydrophilic and magnetic molecularly imprinted polymer microspheres with excellent molecular recognition ability in a real biological sample. Chem Commun 50:2208–2210
Gao R, Kong X, Wang X, He X, Chen L, Zhang Y (2011) Preparation and characterization of uniformly sized molecularly imprinted polymers functionalized with core–shell magnetic nanoparticles for the recognition and enrichment of protein. J Mater Chem 21:17863–17871
Serrate D, De Teresa JM, Marquina C, Marzo J, Saurel D, Cardoso FA, Cardoso S, Freitas PP, Ibarra MR (2012) Quantitative biomolecular sensing station based on magnetoresistive patterned arrays. Biosens Bioelectron 35:206–212
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interf 140:114–131
Abolghasemi MM, Parastari S, Yousefi V (2015) Microextraction of phenolic compounds using a fiber coated with a polyaniline-montmorillonite nanocomposite. Microchim Acta 182:273–280
Abolghasemi MM, Parastari S, Yousefi V (2014) Polypyrrole-montmorillonite nanocomposite as sorbent for solid-phase microextraction of phenolic compounds in water. J Sep Sci 37:3526–3532
Zarpon L, Abate G, dos Santos LBO, Masini JC (2006) Montmorillonite as an adsorbent for extraction and concentration of atrazine, propazine, deethylatrazine, deisopropylatrazine, and hydroxyatrazine. Anal Chim Acta 579:81–87
Wu PX, Dai YP, Long H, Zhu NW, Jai P, Wu JH, Dang Z (2012) Characterization of organo-montmorillonites and comparison for Sr (II) removal: equilibrium and kinetic studies. Chem Eng J 191:288–296
Jafari MT, Saraji M, Sherafatm H (2014) Polypyrrole/montmorillonite nanocomposite as a new solid phase microextraction fiber combined with gas chromatography-corona discharge ion mobility spectrometry for the simultaneous determination of diazinon and fenthion organophosphorus pesticides. Anal Chim Acta 814:69–78
Liu X, Yin J, Zhu L, Zhao G, Zhang H (2011) Evaluation of a magnetic polysulfone microcapsule containing organic modified montmorillonite as a novel solid-phase extraction sorbent with chlorophenols as model compounds. Talanta 85:2451–2457
Larraza I, López-Gónzalez M, Corrales T, Marcelo G (2012) Hybrid materials: magnetite–polyethylenimine–montmorillonite, as magnetic adsorbents for Cr (VI) water treatment. J Colloid Interface Sci 385:24–33
Chen D, Li W, Wu Y, Zhu Q, Lu Z, Du G (2013) Preparation and characterization of chitosan/montmorillonite magnetic microspheres and its application for the removal of Cr (VI). Chem Eng J 221:8–15
Ho C, Sin DWM, Tang HPO, Chung PK, Siu SMP (2004) Determination and on-line clean-up of (fluoro)quinolones in bovine milk using column-switching liquid chromatography fluorescence detection. J Chromatogr A 1061:123–131
He H, Dong C, Li B, Dong J, Bo T, Wang T, Yu Q, Feng Y (2014) Fabrication of enrofloxacin imprinted organic–inorganic hybrid mesoporous sorbent from nanomagnetic polyhedral oligomeric silsesquioxanes for the selective extraction of fluoroquinolones in milk samples. J Chromatogr A 1361:23–33
Ibarra IS, Rodríguez JA, Páez-Hernández ME, Santos EM, Miranda JM (2012) Determination of quinolones in milk samples using a combination of magnetic solid-phase extraction and capillary electrophoresis. Electrophoresis 33:2041–2048
Springer V, Jacksén J, Ek P, Lista AG, Emmer Å (2014) Determination of fluoroquinolones in bovine milk samples using a pipette-tip SPE step based on multiwalled carbon nanotubes prior to CE separation. J Sep Sci 37:158–164
Xiao J, Chen Y, Xu J (2014) Plasma grafting montmorillonite/iron oxide composite with b-cyclodextrin and its application for high-efficient decontamination of U(VI). J Ind Eng Chem 20:2830–2839
Sun Y, Wang Q, Chen C, Tan X, Wang X (2012) Interaction between Eu(III) and graphene oxide nanosheets investigated by batch and extended X-ray absorption fine structure spectroscopy and by modeling techniques. Environ Sci Technol 46:6020–6027
Hsieh S, Huang BY, Hsieh SL, Wu CC, Wu CH, Lin PY, Huang YS, Chang CW (2010) Green fabrication of agar-conjugated Fe3O4 magnetic nanoparticles. Nanotechnology 21:445601
Hao Y, Gao R, Shi L, Liu D, Tang Y, Guo Z (2015) Water-compatible magnetic imprinted nanoparticles served as solid-phase extraction sorbents for selective determination of trace 17 beta-estradiol in environmental water samples by liquid chromatography. J Chromatogr A 1396:7–16
Xie X, Pan X, Han S, Wang S (2015) Development and characterization of magnetic molecularly imprinted polymers for the selective enrichment of endocrine disrupting chemicals in water and milk samples. Anal Bioanal Chem 407:1735–1744
Anirudhan TS, Divya PL, Nima J (2013) Silylated montmorillonite based molecularly imprinted polymer for the selective binding and controlled release of thiamine hydrochloride. React Funct Polym 73:1144–1155
Giakisikli G, Anthemidis AN (2013) Magnetic materials as sorbents for metal/ metalloid preconcentration and/or separation: a review. Anal Chim Acta 789:1–16
Wagenlehner FME, Weidner W, Sörgel F, Naber KG (2005) The role of antibiotics in chronic bacterial prostatitis. Int J Antimicrob Agents 26:1–7
Gao W, Chen G, Chen Y, Zhang X, Yin Y, Hu Z (2011) Application of single drop liquid–liquid–liquid microextraction for the determination of fluoroquinolones in human urine by capillary electrophoresis. J Chromatogr B 879:291–295
Acknowledgments
This study was supported by the National Natural Science Foundation of China (21464006), the Major Projects of Department of Education of Guangdong Province (2014KZDXM074), the Project of Department of Education of Guangdong Province (2013KJCX0191 and 2014KTSCX193), the Project of Science and Technology Innovation Project of Zhaoqing City (2013 F013), the Special Projects of University Talent Introduction of Guangdong Province(2013197), the Science and Technology Planning Project of Zhaoqing High-Technology Zone (2012B01003002), and the Natural Science Foundation of Zhaoqing University (201318).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Wang, H., Liu, Y., Wei, S. et al. Selective extraction and determination of fluoroquinolones in bovine milk samples with montmorillonite magnetic molecularly imprinted polymers and capillary electrophoresis. Anal Bioanal Chem 408, 589–598 (2016). https://doi.org/10.1007/s00216-015-9140-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9140-1