Abstract
Rationale
The nucleus accumbens (NAc) core gates motivationally relevant behavioral action sequences through afferents from cortical and subcortical brain regions. While the role of the NAc core in reward and effort-based decision making is well established, its role in working memory (WM) processes is incompletely understood. The odor span task (OST) has been proposed as a measure of non-spatial working memory capacity (WMC) as it requires rodents to select a novel odor from an increasing number of familiar odors to obtain a food reward.
Objective
To assess the role of the NAc core in the OST using (1) reversible chemical inactivation and (2) selective blockade of dopamine D1 and D2 receptors in the area.
Methods
Well-trained male rats were tested on the OST following intra-NAc core infusions of muscimol/baclofen, the D1 receptor antagonist SCH-23390 (1 μg/hemisphere) and the D2 receptor antagonist eticlopride (1 μg/hemisphere). Behavioral measurements included the average odor span, maximum odor span, choice latency, searching vigor, and patterns of responding during foraging that may relate to impulsivity.
Results
Chemical inactivation of the NAc core significantly decreased odor span relative to sham and vehicle conditions. Selective antagonism of D2, but not D1, receptors in the NAc core also produced deficits in odor span. We found that secondary behavioral measures of choice latency, searching vigor, and responding to the first odor stimulus encountered were largely unaffected by treatment.
Conclusions
These findings suggest that D2 receptors in the NAc core are required for OST performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A clinical manifestation of psychiatric disorders, including Alzheimer’s disease, Parkinson’s disease, and substance use disorders (SUD), is impaired working memory (WM) and working memory capacity (WMC) (Bechara and Martin 2004; Barch et al. 2012; Kirova et al. 2015; Lewis et al. 2003). WM is an essential cognitive ability that allows maintenance and active manipulation of a finite amount of information to support goal-directed behavior (Baddeley and Hitch 1974; Constantinidis and Klingberg 2016). In rodents, WM is typically assessed by presenting stimuli, be they objects, odors, or spatial cues, that must be used after a short delay to guide behavior (Dudchenko 2004; Ennaceur and Delacour 1988; Olton and Samuelson 1976). While many tasks measure WM in rodents, few specifically measure WMC (Dudchenko et al. 2013).
The odor span task (OST) has been proposed as a measure of non-spatial WMC that relies on rodents’ ability to discriminate between, and remember, distinct scents to locate a hidden food reward after a delay (Dudchenko et al. 2000, 2013). While the OST has similarities with span tasks used to assess WMC in humans, debate exists over whether it truly taps a limited capacity as rats have to been shown to exhibit high spans under some conditions (April et al. 2013; Bratch et al. 2016). Previous work from our laboratory has established a cortico-striatal-thalamic circuit subserving task performance (Davies et al. 2013a, b, 2017; Scott et al. 2020), and that the rat medial prefrontal cortex (mPFC) exhibits dynamic spiking activity during the delay period (De Falco et al. 2019), which is thought to be a biological correlate of WM in rodents and humans (Constantinidis et al. 2018; Fuster and Alexander 1971). Davies et al. (2017) show that a glutamatergic projection from the mPFC to the dorsomedial striatum (dmSTR) is required for OST performance; however, the role of the ventral striatum has not been investigated despite a dense projection from the prelimbic (PL) region of the mPFC to the nucleus accumbens (NAc) core that mediates attention and visuospatial WM (Christakou et al. 2004; Floresco et al. 1999; Howland et al. 2022).
Anatomically, the NAc core primarily receives glutamatergic afferents from the PL, basolateral amygdala (BLA), and the ventral hippocampus (vHipp), with a dopaminergic afferent from the ventral tegmental area (VTA), which each encode dissociable types of information that can bias behavior in specific ways (Deroche et al. 2020; Floresco 2015). Approximately 95% of neurons within the NAc core are GABAergic medium spiny neurons (MSNs) which are segregated based on expression of dopamine (DA) D1 or D2 receptors (Kawaguchi 1997; Li et al. 2018). Recent evidence suggests that different MSN populations preferentially respond to specific excitatory afferents, where the BLA and the PFC are the main source of synaptic excitation on D1- and D2-expressing MSN’s, respectively (Deroche et al. 2020).
Functionally, a prevailing view is that the NAc core integrates cognitive and limbic information to gate approach behavior towards reward-predicting stimuli (Floresco 2015; Nicola 2010; Saunders and Robinson 2012). As a result, the NAc core has been extensively studied in animal models of addiction given its role in acquiring drug-reward associations and facilitating cue-elicited drug-seeking behaviors driven by plastic changes from repeated drug exposure (Koob and Volkow 2010; McFarland et al. 2003; Stefanik et al. 2013; Wang et al. 2012). While the NAc core is implicated in cognitive constructs including selective attention and cognitive control (Bryce and Floresco 2019; Christakou et al. 2004), to our knowledge, only one study has reported a role of the NAc in mediating visuospatial WM on a delayed spatial win–shift version of the radial arm maze task (Floresco et al. 1999), and no studies have assessed its role in mediating non-spatial WM or WMC.
The OST is uniquely positioned to allow an assessment of the role of the NAc core in non-spatial WM as performance is independent of the hippocampus (Dudchenko et al. 2000), it includes a foraging period where searching vigor can be inferred (McGinty et al. 2013), and the capacity of rats’ olfactory WM can be quantified (Scott et al. 2020). In the present study, we conducted two experiments to assess: (1) the effect of temporarily inactivating the NAc core on odor span capacity and searching vigor and (2) if DA receptor-mediated regulation of MSN activity is required for performance on the OST. Findings show that the NAc core serves a critical role in OST performance, and this function is primarily regulated by signaling through D2 receptors. Chemical inactivation and dopamine receptor-specific antagonism had little effect on choice latency, searching vigor, and patterns of responding during foraging that may relate to impulsivity.
Materials and methods
Animals
Adult, male Long-Evans rats (n=12; Charles Rivers Laboratories, Kingston, NY) were used in this experiment. Four rats were initially tested in the chemical inactivation experiment and a probe session. An additional cohort of eight rats were tested in the chemical inactivation experiment, probe session, and dopamine receptor antagonist experiment. Upon arrival, rats were given 7 days to acclimatize to the vivarium and were provided with ad libitum access to food (Purina rat chow) and water. Rats were maintained on a 12-h light:dark cycle (with lights on at 0700) and were restricted to 85–90% of their free feeding weight to motivate reward-seeking behavior. Throughout training and testing procedures, rats were individually housed in standard ventilated cages with ad libitum access to water and an environmental enrichment object. All experimental procedures were approved by the University of Saskatchewan Animal Research Ethics Board and were conducted in accordance with the guidelines established by the Canadian Council on Animal Care.
Apparatus
Behavioral training and testing took place on a custom-made wooden table (plywood pained white, area = 0.84 m2, height = 0.95 m). The testing platform contained 24 identical circular cut-outs (6 cm2) evenly spaced along the table border (circle origin 6 cm from the table edge). Circular cut-outs were lined with white corrugated plastic at the base to allow for efficient cleaning. The table was surrounded by a retractable silk curtain to minimize visual distractors from the environment during behavioral assessment. Between trials, rats were placed in a plastic transport cage (35 cm W × 35 cm L × 18cm H) surrounded by a black corrugated plastic enclosure (40 cm W × 40 cm L × 40 cm H) to avoid visual cueing to the location of the novel stimulus between trials. A webcam (Logitech Brio, Logitech) was mounted to a wooden support arm above the testing platform to collect behavior videos from an overhead perspective.
Odors
This experiment employed an adapted version of the OST from that originally presented by Dudchenko et al. (2000), where odors are presented in the form of scented plastic lids (40 cm2, Polar Pak #55983) covering translucent portion cups (59 ml, Dixie #P020XXTRANSL) containing food rewards (Dustless Precision Pellets, 45 mg, Rodent Purified Diet; BioServ, NJ, USA) located in circular cut-outs on the testing platform (MacQueen et al. 2011; Scott et al. 2020). Common household spices purchased at a local supermarket were stored in plastic food containers and used to scent the lids. We used 23 different spices as odorants: sage, cocoa, basil, clove, lemon, all spice, paprika, thyme, garlic, celery, fennel, marjoram, cumin, dill, cinnamon, oregano, onion, anise, caraway, coffee, mustard, nutmeg, and ginger.
Odor span task training
Prior to training on the OST, rats completed three stepwise pretraining phases: (1) habituation; (2) response shaping; (3) delayed non-match-to-sample (DNMTS) training. First, rats were each handled for three consecutive days for 5 min and habituated to the intended route of transport, experimental room, and testing platform. Next, rats were shaped to remove unscented lids covering a portion cup to collect a food reward. As outlined by Scott et al. (2020), rats were first presented with an uncovered portion cup containing two food pellets, then following each correct response (collection of the food reward within 5 min), the portion cup was covered by an unscented lid to an incrementally greater degree (50% covered, 75% covered, 99% covered, 100% covered) on the subsequent trial. If a rat failed to collect the food reward within the 5-min trial duration, the degree of lid coverage on the subsequent trial was decreased by one increment. Rats were allowed to complete three trials per day until mastery was demonstrated by successfully completing three trials on fully covered portion cups in a single training day.
Next, rats learned to discriminate between odors and respond to novelty through a DNMTS training phase. In the sample phase of a single trial, rats were presented with a portion cup that was fully covered by a scented lid and contained two hidden food reward pellets. Upon removal of the scented lid and collection of the food reward, the rat was moved into a shielded holding cage for the 1-min delay. During the delay, the spatial position of the initially encountered cup-lid pairing was changed, and a novel odor was introduced through the random placement of an additional reward-containing cup-lid pairing. In the test phase, a correct response was defined as removal of the novel lid and collection of the food reward within 5 min. Rats were allowed to complete six trials per training day until mastery was demonstrated by correctly responding on five to six trials for two consecutive training days. Throughout DNMTS training, the position of cup-lid pairings was randomized, and the scented lid presented in the sample phase was replaced by an identically scented lid in the test phase to prevent the development of a scent marking mitigating strategy.
A visualization of the procedure used for OST training and testing is shown in Fig. 1A. For OST training and testing, the term trial refers to a single foraging episode, a session refers to a series of trials before an error is made (i.e., one span), and a training/testing day refers to all OST sessions conducted on a single day. The first two trials within the OST are identical to the sample and test phases completed in DNMTS training; however, continuing beyond the second trial, a novel scented cup-lid pairing was added to the subsequent trial following each correct response until an incorrect response was made. Said differently, rats were allowed to serially complete discrimination trials, where advancement to the next trial was contingent on correctly responding to the novel cup-lid pairing, and touching or removing a lid scented with a familiar odor marked the end of a given training session. Odor span capacity for a single session was defined as the number of trials completed minus 1. As with DNMTS training, each trial was separated by a 1-min delay where the rat was temporarily held in a shielded holding cage, the spatial position of cup-lid pairings was continuously shuffled within sessions, and the order of odor presentation was randomized between training days. Each day, rats were allowed to complete a maximum of three sessions (with no repetition of odors between sessions) in a maximum of 30 min. Rats were trained until mastery was demonstrated by achieving an average odor span of eight across three consecutive training days. Between trials and sessions, the testing platform was thoroughly cleaned with 70% ethanol. For all training phases, rats were tested 5 days per week between the hours of 0800 and 1600. The number of training sessions required to achieve mastery criterion for each stepwise training phase is shown in Fig. 1B. As changes in impulsivity or locomotion could drive changes in task performance, we calculated secondary behavioral measures of choice latency, searching vigor, and 1st approach responses and errors.
A Schematic representation of the odor span task. The OST is an odor-based incrementing DNMTS test that relies on rodents’ ability to discriminate between, and remember, distinct scents. Colored circles represent distinct odors, and novelty is denoted by the red dot. After completing an initial trial where a single odor is presented, every subsequent trial introduces one novel scent that is unique from previously encountered odors, and advancement to the next trial was contingent on removing the lid of the novel scent and collecting a food reward. Between trials, rats were placed in an opaque holding cage for approximately 1 min. Discrimination trials were sequentially completed until the lid of a previously experienced scent was moved. B Training timeline and stepwise progression to OST mastery criteria. After being habituated to experimental conditions, rats were taught to remove plastic lids of portion cups containing odorless food pellets. Before progressing to the full OST, rats were trained on an odor-based DNMTS task to create a strong association between novelty and a food reward after a delay. Finally, rats were trained on the full OST until mastery criteria were achieved by demonstrating an average odor span of eight odors for across three consecutive training days. C Mean group odor span capacity eight training days preceding stereotaxic cannulation surgery. Surgery was performed after demonstration of mastery
Probe sessions
To ensure that rats were not cueing to the scent of the food reward instead of the novel odor, we conducted a single probe experiment that assessed rat behavior when presented with an unbaited novel cup-lid pairing within the OST. The probe testing occurred for all rats (n=12) upon completion of all other OST testing. Following the probe experiment protocol described by Davies et al. (2013b), we conducted standard OST testing until rats achieved an odor span of 2, then in the subsequent trial, no food reward pellets were placed in the novel cup-lid pairing. We found that rats responded with 100% accuracy on probe trials with no significant change in choice latency (data not shown). Studies utilizing the OST typically conduct a second probe experiment that tests for evidence of a scent marking mitigating strategy; however, as scented lids were exchanged following physical contact with the rat, this experiment was deemed unnecessary.
Surgery and intracranial drug infusions
Upon demonstration of mastery on the OST (Fig. 1C), rats were provided ad libitum access to food and water for three days, and then were subjected to stereotaxic surgery. Rats were anesthetized with aerosolized isoflurane (~3% isoflurane in oxygen, Janssen, Toronto, Ontario) throughout the procedure. Rats were positioned in the stereotaxic frame and secured by ear bars to maintain a flat skull. Guide cannulae (23 Ga) were bilaterally inserted above the NAc core (AP + 1.60 mm; ML ± 1.80 mm; DV −6.20 mm) (Bryce and Floresco 2019). Cannulae were secured using six surgical screws and dental acrylic and were sealed by insertion of dummy stylets. Post-operatively, rats were treated with buprenorphine and Anafen, and allowed to recover from surgery for at least 7 days prior to the commencement of behavioral training.
Rats were then trained on the OST until stable performance was demonstrated by achieving an average odor span of 8 for 3 consecutive training days. We tested rats on two separate intra-NAc core drug manipulation experiments using a within-subjects design: (1) chemical inactivation of the NAc core using a combination of Muscimol (M, GABA-A receptor agonist, Sigma-Aldrich) and Baclofen (B, GABA-B receptor agonist, Sigma-Aldrich), with vehicle (0.9% saline) and sham controls; and (2) DA receptor-specific antagonism with R(+)-SCH-23390 hydrochloride (SCH, D1 receptor antagonist, Sigma-Aldrich), S-(−)-Eticlopride hydrochloride (ETI, D2 receptor antagonist, Sigma-Aldrich), and a vehicle control. Prior to drug testing, rats were habituated to the microinfusion procedure and experimental conditions. Specifically, on the 2 days preceding the initial microinfusion test day, rats were transported to the infusion room and allowed to acclimatize for 15 min alone, then the infusion pump (Harvard Apparatus) was powered on for 1 min with shortened infusion needles inserted into cannulae, and then rats were trained on the OST approximately 15 min later. Microinfusions were conducted using 30-gauge stainless steel infusion needles connected to Hamilton syringes with PE50 tubing. The tips of the infusion needles extended 1 mm past the tips of the cannulae when fully inserted. Infusion needles were left in the cannulae for 1-min post-infusion to allow for adequate drug diffusion. Behavioral assessment was conducted approximately 15 min post-infusion and one infusion was conducted per test day. Rats were required to achieve an average odor span of 6 on washout days to receive the next treatment.
All rats (n=12) were tested on the chemical inactivation experiment, where treatments were counterbalanced and administered in a quasi-randomized order. M/B (mixed at a 1:1 ratio, 0.15 μg/hemisphere each) was infused at a rate of 0.3 μL/min to a total infusion volume of 0.3 μL (Davies et al. 2013; Scott et al. 2018, 2020). This dose was selected it has been shown to chemically inactivate both cortical and subcortical brain regions to produce cognitive impairment, without causing severe locomotor impairment (Scott et al. 2020). The vehicle condition was delivered at the same infusion rate to the same infusion volume, and the sham condition consisted of inserting shortened needles into cannulae, but not infusing liquid.
A cohort of rats (n=8) were re-tested using DA receptor-specific antagonists following a 3-week washout period. Using an identical microinfusion procedure as described above, SCH (1 μg/hemisphere), ETI (1 μg/hemisphere), and the vehicle control were infused in a counterbalanced and quasi-randomized order. Antagonist dosages were selected based on the higher end of previous dose response curves assessing their effects on rat behaviors mediated by NAc subregions (Anderson et al. 2003; Goto and Grace 2005; Haluk and Floresco 2009; Pattij et al. 2007; Stopper et al. 2013).
Histology
Rats were deeply anesthetized with isoflurane and transcardial perfusions were completed by infusing physiological saline (0.9%, 150 mL) followed by a mixture of formalin, glacial acetic acid, and methanol (FAM, 150 mL). Brains were then removed and post-fixed in paraformaldehyde before being transferred to a solution containing 30% sucrose and 0.1% sodium azide. Brains were sectioned coronally on a sliding microtome at 50 μm, mounted on glass slides, and Nissl stained with cresyl violet. Cannula and infusion site placements (Fig. 2) were confirmed in reference to a rat brain atlas (Paxinos and Watson 1997). Poor sectioning made it impossible to accurately determine the infusion sites from three of the four rats tested in only the chemical inactivation experiment. These rats are included in the “Results” given similarities in their performance to those rats with confirmed placements following chemical inactivations.
Locations of the confirmed infusion sites in the NAc core for both experiments. Placements of one rat from the cohort tested only in the chemical inactivation experiment and seven rats tested in both the chemical inactivation and dopamine receptor experiments are plotted. See “Methods” for additional details. Numbers refer to the anterior–posterior location of the plates relative to bregma
Statistical analysis
Statistical analysis was performed using Prism software (9.5.1) with significance set at p < 0.05. For both experiments, performance measures of odor span capacity, rat-stimulus interaction bout count, and choice latency were measured by session. Choice latency was defined as the time interval between the rat being placed on the test platform to removing the lid from a cup-lid pairing on both correct and incorrect trials. For analysis, we transformed some performance measures to calculate average odor span capacity, maximum odor span capacity, searching vigor, and 1st approach responses and errors. Average odor span was defined as the mean odor span capacity achieved across the maximum of three sessions conducted on a given testing day. Maximum odor span capacity was defined as the highest odor span capacity achieved across the maximum of three sessions conducted on a given testing day. Searching vigor was defined as the normalization of choice latency to rat-stimulus interaction bout count (choice latency/interaction bout count, by trial). First approach responses were defined as the frequency in which rats remove a lid on the initial rat-stimulus interaction bout following chemical manipulation relative to the vehicle condition. Said differently, we identified the OST session where each rat achieved their maximum odor span following each chemical intervention (M/B, ETI, SCH), then compared the number of 1st approach responses following intervention to the corresponding vehicle condition matched by session number and trial number, where possible (1st approach responses = (# responses following chemical intervention on the max span session)/(# responses following saline infusion, matched by session number and trial count). First approach errors were defined as the percentage of sessions where a rat made an incorrect 1st approach response. We chose to analyze these measures separately as 1st approach responses may relate to an impulsive phenotype that is evident across multiple trials (increased probability of selecting the first cup-lid pairing, regardless of correctness), while 1st approach errors may relate to an impulsive phenotype that negatively impacts task performance (increased probability of selecting an incorrect cup-lid pairing, resulting in a lower odor span), but does not capture how many trials were completed prior to making an incorrect 1st approach response. Taken together, we suggest that 1st approach responses and errors reported together more completely assess a behavioral phenotype that may related to treatment-mediated changes in impulsivity.
For analysis of average span, max span, choice latency, and searching vigor, we checked assumptions of normality (Shapiro-Wilk test) and sphericity (Geisser-Greenhouse correction applied), then conducted 1-way repeated measures (RM) ANOVA or the non-parametric Friedman test, where appropriate. For parametric testing, multiple comparisons were performed using Tukey’s test, and for non-parametric testing, Dunn’s multiple comparisons test was used, where appropriate. We conducted a paired t-test for analysis of average odor span on baseline and washout days. Analyses of 1st approach responses were conducted using a one-sample t-test with a hypothetical value of 1 (representing the same number of 1st approach responses following a treatment and vehicle condition, matched for session number and trial count). For analysis of 1st approach errors, we conducted the non-parametric Wilcoxon test to compare the percentage of 1st approach errors following a treatment to the corresponding vehicle condition. Post hoc power analysis was conducted by calculating the standardized effect size (Lakens 2013) and achieved power for dependent measures with the paired t-test configuration within G*Power (3.1) (Faul et al. 2007).
Results
Inactivation of the NAc core impairs odor span capacity but not searching vigor
Odor span capacity
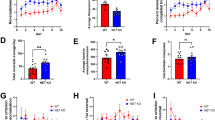
Twelve rats implanted with guide cannulae were tested on this experiment; however, one rat was removed for off-target cannula placement, and one rat was removed for not completing behavioral testing, leaving 10 rats for the final analysis. We found that average odor span capacity did not differ between baseline and washout test days (t(9) = 1.08, p = 0.31, Fig. 3A). To assess the effect of reversible chemical inactivation on odor span, we conducted a series of 1-way RM ANOVAs on average odor span and maximum odor span (Fig. 4A, B). We found a significant effect of treatment on both average odor span (F(2, 17.93) = 10.59, p < 0.001) and maximum odor span (F(1.708, 15.38) = 23.93, p < 0.0001). The average odor span of rats following treatment with M/B was significantly lower than vehicle (p = 0.013) and sham (p < 0.01) conditions. On average, the M/B treatment lowered average odor span capacity by 8.9 odors and 7.7 odors relative to sham and vehicle conditions, respectively. Similarly, the maximum odor span of rats following treatment with M/B was significantly lower than both vehicle (p < 0.01) and sham (p < 0.0001) conditions. On average, the M/B treatment lowered maximum odor span capacity by 10.7 odors and 8.7 odors relative to sham and vehicle conditions, respectively. For average span, a large effect size (Cohen’s d = 1.13) with high achieved power (0.89) was found between vehicle and M/B conditions. For maximum odor span, a large effect size (Cohen’s d = 1.43) with high achieved power (0.98) was found between vehicle and M/B conditions.
Average odor span between baseline and washout test days. Baseline average odor span was defined as the mean odor span capacity achieved in the three test sessions preceding the initial microinfusion test day. Washout average odor span was defined as the mean odor span achieved in the two washout test sessions between microinfusion test days, where rats were required to achieve an average odor span of six to receive the next treatment. No differences between baseline and washout were observed for both the chemical inactivation (A) and dopamine receptor-specific antagonist (B) experiments
Effects of NAc core inactivation on OST performance. A The average odor span achieved across a maximum of three sessions. Infusion of M/B significantly reduced average odor span relative to both sham and vehicle conditions. B The maximum odor span achieved across a maximum of three sessions. Infusion of M/B significantly reduced average odor span relative to both sham and vehicle conditions. C Average choice latency across test trials. Infusion of M/B significantly reduced choice latency relative to sham, but not vehicle, conditions. D Average searching vigor across test trials. No differences in searching vigor were observed between conditions. Data are presented as mean±SEM
Searching vigor
Given that manipulation of the NAc core has been shown to affect approach behavior to reward-related stimuli, and that an extended choice latency on the OST may impact WM performance by extending the delay period, we inferred rat foraging behavior by analyzing choice latency and searching vigor (McGinty et al. 2013; Scott et al. 2020). To assess the effect of reversible chemical inactivation on trial duration, we conducted a Friedman test on choice latency (Fig. 4C). We found a significant effect of treatment on choice latency (κ = 11.40, p < 0.01), where the M/B treatment produced shorter choice latencies relative to sham (p < 0.01), but not saline (p = 0.13), conditions. As choice latency as an indicator of locomotion may be confounded by the number of stimuli on the testing platform, we also analyzed searching vigor, which we define as the average duration between rat-stimulus interaction bouts (Fig. 4D). We found no effect of treatment on searching vigor using a Friedman test (κ = 4.20, p = 0.14). For choice latency, power analysis revealed a medium effect size (Cohen’s d = 0.66) with low achieved power (0.33) between sham and M/B conditions. For searching vigor, power analysis revealed a medium effect size (Cohen’s d = 0.57) but low achieved power (0.24) between sham and M/B conditions, although this effect failed to reach significance (p = 0.13).
Patterns of responding during foraging
As impulsive responding could have driven the observed odor span impairments, we analyzed both impulsive responses and impulsive errors following chemical inactivation. To assess the possibility of an impulsive-like phenotype that is evident across multiple trials following treatment with M/B, we conducted a one-sample test on the ratio of 1st approach responses following chemical inactivation relative to the vehicle condition (Fig. 6A). We found that treatment with M/B did not significantly alter 1st approach responses (t=0.75, df=9, p = 0.47). Next, to evaluate if the proportion of sessions that were terminated due to a 1st approach error differed following chemical inactivation relative to vehicle, we conducted a Wilcoxon test (Fig. 6B). We found that chemical inactivation with M/B did not significantly change 1st approach errors (p = 0.5); however, power analysis revealed a small effect size (Cohen’s d = 0.39) and low achieved power (0.20) associated with this effect.
Selective antagonism of D2, but not D1, receptors in the NAc core impairs odor span but not searching vigor
Odor span capacity
Eight rats were re-tested using DA receptor-specific antagonists following a 3-week washout period; however, one rat was removed for off-target cannula placement, leaving seven rats for the final analysis. We found that average odor span did not differ between baseline and washout test days (t(6) = 0.38, p = 0.72, Fig. 3B). To assess the effect of D1- (SCH) and D2- (ETI) specific antagonists on odor span, we conducted a series of Friedman tests on average odor span and maximum odor span values (Fig. 5A, B). We found a significant effect of treatment on both average odor span (κ = 8.86, p < 0.01) and maximum odor span (κ = 9.85, p < 0.01). The average odor span of rats following treatment with ETI was significantly lower than vehicle (p < 0.01), but not SCH (p = 0.18) conditions. However, on average, the ETI treatment lowered average odor span by 7.9 odors and 5.9 odors relative to vehicle and SCH conditions, respectively. The maximum odor span of rats following treatment with ETI was significantly lower than vehicle (p < 0.01), but not SCH (p = 0.25) conditions. However, on average, the ETI treatment lowered average odor span by 9.4 odors and 6.3 odors relative to vehicle and SCH conditions, respectively. For both average and maximum odor span, a large effect size (1.7 < Cohen’s d < 1.9) and high achieved power (0.96 < power < 0.99) was found between vehicle and ETI conditions. Although no statistical difference was found between SCH and ETI conditions, power analysis performed on both average and maximum odor span values revealed a large effect size (0.84 < Cohen’s d < 0.93) but low achieved power (0.42 < power < 0.54) between antagonists.
Effects of dopamine receptor-specific antagonism using SCH-23390 (D1) and eticlopride (D2) in the NaC core on OST performance. A The average odor span achieved across a maximum of three sessions. Average odor span was significantly reduced following ETI, but not SCH, treatment relative to vehicle. B The maximum odor span achieved across a maximum of three sessions. Maximum odor span was significantly reduced following ETI, but not SCH, treatment relative to vehicle. C Average choice latency across test trials. No differences in choice latency were observed between conditions. D Average searching vigor across test trials. No differences in searching vigor were observed between conditions. Data are presented as mean±SEM
Searching vigor
To assess the effect of D1- (SCH) and D2- (ETI) specific antagonists on choice latency and searching vigor, we conducted a 1-way RM ANOVA and a Friedman test, respectively. We found no effect of treatment on choice latency (κ = 0.85, p = 0.76) or searching vigor (F(1.22, 7.35) = 2.18, p = 0.18). Given marginal mean differences and high variability between treatments in choice latency, we did not conduct a power analysis; however, as we observed a subtle decrease in searching vigor following chemical inactivation of the NAc core, and significantly impaired average and maximum odor span following the intra-NAc core ETI microinfusion, we conducted a power analysis between vehicle and ETI for searching vigor. We found a medium effect size (0.74) with low achieved power (0.38) between vehicle and ETI conditions.
Patterns of responding during foraging
Dopamine receptor-specific antagonist effects on patterns of responding during foraging that may relate to impulsivity were evaluated by analyzing 1st approach responses and 1st approach errors. To assess the possibility of an impulsive-like phenotype that is evident across multiple trials following treatment with SCH and ETI, we conducted one-sample tests on the ratio of 1st approach responses following treatments relative to the vehicle control condition (Fig. 6A). We found that neither treatment with SCH (t=1.07, df=6, p=0.32) nor ETI (t=1.01, df=6, p=0.35) significantly altered 1st approach responses. Next, to evaluate if the proportion of sessions that were terminated due to a 1st approach error differed following DA receptor-specific antagonism relative to vehicle, we conducted a series of Wilcoxon tests (Fig. 6C). We found that neither treatment with SCH (p=0.31) nor ETI (p=0.75) significantly altered 1st approach errors.
Effects of the intra-NAc core manipulations on measures that may relate to impulsivity. A Relative to their control performance, rats made a similar number of responses to the first odor stimulus they approached regardless of treatment. In addition, rats did not differ in the number of errors made on the first approach in either the inactivation (B) or dopamine receptor antagonist experiments (C). Data are presented as mean±SEM
Discussion
In the present study, we investigated the role of signaling through the NAc core in performance on the OST. We show that chemical inactivation of the NAc core profoundly impaired odor span but did not disrupt odor discrimination (Fig. 4). Selective antagonism of DA receptors in the NAc core revealed that blockade of D2, but not D1, receptors impaired odor span, but did not impair odor discrimination (Fig. 5). Chemical inactivation and DA receptor-specific antagonism had little effect on searching vigor or measures related to impulsive responding (Fig. 6). Taken together, these findings implicate NAc core D2-mediated signaling in the neural circuitry subserving OST performance.
Performance on the OST is orchestrated by cortico-striatal-thalamic circuits with tuning by several neurotransmitter systems (Davies et al. 2013a, b, 2017; MacQueen et al. 2011; Scott et al. 2020). We found that chemical inactivation of the NAc core during behavioral assessment profoundly impaired odor span, which may represent a deficit in olfactory WMC. However, decreased locomotion or odor discrimination caused by pharmacological manipulation could explain this impairment given the role of the NAc core in these functions (Maldonado-Irizarry and Kelley 1994; Setlow et al. 2003). To indirectly assess changes in locomotion, we normalized choice latency to the number of rat-stimulus interaction events to yield a novel behavioral measure termed searching vigor. Previous work has used choice latency as a proxy for psychomotor function (Davies et al. 2013); however, as choice latency is partially confounded by the number of stimuli presented on a given trial, we contend that choice latency and searching vigor are dissociable but complementary measures.
Psychomotor changes likely did not confound results in this study as we observed marginal changes in searching vigor and choice latency following inactivation. To assess changes in odor discrimination, we considered the number of rats that successfully completed the initial odor discrimination trial, indicated by attaining a maximum odor span of at least 1. Deficits in odor discrimination likely did not confound results in this study as all rats achieved a maximum odor span of at least 1 across conditions (Fig. 4B, Fig. 5B). Marginal changes in searching vigor are somewhat unexpected given the well-established role of the NAc core in invigorating approach behavior toward reward-predicting stimuli (Floresco 2015; McGinty et al. 2013). A potential explanation for this observation is that searching vigor only accounts for behavioral action during the foraging period, where all stimuli are visually identical, and the critical reward-predicting cue is the lid odor. Additionally, lesions of the NAc do not affect non-delayed random foraging on the radial arm maze (Floresco et al. 1997). Taken together, while we observed little change in searching vigor during the foraging period, invigoration of the motor sequence involved in reward collection following exposure to the novel odor cue may have occurred and should be directly measured in future work. We also considered patterns of responding to the first odor stimulus encountered when the rats were foraging (Fig. 6). Neither NAc inactivations nor DA receptor manipulations significantly affected the number of responses or errors made to the first stimulus, suggesting that rats were not responding more impulsively after these manipulations.
Differential expression of DA receptor subtypes anatomically distinguishes MSN populations, and functionally serves to regulate afferent excitatory neurotransmission (Wang et al. 2012). In the present experiment, we selectively blocked signaling through D1 and D2 receptors to assess receptor-specific regulation of MSN activity for performance on the OST. We found that antagonism of D2 receptors with ETI, but not D1 receptors with SCH, profoundly impaired odor span without affecting odor discrimination, searching vigor, or choice latency. An inherent limitation of employing DA receptor antagonists to investigate their local function is that DA receptors are expressed presynaptically, postsynaptically, and on cholinergic interneurons in the NAc core, where functional consequences may depend on spatial localization (Alcantara et al. 2003; Wang et al. 2012). Additionally, while SCH is a highly specific D1 antagonist (Hyttel 1983), ETI shows affinity at both D2 and D3 receptors (Shaik et al. 2021) which may affect DA signaling in the NAc core (Congestri et al. 2008). While we cannot rule out a role for D1 receptors, our results point to a substantial role of D2 receptors in mediating performance on the OST.
Several lines of research substantiate a role for the NAc core in olfactory non-spatial WM processes in rats. Existing work from our laboratory and others show that the OST requires engagement of the mediodorsal thalamus (mdThal) (Scott et al. 2020), mPFC (Davies et al. 2013), and dmSTR (Davies et al. 2017), but not engagement of the posterior parietal cortex (PPC) (Scott et al. 2018) or the hippocampus (Dudchenko et al. 2000). This network contains several nodes that are homologous to those within established networks underlying visuospatial WM in primates and humans (Constantinidis and Klingberg 2016). As the PPC and hippocampus are not required for performance on the OST but are well established in primate and human visuospatial WM processes, network differences may reflect modality-specific processing by the PPC and the preferential involvement of the hippocampus in tasks requiring spatial memory. To our knowledge, no studies have investigated the involvement of the NAc core in non-spatial WM; however, Floresco et al. (1999) showed that functional disconnection of the PL and the NAc caused impaired performance on a delayed spatial win–shift version of the radial arm maze task, reflecting spatial WM deficits. Our results support the notion that the NAc core is required for multiple forms of WM, given that performance on the OST does not rely on spatial cues and uses odor-based stimuli.
Our finding that D2 receptors in the NAc core mediate performance on the OST supports evidence of a projection from the PFC to the NAc core in mediating goal-directed behavior. As the OST is hippocampal independent, the two primary glutamatergic afferents to the NAc core that may direct task performance are the BLA and the PFC (Floresco 2015). Recent evidence suggests that the BLA and the PFC are the main sources of synaptic excitation on D1 and D2 expressing MSN’s, respectively (Deroche et al. 2020). Additionally, Brady and O’Donnell (2004) show selective modulation of prefrontal inputs by D2 receptors and limbic inputs by D1 receptors in vivo. Behaviorally, a projection from the PFC to the NAc core has been implicated in goal-directed behavior on a reward-driven plus maze task (Goto and Grace 2005), in spatial WM on a delayed radial arm maze task (Floresco et al. 1999), and in selective attention (Christakou et al. 2004). Taken together, the results of the present experiment and previously published work provide support for a PFC to NAc core projection in mediating non-spatial WM processes in rats; however, this hypothesis must be directly tested in future work.
References
Alcantara AA, Chen V, Herring BE, Mendenhall JM, Berlanga ML (2003) Localization of dopamine D2 receptors on cholinergic interneurons of the dorsal striatum and nucleus accumbens of the rat. Brain Res 986(1):22–29
Anderson SM, Bari AA, Pierce RC (2003) Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology 168(1–2):132–138
April LB, Bruce K, Galizio M (2013) The magic number 70 (plus or minus 20): variables determining performance in the rodent odor span task. Learn Motiv 44:143–158
Baddeley AD, Hitch G (1974) Working memory. Psychol Learn Motiv 8:47–89
Barch DM, Moore H, Nee DE, Manoach DS, Luck SJ (2012) CNTRICS imaging biomarkers selection: working memory. Schizophr Bull 38:43–52
Bechara A, Martin EM (2004) Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology 18(1):152–162
Brady AM, O’Donnell P (2004) Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J Neurosci 24(5):1040–1049
Bratch A, Kann S, Cain JA, Wu J-E, Rivera-Reyes N, Dalecki S, Arman D, Dunn A, Cooper S, Corbin HE, Doyle AR, Pizzo MJ, Smith AE, Crystal JD (2016) Working memory systems in the rat. Curr Biol 26:351–355
Bryce CA, Floresco SB (2019) Alterations in effort-related decision-making induced by stimulation of dopamine D1, D2, D3, and corticotropin-releasing factor receptors in nucleus accumbens subregions. Psychopharmacology 236(9):2699–2712
Christakou A, Robbins TW, Everitt BJ (2004) Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci 24(4):773–780
Congestri F, Formenti F, Sonntag V, Hdou G, Crespi F (2008) Selective D3 receptor antagonist SB-277011-A potentiates the effect of cocaine on extracellular dopamine in the nucleus accumbens: a dual core-shell voltammetry study in anesthetized rats. Sensors 8(11):6936–6951
Constantinidis C, Funahashi S, Lee D, Murray JD, Qi X-L, Wang M, Arnsten AFT (2018) Persistent spiking activity underlies working memory. J Neurosci 38(32):7020
Constantinidis C, Klingberg T (2016) The neuroscience of working memory capacity and training. Nat Rev Neurosci 17(7):438–449
Davies DA, Greba Q, Howland JG (2013b) GluN2B-containing NMDA receptors and AMPA receptors in medial prefrontal cortex are necessary for odor span in rats. Front Behav Neurosci 7:183
Davies DA, Molder JJ, Greba Q, Howland JG (2013a) Inactivation of medial prefrontal cortex or acute stress impairs odor span in rats. Learn Mem 20(12):665–669
Davies DA, Greba Q, Selk JC, Catton JK, Baillie LD, Mulligan SJ, Howland JG (2017) Interactions between medial prefrontal cortex and dorsomedial striatum are necessary for odor span capacity in rats: role of GluN2B-containing NMDA receptors. Learn Mem 24(10):524–531
De Falco E, An L, Sun N, Roebuck AJ, Greba Q, Lapish CC, Howland JG (2019) The rat medial prefrontal cortex exhibits flexible neural activity states during the performance of an odor span task. eNeuro 6(2):ENEURO.0424-18.2019
Deroche MA, Lassalle O, Castell L, Valjent E, Manzoni OJ (2020) Cell-type- and endocannabinoid-specific synapse connectivity in the adult nucleus accumbens core. J Neurosci 40(5):1028
Dudchenko PA (2004) An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev 28(7):699–709
Dudchenko PA, Talpos J, Young J, Baxter MG (2013) Animal models of working memory: a review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neurosci Biobehav Rev 37:2111–2124
Dudchenko PA, Wood ER, Eichenbaum H (2000) Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. J Neurosci 20(8):2964
Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31(1):47–59
Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191
Floresco SB (2015) The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol 66(1):25–52
Floresco SB, Braaksma DN, Phillips AG (1999) Thalamic–cortical–striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci 19(24):11061–11071
Floresco SB, Seamans JK, Phillips AG (1997) Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci 17(5):1880–1890
Fuster JM, Alexander GE (1971) Neuron activity related to short-term memory. Science 173(3997):652–654
Goto Y, Grace AA (2005) Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci 8(6):805–812
Haluk DM, Floresco SB (2009) Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacol 34:2041–2052
Howland JG, Ito R, Lapish CC, Villaruel FR (2022) The rodent medial prefrontal cortex and associated circuits in orchestrating adaptive behavior under variable demands. Neurosci Biobehav Rev 135:104569
Hyttel J (1983) SCH 23390—the first selective dopamine D-1 antagonist. Eur J Pharmacol 91(1):153–154
Kawaguchi Y (1997) Neostriatal cell subtypes and their functional roles. Neurosci Res 27(1):1–8
Kirova A-M, Bays RB, Lagalwar S (2015) Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed Res Int 2015:1–9
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacol 35(1):217–238
Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:863
Lewis SJG, Cools R, Robbins TW, Dove A, Barker RA, Owen AM (2003) Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. Neuropsychologia 41(6):645–654
Li Z, Chen Z, Fan G, Li A, Yuan J, Xu T (2018) Cell-type-specific afferent innervation of the nucleus accumbens core and shell. Front Neuroanat 12:84
MacQueen DA, Bullard L, Galizio M (2011) Effects of dizocilpine (MK801) on olfactory span in rats. Neurobiol Learn Mem 95(1):57–63
Maldonado-Irizarry CS, Kelley AE (1994) Differential behavioral effects following microinjection of an NMDA antagonist into nucleus accumbens subregions. Psychopharmacology 116(1):65–72
McFarland K, Lapish CC, Kalivas PW (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23(8):3531
McGinty VB, Lardeux S, Taha SA, Kim JJ, Nicola SM (2013) Invigoration of reward seeking by cue and proximity encoding in the nucleus accumbens. Neuron 78(5):910–922
Nicola SM (2010) The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci 30(49):16585–16600
Olton DS, Samuelson RJ (1976) Remembrance of places passed: spatial memory in rats. J Exp Psychol Anim Behav Process 2(2):97–116
Pattij T, Janssen MCW, Vanderschuren LJMJ, Schoffelmeer ANM, van Gaalen MM (2007) Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology 191:587–598
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates. Elsevier, Amsterdam
Saunders BT, Robinson TE (2012) The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci 36(4):2521–2532
Scott GA, Liu MC, Tahir NB, Zabder NK, Song Y, Greba Q, Howland JG (2020) Roles of the medial prefrontal cortex, mediodorsal thalamus, and their combined circuit for performance of the odor span task in rats: analysis of memory capacity and foraging behavior. Learn Mem 27(2):67–77
Scott GA, Zabder NK, Greba Q, Howland JG (2018) Performance of the odour span task is not impaired following inactivations of parietal cortex in rats. Behav Brain Res 341:181–188
Setlow B, Schoenbaum G, Gallagher M (2003) Neural encoding in ventral striatum during olfactory discrimination learning. Neuron 38(4):625–636
Shaik AB, Boateng CA, Battiti FO, Bonifazi A, Cao J, Chen L, Chitsazi R, Ravi S, Lee KH, Shi L, Newman AH (2021) Structure activity relationships for a series of eticlopride-based dopamine D2/D3 receptor bitopic ligands. J Med Chem 64(20):15313–15333
Stefanik MT, Kupchik YM, Brown RM, Kalivas PW (2013) Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. J Neurosci 33(34):13654
Stopper CM, Khayambashi S, Floresco SB (2013) Receptor-specific modulation of risk-based decision making by nucleus accumbens dopamine. Neuropsychopharmacology 38:715–728
Wang W, Dever D, Lowe J, Storey GP, Bhansali A, Eck EK, Nitulescu I, Weimer J, Bamford NS (2012) Regulation of prefrontal excitatory neurotransmission by dopamine in the nucleus accumbens core. J Physiol 590(16):3743–3769
Acknowledgements
Authors thank Faith V. Austin-Scott for her assistance with behavioural testing.
Funding
Research was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to JGH. TJO, ALH, and SNO were supported by scholarship funding from NSERC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Onofrychuk, T.J., Heidt, A.L., Orvold, S.N. et al. Nucleus accumbens core dopamine D2 receptors are required for performance of the odor span task in male rats. Psychopharmacology 241, 963–974 (2024). https://doi.org/10.1007/s00213-023-06522-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06522-4