Abstract
Laser-induced breakdown spectroscopy (LIBS) has become an established analytical atomic spectrometry technique and is valued for its very compelling set of advantageous analytical and technical characteristics. It is a rapid, versatile, non-contact technique, which is capable of providing qualitative and quantitative analytical information for practically any sample, in a virtually non-destructive way, without any substantial sample preparation. The instrumentation is simple, robust, compact, and even enables remote analysis. This review attempts to give a critical overview of the diverse progress of the field, focusing on the results of the last five years. The advancement of LIBS instrumentation and data evaluation is discussed in detail and selected results of some prominent applications are also described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It seems safe to say now that Winefordner et al. were right in 2004 to call laser-induced breakdown spectrometry (LIBS, sometimes also called laser-induced plasma spectroscopy, or LIPS) the “next superstar” within the group of analytical atomic spectrometry methods [1]. Today, this field produces the vast majority of the fundamental research results within this group, and the number of analytical applications is also exponentially growing. The reason for the popularity of LIBS is obvious: it offers a very compelling set of advantageous analytical and technical characteristics. It is a versatile, non-contact, powerful atomic spectrometry method, which is capable of providing elemental composition data (either at the level of trace, minor, or major components) for practically any sample in a virtually non-destructive way and without any substantial sample preparation, meaning the analysis is very fast. The instrumentation needed is reasonably simple, very robust, and is also available in such a compact format that it enables not only portable operation, but even space applications. In addition to this, remote analysis is also possible, in several configurations, because of the unique principle of operation which requires no material transfer but only photons, both for the sample interrogation (in the form of a high-powered, focused laser beam, which produces the atom or excitation source) and for detection (photons emitted by the microplasma are collected).
The LIBS literature has grown so enormously that today an interested reader must read several books and reviews in an attempt to process the immense number of results produced by this field. A good indicator of the rapid growth of the literature is that thematic LIBS reviews are also produced in a great number, each covering special application fields, for example geology, archeology, biology, or materials science. LIBS has even established itself as an instrumental analytical technique appearing in university teaching labs [2]. Although it is obviously hard to shortlist recommended publications when there is such a wealth of information, newcomers to the field are recommended to start with Refs. [3–5], whereas LIBS experts can find excellent advanced scientific information in Refs. [6–8], to name a few select sources.
The objective of this paper is to provide a critical overview of the most analytically relevant recent results and trends, focusing on the literature of the last five years (2010–2015). Because of the vast and diverse literature on LIBS, and to restrain the length of the paper, a subjective selection of sources had to be regretfully made by the author. The analytical character is maintained throughout the paper, and thus no strictly theoretical results are covered.
This review is divided into three large sections. The topics of these sections are meant to represent the three large topics in which most of the progress has been made and which, at least in the view of the author, will continue to produce important fundamental results in analytical LIBS spectroscopy in the near future. The first section discusses instrumental developments, which has been a very diverse and productive field. This section is somewhat more descriptive than the other two, because it also has an introductory character. The second section discusses the progress in LIBS data evaluation and data processing, which is an emerging field and heavily builds on concurrent results in chemometry. The third section is meant to provide an overview of the fields of application that have been most reported in recent years. This last section is interrelated with the other two sections, because these applications were partially made possible by progress described in the instrumentation and data-evaluation sections.
Instrumental progress
The status of LIBS instrumentation and performance
In essence, a LIBS spectrometer is an atomic emission spectrometer that uses the microplasma generated by a pulsed, focused laser beam (with the irradiance in the GW cm−2 range) on the surface of or inside the sample (for solid and fluid samples, respectively) as an atom and excitation source. The laser-induced breakdown (LIB) plasma is a strongly transient phenomenon with a typical lifetime of ca. 0.1 to 100 μs (depending on experimental conditions), and thus the practical recording of its emission spectrum necessitates the use of a fast spectrometer, the operation of which is tightly synchronized with the laser pulse. The gate delay and width of the spectrometer need to be controlled and/or optimized with sub-μs accuracy, so that the radiation of the analyte species (atoms, ions, or molecules) can be captured when they are dominant within the plasma lifetime and the continuum background radiation is already low.
Laser sources
LIBS requires a more powerful laser source than laser ablation, because the breakdown-threshold fluence is several orders of magnitude larger than the ablation-threshold fluence [9, 10]. In practice, this usually translates to the need for a laser source with at least some tens of mJ pulse energy and a well-focused beam (with a focal spot diameter of ca. 1–200 μm). Increasing the pulse energy, while keeping all other laser properties constant, usually strongly increases the LIBS signal (the ablation rate linearly increases with the fluence), but only up to a specific point, because a roll-off is caused by plasma shielding [10, 11].
The mass of ablated material and plasma shielding are both important for efficient LIBS signal generation, and for these reasons ns-duration laser pulses are generally regarded as optimum for LIBS in terms of limits of detection [7]. As recent laser-ablation research reveals (e.g. Ref. [12], Chapter 6 in Ref. [8]), the application of ultrashort (femtosecond, fs) laser pulses seems to have distinct analytical advantages, including more stoichometric sample ablation and thus more accurate analyses, reduced continuum radiation, and cleaner crater rims in solids and thus better spatial resolution. Thus, more and more fs LA applications can be expected in the near future. These ablation-related advantages can potentially also be exploited in LIBS, and the community started to investigate these in single and double-pulse analytical applications [13–15]. However, at the moment it seems very questionable whether the analytical benefits realized in LIBS by the use of fs laser pulses would justify the need to replace the conventional compact, robust, and economical ns laser sources with the substantially bulkier and costlier fs laser systems other than in some special applications. In addition, studies that compare the analytical performance of ns and fs-LIBS using the same fluence are extremely scarce [16], and thus it is too early to draw any conclusion on the matter.
The laser wavelength has a complex effect on LIBS performance. First, one can argue that laser wavelength should generally be selected so that the light absorption of the sample is large at this wavelength. On the basis of this condition, UV lasers should usually be preferred. However, plasma shielding, important for a high-temperature LIB plasma, was revealed to increase with λ 3, thus being significantly stronger in the infrared (IR) range [8], and most of the economic and powerful pulsed solid-state lasers also work at near-infrared (NIR) wavelengths (in the range ca. 1.0–1.1 μm). Frequency upconversion by nonlinear techniques (e.g. harmonic generation, sum frequency generation) is a common laser option that enables the production of visible or UV-wavelength radiation from a pulsed, NIR fundamental laser emission, but it comes with a great loss in pulse energy, and thus many LIBS analyses of most solids in air are still typically done at NIR wavelengths. In addition, eye safety is also a concern and this would also dictate the use of a 1.3–1.5 μm laser beam [17]. Exceptions to the use of NIR wavelengths are special cases when the fluid medium in which the solid sample resides absorbs strongly in the NIR region (e.g. bulk-liquid LIBS of aqueous samples) or when the sample has an exceptionally low absorbance in the NIR region (e.g. glasses or crystals). In these instances, 532 nm (optimum for aqueous samples) and 266 or 213 nm wavelengths produced from a single Nd:YAG source, or rarely 193 nm pulses from ArF excimer lasers, are used.
On the basis of the above, the typical LIBS laser source is a (preferentially actively) Q-switched solid-state laser (typically Nd:YAG) releasing some tens of mJ energy, ns-duration pulses. As semiconductor laser technology becomes more and more advanced, diode-pumped solid-state (DPSS) Nd:YAG lasers are slowly taking over, because these lasers have better characteristics in almost every respect; most notably higher repetition rates, better pulse-to-pulse repeatability, improved optical-beam characteristics, durability, much-reduced size, and better energy efficiency. Only in terms of pulse energy (and its cost-efficient scalability) are the flashlamp-pumped Nd:YAG lasers still somewhat better, and thus they are still popular in laboratory-based or industrial LIBS setups where very high fluences are required, e.g. in liquid-sampling LIBS or when frequency upconversion is needed. A variant of DPSS lasers called the micro/macrochip laser was also tested for LIBS [18, 19], but because the pulse energies of these compact and robust lasers are still small, typically sub-mJ, they were found not to be really useful for analytical LIBS. Another, more promising, and most novel laser type for LIBS is the group of Q-switched ns fiber lasers, which have many attractive features [20, 21], including resistance to vibrations, flexible use because of an inherent fiber-coupled light beam, and compactness. Their top pulse energy increases every year and has now reached the 10 mJ range, which is sufficient for most LIBS applications, and thus it is expected that they will be more and more used in the near future.
Spectrometers and detectors
As was alluded to above, the spectrometer used in a LIBS setup has to be robust and needs to have a fast synchronization possibility. It also needs to have a reasonable spectral coverage in the UV and visible range and a resolution suitable for atomic spectroscopy (well below 0.1 nm resolution). The detector array also has to be quite sensitive; even with μs-range gate-width values it should provide good S/N spectra enabling analysis with low detection limits. This stringent set of requirements is not easy to fulfill, but is most closely approached by charge-coupled device (CCD) spectrometers, and thus their use is the most common in LIBS spectroscopy. Back-illuminated CCD detector arrays, either in a linear format (max. 4096 pixels) mounted in a Czerny–Turner arrangement or in a two-dimensional format (usually 1024 × 1024 pixels) mounted in an Echelle arrangement, are mostly found today in these spectrometers. There has been an argument in recent literature as to whether a non-gated (e.g. linear CCD) or a gated (e.g. intensified CCD camera, or iCCD for short) detector is better in a LIBS setup [22, 23]. It has been suggested that linear CCD spectrometers are more robust, and provide better S/N and sensitivity; however, their resolution is poorer in broadband configurations and they do not enable sub-μs gating. At the same time, Echelle spectrographs with ICCD camera detectors are costly, bulky constructions with excellent time and spectral resolution, but mediocre S/N. Not surprisingly, laboratory LIBS setups used for fundamental research most often use an ICCD spectrograph configuration, whereas portable instruments or those mainly used for routine analytical work almost exclusively use linear-array CCD spectrometers [8]. A partial resolution of this dilemma was recently provided by Effenberger and Scott, who suggested the use of a Fabry–Perot etalon in conjunction with a standard imaging spectrometer to substantially enhance the resolution [24].
The optical setup
The light focusing and light collection optics also have an important function and add substantial flexibility to the LIBS setup. Basic LIBS-focusing optics comprises a single, fixed-focal-length lens, and the incident beam is normal to the sample surface. The use of small-magnification, long-working-distance, high-damage-threshold microscope objectives (e.g. 4×, 10×) is also common. The minimum practical working distance of the objective is approximately 20 mm; however, combustible samples (e.g. plastics, organics, and coal) can easily produce flames in air higher than 20 mm. Extremely long focal length (several meters) focusing optics in a telescope arrangement is used in stand-off LIBS setups [25, 26]. Recently reflective focusing optics, for example Schwarzschild objectives, have also sometimes been used [26, 27]. In sophisticated, automated setups (e.g. industrial, military, or space applications), where the lens-to-sample (or instrument-to-sample) distance has to be adjusted frequently, the use of autofocus optics (e.g. based on a time-of-flight laser range finder) has also been described [25, 26]. With tight focusing, high spatial resolutions in LIBS (useful in, e.g., imaging) can be achieved; the best value ever achieved was 450 nm [28]. However, a too-tight focusing is usually disadvantageous regarding signal repeatability because of the very short Rayleigh length, which causes a strong fluctuation of plasma properties. Some defocusing, or focusing below the sample surface, is typically advantageous because it can prevent gas breakdown (air sparks) above a solid sample.
Two direct light-collection geometries are common; coaxial with the incident laser beam, and sideways at an angle (typically ca. 45 °). The use of a large collection solid angle obviously increases sensitivity. Coaxial light collection is more compact, and is more useful for depth-resolved analyses, but is also more prone to self-absorption that deteriorates the linearity of calibration plots. Sideways observation is a more economic and more flexible solution, but typically suffers from a larger signal scatter caused by the positional instability of the plasma plume. Another, often overlooked, condition in LIBS optical setups is that light collection should be optimized for broadband detection, meaning the use of reflective optics (e.g. parabolic mirrors, Schwarzschild objectives) should be preferred; it should also be taken into consideration that achromat optics are only available for wavelengths above ca. 240 nm and for a limited wavelength range. However, most analytical LIBS systems use a simple collection setup with one or two fused-silica lenses, which greatly compromises sensitivity towards the two ends of the collected spectra. Another aspect that should be considered is that light-collection optics that spatially integrate the light from the plasma plume (as opposed to point-to-point imaging arrangements) are advantageous in terms of sensitivity and signal repeatability.
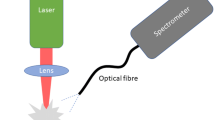
In recent years, the use of fiber optics in LIBS has become almost standard because of the widespread use of fiber-optic-coupled spectrometers and the great flexibility provided by fibers in light guiding. These setups are sometimes called FO-LIBS (fiber-optic LIBS). In such setups, the light-collection arm is built using a multimode, solarization resistant, high-OH optical fiber. In a full FO-LIBS (direct-push FO-LIBS), a high-damage-threshold, large-core optical fiber is also used to direct the laser light to the sample [29]. The direct-push FO-LIBS arrangement enables analysis at hard-to-access locations (e.g. nuclear reactor cores [30] or submarine archeological sites [31]), and also makes handheld, man-portable, point-and-shoot LIBS instruments possible. These are now offered by several commercial companies.
Sample presentation
Despite the fact that one of the appealing features of LIBS is its very simple sample preparation, the means of sample presentation (sample-holder type, how the sample is affixed to the holder, ambient conditions, etc.) is very important. One of the main reasons for this is that LIBS is a microanalytical (microsampling) method, and thus the decisions made by the analyst with regard to sample presentation, in particular the selection of the microliter fraction or sub-mm spot of the sample to be analyzed, have a great effect on the accuracy and precision of analysis. In general, sample presentation has approximately equivalent analytical importance in LIBS to that of sample introduction in atomic spectrometry. It is also very much connected to the phase and form of the sample; different options are available and have different effects on the analytical results for gases, liquids, and solids.
Solids
The most common application of LIBS is the analysis of bulk solid samples in air, in an “open” setup. A closed ablation cell is hardly ever used in a laboratory, because it would greatly limit the sample-presentation options (e.g. size and shape of sample). Other than some commercial instruments, the exceptions are a) if the samples analyzed pose an elevated level of biological or chemical hazard to the analyst, or b) if a special gas atmosphere is used.
Small (<1–2 cm) samples or sub-samples are typically simply fixed to a microscope slide using a double-sided adhesive foam tape, and so are placed on a manual or motorized stage. The stage enables adjustment of the lens-to-sample distance and selection of the analyzed spot on the sample. The selection of the analyzed spot on the sample surface may, in some cases (e.g. with large-surfaced, flat samples), be done by simple visual aiming, but in more sophisticated setups either a complementary, small power, visible-wavelength aiming laser (HeNe or diode laser) or a digital camera, also enabling sample documentation, is used for this purpose.
Powdered or fine-grained solid samples are usually either pelletized in a hydraulic press (with or without a binder) or simply directly smeared onto an adhesive tape (thereby fixing a more or less even layer of grains on the tape) and then handled or presented analogously to bulk solids. The latter presentation approach, although attractively simple and widely used, has several disadvantages which are often overlooked. These include the need to use a microscope camera for aiming to ensure that the excitation laser beam will actually hit a grain, and the use of a high-purity adhesive tape to ensure low background intensity. The shockwave generated by the ablation can also blow away (rearrange) some of the grains on the adhesive tape. These effects can cause problems, especially in distribution studies or when very fine powders (microparticles) are measured.
Recently a novel approach, termed optical catapulting, was suggested and successfully used for microparticle analysis by Laserna et al. [32, 33]. In this approach, solid particles on a thin solid substrate (e.g. microscope slide) are ejected (converted to a dry aerosol) by the shockwave generated by a defocused laser pulse that hits the substrate from the back side. The aerosol is then subjected to LIBS analysis by a second laser pulse coming from the side. The main advantage of the method is that it enables the analysis of very small amounts of microparticles without any contamination or spectral contribution from the substrate.
Liquids
Liquid sample analysis by LIBS is a real challenge for several physical and technical reasons. First, the free surface and the presence of (micro)bubbles or suspended particles within liquids can cause focusing problems, and the collection of the emitted light is also obviously hindered by the liquid. More importantly, a liquid-phase sample also has a significantly higher density and cooling effect, and thus imposes stronger quenching on the LIB plasma than the gaseous environment typical for LIBS. Consequently, LIBS plasmas created in liquids have lower temperature, lower emission intensities, shorter lifetimes, and smaller maximum dimensions than their counterparts generated on solid samples in air [34]. However, the analysis of liquid samples and of solids submerged in liquids is so important in many fields that, despite the difficulties, LIBS research in this subject has recently been much intensified. This is mainly the consequence of the availability of the double-pulse method, which can be used to boost the performance of liquid LIBS analysis (see below).
A liquid sample can be analyzed in bulk form, by focusing the laser beam either on the free surface of the liquid held in an open container or into some depth of the liquid (in the latter case, the liquid has to be transparent at both the laser and the detection wavelength). The bulk approach typically gives poor analytical performance, and thus is avoided if possible. A better, and now often used, sample-presentation option is to convert the liquid sample to a wet aerosol [35] or to a liquid jet [36]. To improve signal repeatability, and hence the limits of detection, a large number of repeated measurements are usually performed, and thus the conservation of sample volume dictates liquid recirculation, unless a nanoflow nebulizer is used [37]. Although these approaches provide a decent analytical performance [34, 38], their general application cannot be expected, because: a.) sample throughput is typically low because of the difficulty of changing from one sample to another; b.) clogging and memory effects may occur, meaning frequent washing of the system has to be performed; c.) the handling of fluid-phase sample requires fragile devices that are relatively impractical for use in the field or on a factory floor; d.) the spraying of hazardous liquid samples (e.g. biological, toxic, or flammable samples) in a partially open system is unacceptable. Thus, the use of these liquid-sample-presentation approaches is expected to remain limited to environmental or synthetic aqueous samples.
An analytically even more beneficial option is to convert the liquid into a solid matrix. The liquid sample is either frozen [39], dried onto a non-permeable and non-wettable substrate, for example a metal plate [40] or a graphite pellet [41], or soaked up by a non-metallic solid matrix (filter paper [42], wood [43], etc.) before LIBS analysis. This conversion approach efficiently eliminates most of the disadvantages associated with bulk-liquid analysis because the plasma is actually generated on a solid sample, representative of the composition of the original liquid. Further advantages of the liquid-to-solid conversion approach include the fact that it requires only a very small sample volume (μL or less) and that it can also be used with hazardous samples by placing the substrate in a closed ablation cell [40]. However, it has to be considered that the substrate is co-ablated with the sample, which can give rise to spectral interferences, although it has also been suggested that spectral lines from the substrate can be used as an internal standard [44]. With respect to the “drying onto a solid substrate” sample-presentation method it also has to be pointed out that no studies have yet been described in the literature which have investigated the important effects of both the thickness and packing of the deposited dry layer on the analytical signal. These effects should be addressed before general application is attempted. Also, a known problem of practically all of the above approaches is that they often produce a “coffee spot” effect, which is an easily observable, uneven lateral distribution of the solid deposits (i.e. more deposit is formed around the rim of the spot) that necessitates careful control of the location of the laser ablation during quantitative analysis. Alternatively, a spatial localization of the deposit, as suggested recently by Metzinger et al. [40], can be performed.
Most recently, some researchers started to realize that conventional macro-scale liquid-sample preparation methods, for example preconcentration or extraction, can be advantageously adapted to pretreat micro-volume liquid samples before LIBS analysis. These efforts can be best exemplified by the latest publications of Hidalgo et al., who performed single-drop microextraction (SDME) [45] and dispersive liquid–liquid microextraction (DLLME) [46, 47] of chelated metal ions. The resulting extracts were then dried onto a solid substrate and subjected to LIBS analysis. Other groups reported successful preconcentrations by the electrodeposition of metal ions [48] and by using ion-exchange polymer membranes [49].
Gases and aerosols
The direct LIBS analysis of gases (gas mixtures) is usually a simple task from the perspective of sample presentation. The analysis is usually performed on a gas stream flowing in a sample cell equipped with a windowed aperture for both the laser beam and the emitted light [50, 51].
The LIBS analysis of aerosols can be performed in a setup similar to the above, which is then called free-stream aerosol analysis. The particles are either randomly sampled, or are aligned to form a particle beam by using a capillary or an aerodynamic lens. An alternative method of sample presentation is substrate-based analysis, when the aerosol is first collected on a filter, as is usual in aerosol chemical analysis, and the filter surface is then analyzed by LIBS. Another option is to repeatedly generate LIB on a substrate while the aerosol particle beam is directed through the plasma [7, 8, 52]. The sampling statistics of the free-stream analysis is usually very poor, and thus it is usually not applicable to the analysis of low-concentration aerosols. The filter-based collection technique significantly improves sampling statistics, but still only a small fraction of the filter surface is analyzed, meaning detection limits are only slightly improved and relatively large pumps are needed, which is not advantageous in a compact instrument. Substrate-based aerosol sampling is also subject to potential spectral interferences from the substrate material. A new aerosol-sampling method was recently developed [53] which involves the charging and subsequent collection of the particles on the flat tip of a microelectrode, followed by LIBS analysis.
Means of improving the analytical performance
Despite its versatility and very useful set of analytical characteristics, there are some aspects of LIBS performance that are not competitive enough with the performance of well-established analytical atomic spectrometry methods. The main characteristics to be improved are sensitivity (limits of detection) and repeatability (precision), but of course there is always room for improvement of other aspects as well.
Plasma conditioning using laser pulses
The double-pulse method
The use of two spatially aligned and time-synchronized laser pulses for LIBS signal generation is an idea that has been around since 1970 (proposed by Piepmeier and Malmstadt and by Scott and Strasheim), but technical difficulties prevented its practical realization until approximately the end of the 1990s. Its use has been mainly suggested for improving sensitivity, but the general concept behind using the double-pulse LIBS (DP-LIBS) method is that with a single laser pulse (single-pulse LIBS, or SP-LIBS) the processes of breakdown and plume excitation cannot be separately controlled and optimized. Today DP-LIBS is a widely accepted and used instrumental approach, which provides substantial (up to a hundredfold) enhancements of signal intensity and some improvement in repeatability, which translates into at least a one order of magnitude reduction in limits of detection. Improvements in the linear dynamic range have also been observed. Detailed analytical performance data can be found in Refs. [54, 55] and in Chapter 5 of Ref. [8].
Double-pulse LIBS can be executed by using two lasers synchronized with some time delay, or by splitting a single pulse using a beam splitter and delaying one of the two produced pulses. In terms of optical arrangement, four common DP-LIBS configurations are described in the literature (Fig. 1). Most of the fundamental studies on DP-LIBS are performed in one of the orthogonal configurations, which offer the greatest level of control of the processes occurring. However, only the collinear configuration is practical for routine or portable analytical LIBS instrumentation. Some very recent setups even enable the use of a variable incident angle cross configuration [56]. The timing of gated data collection starts after the arrival of the second laser pulse, with some delay, and thus the analytical benefits realized in DP-LIBS are always connected to the second pulse. The literature reveals great variations in terms of the characteristics of the laser pulses used; analytical benefits were observed, for different samples, for interpulse delays from ns to ms, different pulse-energy ratios, and different combinations of pulse durations (from ns to fs) or wavelengths.
As well as an increasing number of analytical applications of the DP-LIBS method, recent progress in the field is also related to the understanding of the underlying fundamental processes. Although this is not the focus of this review, it can be mentioned that the results so far suggest that the following sub-processes all contribute to the signal enhancements observed: a.) reheating and/or rekindling of the LIB plasma by the absorption of the second laser pulse in the plume (increase in plasma temperature); b.) increased material ablation resulting from the contribution from the second laser pulse (in the co-linear and cross configurations); and c.) modified conditions on the sample surface or immediately above it (e.g. pressure drop behind the propagating shockwave, increase of sample surface temperature) leading to a decrease in ablation and breakdown thresholds, thereby increasing the material ablation and plasma temperature. There also seems to be a correlation between the excitation energy of the transition investigated and the signal enhancement observed [57, 58]. It also has to be stressed that generalization of the mechanisms occurring is simply not possible, despite the efforts of some plasma modeling studies, because there is a large variety of experimental conditions used in DP-LIBS. The increasing trend of using ultrashort (fs) laser pulses in DP-LIBS also complicates the theoretical discussion, because laser–matter interaction is distinctively different in this time regime. A clear discussion of the state of understanding of DP-LIBS processes was recently provided by Tognoni and Cristoforetti [59].
One particular, related subject that has received much interest from the community recently is the analysis of liquids and of solid samples submerged in liquids (underwater analysis), because it was revealed that in these cases much better analytical performance can be obtained using the double-pulse method. The first laser pulse produces a very weak plasma and vapor (cavitation) bubble, whereas the second pulse can efficiently excite the plasma or—depending on the interpulse delay—can generate a plasma in the gas environment of the bubble. An excellent overview of the processes involved and the analytical performance achievable in this field was recently given by Lazic and Jovicevic [34].
The multi-pulse method
The novel approach known as multi-pulse LIBS (MP-LIBS) can be regarded as an extension of DP-LIBS. The only practical way of realizing the MP-LIBS technique is to use the co-linear configuration with a single laser, thus also using laser pulses of identical wavelength, duration, and energy. Because it requires a special laser source, only a few MP-LIBS setups and studies have been described in the literature in the past few years; the setups differ mainly in the number of pulses and the timing of data collection (e.g. gated or non-gated detection with different timing). Most of these setups use ns pulses, but Zhang et al. recently also investigated fs multi-pulse (pulse-train) excitation [60].
Recent publications on MP-LIBS have mainly been produced by Galbács and co-workers [58, 61–64], who use space and time-integrated, but gated, detection and a controllable number of ns laser pulses (up to 11) with μs-range inter-pulse delays. These studies reported the achievement of a factor of two improvement of repeatability from just five laser bursts, and an order of magnitude reduction in limits of detection with respect to DP-LIBS for small and medium-excitation-energy spectral lines (<7 eV). The ablated mass per laser pulse was found to strongly increase with the number of pulses delivered in a burst. A significantly extended linear dynamic range was also observed (Fig. 2), most probably caused by the reduced self-absorption in a diluted, fast-expanding plasma. The authors pointed out that the improvement achievable decreases with an increase in the number of laser pulses in a burst; the signal enhancement of the pulse-number-normalized net signals was found to peak at approximately 4–7 pulses.
Comparison of SP-LIBS, DP-LIBS (solid circles), and sextuple-pulse MP-LIBS (open circles) calibration curves for a Cu and a Zn atomic line. Fitted lines and curves are only meant to guide the eye. Error bars indicate standard deviations based on using a comparable total number of laser pulses. Reproduced with permission from Ref. [58]
Hoechse et al. recently also successfully demonstrated the analytical improvements achievable by a MP-LIBS method using a DPSS laser working at 200 kHz [65], and multi-pulse excitation has been found to be advantageous in liquid-sample LIBS analysis [34]. Laserna et al. published a study on the MP-LIBS analysis of Si [66], in which it was determined that the signal-enhancement process in the special case of Si is dominantly governed by the pulse duration.
Because of the complex processes involved, further fundamental studies are needed to fully clarify the enhancement mechanisms. The MP-LIBS field has not yet been reviewed, although Ref. [8] provides partial coverage. Data in Table 1 and Fig. 2 illustrate the relative analytical performance of SP-LIBS, DP-LIBS, and MP-LIBS methods.
Plasma conditioning using means other than laser pulses
Gas atmosphere
Although most LIBS experiments are performed in air at atmospheric pressure, the effects of the gas environment (gas composition and pressure) on LIBS performance have long been studied. As well as fundamental physical interest, research in this field has recently also been motivated by the need for method development for important, special applications including space exploration or power-plant monitoring. A thematic review [67] has been written by Effenberger and Scott on the results of this field.
Results indicate that lowering the pressure of the surrounding gas, from 760 to a few Torr, usually increases the LIBS signals, improves the S/N, and increases the resolution of the spectral features. Also, less debris is deposited around the ablation craters. These advantageous effects are caused by the combination of several processes, which can be best exploited by ns laser pulses. Lowering the pressure causes a higher rate of plasma expansion, which in turn gives rise to less plasma shielding, resulting in a significantly increased ablation rate and longer plasma lifetime. However, reduced plasma shielding also means a lower electron concentration and plasma temperature and less collisional excitation, all working against a signal increase, which is only partially compensated by the smaller cooling effect of a thinner surrounding gas atmosphere. The increased spectral resolution is the result of reductions in pressure broadening and in Stark broadening. However, it was also revealed that if the pressure of the surrounding gas is further decreased to vacuum pressures (e.g. below a few Torr), the net result of the combination of processes described above leads to a large signal reduction, although the spectral resolution becomes excellent. Nevertheless, high-pressure gas environments are analytically clearly disadvantageous, because they give rise to poor signal intensities, S/N, and resolution, and strong self-absorption effects.
Another trend in related recent LIBS research is the study of how the type of the gas that surrounds the sample affects the analytical performance. Most of these studies use nitrogen, helium, argon, or carbon dioxide, and also vary the gas pressure. Different gas compositions have different thermal conductivity and ionization potentials and produce different collision rates, thus strongly affecting the ablation and excitation processes and the temporal evolution of plasma, and therefore also having a substantial effect on LIBS signals. Noble gas atmospheres can improve sensitivity, although the data published is contradictory regarding whether the use of, e.g., Ar or He gas is better. It also has to be added that a disadvantage of using a gas environment other than air is that it usually requires the use of a sample chamber and a large amount of pressured gas, with associated inconveniences. To overcome this, Son et al. [68] recently suggested the use of synchronized gas jets to flush the sample.
A further, unwanted effect of the surrounding gas is the chemical reactions induced by the plasma. For example, the apparent result of a LIBS experiment in air is the ablation debris, mainly composed of oxides and nitrides of the metallic elements in the samples, which is deposited in and around the crater. This ring of debris has to be considered when planning the step size (spatial resolution) of a LIBS measurement with moving sample (distribution study). Gornushkin et al. [69] reported that in LIBS experiments performed in nitrogen and oxygen-containing gas, nitrogen oxides (NO, N2O, and NO2) and ozone are produced by the plasma via radiation and pressure-induced chemical reactions. A consequence of the presence of these molecular species around the plasma plume is band absorption (e.g. at approximately 253 nm for ozone), which may result in a change in the observed LIBS analytical line-intensity ratios and thus could be experienced as a matrix effect.
Microwave-assisted LIBS
The lifetime of the LIB plasma can be significantly increased, and its line emission enhanced, if the plasma is exposed to microwave radiation. Pulsed microwave (MW) irradiation can significantly increase the rate of collisions, mediated by electrons. Microwave-assisted LIBS (MA-LIBS) is the basis of a commercial tabletop instrument named LAMPS and offered by the company Ocean Optics. The usefulness of the concept was further revealed in two recent studies. Liu et al. [70] applied ms-pulsed MW radiation of 1 kW power to LIB plasmas on ceramic samples and observed up to 33-fold-enhanced signals and intense molecular emission, mainly for transitions with low excitation energy. The same researchers also successfully improved the sensitivity of Cu measurement in soil samples [71]. The MA-LIBS setups can potentially be made more practical (i.e. the cavity omitted) if the microwave radiation is coupled to the plasma by using an antenna. This idea was most recently exploited in a study by Japanese researchers, who used a 3 mm-diameter loop antenna to generate a localized MW field on a Gd2O3 sample. Gd ionic line intensities were successfully boosted by a factor of 50 [72].
Spatial confinement
Spatial confinement is a cost-effective and simple method of signal enhancement in LIBS. It was first described approximately a decade ago, and today it is more and more often used. The concept essentially means that the plasma is generated inside a confined space (typically of a few mm dimensions), where the expanding shockwave is reflected back from the wall of the cavity and performs work on the plasma, thus compressing it. This increases the plasma temperature, and consequently the emission intensities. The signal enhancement was studied in cavities made of quartz, metals, or PTFE materials and of different shapes and sizes, including rectangular [73], cylindrical [74], hemispheric [75, 76], and other shapes, often fabricated with polished walls to aid light collection. Enhancement factors of up to 10 were observed. An obvious, impractical aspect of this technique is that meticulous cleaning of the chamber walls to remove the microparticles deposited has to be performed after each experiment.
Magnetic confinement
When a strong, steady or pulsed, external magnetic field (0.5–10 T) is applied to the LIB plasma, the movement of electrons and ions will be affected by the Lorentz force. This causes a slower plasma expansion, and thus the volume of the plume will be reduced, resulting in increased electron concentration and consequently leading to a higher collision frequency, higher plasma temperature, and eventually higher emission intensities. This premise, first proposed by Mason and Goldberg in 1991, has only been observed in action by a couple of studies. For example, most recently, ring magnets (which combine the effects of spatial and magnetic confinement) were used to produce many-fold signal enhancements in the detection of V and Mn elements in steel [77].
Hyphenated instruments
A novel research direction is the combination of LIBS with other instruments. In these hyphenated (or tandem) instruments, LIBS either serves as an element-selective detector coupled to a separation technique or it enables obtaining of in-situ extended and/or complementary analytical information about the sample. In the opinion of this author, these instrument combinations should be clearly separated in terminology from those in which LIBS basically functions only as a mean of sample introduction and atomization. In this case the use of the term laser ablation, instead of LIBS, is a more correct description, even if the fluence or irradiance of the ablative laser pulse exceeds the breakdown threshold. The ground for this separation in terminology is that the LIBS acronym describes a technique which directly uses the plasma emission for analytical spectrometry. When the emission is not used, but the plume is further excited and/or conditioned and the analytical signal is derived from absorption or fluorescence measurements, the laser-ablated plume only serves as an atom reservoir. An example is LIBS-LIF, a terminology sometimes used in the literature, which should correctly be called either laser-ablation laser-induced fluorescence (LA-LIF) or resonance-enhanced LIBS (RELIBS). DP-LIBS and MP-LIBS terminologies do not contradict this principle, because in these cases the plasma emission is used.
Raman–LIBS
Raman–LIBS is the most widespread hyphenated LIBS instrument. Complementary use of the molecular compositional information provided by Raman microscopy and the data on the elemental composition provided by LIBS spectroscopy is highly useful, especially in applications when a small amount of solids has to be analyzed. Approximately a decade ago the spectroscopy community realized that this dual analytical approach could also be performed with a single (hyphenated) instrument, on the same spot of the sample. The idea is based on the fact that both of these microsampling techniques require the use of a focused laser beam; the difference is that LIBS uses a beam with orders of magnitude higher irradiance (on the order of GW cm−2, as opposed to 10 mW cm−2 or less for Raman). Also, the wavelength of the laser radiation used is not critical in either technique, e.g. it does not have to fulfill resonance criteria etc., so they can be chosen to be identical (typically 532 or 1064 nm) and thus the analysis can be performed with a single laser. This makes the combined instrument more compact and more cost-effective, especially if the laser is the DPSS type. However, the collection of Raman and of LIBS signals require different time-synchronization settings and optical setups, and thus a (micro)Raman–LIBS instrument typically consists of a common laser and focusing optics, but two separate observation optical arms [65, 78]. The Raman detection arm typically contains a notch filter and a spectrometer with smaller spectral coverage and medium resolution (e.g. 150 nm and 2 cm−1, respectively), whereas the LIBS arm has a spectrometer with wide spectral coverage and high resolution (e.g. 600–700 and 0.01 nm, respectively). The laser irradiation is essentially non-destructive in Raman spectroscopy, so the execution order of the measurements is that first a low-fluence laser pulse is delivered to the spot on the sample surface to generate the Raman signal, and then a high-fluence pulse is delivered for LIB plasma and signal generation. If needed, this procedure can be repeated without moving the sample and thus a depth-resolved (layer-by-layer) analysis is also possible, taking advantage of the ablative nature of LIBS. Stand-off detection by hyphenated Raman–LIBS instruments is also possible, although multiple challenges have to be tackled: most importantly lower inherent sensitivity resulting from the small angle of detection, and increased interference caused by the open-field application and resulting in excessive noise in the Raman spectrum and increased background in the LIBS spectrum. Further information on the technical details and analytical applications of Raman–LIBS instruments can be found in a recent review by Lin [79], and on data-evaluation strategies in a paper by Moros and Laserna [80].
As is revealed by recent LIBS literature, the Raman–LIBS hyphenated instrument has already proved itself useful in several application fields. For example, the analysis of pigments used in artworks is of high importance in cultural heritage. It not only provides historical and artistic information, but also helps with conservation and restoration of artworks, as has been revealed multiple times for different sample types [81, 82]. Popular stand-off contemporary applications for micro-Raman–LIBS include the detection of explosives and suspect materials [83, 84] as part of anti-terrorist activities, and the analysis of geological samples [85, 86], which is of primary interest to planetary investigations [87]. In fact, this hyphenated instrument is one of the most important instruments now in use or planned for use in several space missions (ExoMars, Moon4you, etc.).
Other combinations
Although in smaller numbers, other combined instruments which include LIBS as a detector have also been reported recently. Some of these combinations are quite unusual, and their partner device should be regarded purely as a signal-enhancement accessory (e.g. a spark discharge, an RF plasma jet, or electrodeposition combined with LIBS). These instruments will not be discussed in this section.
In the combination of laser-ablation ICP-MS and LIBS (LIBS–LA-ICP-MS) the same laser is used for breakdown plasma generation and laser ablation, either simultaneously or sequentially [88, 89]. This combination provides substantial advantages, for example: a.) LIBS can perform rapid screening of the elements present in the sample, so the conditions for a consecutive quantitative LA-ICP-MS analysis can be optimized; and b.) the technique can be used to analyze elements which are inaccessible to LA-ICP-MS because they are either present in too high a concentration or are hampered by low sensitivity and/or interference (e.g. light elements in particular). From the perspective of LIBS analysis, the benefit of ICP-MS is that it adds the possibility for isotope analysis. These advantages are now widely available to the community: at least one company has already seen commercial potential in the technique and brought an LA–LIBS tandem instrument to the market (J200, Applied Spectra). The combination of LA (without ICP-MS) and LIBS was also tested and found useful in partially eliminating the matrix effects usually occurring in LIBS [90], on the basis of observation of linear responses during the measurement of Mn and Fe across different alloys.
A novel LIBS tandem instrument incorporating a chromatograph was reported by Aras et al. [91]. A gel electrophoresis–LIBS (SDS-PAGE–LIBS) system was constructed to recognize phosphoproteins (e.g. casein and ovalbumin) in protein mixtures, on the basis of the emission from phosphorus in the stained spots of the dried gel. Both coomassie brilliant blue and silver staining procedures were revealed to work. SDS-PAGE–LIBS was described as a technique of great promise in the determination of the phosphorylation sites of proteins, important in the study of the regulation processes of many biological systems. This tandem instrument is very promising, not only because it offers a combination of two selective instruments for the analysis of organic compounds, but also because these instruments are relatively simple and compact and thus the joint use of these instruments in the field is highly feasible.
Another case worthy of mention is the combination of hydride generation and LIBS (HG-LIBS), which was recently reported in three studies [92–94]. This is a special case because hydride generation has several functions in this combination: it serves as a liquid-to-gas conversion device, it separates the hydride-forming elements from other components, and it generates a signal enhancement. The analytical usefulness of this combined instrument was established for the analysis of Sn, As, Sb, and Se, but it will most probably also be applicable to other hydride-forming elements.
Another newly described combination is optical-coherence-tomography LIBS (OCT-LIBS). In a feasibility study, this combination was suggested for use in depth-resolved multi-layer pigment identification in paintings [95]. An OCT scan can provide high-resolution 3D images of the layers in the paintings, and can assist LIBS in depth-resolved elemental-composition studies by accurately measuring ablation-crater depths and ablation rates.
Trends in data evaluation
The path leading from sample composition to analytical signal in LIBS is a very complex one. This results in difficulty if accurate quantitative analysis is attempted, but also means that LIBS spectra contain a wealth of information related to the qualitative sample composition and experimental conditions. In addition to this, LIBS is a microanalytical technique, and as such each laser pulse generates a spectrum which cannot be obtained again in identical form; thus ideally as much as possible of the information contained in that unique dataset should be used. All these aspects, and more, mean that data processing and evaluation in LIBS is very important. In the limited page length allowed for this critical review, it is only possible to emphasize some current results in this vast field. To aid the discussion, the results are divided in three categories in accordance with conventional analytical spectroscopy, and also in accordance with the broad problems they deal with: namely spectrum correction (signal normalization, denoising, etc.), quantitative analysis (including calibration and matrix effects), and qualitative analysis (e.g. sample classification).
At this point it must be stressed that the current trend, in line with the ongoing processes in analytical spectroscopy, is that data-mining chemometric and statistical methods are more and more often applied to experimental LIBS data. This also means that, whereas data-evaluation tasks and problems were previously handled separately, current approaches tend to be multivariate and attempt to provide an integrated solution (the improvements are linked to each other). For example, the application of chemometric data-evaluation approaches is often found to improve not only precision, but also the accuracy of the analysis, e.g. by correcting for matrix effects. A new way of looking at analytical problems relevant to LIBS also comes with this. For example, matrix effects, which are an inherent and traditionally very important class of problem in LIBS, are becoming less and less important because they need not necessarily be detected and explicitly handled, but are rather automatically corrected for by appropriately training (setting up) a sufficiently flexible calibration model by using standards in different matrices. In addition, with the advance of electronics and computing technology, the pre-processing (correction) of the LIBS data, for S/N improvement, background correction, outlier removal, normalization, etc., is becoming default and standard. These new trends are manifested in the LIBS field because LIBS measurements can be relatively easily automated, and thus the field readily adopts relevant advances in computation science and chemometry. It must be added that all these advances unfortunately come with the danger of applying chemometrics simply as a “black box”, without a working knowledge of the capabilities and limitations of LIBS, which can lead to unreliable analytical results.
Spectrum correction
One disadvantageous characteristic of SP-LIBS is the mediocre repeatability of signals (shot-to-shot signal fluctuation); it is not uncommon for 20–30 % RSD repeatability values, based on a few repetitions, to be observed. One approach to mitigating the problem is to use the double or multi-pulse method, which improves repeatability to less than 10 % RSD, although at the expense of increased complexity and cost of instrumentation. Another, more practical approach is to greatly increase the number of repeated analyses to, e.g., 50–500; but the disadvantages of this method are the increased sample consumption and that the approach cannot be adapted to remote or mapping applications. Thus it is reasonable that a third approach, the correction of the spectrum by normalization, is the most popular in the LIBS community.
The concept of signal normalization is that it normalizes the whole spectrum or the intensity of a spectral line of interest to a related reference quantity (derived from the spectrum or measured independently), which is assumed to have correlated fluctuations, similar to the idea of internal-standard or line-pair methods [96]. The methods described in the literature vary substantially in the reference value used. In the LIBS literature of the past 10–15 years, publications using the acoustic signal originating from the shockwave generated by the ablation under ambient conditions, plasma temperature, electron number density, total integrated background signal, total integrated light, and laser pulse energy are most abundant. The field of signal normalization has been recently reviewed by Zorov et al. [97], with most of the examples taken from laser ablation and LIBS.
The current trend in LIBS is that instead of using just a single reference value in the normalization scheme, as was typical in earlier efforts, the normalization schemes used today are becoming multivariate, because of the realization that they are more efficient. Recent examples include the work of Feng et al. [98], who based their normalization on both plasma temperature and electron density, and Wang et al. [99], who proposed the use of an algorithm based on the combination of the intensities of selected atomic and ionic lines of the analyte. Worthy of mention is a series of papers on a novel scheme called spectrum normalization (standardization), also published by Wang and co-workers [100–102]. Their method defines a standard plasma state and uses Taylor expansion to calculate the theoretical spectral line intensities. The sum of multiple spectral line intensities for the measured element is then assumed to be proportional to the total number density of the analyte. The authors later expanded their method by incorporating partial least squares (PLS), and achieved ca. 3–8 % RSD.
In addition to normalization, research on other spectrum-correction methods has also produced novel results recently. For example, a high-performance background-correction algorithm (adopted from NMR spectroscopy) has been developed for LIBS [103]. The great advantage of this algorithm is that it requires no preliminary input from the analyst (thresholds, decisions, selections, etc.), and thus can be made fully automatic. In other noteworthy publications, algorithms based on wavelet transformation have been described and successfully used to suppress noise in LIBS spectra [104]. The improvement of S/N directly aids both qualitative and quantitative analyses.
Qualitative analysis
The comparison of spectra is a frequent qualitative analytical task in spectroscopy. In practice, the investigation of differences or similarities between sample spectra usually occurs in the context of one of the following scenarios: a.) monitoring of the deviation of the sample spectrum from a reference spectrum (discrimination); b.) sorting of samples, on the basis of their spectra, into predefined classes (classification); and c.) identification of a sample or compound by a database search (identification). For the fulfillment of the above tasks, also known as qualitative discriminant analysis, the statistical and chemometric literature generally suggests the following strategies or their combinations: direct comparison on the basis of the calculation of Euclidean distances, correlation coefficients, or direction cosines; classification of spectra on the basis of their Mahalanobis distances; pattern recognition; principal component analysis; and spectrum transformations (e.g. Fourier, Wavelet, and Hadamard). Before these operations, the spectra under scrutiny are usually corrected—that is, subjected to weighing, filtering, background correction, normalization, and outlier removal—or are reduced to aid the comparative metrics to be used by enhancing the characteristic spectral features.
The simplest predictors in LIBS that can be used for classification are the presence or absence of a spectral line in the spectrum or whether the ratio of spectral line intensities exceeds a specific threshold [105, 106]. The problem with such univariate approaches is that their success is contingent on a successful prior line assignation, which has to be supported by adequate spectral resolution and limits of detection [107]. The benefit of using more advanced (multivariate) chemometric techniques is that they simultaneously consider several features of the spectrum and thus are more robust and more efficient. They are also more useful for automatic applications using compact, portable LIBS systems. The most popular multivariate techniques in LIBS classification literature are still principal component analysis (PCA) and partial least squares (PLS) combined with discriminant analysis (DA), but other, newer chemometric techniques, including random forest (RF), support vector machines (SVM), and artificial neural networks (ANN), also show great promise. A list of classification methods used in recent LIBS literature is given in Table 2. A description or detailed evaluation of these methods cannot be provided here because it would vastly exceed the page limit of this review. Interested readers are therefore recommended to consult current chemometric books, for example a recent monograph, edited by Andrade-Garda [108], specifically written on applications in atomic spectrometry, including LIBS.
The classification model generated should also be correctly validated and its performance documented. As has been pointed out by El Haddad et al. [109], many LIBS publications still provide inadequate data on validation and performance. It has been suggested that the confusion matrix of the results should be presented in full and that figures of merit describing the success of classification and identification by a metric generally recognized (e.g. overall accuracy, robustness, or number of true and false-positive cases) should be described. Systematic studies comparing the performance of several classification methods on the same LIBS datasets are still rare [110–113], although these studies would be indispensable in assisting the selection of chemometric methods for wide use in LIBS.
The application fields where most of the recent LIBS qualitative discriminant analysis results were reported are forensics, biology, archeology, and some industrial fields (e.g. plastics sorting). Some examples of these results are mentioned in the applications section of this review.
Quantitative analysis
The analytical aspect at which LIBS is widely believed to excel over other atomic spectroscopy techniques is its ability to provide quick and reliable qualitative information about the sample composition. The quantitative capabilities of LIBS, however, are often described as modest and are regarded as a limitation hindering widespread analytical use. In reality, much of this sentiment, as has been clearly pointed out by Hahn and Omenetto [7], is usually unfair, because a comparison of the performance with that of other techniques cannot be done without taking into account the unique characteristics of LIBS. For example, the versatility and the lack of sample preparation, which are otherwise regarded as advantages, necessarily compromise the accuracy of the analysis. The absolute limits of detection achievable with LIBS are also far from unacceptable; they are already in the picogram–femtogram range and are soon expected to be further improved by signal enhancement and sub-micrometer sampling techniques. The comparison of the microsampling LIBS with bulk-sampling methods is often also meaningless, but nevertheless it has been repeatedly proved that LIBS can provide similar accuracy to that of either bulk-solid sampling (AAS, ICP-OES, XRF, and ICP-MS [64, 114–116]) or solid microsampling (μ-XRF, LA-ICP-MS [117–119]) methods, with the added benefit of being portable and available for remote measurements, etc.
The calibration-free method of analysis (CF-LIBS), developed by Tognoni et al. [120], is probably the quantitative method that has generated the most interest in the LIBS community over the years. This method attempts to provide concentration data for all elemental sample constituents from the measurements of the sample, without using any calibration standards. The method assumes local thermodynamic equilibrium (LTE) conditions, stoichiometric ablation, and an optically thin and homogeneous plasma, and calculates Boltzmann plots for essentially all (relevant) species in the plasma. The CF-LIBS method has been tested, complemented, and improved many times over the years; a complete list of applications and iterations can be found in two related recent reviews [121, 122]. The consensus is that the accuracy of the method is usually rather good for major components (e.g. those above 10 % concentration), although it only gives estimates for minor and trace components, in line with expectations. Another approach to standard-less LIBS calibration is the use of plasma modeling. Methods in this group essentially generate a simulated spectrum based on an LTE plasma model and search for the best fit between this simulated and experimentally recorded spectra. One of the better known attempts in this category is the method developed by Gornushkin et al. [123], which incorporates the plasma temperature and number densities for selected species as input variables and uses a Monte Carlo approach for the minimization of multivariate functions (MC-LIBS). Its accuracy was found to be somewhat poorer than that of CF-LIBS [124].
Generally speaking, chemometric calibration approaches are essentially analytical data-mining procedures, meaning their approach to calibration is such that they try to find a purely mathematical model which needs as little as possible (ideally none) preliminary information about the unknown sample or the analytical instrument and/or method used. This calibration approach is called soft modeling and is very different from hard modeling, which is the traditional way of developing calibration, in which the analyst sets up the calibration model building upon known physical and chemical relations and limitations of the instrument and uses sample preparation or selected standards to achieve selectivity, etc. (examples of hard-modeling calibration methods in LIBS include the CF-LIBS and MC-LIBS, discussed above). These two (hard and soft) fundamentally different calibration approaches coexist in current LIBS literature. In recent years, the number of LIBS papers on soft-modeling multivariate calibration methods has steadily increased. Table 2 gives a comprehensive list of quantitative chemometric methods reported in the literature. Again, detailed discussion of these methods is impossible here because of lack of space; for a detailed account see Ref. [108]. Most of these calibration methods use linear algebra to describe the relationship between the analyte concentration and the signal, despite the fact that LIBS calibration plots are often non-linear as a result of self absorption. Only a few authors have used non-linear methods, including artificial neural networks [125, 126], generalized linear correlation [63], or nonlinearized dominant-factor-based partial least squares (NDFPLS) [127, 128], and reported on improved accuracy and precision. Non-linear methods will most probably become more popular in the future, because in addition to their better accuracy and precision they also enable a wider concentration range to be used for calibration. This trend will also be motivated by the further expansion of the use of double and multi-pulse methods, which substantially expand the available dynamic range in LIBS.
Matrix effects, as is also general in, e.g., direct solid-sampling atomic spectrometry, have a strong effect on the accuracy of LIBS analysis. The presence of matrix effects is typically indicated when two matrices give calibration plots drawn over mass or mole fractions with different slopes (curvatures). These effects are usually related to the differences in either the ablation or excitation processes in the two matrices. The usual approach in correcting for these differences is signal normalization, and logically the normalization is most often based on reference signals relating either to the ablation process (e.g. the mass ablated) or to plasma variables (e.g. plasma temperature and electron number density). It was suggested by Aguilera et al. [129] that the volume of the plasma region observed and the total number of atoms in this volume also have to be considered when characterizing matrix effects or optimizing for their minimization. It is also worth pointing out that signal normalization using these same reference signals is also used with the purpose of improving the repeatability of LIBS signals, as was discussed in an earlier section. Thus, whenever signal normalization is used, it typically serves both purposes. It can also be stated that the fact that LIBS requires little if any sample preparation also makes the effective application of otherwise well-established methods of sample buffering impractical, especially with solid samples.
As was alluded to above, recent results suggest that multivariate quantitative chemometric strategies can automatically correct for matrix effects, if an appropriately large and diverse training sample set is used for setting up the model. The most systematic recent investigations of this subject were performed by the ChemCam team at Los Alamos [130, 131], because the Curiosity Mars Rover’s LIBS instrument needs to be able to perform highly automated, accurate quantitative analysis of a variety of unknown geological samples. Five different clustering and training selection algorithms were tested on LIBS spectra acquired for 195 rock samples and 31 pressed powder standards, and it was found that root mean squared errors (RMSE) of approximately 3 wt% in the quantitative analysis can be achieved without any prior knowledge about the unknown samples. It has to be added, though, that high-accuracy analyses without matrix-matched standards are still hard to imagine.
In the past couple of years most quantitative LIBS results have been reported in the field of soil, rock, and coal analysis. Some of these are covered in the applications section of this review. A highly useful tutorial paper was recently also published by El Haddad et al. [108], which gives an overview and advice concerning good and bad practice in quantitative (as well as qualitative) LIBS analysis.
Prominent applications
The total number of fields in which LIBS finds application are increasing steadily every year and there are also major applications for which the analytical features of LIBS seems to fit the requirements perfectly. Generally speaking, in the, e.g., biomedical, cultural-heritage, or space-exploration fields LIBS is used mostly because it can do things that other techniques cannot do; whereas in industry LIBS is used more and more because it does things better than other techniques. The following sub-sections give a brief, commented overview of some representative applications, focusing on some selected recent results and novel application fields (e.g. nanoparticle analysis), the investigation of which has just started. It would be impossible to attempt even a comprehensive overview, given that the total number of LIBS papers published per annum in recent years is well over a thousand and most of these are application-oriented studies. Thus the content of the following sections can be regarded as a subjective selection only. Table 3 gives a general overview of the most important application fields and sample types, based on a literature survey involving scientific papers published in 2010–2015. The indicated categories are by no means exclusive; several applications are of interest to more than one sector.
Biological and biomedical applications
In the last decade, LIBS has started to be increasingly applied to biological and biomedical samples. In this exciting application field, also called bio-LIBS, the main appeal of LIBS is quick, reagentless, non-contact, and virtually non-destructive analysis. The fact that LIBS can be easily combined with an optical microscope makes it relatively easy to incorporate it into other biological or medical instruments, and also enables highly localized or mapping-type analyses. Biological and biomedical LIBS applications are primarily qualitative in nature, but quantitative analyses also occur.
One of the most intensively studied subjects in bio-LIBS is the identification of pathogens (bacteria, viruses, spores, etc.) using multivariate chemometric methods. The great potential of LIBS here is that it does not require any a priori knowledge about the nucleic acid sequences or antibodies against known bacterial antigens, in contrast with established clinical diagnostic techniques. In addition to this, little or no sample preparation is needed. Successful discrimination of pathogenic from non-pathogenic bacteria has been achieved, including some multi-drug-resistant strains of bacteria including Staphylococcus aureus and other strains causing hospital-acquired infections (HAI) [112, 132]. Another advantage of LIBS-based identification of pathogens is that bacterial LIBS spectra have been revealed not to change with time as the culture ages, or on abiotic surfaces, or in killed or inactivated specimens [133]. A support vector machine classification model has been developed for the identification of anthrax spores [134].
Experiments on calcified and soft tissues were among the first biomedical LIBS applications, and activity in this field has continued over the past five years, mainly with the objective of tissue classification. Human bones, teeth, and a variety of stones (gallstones, cholesterol stones, kidney stones, etc.) are relatively often analyzed by LIBS and have been found to be uniquely identifiable by their elemental composition, and a recent study revealed that stones also contain carcinogenic elements [106, 135]. Healthy and carious human teeth were also successfully discriminated using PLS-DA data evaluation [136]. Recent studies related to soft tissues take advantage of the compatibility of LIBS with optical microscopy and its sensitivity to light elements, which make it particularly suitable for the analysis of sectioned tissue samples. Examples include the analysis of liver samples from suspected Wilson’s disease patients for their Cu content [137], and elemental mapping of murine kidneys for the study of renal clearance [138]. LIBS has also been used to characterize human malignant cells [139]. Quantitative human blood and urine [40] and hair and fingernail [140, 141] analysis are also among the continuously tested medical LIBS applications intended to provide information on the exposure or health status of patients.
The success of soft-tissue classification and identification by LIBS has recently led to the envisioning of futuristic surgical instruments that would use LIBS-based real-time tissue identification; this idea was also inspired by the concurrent success of a similar pilot instrument based on rapid evaporative mass spectrometry, termed “iKnife” [142]. Today, research into the development of a “LIBS-based iKnife” is becoming more and more intense. Ex-vivo surgical situations are created, and the qualitative performance of LIBS under these conditions is specifically tested [143, 144]. A further boost to LIBS-guided surgery is expected with the future incorporation of fiber lasers as the ablation source, replacing the bulky and inefficient pulsed Na:YAG lasers currently used in surgical devices.
In general, medical results published so far promise the development of novel LIBS-based clinical and point-of-care diagnostic instruments in the near future. LIBS analytical results related to plants can be directly used in other fields, including environmental, agricultural, or food analysis (e.g. accumulation of toxic elements or nutrients), and for the analysis of construction materials in industry (e.g. treated wood). The LIBS analysis of algae is also becoming more and more important because of the potential of algal biomass in biofuels and bioremediation [145]. Biological and medical LIBS studies have also been reviewed in recent, dedicated papers [146, 147].
Environmental and geological applications
Clearly, the main appeal of LIBS for environmental and geological applications is that the instrument can be made portable and provides highly flexible sample presentation, making quick analysis possible in the field. The community has therefore always been keen for environmental and geological applications. Most studies deal with the development of analytical methods for soil, rock and mineral, water, and aerosol analysis. Traditionally, quantitative applications dominate the field, but qualitative analytical applications (classification) are also becoming popular.
Most of the soil analytical LIBS applications focus on two tasks. One is the measurement of the carbon content of soil, which is also related to soil organic matter. These two quantities not only characterize the overall health and activity of soil (e.g. humification degree), but also determine its carbon-storage capacity, which is now believed to be a major factor affecting global climate. Consequently, a substantial part of LIBS activity in this field has gone into the development of suitable analytical methods for the measurement of total, organic, and/or inorganic carbon content [148] and the degree of humification of soil [149]. The other task related to LIBS soil analysis is the determination of nutrients and toxic constituents (e.g. heavy metals), which is of importance in precision farming and in soil remediation [150, 151]. Recently the analytical performance of LIBS for the determination of mercury content of soil was compared with that of another breakdown-spectroscopy method: spark-induced breakdown spectroscopy (SIBS) [152]. It was found that LIBS yields better results at higher concentrations, whereas SIBS is more suitable at lower concentrations (SIBS had a ca. 20 times lower limit of detection), although powder samples for SIBS and pellet samples for LIBS were compared. Current results from this field indicate that the highly heterogeneous composition and texture of different soil types often affect quantification results via matrix effects and spectral interference. For example, soils are often rich in Fe and Al, and thus spectral interference from these elements can prevent the use of intense carbon spectral lines at 247.86 nm and at 193.03 nm, especially in low-resolution portable LIBS systems. These problems require the use of spectrum correction or advanced calibration models [153]. Nevertheless, one outcome of related LIBS research is the realization that the conventional methods typically used in agrochemical practice (e.g. carbon oxidation by potassium dichromate) probably underestimate the humus content of soils.
Another large group of studies within this field serve geochemical projects. For example, geochemical fingerprinting by LIBS analysis has been recently successfully applied to conflict minerals including coltan [154] and to gemstones [155] to identify their geographic source (origin). Speleothems (e.g. stalagmites) can provide paleoclimatic information [156], which can also obtained in situ in caves by using portable LIBS instrumentation. Quantitative and classification LIBS studies on rock types are also abundant in the literature, especially in stand-off situations or rugged conditions (e.g. discrimination of volcanic rocks and magmatite series [157]), and also in the context of planetary and space exploration (e.g. analysis of meteorites [158, 159]). The most advanced, state-of-the art instrumentation and methods developed in this respect are best represented by the results of the highly successful ChemCam Mars Science Laboratory Rover project, described in the scientific team’s numerous recent publications (ChemCam Mars Science Laboratory Rover Project).
LIBS aerosol analysis is also an important field, mainly for reasons of environmental and health protection. The analysis of aerosols actually dates back to some of the earliest LIBS studies, but the community only acquired some fundamental knowledge of the processes involved in aerosol-LIBS signal generation in the last decade. The progress in this large field was reviewed by Hahn [52] a few years ago. Some noteworthy results of environmental LIBS aerosol analysis include development of methods for more efficient particle collection on a substrate [53], differentiation between gas-phase and particulate analytes [160], and a correlation study of heavy-metal concentrations in Asian dust and local pollution events [161].
The analysis of environmental water samples by LIBS is a difficult task, as was outlined in the instrumental section. Nevertheless, research into this subject is continuous because of the need for such data. The typical approach is not direct liquid analysis, but instead to soak up the water sample using sorbents (e.g. wood or paper) to convert the liquid matrix to a solid one and enhance sensitivity [162, 163]. Unfortunately, the limit of detection achieved in these studies is still only in the 0.1–1 ppm range, which may enable the monitoring of wastewater but is not sufficiently low for general environmental monitoring of water quality.
A comprehensive review on LIBS geochemical and environmental analysis was recently written by Harmon, Russo, and Hark [164].
Industry-oriented applications
LIBS publications related to (mainly) industrial applications are now very abundant; by the estimation of this author, nearly half of all papers discuss applications initiated by real or expected industrial needs. This also indicates how quickly LIBS technology is becoming an established, mainstream analytical technique. True industrial (factory floor) applications usually require specialized LIBS instrumentation, specifically designed for operation in harsh environments in a highly automated fashion, but most applications described in publications typically involve proof-of-concept procedures and instruments. Because of the huge amount of diverse industry-oriented publications, only selected topics can be briefly discussed here. Some topics have been omitted because they deal with sample types and industrial tasks which have been continually addressed by LIBS studies over the years. In this category is the analysis of alloys, plastics, wastes, ceramics and glasses, and nuclear plants. At the same time, there are a few topics in which the number of publications has strongly and steadily increased in recent years; only these topics will be mentioned here.
For example, coal and fly-ash analysis has become an increasingly popular topic in recent years. These samples are mainly investigated by Chinese researchers, and with reason. China is the largest consumer of coal in the world, and it is also the largest user of coal-derived electricity, both in absolute (kilowatt-hours) and relative measures (ca. 69 % of its electricity comes from coal). There is therefore an immediate need to implement instrumentation to enable a fast or online coal-property analysis to optimize combustion processes for power generation, and to provide a more reliable basis for coal pricing (not to mention related efforts towards the monitoring of carbon emissions). Several investigations have been performed with the objective of developing reliable, LIBS-based automatic instrumentation and quantitative analytical methods. The primary analytical objective was the determination of carbon in different sorts of coal and of the unburned carbon content of fly-ash samples, but methods have also been developed for the estimation of the calorific value, volatile content, and ash content [165, 166]. The best results have been achieved by using multivariate calibration methods (mainly PLS) and spectrum standardization, combined with the intensity correction of atomic carbon spectral line intensities by molecular emission for high-volatile-content coal samples [167]. The present general level of relative error achievable by these proximate LIBS analytical methods is approximately 2–10 %, which is promising but is still inferior to standard methods.
LIBS analysis of layers and coatings is of importance in many industrial sectors, but it is also a challenging task. Interestingly, thin-layer analytical publications in recent years have almost exclusively focused on the depth profiling of thin-film solar cells. These modern solar cells typically contain a copper, indium, gallium, and selenide (abbreviated as CIGS) light-absorbing layer of only 1–4 μm thickness, deposited on a glass or a flexible plastic substrate. LIBS is seen as a potential tool useful for monitoring the manufacturing process. Depth-profiling ns LIBS measurements of CIGS layers were successfully performed in several publications with better than 100 nm resolution, resulting from the strong light-absorption coefficient of CIGS layers [168, 169]. The depth sensitivity was further improved to approximately 3 nm by using a fs laser [170]. The elemental profiles recorded had the best agreement with profiles produced by other methods (e.g. secondary-ion mass spectrometry) when a 532 nm laser was used, as opposed to a 1064 nm laser.
Other industrial fields that have recently tended to use LIBS more and more include the food, healthcare, and other consumer-product industries. Quantitative and qualitative (identification and classification) analyses both occur in the literature, and the sample types have great variety. For example, toxic heavy metals and nutrients were determined in, e.g., orange, potato, wheat, milk powder, gelatin, breakfast cereals, and bakery products [171–173]. In addition, toys, hair dyes, talcum, coffee, and tobacco were analyzed for toxic elements. Organic contaminants, for example pesticides or salmonella, were also successfully detected in and on food products including spinach, rice, olive oil, cooking oil, and apple, and in personal-care products including lipstick [174, 175]. Pharmaceutical LIBS applications, for example the test of uniformity or migration of ingredients in tablets, continue to be relatively frequent.
It is also worth mentioning that combustion diagnostics also seems to be an upcoming field of application. Thermometry, mixture fraction determination, and gas-density measurement in different flames and injection engines have recently been successfully performed by LIBS [176, 177].
Application to nanomaterials
Nanomaterial synthesis and characterization and finding novel uses of nanomaterials is a research field that has been thriving in the past one or two decades worldwide. The unique physical and chemical characteristics of nanomaterials have already been exploited in many ways in science, and LIBS research is not an exception. Recently, LIBS and nanomaterials have started to be used jointly in some applications. Interestingly, the merging of the two fields is reciprocal: the scenario is either that LIBS is applied to the analysis of nanomaterials or that nanomaterials enhance and make possible specific LIBS applications.
Aerosol analysis, as alluded to before, was one of the first uses of LIBS, and therefore the analysis of ultrafine (≤0.1 μm) aerosols started relatively early [178]. The investigation of LIBS specifically for the online monitoring of nanoparticle (NP) streams in gas phase was reported by Amodeo et al. [179], successfully achieving quantitative analysis of silicon carbide NPs in the size range of 20 to 100 nm. Noll et al. reported size-resolved (20 to 800 nm) measurements of ultrafine particulate matter using LIBS for the analysis of calcium chloride particles [180]. More recently, Zhang et al. described a novel, low-fluence LIBS-based approach to monitor the growth of TiO2 NPs during flame synthesis [181]. A new electrostatic sampling method was described by Diwakar et al. [53], who pre-concentrated the aerosol NPs by first electrostatically charging them in a corona discharge created between microneedles and then depositing them on one of the microneedles. By changing the collection time, mass loading on the micro-needle tip could also be varied.
Laser-induced breakdown detection (LIBD) is an established technique used for counting NPs in colloidal suspensions. LIBD calculates the concentration of NPs by counting the breakdown probability when a focused laser pulse is directed into a colloidal suspension. Information on particle size is also obtained by measuring the breakdown threshold energy, which is determined from the breakdown probability measured as a function of the laser pulse energy. Common LIBD detection schemes include counting the plasma flashes captured by a CCD camera (optical LIBD) or detecting the shockwave generated in the sample cell (acoustic LIBD). Günther and co-workers recently studied the effect of dispersion agents on particle size and concentration data obtained by LIBD [182], and found a significant effect on the particle concentration when the particle concentration was below ca. 3 × 108 mL−1; particle size data was found to be less affected. LIBD has been coupled to asymmetric-field flow-field flow fractionation (A4F) for the detection of NPs and proteins [183]. The acoustic LIBD technique has also been successfully used as an on-line membrane-integrity-monitoring system during ultrafiltration processes [184]. The construction and application of mobile instrumentation has also been reported [185]. In general, it can be said that LIBD is a promising technique for NP detection, but further improvements and systematic fundamental studies are needed under more realistic conditions to fully assess its potential. For example, LIBD studies on polydisperse suspensions containing NPs of different composition (e.g. other than standard polystyrene beads) or in the presence of dissolved components are still very scarce. It is expected that with the integration of the DP-LIBS method and wavelength-resolved imaging, LIBD could evolve into a very powerful detection technique.
De Giacomo et al. have recently proposed nanoparticle-enhanced LIBS (NELIBS) to increase the LIBS signal [186]. NELIBS is based on the simple deposition of a noble metal NP suspension on the solid sample surface. For conducting samples, a signal enhancement of up to two orders of magnitude was observed. For transparent media, for example glasses, the authors propose that it is possible to obtain enhanced spectra by this approach without damaging or cracking the sample itself. In addition, a method to determine NP concentration and size by use of non-interacting substrates was also proposed [187]. Several fundamental aspects have already been studied by the authors, but further investigations are needed to elucidate the exact mechanisms behind the effect.
NPs have also been recently applied in conjunction with LIBS in biological samples for novel purposes. These applications indirectly exploit the fact that the breakdown threshold of NPs is orders of magnitude lower than that of water. For example, the injection of NPs into organ tissue facilitates the imaging of these samples, because the distribution of inorganic materials can be mapped without using any labeling. This concept has been successfully demonstrated by Motto-Roos and co-workers [138, 188, 189], who studied sectioned, epoxy-embedded animal kidney and tumor tissues which were chemically imaged with μm-range resolution by LIBS after the intravenous injection of Gd-containing ultrasmall (<5 nm) NPs into the animals.
The analysis of NP powders has also been reported recently. For example, a commercial, epoxy-urethane-based polymer has been proposed as sample-presentation solid matrix [190]. The authors found two characteristics of this matrix very useful for LIBS analysis: the polymer remains in a semi-liquid form until it is cured by applying UV light, meaning the NP powder can be thoroughly mixed with it to obtain a homogenous liquid; and the polymer gives very low spectral background in a wide wavelength range. The method was tested with magnetite and gold-coated magnetite NPs. In another interesting application, LIBS was successfully used to differentiate between carbon nanomaterials including multi and few-layer graphene, graphene oxide, and graphite [191] on the basis of their emission spectra, particularly their CN and C2 band emission.
Archeological and cultural-heritage applications
The use of LIBS in the archeological and cultural-heritage field also offers particular benefits. It is a rapid and portable technique, meaning that a large sample throughput can be achieved and artefacts in the field (e.g. on excavations) can also be analyzed. It is an in-situ, non-contact, and nearly non-destructive technique, and these are all important features in the case of precious art items (paintings, antique jewelry, pottery, etc.) kept in museums or art collections. Qualitative analytical applications are most abundant in this field. In addition, laser ablation can also be used for cleaning artworks, and LIBS can not only provide the laser beam for this purpose, but can also be used as a means of real-time monitoring of the process to avoid over-cleaning.
Pigment analysis is a continuing application in this field, because localized identification of colorants can not only help restoration but can also provide chronological information, because some pigments have been in use only since specific dates. Pigments are usually inorganic compounds, because these have higher stability than organic dyes, and thus give rise to line-rich, characteristic LIBS spectra that facilitate their identification. Over the years many successful applications have been described on this topic. In recent years the number of LIBS papers on pigment analysis has decreased, but they are still present in the literature. The interest in the field seems to have somewhat shifted towards porcelain pigments [192] and glass chromophores [193]. Novel tandem instruments, for example Raman–LIBS, have also found well-suited applications in this field, as already alluded to in the instrumental section of this review. A recent example of this trend is the state-of-the-art combined instrument incorporating optical-coherence tomography and LIBS (OCT-LIBS) [95], which was specifically constructed to aid multilayer pigment identification in paintings. This instrument enables the fast, high-resolution, non-destructive visualization of stratigraphy in paintings and makes depth-profiling substantially more precise.
Another topic recently becoming popular is the provenance or chronological study of archeological construction materials [194, 195]; such studies increasingly use multivariate statistical methods, mainly PCA, DA, or SIMCA, to improve their accuracy. Archeological bone and teeth analysis by LIBS is also frequent in the literature; in recent years these studies have tended to investigate the degradation and/or diagenesis processes of such artefacts [196, 197].
Although LIBS is more and more becoming an established technique in the archeological and cultural-heritage fields, studies that compare LIBS results with those produced by standard instruments, for example XRF, LA-TOFMS, and EDS, are still frequent [193, 198]. The comparisons are generally positive for LIBS, and the studies always mention that an added benefit of LIBS is that it can be made portable. A further development in this direction was made in a study [199] that suggested and tested the use of stand-off LIBS directly on an excavation site in an attempt to make archeological field work less damaging and time-consuming by quickly classifying the type of objects found.
An upcoming, novel branch of archeological LIBS applications is the study of artefacts (e.g. shipwrecks) at submarine sites. The Laserna group at the University of Malaga (Spain) are most active in this field. Their first, prototype LIBS spectrometer was onboard a ship, where it was connected to a handheld probe underwater by a 50 m optical fiber. The probe was kept in contact with the artefact, and a jet of compressed air was used to remove water from between the probe and the sample, thereby creating an environment in which sensitive analysis was possible [31]. For deep-sea archeological sites, where the use of a fiber-optic umbilical is not feasible, the complete instrument will have to be sent underwater and a telescopic stand-off LIBS setup will have to be used. This concept was recently successfully demonstrated in the laboratory by using underwater targets in a tank at distances up to 80 cm. Multi-pulse excitation was also tested, and it was found that this mode results in improved performance of the equipment in terms of extended range of analysis, fifteen-times-enhanced signals, and the capability of analyzing a broader variety of samples [200, 201].
Results of the archeological LIBS field were very recently reviewed by Spizzichino and Fantoni [202].
Forensic analysis
Several different LIBS applications, all related to the chemical evaluation of materials in forensic cases, fraud detection, anti-terrorism efforts, post-disaster activities, etc., can be considered here under the inclusive heading of forensic analysis. Among the main advantages of LIBS for these applications are quickness of analysis, potential field portability, the ability to provide characteristic spectral fingerprint information for classification and/or identification purposes, and, last but not least, the potential for the investigation of dangerous materials from a safe distance and/or in safe microscopic quantities. By their nature, these applications typically require qualitative analysis.
The overwhelming majority of publications in the field in the past few years focus on the detection and classification of residues of explosives and other high-energy materials. A series of studies investigated the elimination of the effects imposed by the substrate (e.g. organic, metallic, or polymer) and conditions used in LIBS analysis on the success of classification [203, 204]. The discrimination capabilities and robustness of a range of multivariate chemometric methods were tested. Stand-off and mobile LIBS instruments, tandem Raman–LIBS instrumentation [83, 205], and novel sample-presentation methods (e.g. optical catapulting [32]) were also developed for this specific application. It was suggested that the LIBS identification of the origin of explosives was facilitated by the addition of coding additives (e.g. aluminium alloy powders) containing rare earth elements in dosed amounts at the time of explosive manufacture [206]. Nuclear threats were also targeted by developing field-deployable instrumentation [207] and isotope-selective data-evaluation methods for low-resolution spectrometers [208].
LIBS has also been tested in a variety of other forensic investigations, including document discrimination [88, 209, 210], fraud detection of a variety of industrial products [211], soil fingerprinting [212, 213], and the study of human remains [214, 215] or of samples taken from suspects [216]. The success of all these applications heavily relies on the performance of the multivariate chemometric methods used.
Applications under extreme conditions
Some LIBS applications require the elemental analysis to be performed under extreme conditions (e.g. at extreme pressures and/or temperatures), or in hard-to-reach locations, where remote (also called stand-off) analysis is the only option. These analytical situations can occur, e.g., in some industrial fields, oceanography, and space and planetary exploration. The fact that LIBS spectrometers can be made robust, compact, and lightweight and that they can offer remote analysis makes them very valuable for such applications.
The peak of these applications is LIBS space probes. Several space missions in progress or in planning include a LIBS (or Raman–LIBS) instrument placed on board rovers. Most of the documented LIBS work on space applications to date has been performed in laboratories, under conditions simulating the surface and atmosphere of the extraterrestrial objects to be visited by the probe. These conditions include, for example, ca. 25 °C to −60 °C temperature and 7 Torr CO2 for Mars, ca. 125 °C to −230 °C temperature and near vacuum for the Moon, and ca. 460 °C and 90 atm CO2 for Venus. Some of the findings of these studies related to the gas atmosphere were alluded to in the “Gas atmosphere” section. Increased sample temperature was generally found to increase LIBS signal intensities and improve S/N ratios [217]. The instrumentation and methods developed for space LIBS applications are best represented in the numerous publications related to the ChemCam Mars Science Laboratory Rover Project.
Submarine and deep-ocean applications require measurements in a high-pressure liquid environment. The conditions under which the double-pulse method can be best exploited were recently investigated in detail by Lawrence-Snyder et al. [218] for up to 50 bar liquid pressures. It was found that DP-LIBS emission is inversely proportional to the pressure of the liquid, and the maximum emission can be obtained when the interpulse delay is set in such a way that the second laser pulse hits the vapor bubble generated by the first pulse when it has the largest diameter. Recent carbon-sequestration studies assess the possibility of using LIBS for monitoring purposes in deep saline aquifers, which requires measurements in a high-pressure water–CO2 mixture [219, 220]. It was found that elemental emissions in the liquid were not significantly affected by the presence of dissolved of CO2, and thus LIBS was regarded as a viable tool for this monitoring task.
Concluding remarks
Current trends in the literature suggest that future progress in instrumentation will focus on the use of the double and multiple-pulse methods, femtosecond laser pulses, and the incorporation of fiber lasers into setups. LIBS instrumentation will perhaps also benefit from new optoelectronic detectors, including electron-multiplying CCD and scalable complementary metal-oxide semiconductors. Tandem LIBS instrumentation will become more common as a result of the expected commercialization. It also seems certain that chemometric multivariate data-evaluation methods will soon become standard in analytical LIBS, for both quantitative and qualitative purposes. In terms of applications, industrial and biomedical implementations will most probably expand rapidly, and nanomaterial analysis, the development of microscale sample-preparation methods for selectivity improvement, chemical imaging, and remote measurements are also expected to make major contributions to the progress of the field in coming years.
I vividly remember the 1990s, when LIBS was an almost pitied technique in atomic spectrometry because of the general view that it is an unreliable and insensitive technique. In those years, the LIBS field was much overshadowed by the popular techniques of the time, e.g. ICP-AES and ICP-MS. As is hopefully reflected in this review, those years are long over; nowadays LIBS is the hot topic in atomic spectrometry. It is estimated that LIBS easily produces more publications per annum than any other technique in elemental analysis. The future also looks bright for LIBS, because its unique set of analytical features will continue to enable the development of novel applications and new methods for many years to come.
References
Winefordner JD, Gornushkin IB, Correll T, Gibb E, Smith BW, Omenetto N (2004) Comparing several atomic spectrometric methods to the super stars: special emphasis on laser induced breakdown spectroscopy, a future super star. J Anal At Spectrom 19:1061–1083
Randall DW, Hayes RT, Wong PA (2013) A simple laser induced breakdown spectroscopy (LIBS) system for use at multiple levels in the undergraduate chemistry curriculum. J Chem Educ 90:456–462
Cremers LJ, Radziemski DA (2006) Handbook of laser-induced breakdown spectroscopy. Wiley
Miziolek AW, Palleschi V, Schechter I (eds) (2006) Laser-induced breakdown spectroscopy: fundamentals and applications. Cambridge University Press
Fortes FJ, Moros J, Lucena P, Cabalin LM, Laserna JJ (2013) Laser-induced breakdown spectroscopy. Anal Chem 85:640–669
Hahn DW, Omenetto N (2010) Laser-induced breakdown spectroscopy (LIBS), Part I: review of basic diagnostics and plasma-particle interactions: still challenging issues within the analytical plasma community. Appl Spectrosc 64:335A–366A
Hahn DW, Omenetto N (2012) Laser-induced breakdown spectroscopy (LIBS), Part II: review of instrumental and methodological approaches to material analysis and applications to different fields. Appl Spectrosc 66:347–419
Musazzi S, Perini U (eds) (2014) Laser-induced breakdown spectroscopy: theory and applications. Series in Optical Sciences 182, Springer
Cabalin LM, Laserna JJ (1998) Experimental determination of laser induced breakdown thresholds of metals under nanosecond Q-switched laser operation. Spectrochim Acta B 53:723–730
Cremers DA, Multari RA, Knight AK (2011) Laser-induced breakdown spectroscopy. In: Meyers RA (ed) Encyclopedia of analytical chemistry. Wiley
Mao X, Russo RE (1997) Observation of plasma shielding by measuring transmitted and reflected laser pulse temporal profiles. Appl Phys A 64:1–6
Koch J, Günther D (2011) Review of the state-of-the-art of laser ablation inductively coupled plasma mass spectrometry. Appl Spectrosc 65:155–162
Gill RK, Knorr F, Smith ZJ, Kahraman M, Madsen D, Larsen DS, Wachsmann-Hogiu S (2014) Characterization of femtosecond laser-induced breakdown spectroscopy (fs-LIBS) and applications for biological samples. Appl Spectrosc 68:949–954
Almaviva S, Fantoni R, Caneve L, Colao F, Fornarini L, Santagata A, Teghil R (2014) Use of ns and fs pulse excitation in laser-induced breakdown spectroscopy to improve its analytical performances: a case study on quaternary bronze alloys. Spectrochim Acta B 99:185–192
Lu Y, Zorba V, Mao X, Zheng R, Russo RE (2013) UV fs-ns double-pulse laser induced breakdown spectroscopy for high spatial resolution chemical analysis. J Anal At Spectrom 28:743–748
Harilal SS, Freeman JR, Diwakar P, Hassanein A (2013) Comparison of nanosecond and femtosecond LIBS, CLEO: science and innovation, Technical Digest, Paper CTu2H.8, doi: 10.1364/CLEO_SI.2013.CTu2H.8
Zuclich JA, Lund DJ, Stuck BE (2007) Wavelength dependence of ocular damage thresholds in the near-IR to far-IR transition region: proposed revisions to MPES. Health Phys 92:15–23
Gornushkin IB, Amponsah-Manager K, Smith BW, Omenetto N, Winefordner JD (2004) Microchip laser-induced breakdown spectroscopy: a preliminary feasibility investigation. Appl Spectrosc 58:762–769
Freedman A, Iannarilli FJ Jr, Wormhoudt JC (2005) Aluminum alloy analysis using microchip-laser induced breakdown spectroscopy. Spectrochim Acta B 60:1076–1082
Huang H, Yang LM, Liu J (2012) Qualitative assessment of laser-induced breakdown spectra generated with a femtosecond fiber laser. Appl Opt 51:8669–8676
Gravel JFY, Doucet FR, Bouchard P, Sabsabi M (2011) Evaluation of a compact high power pulsed fiber laser source for laser-induced breakdown spectroscopy. J Anal At Spectrom 26:1354–1361
Mueller M, Gornushkin IB, Florek S, Mory D, Panne U (2007) Approach to detection in laser-induced breakdown spectroscopy. Anal Chem 79:4419–4426
Sabsabi M, Heon R, St- Onge L (2005) Critical evaluation of gated CCD detectors for laser-induced breakdown spectroscopy analysis. Spectrochim Acta B 60:1211–1216
Effenberger AJ, Scott JR (2012) Practical high-resolution detection method for laser-induced breakdown spectroscopy. Appl Opt 51:B165–B170
Melikechi N, Mezzacappa A, Cousin A, Lanza NL, Lasue J, Clegg SM et al (2014) Correcting for variable laser-target distances of laser-induced breakdown spectroscopy measurements with ChemCam using emission lines of Martian dust spectra. Spectrochim Acta B 96:51–60
Han ZY, Pan CY, An N, Du XW, Yu YS, Du LL, Wang SB, Wang QP (2015) The auto-focusing remote laser-induced breakdown spectroscopy system. Spectrosc Spectral Anal 35:304–308
Banerjee SP, Chen Z, Fedosejevs R (2014) High resolution scanning microanalysis on material surfaces using UV femtosecond laser induced breakdown spectroscopy. Opt Lasers Eng 68:1–6
Zorba V, Mao X, Russo RE (2011) Ultrafast laser induced breakdown spectroscopy for high spatial resolution chemical analysis. Spectrochim Acta B 66:189–192
Zeng Q, Guo L, Li X, He C, Shen M, Li K, Duan J, Zeng X, Lu Y (2015) Laser-induced breakdown spectroscopy using laser pulses delivered by optical fibers for analyzing Mn and Ti elements in pig iron. J Anal At Spectrom 30:403–409
Saeki M, Iwanade A, Ito C, Wakaida I, Thornton B, Sakka T, Ohba H (2014) Development of a fiber-coupled laser-induced breakdown spectroscopy instrument for analysis of underwater debris in a nuclear reactor core. J Nucl Sci Technol 51:930–938
Guirado S, Fortes FJ, Lazic V, Laserna JJ (2012) Chemical analysis of archeological materials in submarine environments using laser-induced breakdown spectroscopy. On-site trials in the Mediterranean Sea. Spectrochim Acta B 74–75:137–143
Abdelhamid M, Fortes FJ, Harith MA, Laserna JJ (2011) Analysis of explosive residues in human fingerprints using optical catapulting–laser-induced breakdown spectroscopy. J Anal At Spectrom 26:1445–1450
Fortes FJ, Fernandez-Bravo A, Laserna JJ (2014) Chemical characterization of single micro- and nano-particles by optical catapulting-optical trapping-laser-induced breakdown spectroscopy. Spectrochim Acta B 100:78–85
Lazic V, Jovicevic S (2014) Laser induced breakdown spectroscopy inside liquids: processes and analytical aspects. Spectrochim Acta B 101:288–311
Aras N, Yesiller SU, Ates DA, Yalcin S (2012) Ultrasonic nebulization-sample introduction system for quantitative analysis of liquid samples by laser-induced breakdown spectroscopy. Spectrochim Acta B 74–75:87–94
Cheri MS, Tavassoli SH (2011) Quantitative analysis of toxic metals lead and cadmium in water jet by laser-induced breakdown spectroscopy. Appl Opt 50:1227–1233
Groh S, Diwakar PK, Garcia CC, Murtazin A, Hahn DW, Niemax K (2010) 100% efficient sub-nanoliter sample introduction in laser-induced breakdown spectroscopy and inductively coupled plasma spectrometry: implications for ultralow sample volumes. Anal Chem 82:2568–2573
Yu X, Li Y, Gu X, Bao J, Yang H, Sun L (2014) Laser-induced breakdown spectroscopy application in environmental monitoring of water quality: a review. Environ Monit Assess 186:8969–8980
Al-Adel FF, Dastageer MA, Gasmi K, Gondal MA (2013) Optimization of a laser induced breakdown spectroscopy method for the analysis of liquid samples. J Appl Spectrosc 80:767–770
Metzinger A, Kovács‐Széles É, Almási I, Galbács G (2014) An assessment of the potential of laser induced breakdown spectrometry for the analysis of cesium in liquid samples of biological origin. Appl Spectrosc 68:789–793
Sarkar A, Aggarwal SK, Sasibhusan K, Alamelu D (2010) Determination of sub-ppm levels of boron in ground water samples by laser induced breakdown spectroscopy. Microchim Acta 168:65–69
Lee Y, Oh SW, Han SH (2012) Laser-induced breakdown spectroscopy (LIBS) of heavy metal ions at the subparts per million level in water. Appl Spectrosc 66:1385–1396
Sawaf S, Tawfik W (2014) Analysis of heavy elements in water with high sensitivity using laser induced breakdown spectroscopy. Optoelectron Adv Mater 8:414–417
Aguirre MA, Legnaioli S, Almodóvar F, Hidalgo M, Palleschi V, Canals A (2013) Elemental analysis by surface-enhanced laser-induced breakdown spectroscopy combined with liquid-liquid microextraction. Spectrochim Acta B 79–80:88–93
Aguirre MA, Nikolova H, Hidalgo M, Canals A (2015) Hyphenation of single-drop microextraction with laser-induced breakdown spectrometry for trace analysis in liquid samples: a viability study. Anal Methods 7:877–883
Aguirre MA, Selva EJ, Hidalgo M, Canals A (2015) Dispersive liquid-liquid microextraction for metals enrichment: a useful strategy for improving sensitivity of laser-induced breakdown spectroscopy in liquid samples analysis. Talanta 131:348–353
de Jesus AMD, Angel Aguirre M, Hidalgo M, Canals A, Pereira-Filho ER (2014) The determination of V and Mo by dispersive liquid-liquid microextraction (DLLME) combined with laser-induced breakdown spectroscopy (LIBS). J Anal At Spectrom 29:1813–1818
Zhao F, Chen ZM, Zhang FP, Li RH, Zhou JY (2010) Ultra-sensitive detection of heavy metal ions in tap water by laser-induced breakdown spectroscopy with the assistance of electrical-deposition. Anal Methods 2:408–414
Dockery CR, Pender JE, Goode SR (2010) Speciation of chromium via laser-induced breakdown spectroscopy of ion exchange polymer membranes. Appl Spectrosc 59:252–257
Zhang X, Deguchi Y, Wang Z, Yana J, Liu J (2014) Sensitive detection of iodine by low pressure and short pulse laser-induced breakdown spectroscopy (LIBS). J Anal At Spectrom 29:1082–1089
Dikshit V, Yueh FY, Singh JP, McIntyre DL, Jain JC, Melikechi N (2012) Laser induced breakdown spectroscopy: A potential tool for atmospheric carbon dioxide measurement. Spectrochim Acta B 68:65–70
Hahn DW (2009) Laser-induced breakdown spectroscopy for analysis of aerosol particles: the path toward quantitative analysis. Spectroscopy 24:26–33
Diwakar K, Kulkarni P, Birch ME (2012) New approach for near-real-time measurement of elemental composition of aerosols using laser induced breakdown spectroscopy. Aerosol Sci Technol 46:316–332
Babushok V, Delucia FC Jr, Gottfried JL, Munson CA, Miziolek AW (2006) Double pulse laser ablation and plasma: laser induced breakdown spectroscopy signal enhancement. Spectrochim Acta B 61:999–1014
Scaffidi J, Angel SM, Cremers DA (2006) Emission enhancement mechanisms in dual-pulse laser-induced breakdown spectroscopy. Anal Chem 78:24–32
Yang G, Lin Q, Ding Y, Tian D, Duan Y (2015) Laser induced breakdown spectroscopy based on single beam splitting and geometric configuration for effective signal enhancement. Sci Rep 5:7625
Coons RW, Harilal SS, Hassan SM, Hassanein A (2012) The importance of longer wavelength reheating in dual-pulse laser-induced breakdown spectroscopy. Appl Phys B 107:873–880
Jedlinszki N, Galbacs G (2011) An evaluation of the analytical performance of collinear multi-pulse laser induced breakdown spectroscopy. Microchem J 97:255–263
Tognoni E, Cristoforetti G (2014) Basic mechanisms of signal enhancement in ns double-pulse laser-induced breakdown spectroscopy in a gas environment. J Anal At Spectrom 29:1318–1338
Zhang K, Jiang L, Li X, Shi X, Yu D, Qu L, Lu Y (2014) Femtosecond laser pulse-train induced breakdown in fused silica: the role of seed electrons. J Phys D 47:435105
Galbács G, Budavári V, Geretovszky ZS (2005) Multi-pulse laser-induced plasma spectroscopy using a single laser source and a compact spectrometer. J Anal At Spectrom 20:974–980
Galbács G, Jedlinszki N, Herrera K, Omenetto N, Smith BW, Winefordner JD (2010) A study of ablation, spatial, and temporal characteristics of laser-induced plasmas generated by multiple collinear pulses. Appl Spectrosc 64:161–172
Galbács G, Jedlinszki N, Cseh G, Galbács Z, Túri L (2008) Accurate quantitative analysis of gold alloys using multi-pulse laser induced breakdown spectroscopy and a correlation-based calibration method. Spectrochim Acta B 63:591–597
Galbács G, Jedlinszki N, Metzinger A (2013) Analysis and discrimination of soldering tin samples by collinear multi-pulse laser induced breakdown spectrometry, supported by inductively coupled plasma optical emission and mass spectrometry. Microchem J 107:17–24
Hoechse M, Gornushkin I, Merk S, Panne U (2011) Assessment of suitability of diode pumped solid state lasers for laser induced breakdown and Raman spectroscopy. J Anal At Spectrom 26:414–424
Guirado S, Fortes FJ, Cabelin LM, Laserna JJ (2014) Effect of pulse duration in multi-pulse excitation of silicon in laser-induced breakdown spectroscopy (LIBS). Appl Spectrosc 68:1060–1066
Effenberger AJ Jr, Scott JR (2010) Effect of Atmospheric Conditions on LIBS spectra (review). Sensors 10:4907–4925
Son JG, Choi SC, Oh MK, Kang H, Suk H, Lee Y (2010) Application of pulsed buffer gas jets for the signal enhancement of laser-induced breakdown spectroscopy. Appl Spectrosc 64:1289–1297
Gornushkin IB, Stevenson CL, Galbács G, Smith BW, Winefordner JD (2003) Measurement and modeling of ozone and nitrogen oxides produced by laser breakdown in oxygen-nitrogen atmospheres. Appl Spectrosc 57:1442–1450
Liu Y, Baudelet M, Richardson M (2010) Elemental analysis by microwave-assisted laser-induced breakdown spectroscopy: evaluation on ceramics. J Anal At Spectrom 25:1316–1323
Liu Y, Bousquet B, Baudelet M, Richardson M (2012) Improvement of the sensitivity for the measurement of copper concentrations in soil by microwave-assisted laser-induced breakdown spectroscopy. Spectrochim Acta B 73:89–92
Tampo M, Miyabe M, Akaoka K, Oba M, Ohba H, Maruyama Y, Ikuo W (2014) Enhancement of intensity in microwave-assisted laser-induced breakdown spectroscopy for remote analysis of nuclear fuel recycling. J Anal At Spectrom 29:886–892
Yeates P, Kennedy E (2010) Spectroscopic, imaging, and probe diagnostics of laser plasma plumes expanding between confining surfaces. J Appl Phys 108:093306
Yin H, Hou Z, Yuan T, Wang Z, Ni W, Li Z (2015) Application of spatial confinement for gas analysis using laser-induced breakdown spectroscopy to improve signal stability. J Anal At Spectrom 30:922–928
Guo L, Li C, Hu W, Zhou Y, Zhang B, Cai Z, Zeng X, Lu Y (2011) Plasma confinement by hemispherical cavity in laser-induced breakdown spectroscopy. Appl Phys Lett 98:131501
Li C, Guo L, He X, Hao Z, Li X, Shen M, Zeng X, Lu Y (2014) Element dependence of enhancement in optics emission from laser-induced plasma under spatial confinement. J Anal At Spectrom 29:638–643
Hao ZQ, Guo LB, Li CM, Shen M, Zhou XH, Li XY, Lu YF, Zeng XY (2014) Sensitivity improvement in the detection of V and Mn elements in steel using laser-induced breakdown spectroscopy with ring-magnet confinement. J Anal At Spectrom 29:2309–2314
Hoehse M, Mory D, Florek S, Weritz F, Gornushkin I, Panne U (2009) A combined laser-induced breakdown and Raman spectroscopy Echelle system for elemental and molecular microanalysis. Spectrochim Acta B 64:1219–1227
Lin Q, Niu G, Wang Q, Yu Q, Duan Y (2013) Combined laser-induced breakdown with Raman spectroscopy: historical technology development and recent applications. Appl Spectrosc Rev 48:487–508
Moros J, Laserna JJ (2015) Unveiling the identity of distant targets through advanced Raman-laser-induced breakdown spectroscopy data fusion strategies. Talanta 134:627–639
Bicchieri M, Nardone M, Russo P, Sodo A, Corsi M, Cristoforetti G, Palleschi V, Salvetti A, Tognoni E (2011) Characterization of azurite and lazurite based pigments by laser induced breakdown spectroscopy and micro-Raman spectroscopy. Spectrochim Acta B 56:915–922
Osticioli I, Mendes NF, Porcinai S, Cagnini A, Castellucci E (2009) Spectroscopic analysis of works of art using a single LIBS and pulsed Raman setup. Anal Bioanal Chem 394:1033–1041
Moros J, Lorenzo JA, Lucena P, Tobaria LM, Laserna JJ (2010) Simultaneous Raman spectroscopy − laser-induced breakdown spectroscopy for instant standoff analysis of explosives using a mobile integrated sensor platform. Anal Chem 82:1389–1400
Moros J, Lorenzo JA, Laserna JJ (2011) Standoff detection of explosives: critical comparison for ensuing options on Raman spectroscopy–LIBS sensor fusion. Anal Bioanal Chem 400:3353–3365
Matroodi F, Tavassoli SH (2015) Experimental investigation on concurrent laser-induced breakdown spectroscopy Raman spectroscopy. Appl Opt 54:400–407
Sharma SK, Misra AK, Lucey PG, Lentz RCF (2009) A combined remote Raman and LIBS instrument for characterizing minerals with 532 nm laser excitation. Spectrochim Acta A 73:468–476
Clegg SM, Wiens R, Misra AK, Sharma SK, Lambert J, Bender S, Newell R, Nowak-Lovato K, Smrekar S, Dyar MD, Maurice S (2014) Planetary geochemical investigations using Raman and laser-induced breakdown spectroscopy. Appl Spectrosc 68:925–936
Subedi K, Trejos T, Almirall J (2015) Forensic analysis of printing inks using tandem laser induced breakdown spectroscopy and laser ablation inductively coupled plasma mass spectrometry. Spectrochim Acta B 103–104:76–83
Chirinos JR, Oropeza DD, Gonzalez JJ, Hou H, Morey M, Zorba V, Russo RE (2014) Simultaneous 3-dimensional elemental imaging with LIBS and LA-ICP-MS. J Anal At Spectrom 29:1292–1298
Glaus R, Hahn DW (2014) Double-pulse laser ablation coupled to laser-induced breakdown spectroscopy. Spectrochim Acta B 98:48–53
Aras N, Yalcın S (2014) Rapid identification of phosphorus containing proteins in electrophoresis gel spots by laser-induced breakdown spectroscopy. J Anal At Spectrom 29:545–552
Yesiller SU, Yalcin S (2013) Optimization of chemical and instrumental parameters in hydride generation laser-induced breakdown spectrometry for the determination of arsenic, antimony, lead and germanium in aqueous samples. Anal Chim Acta 770:7–17
Simeonsson JB, Williamson LJ (2011) Characterization of laser induced breakdown plasmas used for measurements of arsenic, antimony and selenium hydrides. Spectrochim Acta B 66:754–760
Unal S, Yalcin S (2010) Development of a continuous flow hydride generation laser-induced breakdown spectroscopic system: determination of tin in aqueous environments. Spectrochim Acta B 65:750–757
Kaszewska EA, Sylwestrzak M, Marczak J, Skrzeczanowski W, Iwanicka M, Szmit-Naud E, Anglos D, Targowski P (2013) Depth-resolved multilayer pigment identification in paintings: combined use of laser-induced breakdown spectroscopy (LIBS) and optical coherence tomography (OCT). Appl Spectrosc 67:960–972
Labutin TA, Zaytsev SM, Popov AM, Seliverstova IV, Bozhenko SE, Zorov NB (2013) Comparison of the thermodynamic and correlation criteria for internal standard selection in laser-induced breakdown spectrometry. Spectrochim Acta B 87:57–64
Zorov NB, Gorbatenko AA, Labutin TA, Popov AM (2010) A review of normalization techniques in analytical atomic spectrometry with laser sampling: from single to multivariate correction. Spectrochim Acta B 65:642–657
Feng J, Wang Z, Li Z, Ni W (2010) Study to reduce laser-induced breakdown spectroscopy measurement uncertainty using plasma characteristic parameters. Spectrochim Acta B 65:549–556
Hou Z, Wang Z, Lui SL, Yuan T, Li L, Li Z, Ni W (2013) Improving data stability and prediction accuracy in laser-induced breakdown spectroscopy by utilizing a combined atomic and ionic line algorithm. J Anal At Spectrom 28:107–113
Wang Z, Li L, West L, Li Z, Ni W (2012) A spectrum standardization approach for laser-induced breakdown spectroscopy measurements. Spectrochim Acta B 68:58–64
Li L, Wang Z, Yuan T, Hou Z, Li Z, Ni W (2011) A simplified spectrum standardization method for laser-induced breakdown spectroscopy measurements. J Anal At Spectrom 26:2274–2280
Li X, Wang Z, Lui SL, Fu Y, Li Z, Liu J, Ni W (2013) A partial least squares based spectrum normalization method for uncertainty reduction for laser-induced breakdown spectroscopy measurements. Spectrochim Acta B 88:180–185
Yaroshchyk P, Eberhardt JE (2014) Automatic correction of continuum background in laser-induced breakdown spectroscopy using a model-free algorithm. Spectrochim Acta B 99:138–149
Schlenke J, Hildebrand L, Moros J, Laserna JJ (2012) Adaptive approach for variable noise suppression on laser-induced breakdown spectroscopy responses using stationary wavelet transform. Anal Chim Acta 754:8–19
Aono Y, Ando K, Hattori N (2012) Rapid identification of CCA-treated wood using laserinduced breakdown spectroscopy. J Wood Sci 58:363–368
Oztoprak BG, Gonzalez J, Yoo J, Gulecen T, Mutlu N, Russo RE, Gundogdu O, Demir A (2012) Analyis and classification of heterogeneous kidney stones using laser induced breakdown spectroscopy 66:1353–1361
Amato G, Cristoforetti G, Legnaioli S, Lorenzetti G, Palleschi V, Sorrentino F, Tognoni E (2010) TI Progress towards an unassisted element identification from Laser Induced breakdown spectra with automatic ranking techniques inspired by text retrieval. Spectrochim Acta B 65:664–670
Andrade-Garda JM (2013) Basic chemometric techniques in atomic spectroscopy, 2nd edition, RSC Analytical Spectroscopy Monographs No. 13
El Haddad J, Canioni L, Bousquet B (2014) Good practices in LIBS analysis: review and advices. Spectrochim Acta B 101:171–182
Aquino FWB, Pereira-Filho ER (2015) Analysis of the polymeric fractions of scrap from mobile phones using laser-induced breakdown spectroscopy: chemometric applications for better data interpretation. Talanta 134:65–73
Pokrajac D, Lazarevic A, Kecman V, Marcano A, Markushin Y, Vance T, Reljin N, McDaniel S, Melikechi N (2014) Automatic classification of laser-induced breakdown spectroscopy (LIBS) data of protein biomarker solutions. Appl Spectrosc 68:1067–1075
Putnam RA, Mohaidat QI, Daabous A, Rehse SJ (2013) A comparison of multivariate analysis techniques and variable selection strategies in a laser-induced breakdown spectroscopy bacterial classification. Spectrochim Acta B 87:161–167
Moros J, Serrano J, Sanchez C, Macias J, Laserna JJ (2012) New chemometrics in laser-induced breakdown spectroscopy for recognizing explosive residues. J Anal At Spectrom 27:2111–2122
Shaltout AA, Abdel-Aal MS, Mostafa NY (2011) The validity of commercial LIBS for quantitative analysis of brass alloy — comparison of WDXRF and AAS. J Appl Spectrosc 78:594–600
Ctvrtnickova T, Mateo MP, Yañez A, Nicolas G (2010) Laser induced breakdown spectroscopy application for ash characterisation for a coal fired power plant. Spectrochim Acta B 65:734–737
Gomes MS, de Carvalho GGA, Santos D Jr, Krug FJ (2013) A novel strategy for preparing calibration standards for the analysis of plant materials by laser-induced breakdown spectroscopy: a case study with pellets of sugar cane leaves. Spectrochim Acta B 86:137–141
Arafat A, Na’esb M, Kantarelou V, Haddad N, Giakoumaki A, Argyropoulos V, Anglos D, Karydas A-G (2013) Combined in situ micro-XRF, LIBS and SEM-EDS analysis of base metal and corrosion products for Islamic copper alloyed artefacts from Umm Qais museum, Jordan. J Cult Herit 14:261–269
Alberghina MF, Barraco R, Brai M, Schillaci T, Tranchina L (2011) Comparison of LIBS and μ-XRF measurements on bronze alloys for monitoring plasma effects. J Phys Conf Ser 275:012017
Meissner K, Lippert K, Wokaun A, Guenther D (2004) Analysis of trace metals in comparison of laser-induced breakdown spectroscopy with LA-ICP-MS. Thin Solid Films 453–454:316–322
Ciucci A, Corsi M, Palleschi V, Rastelli S, Salvetti A, Tognoni E (1999) New procedure for quantitative elemental analysis by LIPS. Appl Spectrosc 53:960–964
Tognoni E, Cristoforetti G, Legnaioli S, Palleschi V (2010) Calibration-free laser-induced breakdown spectroscopy: state of the art. Spectrochim Acta B 65:1–14
Gornushkin IB, Panne U (2010) Radiative models of laser-induced plasma and pump probe diagnostics relevant to laser-induced breakdown spectroscopy. Spectrochim Acta B 65:345–359
Gornushkin IB, Kazakov AY, Omenetto N, Smith BW, Winefordner JD (2005) Experimental verification of a radiative model of laser induced plasma expansion into vacuum. Spectrochim Acta B 60:215–230
Herrera KK, Tognoni E, Gornushkin IB, Omenetto N, Smith BW, Winefordner JD (2009) Comparative study of two standard-free approaches in laser-induced breakdown spectroscopy as applied to the quantitative analysis of aluminum alloy standards under vacuum conditions. J Anal At Spectrom 24:426–438
El Haddad J, Villot-Kadri M, Ismaël A, Gallou G, Michel K, Bruyère D, Laperche V, Canioni L, Bousquet B (2013) Artificial neural network for on-site quantitative analysis of soils using laser induced breakdown spectroscopy. Spectrochim Acta B 79–80:51–57
Mukhono PM, Angeyo KH, Dehayem-Kamadjeu A, Kaduki KA (2013) Laser induced breakdown spectroscopy and characterization of environmental matrices utilizing multivariate chemometrics. Spectrochim Acta B 87:81–85
Li X, Wang Z, Fu Y, Li Z, Ni W (2014) A model combining spectrum standardization and dominant factor based partial least square method for carbon analysis in coal using laser-induced breakdown spectroscopy. Spectrochim Acta B 99:82–86
Feng J, Wang Z, Li L, Li Z, Ni W (2013) A Nonlinearized multivariate dominant factor-based partial least squares (PLS) model for coal analysis by using laser-induced breakdown spectroscopy. Appl Spectrosc 67:291–300
Aguilera JA, Aragon C, Madurga V, Manrique J (2009) Study of matrix effects in laser induced breakdown spectroscopy on metallic samples using plasma characterization by emission spectroscopy. Spectrochim Acta B 64:993
Wiens RC, Maurice S, Lasue J, Forni O, Anderson RB, Clegg S et al (2013) Pre-flight calibration and initial data processing for the Chem Cam laser-induced breakdown spectroscopy instrument on the Mars Science Laboratory rover. Spectrochim Acta B 82:1–27
Anderson RB, Bell JF III, Wiens RC, Morris RV, Clegg SM (2012) Clustering and training set selection methods for improving the accuracy of quantitative laser induced breakdown spectroscopy. Spectrochim Acta B 70:24–32
Manzoor S, Moncayo S, Navarro-Villoslada F, Ayala JA, Izquierdo-Hornillos R, Manuel de Villena FJ, Caceres JO (2013) Rapid identification and discrimination of bacterial strains by laser induced breakdown spectroscopy and neural networks. Talanta 121:65–70
Mohaidat Q, Palchaudhuri S, Rehse SJ (2011) The effect of bacterial environmental and metabolic stresses on a LIBS-based identification of Escherichia coli and Streptococcus viridans. Appl Spectrosc 65:386–392
Cisewski J, Snyder E, Hannig J, Oudejans L (2012) Support vector machine classification of suspect powders using laser-induced breakdown spectroscopy (LIBS) spectral data. J Chemom 26:143–149
Khalil AA, Gondal MA, Shemis M, Khan IS (2015) Detection of carcinogenic metals in kidney stones using ultraviolet laser-induced breakdown spectroscopy. Appl Opt 54:2123–2131
Gazmeh M, Bahreini M, Tavassoli SH (2015) Discrimination of healthy and carious teeth using laser-induced breakdown spectroscopy and partial least square discriminant analysis. Appl Opt 54:123–131
Grolmusova Z, Hornackova M, Plavcan J, Kopani M, Babal P, Veis P (2013) Laser induced breakdown spectroscopy of human liver samples with Wilson’s disease. Eur Phys J Appl Phys 63:20801
Sancey L, Motto-Ros V, Busser B, Kotb S, Benoit JM, Piednoir A, Lux F, Tillement O, Panczer G, Yu J (2014) Laser spectrometry for multi-elemental imaging of biological tissues. Sci Rep 4(6065):1–7
El-Hussein A, Kassem AK, Ismail H, Harith MA (2010) Exploiting LIBS as a spectrochemical analytical technique in diagnosis of some types of human malignancies. Talanta 82:495–501
Emara EM, Imam H, Hassan M, Elnaby SH (2013) Biological application of laser induced breakdown spectroscopy technique for determination of trace elements in hair. Talanta 117:176–183
Bahreini M, Ashrafkhani B, Tavassoli SH (2013) Discrimination of patients with diabetes mellitus and healthy subjects based on laser-induced breakdown spectroscopy of their fingernails. J Biomed Opt 18:107006
Balog J, Sasi-Szabó L, Kinross J, Lewis MR, Muirhead LJ, Veselkov K, Mirnezami R, Dezső B, Damjanovich L, Darzi A, Nicholson JK, Takáts Z (2013) Intraoperative tissue identification using rapid evaporative ionization mass spectrometry. Sci Transl Med 5:194ra93
Kanawade R, Mahari F, Klaempfl F, Rohde M, Knipfer C, Tangermann-Gerk K, Adler W, Schmidt M, Stelzle F (2015) Qualitative tissue differentiation by analysing the intensity ratios of atomic emission lines using laser induced breakdown spectroscopy (LIBS): prospects for a feedback mechanism for surgical laser systems. J Biophotonics 8:153–161
Mehari F, Rohde M, Knipfer C, Kanawade R, Klaempfl F, Adler W, Stelzle F, Schmidt M (2014) Laser induced breakdown spectroscopy for bone and cartilage differentiation - ex vivo study as a prospect for a laser surgery feedback mechanism. Biomed Opt Exp 5:4013–4023
Pořízka P, Prochazka D, Pilát Z, Krajcarová L, Kaiser J, Malina R, Novotný J, Zemánek P, Ježek J, Šerý M et al (2012) Application of laser-induced breakdown spectroscopy to the analysis of algal biomass for industrial biotechnology. Spectrochim Acta B 74–75:169–176
Kaiser J, Novotny K, Martin MZ, Hrdlicka A, Malina R, Hartl M, Adam V, Kizek R (2012) Trace elemental analysis by laser-induced breakdown spectroscopy—biological applications. Surf Sci Rep 67:233–243
Rehse SJ, Salimnia H, Miziolek AW (2012) Laser-induced breakdown spectroscopy (LIBS): an overview of recent progress and future potential for biomedical applications. J Med Eng Technol 36:77–89
Ayyalasomayajula KK, Fang YY, Singh JP, McIntyre DL, Jain J (2012) Application of laser-induced breakdown spectroscopy for total carbon quantification in soil samples. Appl Opt 51:B149–B154
Ferreira EC, Ferreira EJ, Villas-Boas PR, Senesi GS, Carvalho CM, Romano RA, Martin-Neto L, Milori BP, Marcondes D (2014) Novel estimation of the humification degree of soil organic matter by laser-induced breakdown spectroscopy. Spectrochim Acta B 99:76–81
Bricklemyer RS, Brown DJ, Turk PJ, Clegg SM (2013) Improved intact soil-core carbon determination applying regression shrinkage and variable selection techniques to complete spectrum laser-induced breakdown spectroscopy (LIBS). Appl Spectrosc 67:1185–1199
Diaz D, Hahn DW, Molinat A (2012) Evaluation of laser-induced breakdown spectroscopy (LIBS) as a measurement technique for evaluation of total elemental concentration in soils. Appl Spectrosc 66:99–106
Srungaram PK, Ayyalasomayajula KK, Yu-Yueh F, Singh JP (2013) Comparison of laser induced breakdown spectroscopy and spark induced breakdown spectroscopy for determination of mercury in soils. Spectrochim Acta B 87:108–113
Segnini A, Xavier AAP, Otaviani-Junior PL, Ferreira EC, Watanabe AM, Sperança MA, Nicolodelli G, Villas-Boas PR, Oliveira PPA, Milori DMBP (2014) Physical and chemical matrix effects in soil carbon quantification using laser-induced breakdown spectroscopy. Am J Anal Chem 5:722–729
Hark RR, Remus JJ, East LJ, Harmon RS, Wise MA, Tansi BM, Shughrue KM, Dunsin KS, Liu C (2012) Geographical analysis of “conflict minerals” utilizing laser-induced breakdown spectroscopy. Spectrochim Acta B 74–75:131–136
McMillan NJ, Rees S, Kochelek K, McManus C (2014) Geological applications of laser-induced breakdown spectroscopy. Geostand Geoanal Res 38:329–343
Fortes FJ, Vadillo I, Stoll H, Jimenez-Sanchez M, Moreno A, Laserna JJ (2012) Spatial distribution of paleoclimatic proxies in stalagmite slabs using laser-induced breakdown spectroscopy. J Anal At Spectrom 27:868–873
Roux CPM, Rakovsky J, Musset O, Monna F, Buoncristiani JF, Pellenard P, Thomazo C (2015) In situ laser induced breakdown spectroscopy as a tool to discriminate volcanic rocks and magmatic series, Iceland. Spectrochim Acta B 103:63–69
Dell’Aglio M, De Giacomo A, Gaudiuso R, De Pascale O, Longo S (2014) Laser induced breakdown spectroscopy of meteorites as a probe of the early solar system. Spectrochim Acta B 101:68–75
Hornackova M, Plavcan J, Rakovsky J, Porubcan V, Ozdin D, Veis P (2014) Calibration-free laser induced breakdown spectroscopy as an alternative method for found meteorite fragments analysis. Eur Phys J Appl Phys 66:10702
Asgill ME, Brown MS, Frische K, Roquemore WM, Hahn DW (2010) Double-pulse and single-pulse laser-induced breakdown spectroscopy for distinguishing between gaseous and particulate phase analytes. Appl Opt 49:C110–C119
Kwak J, Kim G, Kim YJ, Park K (2012) Determination of heavy metal distribution in PM10 during Asian dust and local pollution events using laser induced breakdown spectroscopy (LIBS). Aerosol Sci Technol 46:1079–1089
Yu Y, Zhou W, Qian H, Su X, Ren K (2014) Simultaneous determination of trace lead and chromium in water using laser-induced breakdown spectroscopy and paper substrate. Plasma Sci Technol 16:683–687
Haider AFMY, Ullah M, Khan ZH, Kabir F, Abedin KM (2014) Detection of trace amount of arsenic in groundwater by laser-induced breakdown spectroscopy and adsorption. Opt Laser Technol 56:299–303
Harmon RS, Russo RE, Hark RR (2013) Applications of laser-induced breakdown spectroscopy for geochemical and environmental analysis: a comprehensive review. Spectrochim Acta B 87:11–26
Yuan T, Wang Z, Lui S, Fu Y, Li Z, Liu J, Ni W (2013) Coal property analysis using laser-induced breakdown spectroscopy. J Anal At Spectrom 28:1045–1053
Zhang L, Hu Z, Yin W, Huang D, Ma W, Dong L, Wu H, Li Z, Xiao L, Jia S (2012) Recent progress on laser-induced breakdown spectroscopy for the monitoring of coal quality and unburned carbon in fly ash. Front Phys 7:690–700
Li X, Wang Z, Fu Y, Li Z, Liu J, Ni W (2014) Application of a spectrum standardization method for carbon analysis in coal using laser-induced breakdown spectroscopy (LIBS). Appl Spectrosc 68:955–962
Kim CK, Lee SH, In JH, Lee HJ, Jeong S (2013) Depth profiling analysis of CuIn1-xGaxSe2 absorber layer by laser induced breakdown spectroscopy in atmospheric conditions. Opt Express 21:A1018–A1027
Kim CK, In JH, Lee SH, Jeong S (2013) Influence of laser wavelength on the laser induced breakdown spectroscopy measurement of thin CuIn1-xGaxSe2 solar cell films. Spectrochim Acta B 88:20–25
Banerjee SP, Fedosejevs R (2014) Single shot depth sensitivity using femtosecond laser induced breakdown spectroscopy. Spectrochim Acta B 92:34–41
Bilge G, Boyaci IH, Eseller KE, Tamer U, Cakir S (2015) Analysis of bakery products by laser-induced breakdown spectroscopy. Food Chem 181:186–190
Li W, Huang L, Yao M, Liu M, Chen T (2014) Investigation of Pb in Gannan navel orange with contaminant in controlled conditions by laser-induced breakdown spectroscopy. J Appl Spectrosc 81:850–854
Lei WQ, El Haddad J, Motto-Ros V, Gilon-Delepine N, Stankova A, Ma QL, Bai XS, Zheng LJ, Zeng HP, Yu J (2011) Comparative measurements of mineral elements in milk powders with laser-induced breakdown spectroscopy and inductively coupled plasma atomic emission spectroscopy. Anal Bioanal Chem 400:3303–3313
Ma F, Dong D (2014) A measurement method on pesticide residues of apple surface based on laser-induced breakdown spectroscopy. Food Anal Methods 7:1858–1865
Multari RA, Cremers DA, Dupre JAM, Gustafson JE (2013) Detection of biological contaminants on foods and food surfaces using laser-induced breakdown spectroscopy (LIBS). J Agric Food Chem 61:8687–8694
Lee SH, Hahn HT, Yoh JJ (2013) Towards a two-dimensional laser induced breakdown spectroscopy mapping of liquefied petroleum gas and electrolytic oxy-hydrogen flames. Spectrochim Acta B 88:63–68
Kiefer J, Troeger JW, Li Z, Seeger T, Alden M, Leipertz A (2012) Laser-induced breakdown flame thermometry. Combust Flame 159:3576–3582
Diwakar PK, Loper KH, Matiaske AM, Hahn DW (2012) Laser-induced breakdown spectroscopy for analysis of micro and nanoparticles. J Anal At Spectrom 27:1110–1119
Amodeo T, Dutouquet C, Tenegal F, Guizard B, Maskrot H, Le Bihan O, Frejafon E (2008) On-line monitoring of composite nanoparticles synthesized in a pre-industrial laser pyrolysis reactor using laser-induced breakdown spectroscopy. Spectrochim Acta B 63:1183–1190
Strauss N, Fricke-Begemann C, Noll R (2010) Size-resolved analysis of fine and ultrafine particulate matter by laser-induced breakdown spectroscopy. J Anal At Spectrom 25:867–874
Zhang Y, Li S, Ren Y, Yao Q, Tse SD (2015) A new diagnostic for volume fraction measurement of metal-oxide nanoparticles in flames using phase-selective laser-induced breakdown spectroscopy. Proc Combust Inst 35:3681–3688
Fedotova N, Kaegi R, Koch J, Günther D (2015) Influence of dispersion agents on particle size and concentration determined by laser-induced breakdown detection. Spectrochim Acta B 103:92–98
Fedotova N, Gogos A, Kägi R, Koch J, Günther D (2015) Asymmetric flow field-flow fractionation (A4F) coupled to laser induced breakdown detection for nanoparticles and proteins detection, European Winter Plasma Conference 2015, Münster, Germany, Paper No. NP3B-OP04
Troester M, Lipp P, Sacher F, Brauch H-J, Hofmann T (2014) Laser-induced breakdown-detection for reliable online monitoring of membrane integrity. J Membr Sci 466:313–321
Latkoczy C, Kaegi R, Fierz M, Ritzmann M, Guenther D, Boller M (2010) Development of a mobile fast-screening laser-induced breakdown detection (LIBD) system for field-based measurements of nanometre sized particles in aqueous solutions. J Environ Monit 12:1422–1429
De Giacomo A, Gaudiuso R, Koral C, Dell’Aglio M, De Pascale O (2013) Nanoparticle-enhanced laser-induced breakdown spectroscopy of metallic samples. Anal Chem 85:10180–10187
De Giacomo A, Gaudiuso R, Koral C, Dell’Aglio M, De Pascale O (2014) Nanoparticle enhanced laser induced breakdown spectroscopy: effect of nanoparticles deposited on sample surface on laser ablation and plasma emission. Spectrochim Acta B 98:19–27
Sancey L, Motto-Roos V, Kotb S, Wang X, Lux F, Panczer G, Yu J, Tillement O (2014) Laser-induced breakdown spectroscopy: a new approach for nanoparticle’s mapping and quantification in organ tissue. J Vis Exp 88, e51353
Motto-Ros V, Sancey L, Wang XC, Ma QL, Lux F, Bai XS, Panczer G, Tillement O, Yu J (2013) Mapping nanoparticles injected into a biological tissue using laser-induced breakdown spectroscopy. Spectrochim Acta B 87:168–174
Borowik T, Przybylo M, Pala K, Otlewski J, Langner M (2011) Quantitative measurement of Au and Fe in ferromagnetic nanoparticles with laser induced breakdown spectroscopy using a polymer-based gel matrix. Spectrochim Acta B 66:726–732
Serrano J, Cabalin LM, Moros J, Laserna JJ (2014) Potential of laser-induced breakdown spectroscopy for discrimination of nano-sized carbon materials. Insights on the optical characterization of graphene. Spectrochim Acta B 97:105–112
Sadek H, Simileanu M, Radvan R, Goumaa R (2012) Identification of porcelain pigments by laser induced breakdown spectroscopy. J Optoelectron Adv Mater 14:858–862
Palomar T, Oujja M, Garcia-Heras M, Villegas MA, Castillejo M (2013) Laser induced breakdown spectroscopy for analysis and characterization of degradation pathologies of Roman glasses. Spectrochim Acta B 87:114–120
Syvilay D, Texier A, Arles A, Gratuze B, Wilkie-Chancellier N, Martinez L, Serfaty S, Detalle V (2015) Trace element quantification of lead based roof sheets of historical monuments by laser induced breakdown spectroscopy. Spectrochim Acta B 103:34–42
Vitkova G, Prokes L, Novotny K, Porizka P, Novotny J, Vsiansky D, Celko L, Kaiser J (2014) Comparative study on fast classification of brick samples by combination of principal component analysis and linear discriminant analysis using stand-off and table-top laser-induced breakdown spectroscopy. Spectrochim Acta B 101:191–199
Kasem MA, Gonzalez JJ, Russo RE, Harith MA (2014) Effect of the wavelength on laser induced breakdown spectrometric analysis of archaeological bone. Spectrochim Acta B 101:26–31
Kasem MA, Russo RE, Harith MA (2011) Influence of biological degradation and environmental effects on the interpretation of archeological bone samples with laser-induced breakdown spectroscopy. J Anal At Spectrom 26:1733–1739
Kokkinaki O, Mihesan C, Velegrakis M, Anglos D (2013) Comparative study of laser induced breakdown spectroscopy and mass spectrometry for the analysis of cultural heritage materials. J Mol Struct 1044:160–166
Vitkova G, Novotny K, Prokes L, Hrdlicka A, Kaiser J, Novotny J, Malina R, Prochazka D (2012) Fast identification of biominerals by means of stand-off laser-induced breakdown spectroscopy using linear discriminant analysis and artificial neural networks. Spectrochim Acta B 73:1–6
Guirado S, Fortes FJ, Laserna JJ (2015) Elemental analysis of materials in an underwater archeological shipwreck using a novel remote laser-induced breakdown spectroscopy system. Talanta 137:182–188
Fortes FJ, Guirado S, Metzinger A, Laserna JJ (2015) A study of underwater stand-off laser-induced breakdown spectroscopy for chemical analysis of objects in the deep ocean. J Anal At Spectrom 30:1050–1056
Spizzichino V, Fantoni R (2014) Laser induced breakdown spectroscopy in archeometry: a review of its application and future perspectives. Spectrochim Acta B 99:201–209
Gottfried JL (2013) Influence of metal substrates on the detection of explosive residues with laser-induced breakdown spectroscopy. Appl Opt 52:B10–B19
De Lucia FC, Gottfried JL Jr (2012) Classification of explosive residues on organic substrates using laser induced breakdown spectroscopy. Appl Opt 51:B83–B92
Gaona I, Serrano J, Moros J, Laserna JJ (2014) Range-adaptive standoff recognition of explosive fingerprints on solid surfaces using a supervised learning method and laser-induced breakdown spectroscopy. Anal Chem 86:5045–5052
Karasevich YK, Kulagin AZ, Skripkin AM, Khatyushin PA, Karpov YA (2010) Laser-induced breakdown method for code detection (identification) in explosion products of coded explosives. Inorg Mater 46:1487–1492
Cremers DA, Beddingfield A, Smithwick R, Chinni RC, Jones CR, Beardsley B, Larry K (2012) Monitoring uranium, hydrogen, and lithium and their isotopes using a compact laser-induced breakdown spectroscopy (LIBS) probe and high-resolution spectrometer. Appl Spectrosc 66:250–261
Doucet FR, Lithgow G, Kosierb R, Bouchard P, Sabsabi M (2011) Determination of isotope ratios using laser-induced breakdown spectroscopy in ambient air at atmospheric pressure for nuclear forensics. J Anal At Spectrom 26:536–541
Metzinger A, Rajkó R, Galbács G (2014) Discrimination of paper and print types based on their laser induced breakdown spectra. Spectrochim Acta B 94–95:48–57
Kula A, Wietecha-Posluszny R, Pasionek K, Krol M, Wozniakiewicz M, Koscielniak P (2014) Application of laser induced breakdown spectroscopy to examination of writing inks for forensic purposes. Sci Justice 54:118–125
Schenk E, Almirall JR (2010) Elemental analysis of cotton by laser-induced breakdown spectroscopy. Appl Opt 49:C153–C160
Jantzi SC, Almirall JR (2014) Elemental analysis of soils using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and laser-induced breakdown spectroscopy (LIBS) with multivariate discrimination: tape mounting as an alternative to pellets for small forensic transfer specimens. Appl Spectrosc 68:963–974
Jantzi SC, Almirall JR (2011) Characterization and forensic analysis of soil samples using laser-induced breakdown spectroscopy (LIBS). Anal Bioanal Chem 400:3341–3351
Moncayo S, Manzoor S, Ugidos T, Navarro-Villoslada F, Caceres JO (2014) Discrimination of human bodies from bones and teeth remains by laser induced breakdown spectroscopy and neural networks. Spectrochim Acta B 101:21–25
Marin-Roldan A, Manzoor S, Moncayo S, Navarro-Villoslada F, Izquierdo-Hornillos RC, Caceres JO (2013) Determination of the postmortem interval by laser induced breakdown spectroscopy using swine skeletal muscles. Spectrochim Acta B 88:186–191
Bahreini M, Ashrafkhani B, Tavassoli SH (2014) Elemental analysis of fingernail of alcoholic and doping subjects by laser-induced breakdown spectroscopy. Appl Phys B Lasers Opt 114:439–447
Darbani SMR, Ghezelbash M, Majd AE, Soltanolkotabi M, Saghafifar H (2014) Temperature effect on the optical emission intensity in laser induced breakdown spectroscopy of super alloys. J Eur Opt Soc Rapid Publ 9:14058
Lawrence-Snyder M, Scaffidi JP, Pearman WF, Gordon CM, Angel SM (2014) Issues in deep ocean collinear double-pulse laser induced breakdown spectroscopy: dependence of emission intensity and inter-pulse delay on solution pressure. Spectrochim Acta B 99:172–178
Goueguel C, McIntyre DL, Singh JP, Jain J, Karamalidis AK (2014) Laser-induced breakdown spectroscopy (LIBS) of a high-pressure CO2-water mixture: application to carbon sequestration. Appl Spectrosc 68:997–1003
Jain J, Mcintyre D, Ayyalasomayajula K, Dikshit V, Goueguel C, Yu-Yueh F, Singh J (2014) Application of laser-induced breakdown spectroscopy in carbon sequestration research and development. Pramana J Phys 83:179–188
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galbács, G. A critical review of recent progress in analytical laser-induced breakdown spectroscopy. Anal Bioanal Chem 407, 7537–7562 (2015). https://doi.org/10.1007/s00216-015-8855-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8855-3