Abstract
The success of carbon capture and storage (CCS) programme relies on the long-term isolation of CO2 from the atmosphere. Therefore, technologies concomitant to physical storage of CO2 such as reliable measurement, monitoring, and verification (MMV) techniques are needed to ensure that the integrity of the storage site is maintained. We propose the use of laser-induced breakdown spectroscopy (LIBS) analytical technique to detect carbon dioxide leaks to aid in the successful application of CCS. LIBS has a real-time monitoring capability and can be reliably used for the elemental and isotopic analysis of solid, liquid, and gas samples. The flexibility of probe design and use of fibre optics make it a suitable technique for real time measurements in harsh conditions and at hard-to-reach places. Proposed monitoring with LIBS includes terrestrial soil samples, water samples from monitoring wells or from different formations, air samples from monitoring wells or suspected leakage areas. This work details the laboratory scale experiments to measure carbon contents in rock, soil, aqueous, and air samples. The potential of the technology for measurements in high pressure high-temperature conditions will also be discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The levels of greenhouse gases such as CO2 in the atmosphere have been increasing steadily since the beginning of the industrial revolution and are causing noticeable climate change. Due to the potential harm that the continued release of CO2 may cause to the Earth, many nations are currently looking into ways of separating CO2 from industrial /electrical power generation flue gas streams where the concentrated gas can then be liquefied and pumped underground for permanent storage [1,2]. A significant portion of the carbon sequestration research, performed in the United States, involves the risk assessment of injecting large quantities of CO2 into different underground formations, and the consequences involved if the injected CO2 moves into different formations or if it leaks into aquifers or back into the atmosphere.
When sensing potential leakage sites for carbon sequestration there is need for a wide range of modality such as atmospheric measurements, subsurface air measurements, surface water measurement, subsurface water measurements, and soil measurements, all at varying temperature and pressure conditions. The currently available methods typically do not have the ability to sample and measure the particular constituents at varying pressure and temperature. So a great deal of error can be introduced into the measurement when the sample is prepared at laboratory conditions. Optical systems such as open path Fourier-transform infrared (FTIR) and light detection and ranging (LIDAR) have been developed to scan and observe gases in open areas above suspected leakage sites. These systems have advantages of wide area monitoring. However, they are very expensive, complex, and not necessarily suitable for the diverse environments where sequestration may take place. Single point measurements can be made to very high precision with gas chromatographs (GC), mass spectrometers (MS), and cavity-ring-down spectrometers (CRDS) but due to their size, cost, and need for constant maintenance it is usually best to retrieve samples from the field and relay them back to these types of analysis devices.
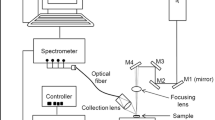
The current technology gap warrants development of new monitoring techniques that are affordable, robust, and easily distributable and deployable in many different locations to study a variety of different aspects of the environment and can analyse solids, liquids, and gases. Laser-induced breakdown spectroscopy (LIBS) has been used extensively for the determination of elemental composition of solid, liquid, and gaseous samples [3–5]. A LIBS set-up essentially comprises a high-intensity laser as an excitation source and a time-gated spectrometer to collect the signal. Generally, a laser beam is focussed onto a solid sample or within a gaseous or liquid sample to create high temperatures, dissociating the sample to form plasma. Radiation from the plasma is then collected by the spectrometer. Subsequent analysis of the radiation gives qualitative and quantitative information of elemental species present in the sample. This paper describes the use of LIBS in CO2 leak monitoring in carbon sequestration.
2 Background
As CO2 is such a versatile molecule and can be cycled between the atmosphere, bodies of water, plants, animals, and soils, it is important to know how much carbon is typically present in a particular scenario and how much you can reliably measure [3,6]. During carbon capture and storage (CCS) operations, the CO2 will be pumped into the storage formations (i.e., brine-filled reservoirs) as a supercritical fluid. If a leakage pathway develops, the CO2 will most likely escape the containment formation as a combination of supercritical and native fluids, with dissolved CO2. Once either the supercritical fluid and /or the CO2-saturated brine reach a portion of the subsurface with appropriate temperature and pressure conditions, the CO2 will become a gas and continue its leakage as a gas. This gas can be detected at a number of locations in the subsurface as well as in the atmosphere once it has left the containment formation. The gas can be measured in water wells, once it has degassed, it can be measured in the atmosphere of mines, in gas and oil production wells as well as in the atmosphere above the leakage point.
The leakage of CO2 into upper formations and possibly into the vadose zone and the plant bearing soil area will need monitoring due to the possible negative effects. Significant natural leakages of CO2 have been known to devastate large areas of forest [7,8]. Expected leakage from an industrial scale injection site is thought to be minimal to negligible. However, to mitigate possible widespread damage it would be prudent to understand the condition of the soils in overlying areas before and during the course of CCS injection.
There is a need for a sampling and measurement system that can reliably measure CO2 in solids such as soil, dissolved in liquids such as groundwater or springs, and in gaseous form as well as in the ambient air or at the bottom of wellbores. This work will describe the progress made using LIBS to measure specific elemental components in solids, liquids, and gases with the emphasis on how this technique can easily and cost-effectively be used for CO2 leak detection and monitoring. This is intended to show the accuracy that can be achieved by using LIBS with little or no sample preparation and with a relatively simple experimental set-up. LIBS has the potential to offer a long-term reliable gas monitoring tool that can be distributed across a wide range of environments to measure CO2 leakage and breakthrough.
3 LIBS experiments
This section will discuss the work recently done in our laboratory, that can easily be geared toward carbon sequestration monitoring and measurement. Although LIBS has been a practical laboratory measurement technique for decades, it has never been used in problems specific to carbon capture and sequestration. A handful of applications listed in this work clearly state that LIBS is a versatile measurement technique that can be applied to multiple measurement modalities and simply needs some engineering and refinement to become a highly useful portable system for routine, widespread monitoring of CO2.
3.1 Analysis of soils
The quantification of the total carbon content in soil can be carried out using many conventional analytical techniques [9]. However, these techniques are laborious, time-consuming, and require a great deal of sample preparation depending on the complexity of the soil matrix [10]. This is true even for LIBS analysis for the most part. However, there is the possibility of improved throughput and screening using LIBS due to the reduced sample preparation and measurement times. One recent study was performed in our lab to see if LIBS was a viable tool for total carbon concentration measurement in soil samples, whereby the ability to measure a wide range of total carbon content values was tested [11]. The study used carbon powder to simulate higher carbon concentrations within the soil samples and was shown to be an appropriate and acceptable simulation technique. The soil samples were finely ground, filtered, mixed with carbon powder in six different known concentrations and made into pellets. Five samples were used to generate a calibration curve and the sixth sample was treated as an unknown sample. LIBS spectra of the pellet samples were collected and analysed with both simple and multiple linear regression techniques to develop the calibration curves for carbon measurement. The ratio of C 247.88 nm line with Fe lines (246.51 and 247.48 nm) was used for the linear regression. Figure 1 shows the calibration curves for carbon using the SLR method; the error bars represent a single standard deviation.
The correlation coefficients of the ratio of the C 247.88 nm line with the Fe 246.51 and Fe 247.48 nm lines were 0.996 and 0.993, respectively. Since R 2 value for the C 247.88-to-Fe 246.51 nm ratios is better, the calibration data from this ratio were used for the analysis of the unknown sample. The unknown sample was also analysed using multiple linear regression (MLR) calibration model (table 1).
The MLR model provided better uncertainty values than the SLR model. The accuracy of the MLR model can depend on the analyte lines and combination of the lines chosen; the best results for the available lines are reported here. The results of this study show that LIBS can be successfully applied to quantitatively measure the total carbon in soil with reasonable accuracy and acceptable uncertainty values.
3.2 Analysis of liquids
Many researchers studied water composition using LIBS analysis [12–16]. Although none of the cited research efforts specifically addresses carbon sequestration issues, they all have the potential to provide adequate measurements to complement and enhance existing methods for sequestration monitoring. The production of laser sparks in water can be a bit more difficult than on the surface of a solid or in a gas due to the lower availability of free electrons and quenching due to the mass and diffusion of the condensed phase (water). In fact, deep saline formations (brine-filled) are viable long-term options for geologic CO2 sequestration (GCS) because of their large storage capacity. Based on field site investigations, sodium is typically the most abundant element in the deepest saline waters, constituting approximately 70–90% of the total cation mass [17,18]. Calcium is generally the second most abundant cation followed by magnesium and potassium, and the sparse trace elements of sulphur, bromine, iodine and carbon [17]. Thus, the effect of brine matrix on LIBS measurements can be significant and must be understood prior to producing meaningful quantitative measurements of the cation concentrations. Understanding how the above-mentioned elements, especially Na, cause interferences and affect the quantitative analysis is important for assessing the feasibility of LIBS to detect metal ions in brines.
Laboratory experiments were conducted to determine the effects of high salinity (in terms of NaCl equivalent) on emission signals of calcium and potassium. The emission signal from Ca I 422.67 nm and K I 766.49 nm lines were investigated as a function of the amount of NaCl dissolved in CaCl 2 and KCl solutions. This study does not address all the issues related to CO2-laden brine monitoring (such as CO2–brine mixture at elevated temperature and pressure) but makes a contribution by analysing the degree in which NaCl-matrix affects LIBS measurements. The overall work was performed in view of the development of a compact and efficient LIBS sensor for in situ monitoring of CO2-laden brine, where appreciable concentrations of NaCl (on an average 2.0–3.0 M) are present. The study, instead of using the liquid–air interface, which is the most common configuration of LIBS analysis for liquids, focussed on the direct measurements of the bulk aqueous solutions, which is more relevant to subsurface CO2 geologic applications and conditions. Figure 2 presents the effect of Na concentration on the measurement of Ca signal. In this experiment, the Ca concentration is held constant and the Na concentration is varied from 0.5 to 3.0 M. It is evident from figure 2 that increasing the concentration of Na not only enhances the Ca signal but also increases the background, potassium follows similar trend.
Calibration curves for constant concentrations of both Ca and K with varying concentrations of Na are shown in figure 3.
Results are shown for solutions with and without NaCl addition. Fitting procedures were applied (full lines) for easy visualization. Inserts in (a) and (b) represent the LIBS spectra obtained for 45 mg · l −1 Ca without NaCl addition, for 7 mg · l −1 K without NaCl addition and with the addition of 0.5 and 3 M NaCl. The results show that abundance of Na in solution does not have a detrimental effect and in fact it enhances the emission spectra for subordinate spectra. The LODs for Ca and K were calculated to be 17 ± 0.6 and 8 ± 1 mg · l −1, respectively.
3.3 Analysis of gases
Measurement of gas is possibly one of the most important and useful aspect of LIBS primarily due to the relatively stable and well-known concentrations of molecular species in air. Currently, gas phase CO2 is measured by various methods [6]; the two most commonly used methods are GC-MS and infrared spectroscopy, but many authors have used LIBS for the detection of gaseous samples as well [19–24]. Recently, there was a study where LIBS was used in the laboratory to directly measure CO2 concentrations in a gas sample cell to simulate the area around a leakage point where concentrations may vary dramatically. This study by Dikshit et al [25] was to investigate whether LIBS can be used as an analytical technique to detect CO2 in air. The working hypothesis was that the CO2 concentration in air can be measured by creating a spark in the air and subsequently measuring the carbon signal at C-247.86 nm; a line used by other researchers [3,26] for carbon measurement in soil. It was assumed that the primary source of the carbon signal in air was from CO2 because the concentrations of CO2, methane, and carbon monoxide in air are about 390, 1.79, and 0.1 ppm respectively [25]. The desired CO2 mixtures for calibration were prepared by diluting 50,000 ppm CO2 mixture with air. The calibration curve utilizing the CO2 concentrations up to 1500 ppm is shown in figure 4. To ensure repeatability of the measurement, the calibration data were collected on five different days and calibration curves were made. It was found that these calibration curves overlap well. To present this fact these datasets were divided into two groups: training and test data. The training dataset comprised data from the first four days and the test dataset comprised data obtained on the fifth day. The training dataset was used to plot the calibration curve shown in figure 4a, which was further used to predict the concentrations of the test dataset. ‘Actual’ vs. ‘predicted’ concentrations from the test data were plotted in figure 4b. The data presented in both the figures show that measurements are repeatable.
The calibration curves showed good linearity (R 2 ∼ 0.99) and were reproducible when the experiment was repeated on different days. Precision and repeatability were ensured by low relative standard deviation (RSD) which was comparable to commercially available IR CO2 sensors (RSD 1–3%). The limit of detection (LOD) was estimated to be 36 ppm. By using an appropriate detection system, it is possible to measure concentrations up to 50,000 ppm with the desired accuracy.
4 New directions
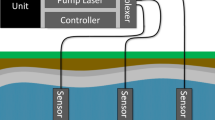
All the measurements discussed thus far have been single-point acquisitions and analysis. A much more useful approach to larger scale detection and measurement would be to connect the measurements from a wide array of locations and measurement modalities across the same injection basin. Rather than deploying a complete LIBS analysis system at each location, researchers can deploy a centrally located computer /controller and then connect it to a number of remotely energized laser spark sources which can be placed in the field, atmosphere, submerged, or downhole. The centrally located controller can then energize each sensor independently inducing sparks and either receive sample spectra to be analysed with an on-board high resolution spectrometer or receive data from miniaturized spectrometers deployed with the spark generator. The centrally located controller can address and activate each sensor through an optical multiplexer and /or electrical distributor which can consolidate the most expensive and /or sensitive pieces of equipment to be utilized for every sensor when activated. Recent advances in the areas of diode end-pumped solid-state passively Q-switched laser systems and miniaturized spectrometers show that the technologies are moving in the direction of smaller, more compact and less costly equipments.
5 Conclusions
This work highlights recent research efforts which show that LIBS is a feasible measurement technique for carbon sequestration leakage detection. Contrary to other measurements and monitoring techniques, LIBS has real-time analysis capability and can be used in harsh environments. With the introduction of new technologies, such as LAMIS which makes LIBS-based isotope measurement easier and more cost-effective, and the application of newly available miniaturization technologies for both lasers and spectrometers and highly portable LIBS analysis system is just on the horizon. New directions introduced by this work propose the optical and /or electrical distribution of LIBS analysis units in the form of optically pumped miniaturized lasers and spectrometers that can be deployed to various extreme environments to perform CCS leakage measurement and monitoring tasks.
References
S Solomon, D Qin, M Manning, Z Chen, M Marquis, K B Averyt, M Tignor and H L Miller (eds), Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2007) p. 996
2010 Carbon Sequestration Atlas of the United States and Canada, Third Edition (Atlas III), http://www.netl.doe.gov/technologies/carbon_seq/refshelf/atlasIII/
R Lal, Forest Ecology Management 220, 242 (2005)
R Lal, Soil Tillage Res. 43, 81 (1997)
R Lal, Prog. Environ. Sci. 1, 307 (1999)
M Z Martin, N Labbe, N Andre, R Harris, M Ebinger, S D Wullschleger and A A Vass, Spectrochim. Acta Part B 62, 1426 (2007)
C D Farrar, M L Sorey, W C Evans, J F Howle, B D Kerr, B M Kennedy, C Y King and J R Southon, Nature 376, 675 (1995)
K A McGee and T M Gerlach, Geology 26, 463 (1998)
R J Gehl and C W Rice, Clim. Change 80, 43 (2007)
Z Sulcek and P Povondra, Methods of decomposition in inorganic analysis, 1st edn (CRC Press, Boca Raton, 1989)
K K Ayyalasomayajula, F Y Yueh, J P Singh, D L McIntyre and J Jain, Appl. Opt. 51, B149 (2012)
M Lawrence-Snyder, J Scaffidi, S M Angel, A P M Michel and A D Chave, Appl. Spectrosc. 60, 786 (2006)
A P M Michel, M Lawrence-Snyder, S M Angel and A D Chave, Appl. Opt. 46, 2507 (2007)
V N Rai, F Y Yueh and J P Singh, Appl. Opt. 42, 2094 (2003)
A P M Michel and A D Chave, Appl. Opt. 47, G131 (2008)
M Lawrence-Snyder, J Scaffidi, S M Angel, A P M Michel and A D Chave, Appl. Spectrosc. 61, 171 (2007)
Y K Kharaka and J S Hanor, Deep fluids in the continents: I. Sedimentary basins, Treatise on geochemistry, J I Drever (ed), Heinrich D Holland and Karl K Turekian (ex. eds) (Elsevier, 2003) Vol. 5, pp. 499–540, ISBN: 0-08-043751-6
K J Breen, C G Angelo, R W Masters and A C Sedam, U.S. Geological Survey: Water Resources Investigation Report (84-4314), Ohio Department of Natural Resources, Division of Oil and Gas (Columbus, OH, 1985)
A J Ball, V Hohreiter and D W Hahn, Appl. Spectrosc. 59, 348 (2005)
M Tran, B W Smith, D W Hahn and J D Winefordner, Appl. Spectrosc. 55, 1455 (2001)
E D McNaghten, A M Parkes, B C Griffiths, A I Whitehouse and S Palanco, Spectrochim. Acta B 64, 1111 (2009)
K E Eseller, F Y Yueh and J P Singh, Appl. Phys. B 102, 963 (2011)
F Ferioli, P V Puzinauskas and S G Buckley, Appl. Spectrosc. 57, 1183 (2003)
V Hohreiter and D W Hahn, Anal. Chem. 77, 1118 (2005)
V Dikshit, F Y Yueh, J P Singh, D L McIntyre, J C Jain and N Melikechi, Spectrochim. Acta B (2012), DOI:10.1016/j.sab.2012.01.009
D A Cremers, M H Ebinger, P J Unkefer, S A Kannerdiener, M J Ferris, K M Catlett and J R Brown, J. Environ. Qual. 30, 2202 (2001)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
JAIN, J., MCINTYRE, D., AYYALASOMAYAJULA, K. et al. Application of laser-induced breakdown spectroscopy in carbon sequestration research and development. Pramana - J Phys 83, 179–188 (2014). https://doi.org/10.1007/s12043-014-0788-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12043-014-0788-4