Abstract
A quadrupole inductively coupled plasma mass spectrometer was evaluated for use in the detection of phosphorus. The influences of nitric acid and methanol (simulating the composition of a sample solution after nitric acid digestion) on phosphorus determination were studied using two different measuring methods at different plasma conditions: detection of phosphorus ions at m/z 31 and detection of phosphorus oxide ions at m/z 47. The existence of polyatomic interferences at m/z 31 and 47 was explored. Nitric acid and methanol are shown to be the sources of polyatomic ions and therefore cause poorer detection limits. Better detection limits were achieved in such matrices when phosphorus was detected as 31P+. The presence of methanol improves the system sensitivity towards phosphorus sevenfold; however, this positive effect is hindered by the high background signal due to carbon-based polyatomic ions. For samples with an organic matrix an appropriate mineralization procedure should be applied (high excess of nitric acid and high temperature) to quantitatively oxidize organic compounds to carbon dioxide, which is easily removed from the sample, in order to achieve correct results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Determination of phosphorus by quadrupole inductively coupled plasma mass spectrometry (ICPMS) at trace levels is difficult as a result of its high ionization potential and due to nitrogen-based, oxygen-based, and carbon-based polyatomic interferences at m/z 31. Despite this, many authors have reported the application of this technique for the determination of phosphorus and its compounds [1–5]. However, due to the abundance of polyatomic interferences, high-resolution [6–9] and reaction/collision cell ICPMS [10–12] instruments are more and more frequently used for the determination of this element. The reaction/collision cell technique, which is more economically viable, requires thorough knowledge about the composition and origin of polyatomic ions to be efficiently used. In addition, the influence of matrix compounds on phosphorus ionization also requires attention, since this effect is important regardless of the type of ICPMS. The influence of organic compounds on the sensitivity of quadrupole ICPMS has not yet been studied with the exception of work by Jiang and Houk [2] who reported a decrease in the sensitivity with increasing concentration of organic solvent. More has been done with high-resolution ICPMS instrumentation: Siethoff et al. [7] reported a decrease in the phosphorus signal with increasing methanol content, and Wind et al. reported [8] an increase in the phosphorus signal of up to threefold due to an increase in the acetonitrile concentration.
Due to the lack of data in this field for a quadrupole ICPMS, we performed a systematic study on the influences of nitric acid and methanol on phosphorus determination. Matrix effects were studied under two measuring conditions: through detection of phosphorus ions at m/z 31 and detection of phosphorus oxide ions at m/z 47. Nitric acid and methanol aqueous solutions were chosen to simulate the chemical composition of sample solutions, since nitric acid is commonly used for sample preparation and methanol is often employed in ion chromatography.
Experimental
Materials, sample preparation, and procedures
Water (18 MΩ cm) was prepared using a Milli-Q system (Millipore, Bedford, MA, USA). Ultrapure nitric acid (69%, Ultrex II, J.T. Baker, Philipsburg, NJ, USA), trace metal analysis grade methanol (>99.9%, Fisher Scientific, Loughborough, UK), acetone, formic acid (both p.a., Kemika, Zagreb, Croatia), 30% hydrogen peroxide, acetic acid, arsenic standard solution, KH2PO4 (all p.a., Merck, Darmstadt, Germany), boron standard solution (CPI International, Santa Rosa, CA, USA), and Rb2CO3 (p.a., Fluka, Buchs, Switzerland) were used.
For the evaluation of matrix effects, solutions containing different concentrations of nitric acid and different concentrations of methanol in 0.05 mol L−1 nitric acid were prepared in duplicate. Phosphorus and internal standards were added to the first solution in the pair, to yield a final concentration of 200 μg L−1 of phosphorus, 100 μg L−1 of boron, 20 μg L−1 of arsenic, and 20 μg L−1 of rubidium. The signal obtained from the solution without phosphorus was used to determine the background. The difference between the signal of the solution containing phosphorus and the signal of the solution without phosphorus was considered as the phosphorus signal. The purity of all chemicals involved in this study was checked by sector-field ICPMS and no significant levels of impurities were found.
Reference materials “pine needles” (1575, NIST, Gaithersburg, MD, USA) and “engine oil” (MO-2, No. 362, 1999/2000, PetroLab, Speyer, Germany) were mineralized in a microwave oven (MLS Mega 1200, Milestone, Bergamo, Italy) in a mixture of 5 mL of nitric acid and 1 mL of hydrogen peroxide. The following program was used: 2 min at 250 W, 2 min at 0 W, 6 min at 250 W, 5 min at 400 W, and 5 min at 600 W (the temperature during the last step was 180°C). Finally, samples were diluted with water up to 50 mL.
Instrumentation
The quadrupole ICPMS HP4500 plus (Agilent, Waldbronn, Germany) was equipped with a Babington type nebulizer and a double-pass spray chamber. The sample intake rate was 0.30 mL min−1 for all tested solutions. The plasma gas and the auxiliary gas flow rates were 15.0 and 0.90 L min−1, the spray chamber temperature was 2°C, and the integration time was 0.1 s with five repetitions per mass. Although aqueous methanol solutions were analyzed, addition of oxygen to plasma was not necessary.
Conditions for 31P+ detection: The initial instrument tuning was performed with solution A (10 μg L−1 of Li, Y, Ce and Tl in 2% nitric acid). It was optimized for high sensitivity towards these elements and a minimum cerium oxide ratio. The following conditions were chosen: RF power 1,600 W, carrier gas flow rate 1.05 L min−1, and torch–interface distance 7 mm. Under these conditions the cerium oxide ratio (140Ce16O+/140Ce+) was 0.004. The mass resolution was optimized using solution B (100 μg L−1 of B, 20 μg L−1 of As and Rb, 2.5 mol L−1 of methanol, and 0.05 mol L−1 of nitric acid) and it was less than 3% (height of valley compared to peak height) for the mass peaks at m/z 10, 31, 75, and 85. Finally, high sensitivity for all measured elements was achieved by tuning the ion optics while nebulizing solution C (200 μg L−1 of P, 100 μg L−1 of B, 20 μg L−1 of As and Rb, and 2.5 mol L−1 of nitric acid).
Conditions for 47[PO]+ detection: The initial instrument tuning was performed with solution A. It was optimized for high sensitivity and high cerium oxide ratio. The following conditions were selected: RF power 1,350 W, carrier gas flow rate 1.20 L min−1, and torch–interface distance 5 mm. Under these conditions the cerium oxide ratio (140Ce16O+/140Ce+) was 9. Further optimization procedures, using solutions B and C, were the same as for optimization of 31P+ detection with the exception that m/z 31 was replaced with m/z 47.
Sample transport efficiencies were determined using solutions containing arsenic (20 μg L−1), boron (100 μg L−1), rubidium (500 μg L−1), and phosphorus (200 μg L−1). The following matrices were tested at carrier gas flow rates of 1.05 and 1.20 L min−1: water, 0.5 and 2.5 mol L−1 nitric acid, and 0.5 and 2.5 mol L−1 methanol. The generated aerosols were collected by passing the carrier gas through a frit immersed in 1% nitric acid and the content of rubidium was determined by ICPMS.
The influence of volatile carbon compounds was investigated using a double-pass spray chamber with a modified end-cap. A 0.5-mm-ID capillary was placed in a hole in the end-cap, which was drilled ≈5 mm under the Babington nebulizer. The capillary reached 1 cm into the interior of the outer region of the spray chamber. Aqueous solutions of methanol were introduced through this capillary at a flow rate of 0.12 mL min−1. It was assumed that no aerosol was produced from this solution and only methanol vapors were swept towards the plasma together with sample aerosol. A solution containing 200 μg L−1 of phosphorus in 0.05 mol L−1 nitric acid was introduced through the nebulizer and was used for phosphorus signal evaluation. A solution containing 0.05 mol L−1 nitric acid was used for evaluation of the background signal.
Polyatomic interferences were identified using a sector-field ICPMS (Element 2, Thermo, Bremen, Germany) in high-resolution mode (10,000). The instrument was equipped with a concentric nebulizer, cyclonic spray chamber, and shield torch. Ionic species in the proximity of m/z 31 (±0.05 m/z) were detected under the following conditions: plasma gas 15.5 L min−1, auxiliary gas 1.4 L min−1, carrier gas 0.97 L min−1, RF power 1,200 W, and torch–interface distance 4.8 mm. Ionic species in the proximity of m/z 47 (±0.05 m/z) were detected under the same conditions except the carrier gas flow rate was increased to 1.09 L min−1 to obtain a maximal phosphorus oxide ratio of 0.27 (PO+/P+).
Determination of residual carbon content in mineralized samples was carried out with a TOC analyzer (Rosemount/Dohrman DC-190, Mason, OH, USA) following the ISO 8245 analytical procedure.
Results and discussion
Matrix effects during 31P+ determination
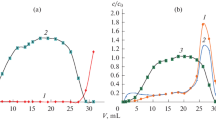
The influence of nitric acid on measurements of 31P+ is presented in Fig. 1. The phosphorus signal decreased from 170,000 CPS in water to 130,000 CPS in 2.5 mol L−1 nitric acid, which could be ascribed to the decreased ionization rate due to cooling of the plasma by additional nitric acid. Similarly, intensities of the internal standards decreased by a factor of 0.9 for 10B, 0.5 for 75As, and 0.6 for 85Rb, which is in agreement with literature data [13]. The background signal increased threefold due to nitrogen-containing polyatomic ions, which were derived from nitric acid. This was confirmed by identification of the following species during introduction of 2.5 mol L−1 nitric acid into the high-resolution ICPMS: 14N16O1H+ (≈65% abundance), 15N16O+ (≈32% abundance), and 14N17O+ (≈3% abundance). High-resolution and quadrupole instruments do not use the same sample introduction and plasma systems. Therefore although these results cannot be directly extrapolated to the quadrupole instrument, they provide at least some information about the composition of the increased background signal. The background signal of ≈1,600 CPS in the solution without nitric acid is caused by polyatomic ions derived from nitrogen and carbon dioxide in the air surrounding the plasma and/or from the plasma gas. To confirm this, the following species were identified during high-resolution ICPMS measurements of water: 14N16O1H+ together with 12C18O1H+ (≈70% abundance), 15N16O+ (≈20% abundance), 12C16O1H +3 (≈8% abundance), and 14N17O+ (≈2% abundance).
In methanol solutions, both the 31P+ and the background signals increased significantly with increasing methanol concentration (Fig. 1). A sevenfold increase in the phosphorus signal was observed when water and 2.5 mol L−1 methanol were compared. It is known from the literature that elements with an ionization potential of 9–11 eV enhance signals in the presence of carbon [14, 15]. However, the sevenfold increase cannot be attributed to this effect alone, since it is also the consequence of improved sample transport efficiencies in the presence of methanol. This was confirmed by measuring sample transport efficiencies, which increased for a factor of 1.05 when water and 0.5 mol L−1 methanol were compared and for a factor of 1.40 when water and 2.5 mol L−1 methanol were compared. An increase in the signals was also observed for the internal standards. The 75As signal increased sixfold, 10B signal 2.3-fold, and 85Rb signal 1.5-fold, which is in accordance with the literature data [14, 16]. The reported enhancement of the phosphorous signal in the presence of methanol is only partially in agreement with the published data, since some authors have reported an increase and others a decrease in sensitivity [2, 7, 8]. However, a direct comparison is difficult as different sample introduction systems and different plasma sources were used. The 28-fold increase in the background signal can be attributed to the carbon-based polyatomic ions. This was confirmed by identification of the following species during introduction of 2.5 mol L−1 methanol into the high-resolution ICPMS: 12C18O1H+ (≈92% abundance), 12C16O1H +3 (≈6% abundance), and 13C18O+ (≈2% abundance).
To further investigate the role of carbon in the enhancement of the phosphorus signal, different methanol solutions were introduced directly into the spray chamber and the phosphorus and background signals were monitored (Fig. 2). In general, the established relationships are similar to the relationships obtained when methanol was present in the sample solution (Fig. 1), confirming the hypothesis that carbon compounds cause the enhancement of the phosphorus signal as well as the background signal, since volatile phosphorus contaminants are not likely to be present in methanol. The highest enhancement factor for phosphorus (12-fold) was obtained at a concentration of approximately 5 mol L−1 of methanol. The decrease in the phosphorus signal at higher concentrations can be explained by the presence of a too high concentration of carbon compounds in the plasma, which disables efficient sample decomposition.
Matrix effects during 47[PO]+ determination
An alternative approach for phosphorus determination is the use of instrumental conditions, which enable detection of phosphorus oxide ions at m/z 47 [17]. Under these plasma conditions, both the phosphorus and background signals increased with increasing nitric acid concentration (Fig. 3). The intensity of the 47[PO]+ signal increased by a factor of 2.7. This effect could be ascribed to more favorable formation of 47[PO]+ ions in the presence of nitric acid owing to additional cooling of the plasma. It could be also the consequence of additional oxygen from nitric acid, since addition of oxygen through a blend gas line (0.04 L min−1) increased the 47[PO]+ signal by a factor of ≈1.5 when the matrix was 0.5 mol L−1 nitric acid. The signal for arsenic (during the experiment described in Fig. 3) was reduced by a factor of 0.67 owing to additional cooling of the plasma by nitric acid [13]. At the same time, the signal intensities of boron and rubidium increased by a factor of 1.3 and 1.5, respectively. The background signal increased by a factor of 4.3 from an initial 2,400 CPS to 10,000 CPS, due to the formation of polyatomic ions. The following species were determined with high-resolution ICPMS measurements during introduction of 2.5 mol L−1 nitric acid: 15N16O16O+ (≈51% abundance), 14N16O17O+ (≈12% abundance), and 14N16O16O1H+ (≈37% abundance).
In the concentration range from 0 to 1.5 mol L−1 of methanol, an increase in the 47[PO]+ and background signals was observed (Fig. 3). The 47[PO]+ signal increased by a factor of 3.7 and the background signal by a factor of approximately 330. The increase of the 47[PO]+ signal may be due to the influence of carbon in the plasma, but thus far no information concerning enhanced ionization of phosphorus oxide has been published. A similar increase (3.5-fold) was also noticed for arsenic, which was as expected, since enhancement of the arsenic signal in the presence of organic solvent is well documented [15]. The signals of the other two internal standards also increased: boron by a factor of 2 and rubidium by a factor of 2.3. This increase could be partially attributed to increased sample transport efficiencies, since they increased by a factor of 1.3 and 1.8 for 0.5 and 2.5 mol L−1 methanol solutions, respectively. The extreme increase in the background signal by a factor of 330 can only be explained by the carbon-based polyatomic ions. Using the high-resolution ICPMS measurements, the following species were detected during introduction of 2.5 mol L−1 methanol: 13C18O16O+ (≈5% abundance) and 12C18O16O1H+ (≈95% abundance). In the range from 1.5 to 2.5 mol L−1 a decrease in the 47[PO]+ and background signals, and also in the signals of the internal standards, was observed. It is likely that such a high concentration of methanol additionally cooled the plasma, which was then unable to sufficiently decompose the matrix and ionize the sample.
To verify the role of carbon compounds in the plasma, an experiment with direct introduction of methanol into the spray chamber was also performed at conditions for 47[PO]+ detection (Fig. 2). An increase in the 47[PO]+ signal by a factor of 1.3 was observed, indicating that the enhancement factor of 3.7, which was determined during the experiment with methanol present in sample solution (Fig. 3), is a combination of increased sample transport efficiency and enhanced formation of PO+ ions. In addition, a large increase in the background signal by a factor of 130 was observed, confirming that carbon compounds are a source of polyatomic ions at m/z 47. The decrease of the PO+ and background signals at higher concentrations (more than 3 mol L−1) is most likely due to a too high concentration of carbon compounds in the plasma, which disables efficient sample decomposition.
Limits of detection
The instrumental limits of detection (LODs) were determined in different concentrations of nitric acid and methanol under both measuring conditions as the background signal plus three times the standard deviation of the background noise (Table 1). The determined LODs were higher at higher concentrations of nitric acid and methanol under both measuring conditions, which is as expected, since in all cases the signal-to-background ratio decreases with increasing matrix concentration. Comparing both measuring methods, lower LODs were achieved with detection of 31P+ ions, especially in the case of methanol. The exception is aqueous solutions for which a better LOD was achieved when phosphorus was detected as 47[PO]+.
Determination of phosphorus in organic samples
In ICPMS measurements, nitric acid is frequently used for mineralization of samples. Since the amount of residual nitric acid after digestion depends on the sample type and its amount [18], systematic errors can occur if the concentration of nitric acid in the samples does not match the nitric acid concentration in the calibration solutions. Furthermore, if the digestion procedure is incomplete and some carbon compounds remain in the measuring solution, these compounds will contribute to the measured signal. When the amount of the remaining carbon is not known, inaccurate results might be obtained even when a standard addition calibration is performed.
Different amounts of the two reference materials were mineralized and phosphorous was determined as 31P+ and 47[PO]+ using calibration solutions with different concentrations of nitric acid (Table 2). In addition, the residual carbon content after mineralization was determined (Table 2). The first set of calibration solutions contained the same concentration of nitric acid as was added to the sample prior to mineralization (10% v/v) and the second one five-times less nitric acid (2% v/v).
The results obtained with detection of 31P+ depend on the mass of sample used in the mineralization process. Higher amounts of sample gave higher results, which is a consequence of the incomplete digestion of organic matter as can be seen from data on the total concentration of carbon. For the same reason, differences between obtained and certified values also increase with the amount of digested sample. Only the results obtained with the lowest amount of sample (100 mg of NIST 1575, 40 mg of engine oil) are in satisfactory agreement with the certified concentrations, since the excess of nitric acid was sufficient to remove enough carbon to prevent interference with measurements. The absence of carbon-based polyatomic ions in the mineralized sample (100 mg of NIST 1575) was also confirmed by high-resolution ICPMS measurements, since only nitrogen-based polyatomic ions originating from nitric acid were detected.
The results for both reference materials, calculated using different concentrations of nitric acid in the calibration solutions, were always lower if calibration with 2% nitric acid was applied (compared to 10% nitric acid), since a higher concentration of nitric acid reduces phosphorus sensitivity (Fig. 1). In the case of the lowest amount of mineralized sample (100 mg of NIST 1575 and 40 mg of engine oil), an approximately original amount of nitric acid should be present in the sample solution (10%); therefore, results calculated using calibrations in 10% nitric acid should be in best agreement with certified values. However, due to the uncertainty in the certified values it is not possible to confirm this hypothesis.
In all experiments determinations of phosphorus as 47[PO]+ gave incorrect (mostly too high) results. This is due to the more pronounced influence of carbon-based polyatomic ions (Fig. 3) and also partially due to the presence of titanium in the reference samples (NIST 1575 ≈7 μg g−1, engine oil ≈0.7 μg g−1). From these findings we can conclude that detection of phosphorus as 47[PO]+ is not suitable for determination of phosphorus in organic samples.
Conclusions
An increase in the concentration of nitric acid and methanol causes a decrease in signal-to-background ratios and a consequent increase in the instrumental detection limits. The main reason for this effect is an increased abundance of polyatomic ions, which are formed in the plasma in the presence of nitric acid and methanol. It was proved that the 31P+ signal is enhanced in the presence of methanol and it behaves like other elements with similar ionization potentials. Comparing measurements of 31P+ and 47[PO]+ ions, the first type of detection is preferable owing to less pronounced polyatomic interferences. As was shown by analyzing two organic certified reference materials containing relatively high concentrations of phosphorus, as high as possible excess of nitric acid should be used for sample mineralization to achieve complete removal of carbon from the digested sample and to maintain approximately the original amount of nitric acid in the sample solution. The described effects have practical consequences in both chromatographic and direct determinations of phosphorus and/or its compounds. If a quadrupole ICPMS is used as a chromatographic detector, nitric acid and methanol containing mobile phases will cause higher baseline and poorer detection limits.
References
Esslemont G, Maher W, Ford P, Krikowa F (2000) At Spectr 21:42–45
Jiang S, Houk RS (1988) Spectrochim Acta B 43:405–411
Fernandez RG, Alonso JIG, Sanz-Medel A (2001) J Anal At Spectrom 16:1035–1039
Divjak B, Novi̇č M, Goessler W (1999) J Chromatogr A 862:39–47
Kovȧčevič M, Gartner A, Novič M (2004) J Chromatogr A 1039:77–82
Wind M, Edler M, Jakubowski N, Linscheid M, Wesch H, Lehmann WD (2001) Anal Chem 73:29–35
Siethoff C, Feldmann I, Jakubowski N, Linscheid M (1999) J Mass Spectrom 34:421–426
Wind M, Eisenmenger A, Lehmann WD (2002) J Anal At Spectrom 17:21–26
Helfrich A, Bettmer J (2004) J Anal At Spectrom 19:1330–1334
Pröfrock D, Leonhard P, Prange A (2003) J Anal At Spectrom 18:708–713
Kovȧčevič M, Leber R, Kohlwein SD, Goessler W (2004) J Anal At Spectrom 19:80–84
Bandura DR, Baranov VI, Tanner SD (2002) Anal Chem 74:1497–1502
Steward II, Olesik JW (1998) J Anal At Spectrom 13:1313–1320
Allain P, Jaunault L, Mauras Y, Mermet JM, Delaporte T (1991) Anal Chem 63:1497–1498
Larsen EH, Stürup S (1994) J Anal At Spectrom 9:1099–1105
Hu Z, Hu S, Gao S, Liu Y, Lin S (2004) Spectrochim Acta B 59:1463–1470
Kozono S, Takahashi S, Haraguchi H (2002) Anal Bioanal Chem 372:542–548
Wasilewska M, Goessler W, Zischka M, Maichin B, Knapp G (2002) J Anal At Spectrom 17:1121–1125
Acknowledgements
The authors thank the Slovenian Research Agency (S1-104-004/19054/2001, P1-0034) and the Ministry of Science, Education and Sport of the Republic of Croatia (0098132) for financial support, Mrs. M. Moder (Petrol) for engine oil reference material and Dr. J. Hinrichs (Thermo) for assistance with SF-ICPMS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kovačevič, M., Goessler, W., Mikac, N. et al. Matrix effects during phosphorus determination with quadrupole inductively coupled plasma mass spectrometry. Anal Bioanal Chem 383, 145–151 (2005). https://doi.org/10.1007/s00216-005-3389-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-3389-8