Abstract
The catecholamine neuromodulators dopamine and norepinephrine are implicated in motor function, motivation, and cognition. Although roles for striatal dopamine in these aspects of behavior are well established, the specific roles for cortical catecholamines in regulating striatal dopamine dynamics and behavior are less clear. We recently showed that elevating cortical dopamine but not norepinephrine suppresses hyperactivity in dopamine transporter knockout (DAT-KO) mice, which have elevated striatal dopamine levels. In contrast, norepinephrine transporter knockout (NET-KO) mice have a phenotype distinct from DAT-KO mice, as they show elevated extracellular cortical catecholamines but reduced baseline striatal dopamine levels. Here we evaluated the consequences of altered catecholamine levels in NET-KO mice on cognitive flexibility and striatal dopamine dynamics. In a probabilistic reversal learning task, NET-KO mice showed enhanced reversal learning, which was consistent with larger phasic dopamine transients (dLight) in the dorsomedial striatum (DMS) during reward delivery and reward omission, compared to WT controls. Selective depletion of dorsal medial prefrontal cortex (mPFC) norepinephrine in WT mice did not alter performance on the reversal learning task but reduced nestlet shredding. Surprisingly, NET-KO mice did not show altered breakpoints in a progressive ratio task, suggesting intact food motivation. Collectively, these studies show novel roles of cortical catecholamines in the regulation of tonic and phasic striatal dopamine dynamics and cognitive flexibility, updating our current views on dopamine regulation and informing future therapeutic strategies to counter multiple psychiatric disorders.
Similar content being viewed by others
Introduction
Cognitive flexibility and motivated behavior are essential for survival, and their dysfunction plays an important role in the clinical manifestations of multiple neuropsychiatric and neurodegenerative disorders. The catecholamines norepinephrine and dopamine are important for regulating a range of processes including motivation, reinforcement learning, and movement [1,2,3,4], and abnormal catecholamine function is central to cognitive/motivational dysfunction observed in many neurological disorders, dementias, and psychiatric disorders [5,6,7]. Several studies have highlighted the importance of cortical catecholamines, cortico-striatal circuits, and striatal dopamine signaling in regulating cognitive flexibility and goal-directed behavior [8,9,10,11,12,13,14]; however, the contributions of cortical catecholamines to striatal dopamine regulation and cognitive flexibility are not clear.
Pharmacological inhibition of the norepinephrine transporter (NET) not only elevates cortical norepinephrine but also cortical dopamine, as expression of the dopamine transporter (DAT) is very low in the cortex [15,16,17], and NET serves as the primary reuptake site for cortical dopamine [18,19,20,21]. NET is a common pharmacological target for multiple psychiatric disorders, and drugs targeting NET have been well studied in the context of cognition, anxiety and depression [22,23,24]. These pharmacological agents have mixed pharmacology at monoamine transporters and/or multiple off-target effects [25,26,27,28], however, which renders it difficult to ascertain the true effects of NET-specific inhibition. Genetic deletion of NET via NET knockout (NET-KO) mice represents an alternative tool to study the specific effects of NET dysfunction, given that changes in levels of NET expression and NET genetic polymorphisms might bear associations with multiple psychiatric conditions [29,30,31,32,33]. Interestingly, NET-KO mice have not only the same alterations in cortical catecholamines as pharmacological NET inhibition, but also have lower striatal DA levels as measured by microdialysis [19], revealing these mice as a unique model for studying the effects of cortico-striatal catecholamine signaling and its effects on cognitive flexibility.
The individual contributions of cortical NE and DA to distinct aspects of cognitive flexibility are not clear. Although pharmacological blockade of NET (elevated DA and NE) enhances reversal learning [34,35,36] and attentional set shifting [37], some studies suggest that NE is important for attentional set shifting but not reversal learning [37,38,39,40,41]. In contrast, dopamine and its receptors are sufficient to regulate both set shifting and reversal learning [8, 9, 42,43,44]. Striatal dopamine regulates reversal learning as well [45, 46], but how cortical catecholamines regulate tonic striatal dopamine and modulate cognitive flexibility is unclear. Microdialysis studies show an inverse relationship between dopamine levels in the prefrontal cortex (PFC) and striatum [47, 48]. Moreover, dopamine depletion in the PFC via pharmacological lesion increases striatal dopamine levels and amphetamine-induced locomotor activity compared to non-lesioned animals [47, 49, 50]. A recent study from our group further showed that elevating PFC dopamine but not norepinephrine levels can reverse hyperlocomotion in DAT-KO mice, which have elevated striatal dopamine [21]. To further clarify the role of cortical catecholamine regulation of striatal dopamine and cognitive flexibility, we used genetic (NET-KO mice) and lesion (mPFC NE lesion) models to test the effect of altered cortical catecholamines on cognitive flexibility and phasic striatal dopamine dynamics.
Methods
Animals and drugs
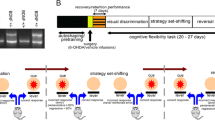
All studies were conducted in accordance with NIH guidelines for animal care and use and with an approved animal protocol from the University of Florida Institutional Animal Care and Use Committee. The NET-KO [19] mice were obtained from Dr. Marc Caron (Duke University). These knockout mice and littermate controls were backcrossed on to a C57BL6/J background for at least 10 generations and maintained on this background. Both male and female mice (3–5 months at testing) were used for experiments and housed in same-sex groups of 2–5 mice/cage, with ad libitum food (chow) and water except as noted below. In experiments that involved food restriction, mice were individually housed and gradually reduced to 85% of their free-feeding weight and maintained at this weight for the duration of these experiments. 6-hydroxydopamine (6-OHDA) was purchased from Sigma and dissolved in saline containing 0.02% ascorbic acid.
Probabilistic reversal learning task (PRL)
The PRL task was performed as described previously [13]. Briefly, the task was conducted in 8 identical operant chambers (15 × 13 × 13 cm, Med Associates) in individual sound-attenuating cubicles with a house light, two illuminated nosepoke holes on the chamber wall opposite the houselight, and a pellet (20 mg, Bio-Serv, Catalog# F0071) delivery trough between the two nosepoke holes. Prior to PRL testing, mice underwent several stages of shaping, including magazine training and nosepoke shaping, first on a FR1 schedule, and then on a FR1 schedule with a 50% probability of food delivery. In the PRL task itself, mice learned to discriminate between the two nosepoke holes that were 80% or 20% reinforced by food reward. On each 15 s trial, the houselight and both nosepoke holes were illuminated, and a response at either hole triggered the respective contingency. After a nosepoke response was recorded, lights turned off for an intertrial interval that lasted until the next trial. Failure to respond within 10 s was counted as an omission. Each trial lasted 15 s in duration, including the intertrial interval.
Once mice responded on 8 consecutive correct trials at the correct nosepoke (excluding omissions), the probabilities of food delivery at the two nosepokes were reversed. Each session was 50 min in duration and contained 200 trials. The primary measure of interest in the PRL task was the number of reversals completed per session. Additional measures in each session included the number of omitted trials, total number of correct responses, total number of errors, total number of inactive nosepokes (i.e., nosepokes during intertrial intervals when the house light was extinguished), latencies to initiate a nosepoke following trial onset, choice strategy (win-stay or lose-shift) [13, 51], errors to initial discrimination, errors to first reversal, trials to first reversal, and perseverative errors.
Surgical procedures
For NE lesions, mice were bilaterally injected with 200 nl of 3 ug/ul 6-hydroxydopamine (6-OHDA) in the dorsomedial prelimbic PFC (AP + 2.4 ML ± 0.6 DV −1.8 from Bregma). Two-three weeks later, the mice underwent training and testing in the PRL task as described above.
For fiber photometry, mice were unilaterally injected with 200 nl of AAV5-CAG-dLight 1.3b (Addgene) in the dorsomedial striatum (DMS) (AP + 1.0 ML ± 1.5 DV −3.0 from Bregma). A fiber optic cannula (400 μm, 0.66NA, Doric Lenses) was inserted into the brain 200 μm above the dLight injection site in the DMS and secured using dental cement with a bone anchor screw. Two mice from each genotype were also co-injected with AAV8-hSyn-mCherry-FlpO (Addgene) in the DMS as an expression control. Two-three weeks later the mice underwent training and testing for the PRL task as described above.
Fiber photometry
Dopamine transients were measured using a RZ10X TDT fiber photometry LED processor (Tucker Davis Technologies), which delivered 465 nm and 405 nm (isosbestic, control for motion artifacts) excitation light through a low autofluorescence optical fiber patch cord connected through a bronze sleeve to the metal ferrule of the fiber optic cannula implanted in the DMS. Excitation and emission signals were separated using a 6-port fluorescence minicube (FMC, Doric Lenses). TTLs from Med Associates hardware were transferred through a superport card connected to the RZ10X digital I/O port. Photometry signals were analyzed using pMAT software from the Barker lab [52].
Progressive ratio task (PRT)
A progressive ratio task was used to assess motivation to work for food as described previously [53, 54]. Briefly, mice underwent similar shaping in the same Med Associates operant chambers (magazine training, FR1) used in the PRL task but also underwent FR3 and FR5 training to acclimatize them to exerting increased effort to earn food. The mice were then tested in the progressive ratio task for 11 days, using the formula x = (5*e^reward*0.24))−5, in which x = 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95,118, 145, 178, 219, 268, 328…..901 nosepokes required to earn a single food pellet in each daily session. Failure to earn a food pellet within 20 min terminated the session and the previous ratio achieved was considered the “breakpoint”. This breakpoint was the primary measure of interest in the task, but the total number of nosepokes and the total number of food pellets earned were also assessed.
Monoamine tissue level - HPLC analysis
After behavioral testing, 6-OHDA- and vehicle-injected mice were sacrificed, brains isolated, and the mPFC was dissected to assess monoamine content. Monoamine levels were determined by HPLC analysis with electrochemical detection (EICOM) as described previously [21].
Nestlet shredding assay
The nestlet shredding assay was performed as described previously [55]. Mice were placed in fresh cages with a single nestlet (square-shaped compressed cotton) that was weighed prior to testing. Mice were left in the cages for 4 h, and the percent weight of unshredded nestlet remaining was calculated each hour. Data are represented as percent nestlet shredded (inverse of the percent nestlet remaining).
Locomotor activity
Locomotor activity was measured in a Versamax activity monitor (16 × 16 in, Omnitech Electronics, Columbus, OH) as described previously [56]. Mice were placed in the activity monitor, and activity (total distance traveled) was measured in 5 min bins for a total of 90 min.
Statistical analyses
Statistical analyses were performed using Graphpad Prism 10.0. Group sizes were based on our previously published studies, as well as on power analyses suggesting that they would yield power to detect significant (alpha = 0.05) effects of 0.8 and above. Data were analyzed using unpaired t-test, two-way ANOVA, or mixed-effects model with repeated measures (behavior), for comparison between genotypes, day, injection pairs, or lesion and monoamine content as described in figure legends. Individual factors were compared using post hoc Tukey tests described in figure legends. Sex was included as a variable in all analyses, but as no main effects or interactions involving sex were significant, they are not reported further. Data are presented as means ± SEM. No data points were excluded. Values of p < 0.05 were considered significant.
Results
As NET-KO mice have elevated cortical catecholamines and lower striatal dopamine [19], we reasoned that these mice should also show altered performance on cognitive tasks mediated by cortico-striatal catecholamine signaling. To address this question, we used a cognitive flexibility task (probabilistic reversal learning) that assesses the ability of mice to rapidly switch between response contingencies to earn food rewards [57]. In this task, analysis of number of reversals per session in WT and NET-KO mice revealed significant main effects of genotype and days but no interaction effects were observed during the 10 days of testing (Fig. 1A, Mixed-effects model Repeated measures, Genotype, F (1, 22) = 8.292, P = 0.0087; Day, F (4.986, 109.7) = 2.604, P = 0.0290; Day × Genotype, F (7, 154) = 0.9720, P = 0.4537). The NET-KO mice also made fewer trial omissions than WT mice (i.e., they completed a greater number of trials per session; unpaired t-test, WT vs NET-KO, Two-tailed, t = 5.089, df = 14, P = 0.0002, Fig. 1B). This greater number of completed trials did not account for the greater number of reversals, however, as normalization of the number of reversals by the number of completed trials per session (percent reversals per 200 trials) in WT and NET-KO mice still revealed significant main effects of genotype and days (Fig. 1C, Mixed-effects model Repeated measures, Genotype F (1, 21) = 9.436, p = 0.0058; Days, F (5.722, 115.2) = 3.042, p = 0.0095; Days × Genotype F (7, 141) = 1.010, p = 0.4268). Total reversals averaged over all 10 days also revealed significant genotype effects (unpaired t-test, Two-tailed, t = 2.883, df = 21, p = 0.0089, WT vs NET-KO, Fig. 1D). NET-KO mice had a greater number of nosepokes during intertrial intervals when the nosepokes were inactive (inactive nosepokes; unpaired t-test, WT vs NET-KO, Two-tailed, t = 2.609, df = 14, P = 0.0206, Fig. 1E), but no differences were observed when inactive nosepokes were normalized to total no. of nosepokes (Fig. 1F). Although there was a trend for the NET-KO mice to be lower than WT mice, no significant main effects were observed in the number of errors to criterion on the initial discrimination (Fig. 1G) or trials to first reversal (Fig. 1H) in each daily session. However, a significant main effect of days for errors to criterion after first reversal (Fig. 1I, days, p = 0.0017, F (3.484, 68.91) = 5.198) was observed with no genotype differences. Additionally, NET-KO mice made more active correct responses (correct) on the 80% probability nosepoke, earned more food pellets, and had fewer perseverative errors (day 1) compared to WT mice but did not differ in the number of responses at the 20% probability nosepoke (incorrect) or in latencies to respond (Supplementary Fig. S1) averaged over each day of PRL. Furthermore, analysis of choice strategies revealed greater win-stay and less lose-shift behavior in NET-KO mice compared to WT controls (Supplementary Fig. S2), suggesting that NET-KO mice are more sensitive to both positive and negative feedback, which presumably optimizes their subsequent choices to enhance reversal learning.
NET-KO mice have A significantly greater numbers of reversals per session, *p < 0.05 Mixed-effects model main genotype effect and B fewer trial omissions for each genotype averaged over each session for 10 days, ***p < 0.001 t-test, WT vs NET-KO, C greater number of reversals normalized by number of completed trials, from Day 1 through Day 10. **p < 0.01 Mixed-effects model main genotype effect, NET WT vs KO. D More total reversals averaged over each session for 10 days **p < 0.01 t-test, WT vs NET-KO, E more inactive nosepokes averaged over each session for 10 days, *p < 0.05 t-test, NET WT vs KO but F similar normalized inactive nosepokes averaged over each session for 10 days, and similar G errors to criterion initial discrimination, H Trials to 1st reversal and I errors to criterion after 1st reversal, compared to WT controls. n = 11–12 mice per genotype (6/7 WT and 6/4 NET-KO male/female mice).
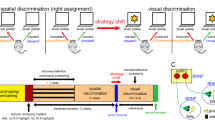
Microdialysis studies show that genetic or pharmacological disruption of NET causes elevated cortical catecholamines but lower baseline levels of striatal dopamine [19, 58, 59]. It is well known that striatal dopamine dynamics play an important role in reinforcement learning. Specifically, phasic striatal dopamine signals encode reward prediction error (RPE)-like signals that inform future choices [1, 60]. We asked whether changes in cortical catecholamines and baseline striatal dopamine play a role in altered phasic striatal dopamine dynamics, which could explain the enhanced cognitive flexibility of the NET-KO mice. We used dLight 1.3b and fiber photometry to measure dopamine dynamics in the dorsomedial striatum in WT and NET-KO mice while performing the PRL task. We focused on dLight measurements in the DMS as the PRL task is an instrumental learning task that relates to action-outcome learning, which is predominantly mediated by the DMS [61,62,63]. We measured phasic dopamine transients at early (day 1) and late (day 10) phases of the PRL task, at specific peri-event timepoints such as cue light, nosepoke, reward delivery, and reward omission (dashed line in figure denotes onset of respective event). We achieved consistent targeting of dLight 1.3b AAV and the optic fiber in the DMS (Fig. 2A) of WT and NET-KO mice. In mice co-injected with an mCherry AAV to control for expression between genotypes, we found that expression of mCherry was similar between genotypes. We were able to consistently measure stable dopamine signals throughout the 50 min behavioral sessions in both genotypes (Fig. 2B). Similar to Fig. 1, we observed genotype differences in reversal learning in mice used for photometry measurements (Supplementary Fig. S3a). During early PRL task training, at the high probability (80%) nosepoke, both WT and NET-KO mice showed an RPE-like pattern of dopamine signals, but NET-KO mice had significantly larger phasic dopamine responses during reward delivery (Fig. 2C, 80% nosepoke reward, i-peri-event histogram (PETH), ii-AUC and iii-peak response), reward omission (Fig. 2D, PETH, 80% nosepoke no reward, i-peri-event histogram (PETH), ii-AUC and iii-peak response), and during cue presentation (Supplementary Fig. S3b). Phasic dopamine responses at the low probability (20%) nosepoke were also greater in NET-KO compared to WT controls during reward delivery (Fig. 2E, 20% nosepoke reward, i-peri-event histogram (PETH), ii-AUC and iii-peak response) and omission (Fig. 2F, 20% nosepoke no reward, i-peri-event histogram (PETH), ii-AUC and iii-peak response). In contrast, during late phases of the PRL task, all phasic dopamine responses were similar in WT and NET-KO mice (Fig. 2G–J). Analysis of AUC and peak values of phasic dopamine responses for daily sessions from day 1 to 10 revealed significant genotype and interaction effects during reward delivery but not reward omission, particularly during early training (Supplementary Fig. S3c–f). These results are consistent with the enhanced initial learning shown by the NET-KO mice in the PRL task.
A Fiber photometry setup and representative images of optical probe implant site and dLight and mCherry AAV expression in the DMS of WT and NET-KO mice. B Dopamine measurements using dLight 1.3b in the 465 nm channel over the course of an entire daily PRL session of 50 min. C–J i -peri-event time histograms (PETH), ii - AUC ΔF/F values (peri-event: 0–2.5 s) and iii – Peak ΔF/F responses (peri-event: 0–2.5 s) of phasic dopamine transients in WT and NET-KO mice for different reward contingencies as mentioned in the figure, at early (C–F) or late (G–J) phases of PRL task. Dashed line denotes onset of reward delivery or omission. *p < 0.05, **p < 0.01 unpaired t-test, WT vs NET-KO; ns not significant. n = 5 mice per genotype (4 male/1 female, WT and NET-KO mice).
In addition to elevated norepinephrine levels, NET-KO mice also have elevated dopamine levels in the prefrontal cortex [18]. To address the specific roles played by cortical norepinephrine and dopamine in reversal learning, we used the toxin 6-OHDA to lesion norepinephrine terminals in the mPFC (prelimbic) of WT mice, followed by PRL testing. Within the PFC the prelimbic and OFC regions have apparently opposing roles in reversal learning performance [13], such that inactivation of the prelimbic region enhances cognitive flexibility. As shown in Fig. 3A, reversal learning in 6-OHDA-injected mice was not different from vehicle-injected controls, and the two groups had similar numbers of trial omissions (Fig. 3B) and inactive nosepoke responses (Fig. 3C). To confirm depletion of norepinephrine in the PFC, we isolated tissue from lesioned mice after behavioral testing and measured catecholamine levels in the PFC using HPLC. As expected, we achieved selective depletion of NE terminals, as expression of DAT in the PFC is very low (Fig. 3D, Two-Way ANOVA, lesion x monoamine F (2, 26) = 19.34 p < 0.0001). These data suggest that mPFC NE and DA play distinct roles in reinforcement learning and cognitive flexibility.
Compared to vehicle (Veh) controls, NE lesioned mice (6-OHDA) showed: A similar numbers of reversals per session from Day 1 through Day 8. p = 0.77 Mixed-effects model Repeated measures, Veh vs 6-OHDA; B similar numbers of trial omissions averaged over each session for 8 days, p = 0.73, t-test, Veh vs 6-OHDA. C similar numbers of inactive nosepokes averaged over each session for 8 days p = 0.82, t-test, Veh vs 6-OHDA. and D lower levels of NE but not DA or 5-HT as measured by HPLC analysis of PFC tissue. **p < 0.01 Two-Way ANOVA, Veh vs 6-OHDA. n = 6 (3 male and 3 females) mice for each treatment group.
To decipher whether NE plays a role in behaviors other than probabilistic reversal learning, we tested NET-KO and mPFC NE-lesioned mice in simple stress- and anxiety-related behavioral assays [64, 65]. Depletion of norepinephrine renders mice more susceptible to stress and anxiety, whereas enhancing NE levels can mimic antidepressant-like effects [66, 67]. The nestlet shredding task, conducted in a novel environment, is useful for measuring stress-induced repetitive, compulsive behavior during physiological stress or anxiogenic situations - i.e., nest building may be a means to actively cope with anxiogenic stimuli [68]. As seen in Fig. 4, NET-KO mice shredded nestlets significantly more than WT controls in the 4-hour testing period (Mixed-effects model Repeated measures, Time x Genotype, F (4, 56) = 4.497, P = 0.0032). In contrast, PFC NE-lesioned mice shredded the nestlets significantly less than vehicle controls (Fig. 4, Mixed-effects model Repeated measures, Time x Lesion, F (4, 40) = 5.165, P = 0.0019). Additionally, as observed previously [19], NET-KO mice showed significantly less novelty-induced activity in an open field compared to WT controls (Fig. 4C, Mixed-effects model Repeated measures, Time x Genotype, F (17, 221) = 2.657, P = 0.0006) and a shorter cumulative total distance traveled (Fig. 4D, unpaired t-test, NET WT vs KO, Two-tailed, t = 3.191, df = 13, P = 0.0071). Thus, NE bidirectionally modulates nestlet shredding, again highlighting distinct roles for cortical DA and NE in specific behaviors.
A NET-KO and WT control, n = 8 per genotype or B PFC NE-lesioned or vehicle (Veh) control, n = 8 per group mice were placed in fresh cages with nestlets, and weights of nestlets were measured every hour for 4 h. Data are presented as percent of the nestlet shredded. *P < 0.05 by Mixed-effects model Repeated measures, post hoc Tukey’s test, comparing NET WT and KO (4 male and 4 female mice for KO and 5 male and 3 females for WT mice) or Veh control and PFC NE lesion (4 male and 4 female mice for WT and KO mice). C, D Mice were placed in an open field chamber and distance traveled (locomotor activity) was measured for 90 min. n = 7–8 per genotype **P < 0.01 by t-test comparing NET WT and KO (4/5 male and 3 female mice for KO/WT mice).
The heightened cognitive flexibility and fewer trial omissions observed in NET-KO mice in the PRL task could imply that these mice are more motivated to work for food. To address this possibility, a separate group of mice was trained to respond for food on a progressive ratio schedule of reinforcement, in which each successive reinforcer earned increases the number of responses required to earn the subsequent reinforcer, until responding ceases altogether (i.e., breakpoint) [53, 69]. Surprisingly, there was no difference in the breakpoint between WT and NET-KO mice (Supplementary Fig. S4A), nor were there differences in the total number of responses or food pellets earned (Supplementary Fig. S4B, C), suggesting equivalent levels of food motivation.
Considered together, these results suggest distinct roles for cortical norepinephrine and dopamine in cognitive flexibility and motivated behavior. Furthermore, in NET-KO mice task performance during reversal learning parallels the magnitude of phasic dopamine transients in the DMS, even though tonic dopamine levels are low.
Discussion
In the current study we show that mice with genetic deletion of NET display enhanced cognitive flexibility as measured by reversal learning, with a simultaneous increase in the magnitude of phasic dopamine transients in the DMS. Furthermore, we show that PFC NE levels are crucial for nestlet shredding but not reversal learning. Cognitive flexibility promotes survival in the natural world by allowing adaptive decision making in an ever-changing environment. Cognitive flexibility also allows switching of responses during cue-response-outcome behavior to enable formation of new associations [45, 70]. Deficits in switching or behavioral flexibility can lead to adverse outcomes, some of which are apparent in conditions such as ADHD, schizophrenia, OCD, and substance use disorders. In ADHD in particular, lack of cognitive flexibility can be in part driven by hyperactivity and/or impulsivity that is reversed by pharmacological treatment with monoamine transporter blockers [71]. One of the mechanisms thought to be involved in reversing ADHD phenotypes is the targeting of norepinephrine signaling via NET or alpha-adrenergic receptors in the PFC [72]. It is not clear, however, if the consequences of pharmacological targeting of NET are due to cortical NE or DA and how they affect striatal dopamine dynamics and cognitive flexibility. To test whether NET blockade can alter cognitive flexibility, we evaluated NET-KO mice in a probabilistic reversal learning task. NET-KO mice made more reversals per session compared to WT controls, suggestive of enhanced cognitive flexibility. NET-KO mice also had significantly fewer trial omissions, but normalization of reversals by the number of completed trials produced results comparable to those with completed reversals alone though, indicating that the reduction in omissions does not account for apparently greater flexibility in NET-KO mice.
NET deletion not only elevates NE but also cortical DA, as NET is the primary site of DA reuptake in the cortex. Selective dorsal PFC NE depletion that maintained PFC DA levels did not affect any measure of reversal learning task performance, consistent with a previous study [38] and suggesting that PFC DA and NE differentially regulate cognitive flexibility. PFC DA and its receptors within both dorsal and ventral regions of the PFC have been shown to be sufficient to regulate cognitive flexibility [8, 9, 73]. In contrast, the specific contributions of NE to reversal learning are less clear, as some studies suggest that NE in the OFC might play a role in action-outcome updating in an outcome devaluation reversal task [40, 41]. PFC NE might also play a role in regulating behavior under stressful or aversive conditions [65]. The nestlet shredding test is often used as a measure of repetitive or compulsive behavior under anxiogenic or stressful situations - i.e., “shred the nestlet to create a safe harbor in an anxiety-inducing novel cage environment” [55]. Moreover, regions of the PFC including the anterior cingulate cortex (ACC) [74, 75] and the OFC [11] have been implicated in compulsive behaviors. Compared to WT mice, the NET-KO mice (which have elevated NE and DA levels in PFC) shredded more nestlet across the 4-hour test, consistent with the idea that these mice perform actions faster to avoid aversive stimuli, which could be on the compulsive behavior spectrum. Conversely, dorsal PFC (ACC and prelimbic) NE depletion (which did not affect DA levels) caused a reduction in nestlet shredding, which is consistent with prior work showing that DBH-KO mice (which have reduced NE but elevated DA levels in PFC) have reduced nestlet shredding [55]. Interestingly, NET KO mice have greater number of inactive nosepokes in the PRL task (noksepokes when the houselight was off and responses had no programmed consequences), which could suggest a compulsive-like behavioral phenotype (although the opposite effect was not evident in the PFC NE-depleted mice). Together, the results from the nestlet shredding task suggest that PFC NE, but not DA, can bidirectionally modulate expression of repetitive/compulsive behaviors, particularly in novel or anxiety-inducing contexts.
In the PRL task, we found larger phasic dopamine transients in the DMS of NET-KO mice in early phases of the task, even though NET-KO mice have lower baseline striatal dopamine levels as shown previously by microdialysis [19], which could explain why they complete more reversals early in the task compared to WT controls. Larger phasic dopamine transients have been thought to translate to stronger reinforcement signals and therefore stronger associations between cues or actions and outcomes [1, 76, 77]. One hypothesis is that NET-KO mice learn at a faster rate than WT mice because they have lower baseline dopamine levels, which allows for larger phasic dopamine bursts relative to baseline—i.e. a higher signal-to-noise ratio [78]. Evidence for this notion comes from a study showing that NET-KO mice have elevated DAT protein but not mRNA expression in the midbrain [79], which could lead to enhanced reuptake and lower extracellular DA, but greater vesicular stores of DA. The elevated vesicular stores of DA would then cause elevated release of DA during phasic activity bursts. The upregulation of only DAT protein but not mRNA levels in the NET KO mice suggests that this is potentially not a compensatory developmental effect but rather a network compensatory effect, since changes in surface DAT protein levels can be induced in an activity-dependent manner - i.e., via depolarization [80]. Mice learn action-outcome associations in the PRL task not only from rewarded trials (positive reinforcement/WinStay) but also from non-rewarded (reward omission/LoseShift) trials. Interestingly, both positive and negative dopamine transients during reward delivery and omission, respectively, are sharper in NET-KO mice in early stages of PRL training, suggesting that NET-KO mice have access to more salient information from both trial types, consistent with their Winstay/Loseshift behavior. However, in WT mice negative dopamine transients are not as sharp as the positive transients, suggesting that WT mice are updating information predominantly from positive reinforcement signals. Intriguingly, in the late phases of PRL training in both NET-KO and WT mice, positive and negative dopamine transients during reward delivery and omission, respectively, were more consistent and sharper, suggesting that unlike early phases, in these later stages of learning WT mice use both positive reinforcement and reward omission signals equally to inform future outcomes.
The larger phasic dopamine signals and enhanced cognitive flexibility could imply that NET-KO mice might have a greater motivational drive to perform tasks. Surprisingly, NET-KO mice show breakpoints similar to WT mice in a progressive ratio task, suggesting that they are not more motivated than WT mice to work for food rewards. Some studies suggest that elevated dopamine levels correlate with enhanced motivation in the progressive ratio task [81, 82], and thus one might expect no change or even reduced food motivation in NET-KO mice, which have lower tonic dopamine levels. One potential explanation for this disconnect between motivation and cognitive flexibility is that the NET-KO mice are more attentive or have greater task engagement (fewer trial omissions) but are not necessarily more motivated. A recent study suggests that greater DMS dopamine terminal phasic activity (as measured by a GCaMP sensor) correlates with punishment-resistant, goal-directed behavior [83]. It remains to be determined if the enhanced DMS phasic dopamine signals in NET-KO mice are associated with greater punishment-resistant, goal-directed behavior.
Although the NET-KO mice provide a unique model in which to study cognitive flexibility, there are possible compensatory developmental mechanisms to consider, owing to chronic changes in catecholamine levels. Developmental changes alone likely do not account for the observed behavioral/neurochemical alterations in these mice, however, as chronic administration of NET blockers induces behavioral changes similar to those observed in the NET-KO mice [19]. In addition, it is important to acknowledge that NET is widely expressed not only in the cortex but also in other regions including the bed nucleus of the stria terminalis, medial shell of nucleus accumbens, midbrain, and hippocampus. Thus, it is possible (and even likely) that the behavioral phenotypes in NET-KO mice result from effects not only in the cortex but also in these other regions. Although we partially addressed this caveat with the specific lesions of mPFC NE terminals, future studies using region-specific NET, PFC NE, or PFC DA manipulations will be important for highlighting the specific role(s) of NET and catecholamines in these other brain regions.
Blockade of NET has been a major pharmacological approach for treating multiple disorders, and it is hypothesized that increased cortical NE is a predominant mechanism of therapeutic action. In our previous work we showed that systemic administration of nepicastat (an inhibitor of the dopamine beta hydroxylase enzyme that converts dopamine to norepinephrine) caused reduced NE but elevated DA levels in PFC, and reduced the hyperactivity phenotype in DAT-KO mice [21]. Thus, the combination of depleted NE and elevated DA in PFC might be a useful pharmacological approach to reduce impulsivity and hyperactivity while maintaining or even enhancing cognitive flexibility. In combination with the current findings, our studies show distinct roles for cortical NE and DA in various aspects of cognitive flexibility. Thus, targeting cortical NE and/or DA will likely have distinct therapeutic actions and should be considered when designing new therapeutic approaches.
Data availability
All data related to this manuscript are stored on a University of Florida OneDrive or Dropbox account and will be made available upon request.
References
Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63.
Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. https://doi.org/10.1196/annals.1417.003.
Cox J, Witten IB. Striatal circuits for reward learning and decision-making. Nat Rev Neurosci. 2019;20:482–94. https://doi.org/10.1038/s41583-019-0189-2.
Aarts E, van Holstein M, Cools R. Striatal Dopamine and the Interface between Motivation and Cognition. Front Psychol. 2011;2:163 https://doi.org/10.3389/fpsyg.2011.00163.
Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–49. https://doi.org/10.1016/j.neuron.2011.02.010.
Abi-Dargham A. From “bedside” to “bench” and back: A translational approach to studying dopamine dysfunction in schizophrenia. Neurosci Biobehav Rev. 2018. https://doi.org/10.1016/j.neubiorev.2018.12.003.
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55.
Hitchcott PK, Quinn JJ, Taylor JR. Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cereb Cortex. 2007;17:2820–7. https://doi.org/10.1093/cercor/bhm010.
Barker JM, Torregrossa MM, Taylor JR. Bidirectional modulation of infralimbic dopamine D1 and D2 receptor activity regulates flexible reward seeking. Front Neurosci. 2013;7:126 https://doi.org/10.3389/fnins.2013.00126.
Natsheh JY, Shiflett MW. Dopaminergic Modulation of Goal-Directed Behavior in a Rodent Model of Attention-Deficit/Hyperactivity Disorder. Front Integr Neurosci. 2018;12:45 https://doi.org/10.3389/fnint.2018.00045.
Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–9. https://doi.org/10.1126/science.1234733.
Burguiere E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–6. https://doi.org/10.1126/science.1232380.
Dalton GL, Wang NY, Phillips AG, Floresco SB. Multifaceted Contributions by Different Regions of the Orbitofrontal and Medial Prefrontal Cortex to Probabilistic Reversal Learning. J Neurosci. 2016;36:1996–2006. https://doi.org/10.1523/JNEUROSCI.3366-15.2016.
Gremel CM, Costa RM. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun. 2013;4:2264 https://doi.org/10.1038/ncomms3264.
Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–708.
Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, et al. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–23.
Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–73. https://doi.org/10.1016/j.neuron.2008.01.022.
Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–95.
Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, et al. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–71. https://doi.org/10.1038/74839.
Tanda G, Carboni E, Frau R, Di Chiara G. Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential? Psychopharmacology. 1994;115:285–8. https://doi.org/10.1007/BF02244785.
Harris SS, Green SM, Kumar M, Urs NM. A role for cortical dopamine in the paradoxical calming effects of psychostimulants. Sci Rep. 2022;12:3129 https://doi.org/10.1038/s41598-022-07029-2.
Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–74. https://doi.org/10.1016/S0893-133X(97)00113-9.
Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. https://doi.org/10.1146/annurev.ne.16.030193.000445.
Shang CY, Lin HY, Gau SS. The norepinephrine transporter gene modulates intrinsic brain activity, visual memory, and visual attention in children with attention-deficit/hyperactivity disorder. Mol Psychiatry. 2021;26:4026–35. https://doi.org/10.1038/s41380-019-0545-7.
Aggarwal S, Mortensen OV. Overview of Monoamine Transporters. Curr Protoc Pharm. 2017;79:12 16 11–12 16 17. https://doi.org/10.1002/cpph.32.
Gainetdinov RR, Sotnikova TD, Caron MG. Monoamine transporter pharmacology and mutant mice. Trends Pharmacol Sci. 2002;23:367–73.
Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. https://doi.org/10.1038/nrn1008.
Wang YM, Xu F, Gainetdinov RR, Caron MG. Genetic approaches to studying norepinephrine function: knockout of the mouse norepinephrine transporter gene. Biol Psychiatry. 1999;46:1124–30. https://doi.org/10.1016/s0006-3223(99)00245-0.
Belfer I, Phillips G, Taubman J, Hipp H, Lipsky RH, Enoch MA, et al. Haplotype architecture of the norepinephrine transporter gene SLC6A2 in four populations. J Hum Genet. 2004;49:232–45. https://doi.org/10.1007/s10038-004-0140-9.
Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, et al. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8. https://doi.org/10.1523/JNEUROSCI.17-21-08451.1997.
Pramod AB, Foster J, Carvelli L, Henry LK. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Asp Med. 2013;34:197–219. https://doi.org/10.1016/j.mam.2012.07.002.
Buttenschøn HN, Kristensen AS, Buch HN, Andersen JH, Bonde JP, Grynderup M, et al. The norepinephrine transporter gene is a candidate gene for panic disorder. J Neural Transm. 2011;118:969–76. https://doi.org/10.1007/s00702-011-0624-7.
Nemoda Z, Angyal N, Tarnok Z, Birkas E, Bognar E, Sasvari-Szekely M, et al. Differential Genetic Effect of the Norepinephrine Transporter Promoter Polymorphisms on Attention Problems in Clinical and Non-clinical Samples. Front Neurosci. 2018;12:1051 https://doi.org/10.3389/fnins.2018.01051.
Seu E, Jentsch JD. Effect of acute and repeated treatment with desipramine or methylphenidate on serial reversal learning in rats. Neuropharmacology. 2009;57:665–72. https://doi.org/10.1016/j.neuropharm.2009.08.007.
Altidor LK, Bruner MM, Deslauriers JF, Garman TS, Ramirez S, Dirr EW, et al. Acute vagus nerve stimulation enhances reversal learning in rats. Neurobiol Learn Mem. 2021;184:107498 https://doi.org/10.1016/j.nlm.2021.107498.
Seu E, Lang A, Rivera RJ, Jentsch JD. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology. 2009;202:505–19. https://doi.org/10.1007/s00213-008-1250-4.
Lapiz MD, Bondi CO, Morilak DA. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set-shifting test. Neuropsychopharmacology. 2007;32:1000–10. https://doi.org/10.1038/sj.npp.1301235.
Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci. 2007;25:3719–24. https://doi.org/10.1111/j.1460-9568.2007.05612.x.
McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. https://doi.org/10.1016/j.neuroscience.2008.01.064.
Cerpa JC, Piccin A, Dehove M, Lavigne M, Kremer EJ, Wolff M et al. Inhibition of noradrenergic signalling in rodent orbitofrontal cortex impairs the updating of goal-directed actions. Elife. 2023;12. https://doi.org/10.7554/eLife.81623.
Sadacca BF, Wikenheiser AM, Schoenbaum G. Toward a theoretical role for tonic norepinephrine in the orbitofrontal cortex in facilitating flexible learning. Neuroscience. 2017;345:124–9. https://doi.org/10.1016/j.neuroscience.2016.04.017.
Bissonette GB, Powell EM. Reversal learning and attentional set-shifting in mice. Neuropharmacology. 2012;62:1168–74. https://doi.org/10.1016/j.neuropharm.2011.03.011.
Izquierdo A, Wiedholz LM, Millstein RA, Yang RJ, Bussey TJ, Saksida LM, et al. Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav Brain Res. 2006;171:181–8. https://doi.org/10.1016/j.bbr.2006.03.029.
Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Front Neurosci. 2013;7:62 https://doi.org/10.3389/fnins.2013.00062.
Bissonette GB, Roesch MR. Neurophysiology of rule switching in the corticostriatal circuit. Neuroscience. 2017;345:64–76. https://doi.org/10.1016/j.neuroscience.2016.01.062.
Clarke HF, Hill GJ, Robbins TW, Roberts AC. Dopamine, but not serotonin, regulates reversal learning in the marmoset caudate nucleus. J Neurosci. 2011;31:4290–7. https://doi.org/10.1523/JNEUROSCI.5066-10.2011.
Ventura R, Alcaro A, Cabib S, Conversi D, Mandolesi L, Puglisi-Allegra S. Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology. 2004;29:72–80. https://doi.org/10.1038/sj.npp.1300300.
Ventura R, Alcaro A, Mandolesi L, Puglisi-Allegra S. In vivo evidence that genetic background controls impulse-dependent dopamine release induced by amphetamine in the nucleus accumbens. J Neurochem. 2004;89:494–502. https://doi.org/10.1111/j.1471-4159.2004.02342.x.
Sokolowski JD, Salamone JD. Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain Res. 1994;642:20–28. https://doi.org/10.1016/0006-8993(94)90901-6.
Pycock CJ, Kerwin RW, Carter CJ. Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980;286:74–76.
Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, et al. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–301. https://doi.org/10.1038/npp.2009.233.
Bruno CA, O'Brien C, Bryant S, Mejaes JI, Estrin DJ, Pizzano C, et al. pMAT: An open-source software suite for the analysis of fiber photometry data. Pharm Biochem Behav. 2021;201:173093 https://doi.org/10.1016/j.pbb.2020.173093.
Fischbach-Weiss S, Reese RM, Janak PH. Inhibiting Mesolimbic Dopamine Neurons Reduces the Initiation and Maintenance of Instrumental Responding. Neuroscience. 2018;372:306–15. https://doi.org/10.1016/j.neuroscience.2017.12.003.
Garman TS, Setlow B, Orsini CA. Effects of a high-fat diet on impulsive choice in rats. Physiol Behav. 2021;229:113260 https://doi.org/10.1016/j.physbeh.2020.113260.
Lustberg D, Iannitelli AF, Tillage RP, Pruitt M, Liles LC, Weinshenker D. Central norepinephrine transmission is required for stress-induced repetitive behavior in two rodent models of obsessive-compulsive disorder. Psychopharmacology. 2020;237:1973–87. https://doi.org/10.1007/s00213-020-05512-0.
Urs NM, Daigle TL, Caron MG. A Dopamine D1 Receptor-Dependent beta-Arrestin Signaling Complex Potentially Regulates Morphine-Induced Psychomotor Activation but not Reward in Mice. Neuropsychopharmacology. 2011;36:551–8. https://doi.org/10.1038/Npp.2010.186.
Dalton GL, Phillips AG, Floresco SB. Preferential involvement by nucleus accumbens shell in mediating probabilistic learning and reversal shifts. J Neurosci. 2014;34:4618–26. https://doi.org/10.1523/JNEUROSCI.5058-13.2014.
Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. https://doi.org/10.1016/S0893-133X(02)00346-9.
Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Local influence of endogenous norepinephrine on extracellular dopamine in rat medial prefrontal cortex. J Neurochem. 1995;65:111–6.
Berke JD. What does dopamine mean? Nat Neurosci. 2018;21:787–93. https://doi.org/10.1038/s41593-018-0152-y.
Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–23. https://doi.org/10.1111/j.1460-9568.2005.04218.x.
Grospe GM, Baker PM, Ragozzino ME. Cognitive Flexibility Deficits Following 6-OHDA Lesions of the Rat Dorsomedial Striatum. Neuroscience. 2018;374:80–90. https://doi.org/10.1016/j.neuroscience.2018.01.032.
Shiflett MW, Balleine BW. Molecular substrates of action control in cortico-striatal circuits. Prog Neurobiol. 2011;95:1–13. https://doi.org/10.1016/j.pneurobio.2011.05.007.
Giustino TF, Maren S. Noradrenergic Modulation of Fear Conditioning and Extinction. Front Behav Neurosci. 2018;12:43 https://doi.org/10.3389/fnbeh.2018.00043.
Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. https://doi.org/10.1146/annurev.neuro.28.061604.135709.
Dziedzicka-Wasylewska M, Faron-Górecka A, Kuśmider M, Drozdowska E, Rogóz Z, Siwanowicz J, et al. Effect of antidepressant drugs in mice lacking the norepinephrine transporter. Neuropsychopharmacology. 2006;31:2424–32. https://doi.org/10.1038/sj.npp.1301064.
Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatr Dis Treat. 2011;7:9–13,. https://doi.org/10.2147/NDT.S19619.
Dorninger F, Zeitler G, Berger J. Nestlet Shredding and Nest Building Tests to Assess Features of Psychiatric Disorders in Mice. Bio Protoc. 2020;10. https://doi.org/10.21769/BioProtoc.3863.
Spear DJ, Katz JL. Cocaine and food as reinforcers: effects of reinforcer magnitude and response requirement under second-order fixed-ratio and progressive-ratio schedules. J Exp Anal Behav. 1991;56:261–75. https://doi.org/10.1901/jeab.1991.56-261.
Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. https://doi.org/10.1038/npp.2009.131.
Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharm Biochem Behav. 2011;99:211–6. https://doi.org/10.1016/j.pbb.2011.01.020.
Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005;1:2 https://doi.org/10.1186/1744-9081-1-2.
Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharm. 1998;42:707–11. https://doi.org/10.1016/s1054-3589(08)60846-7.
Brennan BP, Tkachenko O, Schwab ZJ, Juelich RJ, Ryan EM, Athey AJ, et al. An Examination of Rostral Anterior Cingulate Cortex Function and Neurochemistry in Obsessive-Compulsive Disorder. Neuropsychopharmacology. 2015;40:1866–76. https://doi.org/10.1038/npp.2015.36.
Riffkin J, Yücel M, Maruff P, Wood SJ, Soulsby B, Olver J, et al. A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: comparison with healthy controls and patients with schizophrenia. Psychiatry Res. 2005;138:99–113. https://doi.org/10.1016/j.pscychresns.2004.11.007.
Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–4. https://doi.org/10.1126/science.1168878.
Starkweather CK, Uchida N. Dopamine signals as temporal difference errors: recent advances. Curr Opin Neurobiol. 2021;67:95–105. https://doi.org/10.1016/j.conb.2020.08.014.
Wang Y, Toyoshima O, Kunimatsu J, Yamada H, Matsumoto M. Tonic firing mode of midbrain dopamine neurons continuously tracks reward values changing moment-by-moment. Elife. 2021;10. https://doi.org/10.7554/eLife.63166.
Solich J, Faron-Gorecka A, Kusmider M, Palach P, Gaska M, Dziedzicka-Wasylewska M. Norepinephrine transporter (NET) knock-out upregulates dopamine and serotonin transporters in the mouse brain. Neurochem Int. 2011;59:185–91. https://doi.org/10.1016/j.neuint.2011.04.012.
Richardson BD, Saha K, Krout D, Cabrera E, Felts B, Henry LK, et al. Membrane potential shapes regulation of dopamine transporter trafficking at the plasma membrane. Nat Commun. 2016;7:10423 https://doi.org/10.1038/ncomms10423.
Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006;31:1362–70. https://doi.org/10.1038/sj.npp.1300966.
Sommer S, Danysz W, Russ H, Valastro B, Flik G, Hauber W. The dopamine reuptake inhibitor MRZ-9547 increases progressive ratio responding in rats. Int J Neuropsychopharmacol. 2014;17:2045–56. https://doi.org/10.1017/S1461145714000996.
Seiler JL, Cosme CV, Sherathiya VN, Schaid MD, Bianco JM, Bridgemohan AS, et al. Dopamine signaling in the dorsomedial striatum promotes compulsive behavior. Curr Biol. 2022;32:1175–88.e1175. https://doi.org/10.1016/j.cub.2022.01.055.
Acknowledgements
We would like to thank Dr. Marc Caron for providing us with monoamine transporter knockout mice. We would also like to thank Dr. Stan Floresco (U of British Columbia) for the Med-PC code for the reversal learning task and related advice.
Funding
This work was supported by a NIMH R21 (MH127377) and R01 (MH130778) grant (NMU) and NARSAD/BBRF Young Investigator grant (NMU).
Author information
Authors and Affiliations
Contributions
Jena Delaney –performed experiments, analyzed data. Sanya Nathani - performed experiments. Victor Tan - performed experiments. Alex Orr - performed experiments. Carson Chavez - performed experiments. Joon Paek – Analyzed data. Mojdeh Faraji – analyzed data. Barry Setlow - conceptualized experiments, wrote manuscript. Nikhil Urs - conceptualized experiments, performed experiments, analyzed data, wrote manuscript
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Delaney, J., Nathani, S., Tan, V. et al. Enhanced cognitive flexibility and phasic striatal dopamine dynamics in a mouse model of low striatal tonic dopamine. Neuropsychopharmacol. 49, 1600–1608 (2024). https://doi.org/10.1038/s41386-024-01868-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-024-01868-5
- Springer Nature Switzerland AG