Abstract
A pipette-tip solid-phase extraction (PT–SPE) method using a sol–gel hybrid adsorbent has been developed. The method could be used for rapid screening of vegetable matrices for cyanazine and atrazine; assay of cucumbers is reported as an example. The sol–gel hybrid adsorbent was synthesized from tetraethyl orthosilicate (TEOS) as the precursor and γ-(methacryloyloxy)propyltrimethoxysilane (KH570) as both surface modifying agent and monomer for the polymerization. Under the optimized conditions, good calibration linearity was obtained in the range 0.022–1.65 μg g−1 with correlation coefficients (r) ≥0.9996. Recovery at three spike levels ranged from 87.6 to 93.8 % with relative standard deviations ≤7.8 %. This extraction strategy has several advantages, for example ease of assembly, low cost, and high extraction efficiency, and is a potential pretreatment strategy for rapid screening of cyanazine and atrazine in vegetables.

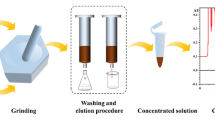
PT–SPE procedure and chromatograms obtained from cucumber
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Throughout the world, cyanazine and atrazine are the most widely used triazine herbicides for protection of crops from weeds, but they and their degradation products can do harm to human health because of their toxicity and persistence in water, soil, and food [1, 2]. The European Union, US, and China have legislated to limit levels of triazine herbicide residues [3–6]. However, amounts of the residues often exceed these levels because of insufficient control or monitoring. Therefore, it is important to monitor and analyze levels of triazine herbicide residues in foodstuffs and the environment.
Because of the trace amounts of analytes and the complexity of biological matrixes, sample pretreatment is often needed to concentrate analytes and eliminate interferences before further instrumental analysis [7, 8]. Miniaturized SPE (mini-SPE) has been widely used, because of its ability to isolate and concentrate analytes from extremely dilute sample solutions and its versatility and flexibility in the preconcentration step of most instrumental analysis [9, 10]. Varieties of mini-SPE techniques have been reported elsewhere, including miniaturized on-line SPE developed in 1999 by Nilsson [11], miniaturized SPE with resin disks described in 2001 by Fritz [12], pipette tip SPE recommended in 2006 by Kumazawa [13], microextraction in packed syringes introduced in 2004 by Abdel-Rehim [14], a similar assembly named a needle-trap device presented in 2009 by Pawliszyn [15], micro-SPE proposed in 2009 by Basheer [16], and dispersive micro-SPE reported in 2009 by Tsai [17]. However, the adsorbents used in these methods are mostly made from silica chemically bonded with different functional groups (for example C18, C8, C2, Ph, CH, CN, NH2, and diol), and they are not stable at extreme pH. Sometimes, some of the silanol groups are not functionalized [18]. Some adsorbents are different forms of carbon, for example activated carbon, graphitized carbon black (GCB), and porous graphitic carbon (PGCs), but some of these adsorb some compounds irreversibly [19, 20]. In addition, polymeric adsorbents are restricted because of their mechanical instability and swelling in some organic solvents or at high operating temperatures [21, 22]. Recently, polymeric/inorganic hybrid adsorbents have emerged as a new class of adsorbent which combines the advantages of inorganic and organic materials and combines large surface area with high mechanical stability [23]. Sol–gel technology, which enables the preparation of multi-component materials, can be used to prepare customized hybrid adsorbents, and enables the preparation of surface-bonded coatings on unbreakable substrates [24, 25]. This technique has potential for preparation of hybrid adsorbents.
In this work, a new hybrid adsorbent was prepared by sol–gel polymerization, using tetraethyl orthosilicate (TEOS) as precursor, and a silane coupling agent γ-(methacryloyloxy)propyltrimethoxysilane (KH570) as both surface-modifying agent and monomer for the polymerization. No other monomers, for example methacrylates, methacrylamides, acrylates, and acrylamides, were added to the polymerization system. The pipette tip SPE (PT–SPE) method using the sol–gel hybrid adsorbent has several advantages, for example easy self-assembly and operation, low cost, and high extraction efficiency, so is a potential pretreatment strategy for screening of vegetables for cyanazine and atrazine.
Experimental
Chemicals and reagents
Cyanazine and atrazine were obtained from Aladdin Chemistry (Shanghai, China). Tetraethyl orthosilicate (TEOS), γ-(methacryloyloxy)propyltrimethoxysilane (KH570), and ethylene glycol dimethacrylate (EGDMA) were purchased from Sigma–Aldrich (St Louis, MO, USA). Acetone and acetic acid were obtained from Guangfu Chemical (Tianjin, China). Dichloromethane and ethyl acetate were ordered from Huadong Chemical Reagent (Tianjin, China). Methanol, 2,2-azobisisobutyronitrile (AIBN), acetonitrile, ethanol, and aqueous ammonia were purchased from Kermel Chemical (Tianjin, China). Multiwalled carbon nanotubes (MCNT; 10–30 μm) were obtained from Chengdu Organic Chemical of the Chinese Academy of Sciences (Chengdu, Sichuan, China). Bakerbond octadecyl C18 adsorbents (40–60 μm) were from Mallinckrodt Baker (Phillipsburg, New Jersey, USA). Oasis HLB adsorbents (30 μm) were from Waters (Milford, MA, USA). All water used was double-deionized and filtered through a 0.45-μm filter membrane.
Instrumentation and conditions
Morphological evaluation was performed with a KYKY-2800B scanning electron microscope (SEM; FEI, Hillsboro, USA; http://www.fei.com). A Shimadzu (Kyoto, Japan) FTIR-8400S Fourier transform infrared spectrometer was used to examine the infrared spectra of the hybrid adsorbent in the range 400–4200 cm−1. The BET specific surface area instrument was from Quantachrome Instruments (Florida, USA). Particle size was measured by use of a laser diffraction analyzer (Beckman Coulter, California, USA). HPLC analysis was performed with a Shimadzu system equipped with two LC-20AT solvent delivery units, an SUS-20A gradient controller, and an SPD-20A UV–visible Detector. An LC solution workstation (Shimadzu) was used for system control and data processing. The analytical column was a C18 column (150 mm × 4.6 mm I.D., 5 μm) from Dalian Institute of Chemical Physics, Chinese Academy of Sciences (Dalian, China). The mobile phase was 52:48, (v/v) methanol–water, containing 0.8 % acetic acid, at a flow rate of 1.0 mL min−1. The detection wavelength of the UV–visible detector was 224 nm, and the injection volume was 20 μL.
Synthesis of the sol–gel hybrid adsorbent
The sol–gel hybrid adsorbent was synthesized as shown in Fig. 1a. TEOS (10 mmol), ethanol (30 mL), aqueous ammonia (1.0 mL), and water (2.0 mL) were mixed in a flask with stirring at 300 rpm for 15 h. Then 10 mL acetonitrile solution containing 4 mmol KH570 was added dropwise to the above solution, and the mixture was continuously stirred at 300 rpm. After 15 h, the solution was adjusted to neutral with acetic acid, and subsequently, EGDMA (20 mmol) and AIBN (100 mg) were added. The polymerization mixture was stirred at 500 rpm in a water bath (60 °C) for 8 h. Finally, the polymer obtained was washed successively with water and ethanol then dried before further experiments.
Preparation of cucumber samples and standard solutions
Cucumbers were purchased from local supermarkets in Baoding, China. Pieces of cucumber were squeezed by use of a juice extractor and the juice obtained was centrifuged at 4000 rpm for 15 min. After homogenization and centrifugation, 2.0 mL of the supernatant solution was used for the PT–SPE procedure.
Stock standard solutions (200 μg mL−1) were prepared by dissolving cyanazine and atrazine in methanol. Working standard solutions of the analytes were prepared by serial dilution of the stock standard solutions with double-deionized water. Spiked juice samples containing the analytes at concentrations of 0.022–1.65 μg g−1 were prepared by adding appropriate microliter amounts of working standard solutions to the supernatant juice after centrifugation.
PT–SPE procedure
The main PT–SPE device and operating procedures are shown in Fig. 2. The device is constructed from an empty polypropylene cartridge (50 mm × 8 mm i.d.), a 100-μL pipette tip, and a syringe. Hybrid adsorbent (3 mg) was packed in a pipette tip, and absorbent cotton was placed at both ends of the adsorbent to fix it in position. After preconditioning with methanol (1.0 mL) then water (1.0 mL), a cucumber sample (2.0 mL) was loaded on to the cartridge. The cartridge was washed with water (0.7 mL), then eluted with methanol (0.9 mL). A syringe was used to adjust the flow rate to one drop per approximately 15 s for all solvents by drawing the liquid from the tip of the pipette. The eluent was collected and evaporated to dryness by gentle nitrogen blowing, and the residues were then redissolved in the mobile phase (0.5 mL) for HPLC analysis.
Results and discussion
Synthesis of the sol–gel hybrid adsorbent
The hybrid adsorbent was synthesized by sol–gel polymerization. This method has several advantages, including low processing temperature, high molecular homogeneity, and self-assembly of individual nanowhiskers [26]. TEOS and water were dispersed quickly in ethanol to form a colloidal solution, known as a “sol”, so molecular homogeneity was rapidly obtained. Reactants were dispersed uniformly at the molecular level during formation of the “gel” matrix, so the reaction was initiated easily. The silica matrix could therefore be formed easily after polycondensation between TEOS molecules at room temperature.
KH570 was used as both surface-modifying agent and monomer in the polymerization process, and promoted firmer conglutination of the outer organic polymer on the silica matrix. The C = C of KH570 can be cross-linked with EGDMA, and the silicate ester bond can be embedded in the silica matrix after condensation. No other functional monomer, for example methacrylates, methacrylamides, acrylates, and acrylamides, were added to the reaction system, so the polymerization method using KH570 to synthesize the sol–gel hybrid adsorbent saves the cost of raw materials to some extent. EGDMA and AIBN are the crosslinker and the initiator, respectively, in synthesis of the outer organic polymer under thermal initiation. Acetonitrile is a good porogenic agent, and its use resulted in an adsorbent with a satisfactory binding capacity [27, 28]; 10 mL acetonitrile was added to the reaction mixture.
Characterization of the sol–gel hybrid adsorbent
Fourier transform infrared (FTIR) spectra (Fig. 1d) revealed the presence of hydrophilic groups on the sol–gel hybrid adsorbent, for example hydroxy (OH) and carbonyl (C = O) groups, corresponding to peaks at 3500 cm−1 and 1700 cm−1, respectively. The peaks of these hydrophilic groups were of similar intensity to those of lipophilic C–C bonds at 1400 cm−1. This indicates the sol–gel hybrid adsorbent is an adsorbent with hydrophilic–lipophilic balance; that is to say, the adsorbent could be used to screen analytes with a wide range of polarity [18].
Scanning electron micrographs of the sol–gel hybrid adsorbent are shown in Fig. 1b, c. Most of the particles obtained are in the size range ≤10 μm (Fig. 1b; magnification × 1,000). It is clearly apparent that the hybrid adsorbent is rough, agglomerate, and irregular (Fig. 1b; magnification × 30,000). The skeleton enlarged the surface area of the adsorbent, and this can embed analyte molecules in the cavities of the adsorbent. Moreover, the adsorption cumulative surface area of the pores on the adsorbent was 40.1 m2 g−1 (BET method), the pore volume was 0.14 cm3 g−1 (BJH adsorption and desorption, cumulative volume of pores between 1.7 nm and 300.0 nm diameter), the pore size of the material is mostly in the range 5–30 nm, and the adsorption average pore diameter is 13.8 nm (BJH adsorption and desorption, average pore diameter, 4 V/A). These could facilitate ready adsorption and desorption of the analytes by the sol–gel hybrid adsorbent.
Figure 3 shows the particle size distribution of the material. It is apparent the diameters of most (91.5 %) of the particles are in the range 1,283.8–4,369.8 nm. The average diameter is 2.96 μm, the polydispersity index is 0.832, and the diffusion constant is 1.662 × 10−9 cm2 s−1 in water at 25 °C. Some particles were probably broken in the ultrasonic dispersion process before detection by use of the laser particle size analyzer, so there are some small particles (8.3 %) in the range 196.2–409.2 nm.
PT–SPE device
An empty polypropylene cartridge (50 mm × 8 mm i.d.) and a 100 μL pipette tip are used to construct the PT–SPE cartridge. The adsorbent (3 mg) was placed in the tip of the homemade device as shown in Fig. 2. The amount of adsorbent was weighed precisely with an electronic balance (to an accuracy of 0.01 mg), to ensure the difference between tips was as small as possible. The size of our hybrid adsorbent material was 1.3–4.4 μm, and the adhesivity of the particles is probably negligible after the complete drying process used in the preparation. The adsorbent was therefore evenly distributed in the PT–SPE cartridge. The operation was performed carefully to achieve the same compaction and same height of the adsorbent column during loading with adsorbent. SPE with a pipette tip is easier and more rapid than with conventional SPE cartridges. The small bed volume and sorbent mass enable use of a reduced volume of solvent, short evaporation time, and higher throughput [13]. The conical shape of the pipette tip (1 mm and 1.5 mm i.d., 7 mm height) could substantially reduce the effect of eddy diffusion, which would help sample solution penetrate the adsorbent sufficiently during an appropriate analysis time. Moreover, because the adsorbent particles are approximately a factor of 10 smaller than those of conventional SPE adsorbents, they increase the mass transfer resistance between liquid and adsorbent, and impede permeation of the adsorbent bed by the liquid. It was difficult for an SPE pump to maintain an invariable flow rate for different liquids with different viscosity during the whole procedure. A syringe was used to control an invariable flow rate (one drop per 15 s, approx.) for all solvents passing through the PT–SPE cartridge, and the liquid was driven downward through the adsorbent by the traction of negative pressure from the tip end of the pipette during the whole PT–SPE procedure.
Optimization of PT–SPE conditions
In accordance with traditional SPE procedures, the volume of sample loaded, washing solvent, elution solvent, and sample pH were investigated to optimize the PT–SPE procedure. On the basis of the minimum loss ratio, the optimized extraction conditions were obtained by use of a series of experiments (the concentration of the spiked sample was 0.55 μg g−1). The results are shown in Figs. 4, 5, and 6. First, when the loading volume was 2.0 mL (from 3.6 g cucumber juice), 98.5 % cyanazine and 97.2 % atrazine in spiked cucumber samples were adsorbed on the sol–gel hybrid adsorbent (3 mg). The more aqueous sample solution functioned as a washing solvent, reducing recoveries for analytes extracted by the adsorbent. If the amount of adsorbent was increased, greater force was needed to drive the sample solution through the bed of adsorbent. This resulted in more difficult operation and the adsorption ratio was not increased substantially. Second, when 0.7 mL water was chosen as washing solvent to remove hydrophilic impurities, 96.5 % of the cyanazine and 96.2 % of the atrazine were retained by the adsorbent. Third, when 0.9 mL of methanol was used to elute the analytes, 90.6 % of the cyanazine and 90.8 % of the atrazine were eluted from the adsorbent, and use of more methanol did not elute more of the analytes. Fourth, impurities were inevitably extracted by the adsorbent and desorbed by the elution solvent, as shown in Fig. 7. The cumulative integral of the area of interference peaks, which accounted for more than 2 % of the total chromatographic peaks was chosen as the Y2 ordinate of Fig. 6, to investigate the effect of pH on the PT–SPE. The results show that sample pH did not need further adjustment, because high recovery and relatively few impurities can be obtained without adjusting sample pH. Finally, when 0.9 mL methanol was loaded on to the cartridge, some methanol molecules evaporated during the several minutes of the procedure, so the actual volume of eluent is reduced. In this work, the eluents were collected and evaporated to dryness, by gentle blowing with nitrogen, and the residues were then redissolved in the mobile phase (0.5 mL) to eliminate interference from differences between the mobile phase solution and the eluent solvent in subsequent HPLC analysis. The final results confirmed the balanced hydrophilic–lipophilic nature of the sol–gel hybrid adsorbent, and a clear chromatogram and satisfactory recovery were obtained.
Validation of the PT–SPE-HPLC method
Under the optimized extraction conditions, experiments were performed to determine linearity, limit of detection (LOD), limit of quantification (LOQ), precision, and accuracy of the PT–SPE–HPLC method. Calibration curves were constructed for seven spiked levels over the range 0.022–1.65 μg g−1; good linearity of the PT–SPE–HPLC method was observed throughout this concentration range for cyanazine and atrazine, with correlation coefficients (r) ≥0.9993, as shown in Table 1. LODs and LOQs were 3.5–5.2 μg kg−1 and 11.6–17.5 μg kg−1 on the basis of signal-to-noise ratios of 3 and 10, respectively. The effect of the cucumber matrix on the accuracy of the PT–SPE–HPLC method was studied by comparing the slopes of calibration graphs in the absence (for aqueous standards) and presence of the cucumber juice; the regression equations are given in Table 1. The results indicate there was little difference between the slopes of two calibration graphs (9.7 × 105 and 9.6 × 105, and 1.2 × 106 and 1.2 × 106, as shown in Table 1), so it could be concluded that the sample matrix would not affect the accuracy of the method. In addition, recovery experiments were conducted for three spiked levels of cucumber samples, and recoveries of the PT–SPE-HPLC method were in the range 87.6–93.8 % for cucumber, with relative standard deviations (RSDs) ≤7.8 %, as shown in Table 2. The recoveries of the two analytes during by the PT–SPE procedure (90.6 % and 90.8 %) are in concordance with this recovery range. Precision and accuracy were evaluated by analyzing five replicates of spiked samples at three levels on the same day and on three different days; intra-assay and inter-assay precision, as RSD, was 3.9–4.1 % and 4.8–8.7 %, respectively. The high recoveries and good reproducibility (low RSDs) all demonstrate that there is little effect of sample matrix components on extraction performance.
Comparison with other adsorbents and pretreatment methods
The purification performance of different adsorbents (MCNTs, C18, HLB, and hybrid adsorbent) was investigated for cucumber samples spiked at concentration of 0.55 μg g−1 by use of previously optimized methods [7, 29, 30] in which these adsorbents were used to screen triazine herbicides. The purification performance is similar for MCNTs, C18, and hybrid adsorbent, as shown by the chromatograms in Fig. 7a. The hydrophobicity of MCNTs and C18 may weaken their compatibility with aqueous sample solution, and reduce recoveries for cyanazine and atrazine. HLB adsorbent resulted in higher recoveries for cyanazine and atrazine than MCNTs and C18, but more impurities from the sample matrix were coextracted. High recoveries (≥90.6 %) and clean chromatograms were obtained by use of the PT–SPE method with the hybrid adsorbent.
Assay of cyanazine and atrazine in cucumber samples
The feasibility of the PT–SPE-HPLC method was evaluated by rapid screening of cucumber samples for cyanazine and atrazine. When cucumbers from four different supermarkets in Baoding were pretreated as described above, one was observed to contain a trace amount of atrazine (0.03 μg g−1 which is less than the MRL, <0.05 μg g−1 [5]). The chromatograms obtained from cucumbers are much cleaner after the PT–SPE process, as seen in Fig. 7b; there are no interfering peaks originating from the cucumber matrix. It can be concluded that PT–SPE-HPLC is a reliable method for screening of in vegetables for triazine herbicides. The low LOQs show sensitive assay can be achieved without the need for expensive equipment.
Conclusions
A PT–SPE method with a sol–gel hybrid adsorbent was developed for screening of cucumbers for cyanazine and atrazine. The sol–gel hybrid adsorbent was obtained by use of the silane coupling agent (KH570) as both surface modifying agent and monomer for the polymerization. The new PT–SPE method with the synthesized hybrid adsorbent has several advantages including ease of assembly, low cost, and high extraction efficiency, and it is a potential method for screening of vegetable samples for herbicide residues.
References
Sathiakumar N, Delzell E (1997) A review of epidemiologic studies of triazine herbicides and cancer. Crit Rev Toxicol 27:599–612
Lee EA, Strahan AP, Thurman EM (2002) Methods of analysis by the US geological survey organic geochemistry research group-determination of triazine and phenylurea herbicides and their degradation products in water using solid-phase extraction and liquid chromatography/mass spectrometry. Geological Survey Denver Co
Kuang H, Wang LB, Xu CL (2011) Overview of analytical techiques for herbicides in food. Herbicides, Theory and Applications, Croatia: In Tech. Europe pp 239–280
Commission Regulation (EC) (2008) No. 149/2008 of 29 January 2008 amending Regulation (EC) No. 396/2005 of the European Parliament and of the Council by establishing Annexes II, III and IV setting maximum residue levels for products covered by Annex I thereto. Off J Eur Commun L58:10–10
National Standards of People's Republic of China GB 2763–2012 (2012) National food safety standard, Maximum residue limits for pesticides in food. pp 86–86
Electronic Code of Federal Regulations (2013) Part 180–Tolerance and Exemptions for Pesticide Chemical Residues in Food, Title 40: Protection of Environment [Internet]. [Updated 27 September 2013; cited 6 October 2013]. Available from: http://www.epa.gov/lawsregs/search/40cfr.html
Pinto GMF, Jardim ICSF (2000) Use of solid-phase extraction and high-performance liquid chromatography for the determination of triazine residues in water: validation of the method. J Chromatogr A 869:463–469
Viñas P, Campillo N, López-García I, Hernández-Córdoba M (2014) Dispersive liquid–liquid microextraction in food analysis: a critical review. Anal Bioanal Chem 406:2067–2099
Tankiewicz M, Fenik J, Biziuk M (2011) Solventless and solvent-minimized sample preparation techniques for determining currently used pesticides in water samples: a review. Talanta 86:8–22
Alves G, Rodrigues M, Fortuna A, Falcão A, Queiroz J (2013) A critical review of microextraction by packed sorbent as a sample preparation approach in drug bioanalysis. Bioanalysis 5:1409–1442
Petersson M, Wahlund KG, Nilsson S (1999) Miniaturised on-line solid-phase extraction for enhancement of concentration sensitivity in capillary electrophoresis. J Chromatogr A 841:249–261
Fritz JS, Masso JJ (2001) Miniaturized solid-phase extraction with resin disks. J Chromatogr A 909:79–85
Hasegawa C, Kumazawa T, Lee XP, Fujishiro M, Kuriki A, Marumo A, Seno H, Sato K (2006) Simultaneous determination of ten antihistamine drugs in human plasma using pipette tip solid-phase extraction and gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 20:537–543
Abdel-Rehim M (2004) New trend in sample preparation: on-line microextraction in packed syringe for liquid and gas chromatography applications: I. Determination of local anaesthetics in human plasma samples using gas chromatography-mass spectrometry. J Chromatogr B 801:317–321
Niri VH, Eom IY, Kermani FR, Pawliszyn J (2009) Sampling free and particle-bound chemicals using solid-phase microextraction and needle trap device simultaneously. J Sep Sci 32:1075–1080
Basheer C, Alnedhary AA, Rao BS, Lee HK (2009) Determination of carbamate pesticides using micro-solid-phase extraction combined with high-performance liquid chromatography. J Chromatogr A 1216:211–216
Tsai WH, Chuang HY, Chen HH, Huang JJ, Chen HC, Cheng SH, Huang TC (2009) Application of dispersive liquid-liquid microextraction and dispersive micro-solid-phase extraction for the determination of quinolones in swine muscle by high-performance liquid chromatography with diode-array detection. Anal Chim Acta 656:56–62
Augusto F, Hantao LW, Mogollón NGS, Braga SCGN (2013) New materials and trends in sorbents for solid-phase extraction. Trends Anal Chem 43:14–23
Fontanals N, Marce RM, Borrull F (2005) New hydrophilic materials for solid-phase extraction. Trends Anal Chem 24:394–406
Fontanals N, Cormack PAG, Marcé RM, Borrull F (2010) Mixed-mode ion-exchange polymeric sorbents: dual-phase materials that improve selectivity and capacity. Trends Anal Chem 29:765–779
Costantini A, Luciani G, Annunziata G, Silvestri B, Branda F (2006) Swelling properties and bioactivity of silica gel/pHEMA nanocomposites. J Mater Sci-Mater Med 17:319–325
Augusto F, Carasek E, Silva RGC, Rivellinoa SR, Batista AD (2010) New sorbents for extraction and microextraction techniques. J Chromatogr A 1217:2533–2542
Pan B, Zhang W, Lv L, Zhang Q, Zheng S (2009) Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem Eng J 151:19–29
Kumar A, Malik AK, Tewary DK, Singh B (2008) A review on development of solid phase microextraction fibers by sol–gel methods and their applications. Anal Chim Acta 610:1–14
Kabir A, Furton KG, Malik A (2013) Innovations in sol–gel microextraction phases for solvent-free sample preparation in analytical chemistry. Trends Anal Chem 45:197–218
Siqueira G, Mathew AP, Oksman K (2011) Processing of cellulose nanowhiskers/cellulose acetate butyrate nanocomposites using sol–gel process to facilitate dispersion. Compos Sci Technol 71:1886–1892
Yan S, Gao Z, Fang Y, Cheng Y, Zhou H, Wang H (2007) Characterization and quality assessment of binding properties of malachite green molecularly imprinted polymers prepared by precipitation polymerization in acetonitrile. Dyes Pigments 74:572–577
Lopez C, Claude B, Morin P, Max J, Pena R, Ribet J (2011) Synthesis and study of a molecularly imprinted polymer for the specific extraction of indole alkaloids from Catharanthus roseus extracts. Anal Chim Acta 683:198–205
Yu Z, Qin Z, Ji H, Du X, Chen Y, Pan P, Wang H, Liu Y (2010) Application of SPE using multi-walled carbon nanotubes as adsorbent and rapid resolution LC-MS-MS for the simultaneous determination of 11 triazine herbicides residues in river water. Chromatographia 72:1073–1081
Carabias-Martınez R, Rodrıguez-Gonzalo E, Domınguez-Alvarez J, Hernandez-Mendez J (2000) Determination of triazine herbicides in natural waters by solid-phase extraction and non-aqueous capillary zone electrophoresis. J Chromatogr A 869:451–461
Acknowledgments
The project was sponsored by the National Natural Science Foundation of China (31301464) and the Natural Science Foundation of Hebei Province (B2012201052).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, M., Yan, H., Yuan, Y. et al. Pipette-tip solid-phase extraction by use of a sol–gel hybrid adsorbent: a new pretreatment strategy for rapid screening of cucumbers for cyanazine and atrazine. Anal Bioanal Chem 407, 1231–1239 (2015). https://doi.org/10.1007/s00216-014-8336-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8336-0