Abstract
Background
Deep brain stimulation (DBS) delivered to the ventromedial prefrontal cortex (vmPFC) induces antidepressant- and anxiolytic-like responses in various animal models. Electrophysiology and neurochemical studies suggest that these effects may be dependent, at least in part, on the serotonergic system. In rodents, vmPFC DBS reduces raphe cell firing and increases serotonin (5-HT) release and the expression of serotonergic receptors in different brain regions.
Methods
We examined whether the behavioural responses of chronic vmPFC DBS are mediated by 5-HT1A or 5-HT1B receptors through a series of experiments. First, we delivered stimulation to mice undergoing chronic social defeat stress (CSDS), followed by a battery of behavioural tests. Second, we measured the expression of 5-HT1A and 5-HT1B receptors in different brain regions with western blot. Finally, we conducted pharmacological experiments to mitigate the behavioural effects of DBS using the 5-HT1A antagonist, WAY-100635, or the 5-HT1B antagonist, GR-127935.
Results
We found that chronic DBS delivered to stressed animals reduced the latency to feed in the novelty suppressed feeding test (NSF) and immobility in the forced swim test (FST). Though no significant changes were observed in receptor expression, 5-HT1B levels in DBS-treated animals were found to be non-significantly increased in the vmPFC, hippocampus, and nucleus accumbens and reduced in the raphe compared to non-stimulated controls. Finally, while animals given vmPFC stimulation along with WAY-100635 still presented significant responses in the NSF and FST, these were mitigated following GR-127935 administration.
Conclusions
The antidepressant- and anxiolytic-like effects of DBS in rodents may be partially mediated by 5-HT1B receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deep brain stimulation (DBS) involves the delivery of electrical current to specific brain targets via surgically implanted electrodes (Awan et al. 2009; Hamani et al. 2010c; Hamani and Temel 2012). To date, several clinical studies have examined the efficacy of DBS for treatment-resistant depression. While open-label trials have shown promising results when DBS was delivered to the subcallosal cingulate gyrus (SCG) (Mayberg et al. 2005; Riva-Posse et al. 2018), ventral capsule/ventral striatum (VC/VS) (Bergfeld et al. 2016; Malone et al. 2009), medial forebrain bundle (MFB) (Fenoy et al. 2018; Schlaepfer et al. 2013), and nucleus accumbens (NAcc) (Schlaepfer et al. 2008), results of randomized clinical trials comparing active versus sham stimulation were quite disappointing (Dougherty et al. 2015; Holtzheimer et al. 2017). Recent improvements in neuroimaging techniques (Coenen et al. 2019, 2020; Riva-Posse et al. 2014, 2018) and the analysis of patients subdivided into responders and non-responders (Bergfeld et al. 2016; Puigdemont et al. 2015) suggest that improvements in outcome are possible and that specific populations of patients may be more suitable candidates for DBS. This urges the investigation of predictors of response and mechanisms for the antidepressant effects of DBS (Brown et al. 2020; Davidson et al. 2020; Frank et al. 2021; Sankar et al. 2020).
Preclinical research has been important for studying potential mechanisms and the development of novel treatments for debilitating psychiatric disorders, including DBS for major depression (Dandekar et al. 2019; Edemann-Callesen et al. 2015; Furlanetti et al. 2015; Hamani and Nobrega 2012; Hamani et al. 2010c; Hamani and Temel 2012; Jimenez-Sanchez et al. 2016a, b; Krishnan and Nestler 2011; Lim et al. 2015a, b; Papp et al. 2019; Rummel et al. 2016; Thiele et al. 2018; Torres-Sanchez et al. 2017, 2018). Despite the complex nature of this disorder, valuable animal models have emerged over the years, including the exposure of animals to chronic social defeat stress (CSDS) (Bartolomucci et al. 2001; Berton et al. 1999, 2006; Hammels et al. 2015; Kudryavtseva et al. 1991; Raab et al. 1986). In a commonly used paradigm, a male “intruder” rodent is placed in the home cage of an unfamiliar male “resident” (Golden et al. 2011). The intruder is often dominated by the resident and, after several exposures, develops anxiety- and depressive-like responses (Berton et al. 2006; Golden et al. 2011; Iniguez et al. 2014; Krishnan et al. 2007; Warren et al. 2014). In addition to a valid behavioural phenotype, susceptible intruders demonstrate physiological changes associated with a depressive-like state, including changes in the hypothalamic–pituitary–adrenal axis (Dallman et al. 2000; Gomez-Lazaro et al. 2011; Kronfeld-Schor and Einat 2012), increases in pro-inflammatory cytokines (Gomez-Lazaro et al. 2011), reduced synaptic plasticity, and neurogenesis (Christoffel et al. 2011; Gomez-Lazaro et al. 2011; Krishnan et al. 2007; Lagace et al. 2010; Warren et al. 2013, 2014).
Chronic stimulation delivered to the ventromedial prefrontal cortex (vmPFC), a region considered to be the rodent homologue of the human SCG (Hamani et al. 2011; Hamani and Nobrega 2012; Hamani and Temel 2012), reverses depressive- and anxiety-like behaviours in various animal models and rat lines (Bambico et al. 2015; Bregman et al. 2018; Bruchim-Samuel et al. 2016; Edemann-Callesen et al. 2015; Furlanetti et al. 2015; Gersner et al. 2010; Hamani et al. 2010a, b, 2012a, b,2014; Jimenez-Sanchez et al. 2016a, b; Lim et al. 2015b; Moshe et al. 2016; Papp et al. 2018; Thiele et al. 2018; Veerakumar et al. 2014). In rodent models, vmPFC DBS has been shown to modulate several neurotransmitters closely related to depression, including the serotonergic system (Bregman et al. 2018; Hamani et al. 2010b; Volle et al. 2018). Serotonin (5-HT)-depleting raphe lesions block the effects of vmPFC stimulation (Hamani et al. 2010b). Likewise, DBS in this target reduces the firing of raphe cells (Lim et al. 2015b; Srejic et al. 2015) and increases serotonin release in the hippocampus (Hamani et al. 2010b; Volle et al. 2018). Despite the fact that vmPFC DBS increases 5-HT1B, but not 5-HT1A receptor expression (Volle et al. 2018), the functional role of specific serotonin receptors in the behavioural effects of DBS remains poorly understood.
We delivered chronic vmPFC stimulation to intruders in a modified CSDS paradigm to test whether the anxiolytic and antidepressant-type effects of DBS were mediated by 5-HT1B receptors.
Materials and methods
All procedures were approved by the Sunnybrook Research Institute Animal Care Committee.
Modified chronic social defeat stress model
C57BL/6 male resident mice (Charles River, Quebec) were pair-housed with tube-ligated females. Three weeks later, residents were screened for aggressive behaviour with training Balb/c males (Charles River, Quebec). During screening, the female was removed, and the training mouse was transferred into the resident’s home cage in a perforated metal barrier for 2 min. The training mouse was then removed from the barrier and placed in the resident’s home cage for a 5-min unprotected encounter. For each resident, two screening sessions were performed daily with different training mice used in consecutive sessions. Daily screening was repeated until residents demonstrated a latency to aggression of 30 s or less and a consistent number of aggressive bouts in consecutive sessions. Once these aggression endpoints were met, screening was terminated.
The modified social defeat stress included 6 days of interactions between C57BL/6 male aggressive residents and Balb/c male intruders. After the female was removed, the intruder mouse was transferred into the resident’s home cage in a perforated metal barrier for 2 min. The intruder was then removed from the barrier and placed in the resident’s home cage for a 5-min contact encounter. Following the interaction, the intruder mouse was returned to its home cage. Each intruder was submitted to one defeat session per day over 3 consecutive days, followed by a 4-day break and another 3 consecutive days of defeat sessions (a total of 6 social defeat sessions per intruder). To avoid individual differences in defeat intensity, intruders were confronted with alternating residents on subsequent days. Non-stressed controls did not undergo social defeat, remaining in their home cage during an equivalent interval.
Behavioural testing
The sequence of tests was chosen so that animals were subjected to the more stressful paradigms as the testing progressed.
Open field test (OFT)
Mice were placed in a 20 cm × 20 cm square plexiglass container and recorded for 5 min. The duration of locomotion and the distance travelled were quantified with a tracking software (Any-Maze; Wood Dale, IL).
Defensive burying test (DBT)
One hour after the OFT, mice were placed in a standard cage containing 5 cm of clean flattened bedding and 8 identical marbles evenly arranged in two columns. The number of marbles buried in a 30-min session was quantified. A marble was considered buried when 50% or less of its surface was visible.
Novel location recognition tests (NLRT) and novel object recognition (NORT)
One day after a 5-min habituation session, mice were allowed to explore a 20 cm × 20 cm square plexiglass arena containing two identical objects for 5 min (familiarization). During the location testing, one of the identical objects was repositioned into a novel quadrant. During object recognition testing, the object in the original location was replaced with a different object. NORT and NLRT sessions lasted 5 min each. Videotaped sessions were analysed with a tracking software (Any-Maze; Wood Dale, IL). The location recognition index was calculated according to the formula: time of novel location exploration divided by the total exploration time × 100. The object recognition index was calculated as follows: duration of novel object exploration divided by total exploration time × 100 (Lueptow 2017).
Novelty suppressed feeding test (NSF)
Two days prior to the test, animals were trained to consume food treats (fruit loops) in their home cage. After a 16-h food deprivation, mice were placed in a plexiglass arena (50 cm × 10 × 60) containing a white platform with the habituated treat. The latency to eat the treat was recorded. Animals that did not eat were excluded from the analysis (n = 1 DBS stress animal in the first experiment; n = 2 stress vehicle, n = 1 DBS stress vehicle; n = 2 DBS stress GR-127935 animals in pharmacological experiments).
Forced swim test (FST)
Mice were placed in an inescapable cylindrical tank (30 cm height × 20 cm diameter) filled 15 cm from the top with 26 °C water for 5 min. The last 3 min of the session were scored, and the total immobility time was quantified. We chose to measure immobility during the last 3 min of testing because this is the timeline in which differences between stimulated and non-stimulated animals are more prominent (Hamani and Nobrega 2012).
DBS surgery and stimulation
A timeline with the behavioural DBS experiments is provided in Fig. 1. Male Balb/c mice (Charles River, Quebec, Canada; 20–25 g) were anesthetized with isoflurane and received bilateral vmPFC stainless-steel implants to be used as cathodes (0.5 mm lead exposure, AP: + 1.9 mm: ML: ± 0.3 mm; DV: − 2.9 mm; Plastics One model 333/3) (Paxinos and Franklin 2012). Screws implanted into the skull over the parietal cortex served as anodes. Sham DBS animals had electrodes implanted but received no stimulation. Control surgeries omitted electrode implants. Exposure to resident mice commenced 5–7 days after the procedure.
Timeline of behavioural experiments. A After 3 days of social defeat, animals went on a break for 4 days, followed by another 3 days of social defeat and then behavioural testing. DBS was started 1 day after the first round of social defeat sessions and continued to the end of the experiment. B Social defeat and behavioural testing conducted during pharmacological experiments. DBS was started 1 day after the first round of social defeat sessions and continued to the end of the experiment. Arrows represent the timepoints of drug administration. D, day; DBS, deep brain stimulation; DBT, defensive burying test; FST, forced swim test; NLRT, novel location recognition test; NORT, novel object recognition test; NSF, novelty suppressed feeding test; OFT, open field test
vmPFC DBS was delivered with a handheld stimulator (ANS model 3510, Plano, TX), connected to the animals through extension cables (Plastics One, model 335–340/3). Stimulation was delivered at 130 Hz, 90 μs, 100 μA (Bregman et al. 2018; Hamani et al. 2012b; Hamani and Nobrega 2012) for 3 h/day prior to behavioural testing/encounters and 5 h/day on non-testing days. No stimulation was delivered during the first 3 days of social encounters. Following the experiments, animals were sacrificed, and the brains of eight animals per group were randomly selected for neurochemical analyses.
Drug administration
WAY-100635 maleate (2.5 mg/kg s.c.; Tocris) and GR-127935 hydrochloride (5 mg/kg i.p.; Tocris) were diluted in saline and administered to the animals 30 min before DBS on behavioural testing days. Selected doses were based on safety profile or previous work suggesting that these drugs antagonized the effects of antidepressant medications (Castro et al. 2008; Cryan et al. 2005b; de Almeida et al. 2001; Hogg and Dalvi 2004; Kaster et al. 2005; Lopez-Mendoza et al. 1998; Mayorga et al. 2001; O’Neill and Conway 2001; Rogoz et al. 2012; Takahashi et al. 2020; Tatarczynska et al. 2002; Zanelati et al. 2010). In pharmacologic experiments, stress animals underwent surgical procedures but were not implanted with electrodes.
Western blot and histology
After removal from the skull, brains were stored at − 80 C°. In animals that did not undergo neurochemical analyses, vmPFC electrode placement was confirmed in cresyl violet stained sections (Fig. 2) (Gidyk et al. 2021; Hamani et al. 2010a, b, 2014). In brains processed for western blot, electrode tracks were visually inspected in thick coronal sections prior to tissue processing. After this step, the following regions were dissected using a biopsy punch tool for analysis: ventromedial prefrontal cortex, nucleus accumbens (NAcc), dorsal hippocampus (HPC), and raphe (all raphe nuclei). Tissue from both hemispheres was collected for analysis. Protein extracts were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis followed by transfer onto polyvinylidene fluoride membranes. These were then exposed to blocking buffer at 4 °C overnight. Primary antibodies were added at the following dilutions: anti-5-HT1B rabbit pAb [Abcam: ab13896] 1:1000 and anti-5-HT1A rabbit pAb [abcam # ab227165] 1:1000. For loading control, the membranes were incubated with anti-tubulin-β3 mouse mAb [BioLegend #801202] 1:2000. After washing, they were incubated with secondary antibodies: 1:2000 HRP-linked, anti-rabbit IgG [CST #7074] and HRP-linked, anti-mouse IgG [CST #7076]. Thereafter, the membranes were washed, incubated in SignalFire™ ECL Reagent substrate solution, and imaged with a MicroChemi 4.2 unit (DNR Bio-Imaging Systems) using GelCapture Chemi software. Representative blots of the groups included in our study may be found in Supplementary Figs. 1 and 2. 5-HT1A and 5-HT1B values in the manuscript refer to protein expression.
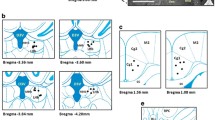
Electrode location. A Photomicrograph of a coronal brain section illustrating the trajectory of an electrode placed in the vmPFC (arrow). B Schematic representation of coronal brain sections showing the location of the tip of the electrodes implanted in animals receiving deep brain stimulation (black circles; n = 9) or sham stimulation (light grey circles; n = 9). Electrodes implanted bilaterally are depicted in a single hemisphere. In this representation, only electrodes associated with the initial set of behavioural experiments were plotted. Electrodes in the remainder experiments were placed in a similar location
Statistical analyses
One-way ANOVA (Tukey post hoc) was used to compare data across groups. Two-way ANOVA (Tukey post hoc) was used to analyse pharmacological data with DBS and drug administration as factors. A Student’s t test was used to compare behavioural data between stressed and non-stressed mice. Results in the text and figures are expressed as means ± standard errors. Statistical significance was set at p ≤ 0.05.
Results
Stress effects
Prior to DBS experiments, we tested the effects of stress in our modified paradigm. In the open field test, stressed animals without any surgical manipulation (n = 16) had a lower locomotion (189.4 ± 6.8 s vs 218.5 ± 6.3 s; p = 0.004; Fig. 3A) and travelled smaller distances (8.2 ± 0.5 s vs 10.9 ± 0.8 s; p = 0.005; Fig. 3B) compared to non-stressed controls (n = 20). In contrast, no group differences were found in the defensive burying test (p = 0.1; Fig. 3C), novel location recognition test (p = 0.4; Fig. 3D), and novel object recognition test (p = 0.4; Fig. 3E). In the NSF, stressed mice had a significantly longer latency to feed (207.7 ± 30.8 s) compared to non-stressed controls (99.2 ± 12.8 s; p = 0.005; Fig. 3F). In the FST, animals exposed to stress spent a higher time in immobility (117.0 ± 7.4 s) compared to non-stressed controls (82.0 ± 5.7 s; p = 0.001; Fig. 3G).
Stress-induced effects in a modified chronic social defeat stress paradigm. A In the open field, A locomotion and the B distance travelled were significantly lower in stress-exposed mice (n = 16) compared to non-stressed controls (n = 20). C In the defensive burying test (DBT), no difference was found in the number of marbles buried by stressed and non-stressed animals. Similarly, no differences between groups were found in the D the novel location recognition test (NLRT) or E the novel object recognition test (NORT). F In the novelty suppressed feeding test (NSF), the latency to feed in stressed animals was significantly higher than in non-stressed controls. G In the forced swim test (FST), stressed mice had significantly more immobility than non-stressed controls. Values represent mean and standard error. *Statistically significant
Chronic vmPFC DBS
Open field test
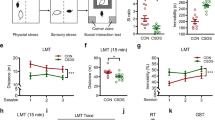
One-way ANOVA revealed a significant treatment effect on locomotion (F [2,25] = 3.96, p = 0.03), due to the higher value recorded in Sham DBS stress mice (216.0 ± 8.4 s; n = 9;) compared to Sham controls (183.4 ± 6.9 s; p = 0.03; n = 10), but not to the DBS stress group (189.6 ± 10.6 s; n = 9; Fig. 4A). In contrast, no significant treatment effect was found for the distance travelled (F [2,25] = 2.90, p = 0.07), with similar values recorded in Stress controls (6.9 ± 0.5 m), Sham stress (8.7 ± 0.5 m), and DBS stress mice (7.8 ± 0.6 m; Fig. 4B).
Antidepressant- and anxiolytic-like effects of deep brain stimulation (DBS) in a modified chronic social defeat stress paradigm. A In the open field, locomotion was significantly higher in Sham DBS stress mice (n = 9) than in animals exposed to stress without implanted electrodes (Stress controls; n = 10), but not to the DBS stress group (n = 9). B In contrast, the distance travelled in the apparatus was similar across groups. C In the defensive burying test (DBT), no difference was found in the number of marbles buried by animals in different groups. Similarly, no differences across groups were found when the indexes calculated for D the novel location recognition test (NLRT) or E the novel object recognition test (NORT) were considered. F In the novelty suppressed feeding test (NSF), the latency to feed in DBS stress animals (n = 8) was significantly lower than in the Sham DBS stress group (n = 9) or in Stress controls (n = 10). G In the forced swim test (FST), both DBS stress and Sham DBS stress animals had significantly less immobility than Stress-exposed controls. Values represent mean and standard error. *Statistically significant
Defensive burying
No significant effect of stimulation was found in the DBT (F [2,25] = 2.34, p = 0.12), despite the lower number of marbles buried by DBS stress animals (4 ± 0.9) compared to Stress controls (5.9 ± 0.5) and the Sham DBS stress group (5.3 ± 0.5; Fig. 4C).
Novel location and novel object recognition tests
One-way ANOVA revealed no significant stimulation effects in the novel location (F [2,25] = 0.91, p = 0.42; Fig. 4D) and novel object recognition tests (F [2,25] = 0.34, p = 0.71; Fig. 4E). Similar NLRT and NORT indices were respectively observed in DBS stress mice (45.3 ± 4.2; 62.7 ± 4.2), Sham DBS stress animals (51.6 ± 3.9; 58.4 ± 4.8) and Stress controls (50.3 ± 2.1; 58.6 ± 3.4).
Novel suppressed feeding
A significant treatment effect (F [2,24] = 9.0, p = 0.001) was observed in the NSF, with DBS stress animals presenting a lower latency to feed (51.6 ± 10.1 s; n = 8) compared to Sham DBS stress animals (178.7 ± 27.3 s; p = 0.0008; n = 9) and Stress controls (125.3 ± 18.7 s; p = 0.048; n = 10; Fig. 4F).
Forced swim test
One-way ANOVA revealed a significant treatment effect (F [2,26] = 12.4, p = 0.0002). Both DBS stress (62.5 ± 7.7 s; p = 0.0002) and Sham DBS stress animals (77.2 ± 9.4 s; p = 0.005) had significantly less immobility than Stress-exposed controls (114.9 ± 6.1 s; p = 0.0002; Fig. 4G).
5-HT1A and 5-HT1B expression
Overall, no significant vmPFC stimulation effects were observed in the expression of 5-HT1A or 5-HT1B (Table 1). In most studied regions, 5-HT1A levels were non-significantly lower, whereas 5-HT1B expression was non-significantly higher in DBS-treated animals compared to controls (Fig. 5).
5-HT1A (upper panel) and 5-HT1B (lower panel) receptor protein expression measured with western blot in the Stress control, Sham DBS stress, and DBS stress groups. A 5-HT1A expression was non-significantly reduced by 11–17% in the vmPFC, hippocampus, and raphe, and increased by 10% in the NAcc of DBS-treated animals, compared to controls. B 5-HT1B expression in the stimulated group was non-significantly increased by 11% in the vmPFC, 19% in the hippocampus, 94% in the NAcc, and reduced by 16% in the raphe, compared to Stress controls. Values represent mean and standard error. n = 8 animals/group
Pharmacological experiments
In our initial experiment, we showed that vmPFC DBS improved anxiety- and depressive-like responses in mice exposed to social defeat stress. To test whether the DBS effects are mediated by 5-HT1A or 5-HT1B receptors, we treated different groups of animals with WAY-100635 (WAY) or GR-127935 (GR). Since DBS did not induce memory changes in our initial experiment, we did not conduct novel location or novel object recognition testing in pharmacological preparations. As Sham DBS stress animals did not differ substantially from non-implanted Stress controls, only the latter group was injected with drugs.
5-HT1A antagonism—WAY-100635
Open field test
Two-way ANOVA revealed a significant effect of DBS (F [1,27] = 6.40, p = 0.02) but no effect of drug (F [1,27] = 0.26, p = 0.61), or a DBS × drug interaction (F [1,27] = 1.52, p = 0.23) on locomotion (Fig. 6A). No effects of DBS (F [1,27] = 3.1, p = 0.09), drug (F [1,27] = 0.01, p = 0.99), or a DBS × drug interaction (F [1,27] = 0.87, p = 0.36) were observed on the distance travelled (Fig. 6B). No significant differences were found when either variable was compared among animals receiving DBS stress WAY (159.2 ± 11.5 s; 6.5 ± 0.7 m.; n = 8), DBS stress vehicle (180.6 ± 13.1 s; 7.3 ± 0.8 m.; n = 10), Stress WAY (205.4 ± 4.6 s; 8.7 ± 0.8 m.; n = 7), and Stress vehicle (196.6 ± 15.1 s; 8.0 ± 1.0 m.; n = 6).
Antidepressant- and anxiolytic-like effects of deep brain stimulation (DBS) are unaffected by the 5-HT1A antagonist WAY-100635 (WAY). A Locomotion in the open field, B the distance travelled, and C the number of buried marbles in the defensive burying test (DBT) were similar in animals receiving Stress vehicle (Veh; n = 6), Stress WAY (n = 7), DBS stress vehicle (n = 10), or DBS stress WAY (n = 8). D) In the novelty suppressed feeding test (NSF), a significant reduction in the latency to feed was observed when DBS stress animals treated with either vehicle (n = 9) or WAY (n = 8) were compared to groups receiving Stress vehicle (n = 5) or Stress WAY (n = 7). E In the forced swim test (FST), a significant decrease in immobility was observed when either DBS stress vehicle animals or DBS stress WAY mice were compared to groups receiving Stress vehicle or Stress WAY. Values represent mean and standard error. *Statistically significant
Defensive burying
No effects of DBS (F [1,27] = 0.50, p = 0.49), drug (F [1,27] = 0.57, p = 0.46), or a DBS × drug interaction (F [1,27] = 0.53, p = 0.47) were noted in the DBT. The number of marbles buried was similar in groups receiving DBS stress WAY (4.9 ± 0.9), DBS stress vehicle (3.6 ± 0.9), Stress WAY (4.9 ± 0.5), or Stress vehicle (4.8 ± 0.9; Fig. 6C).
Novel suppressed feeding
In the NSF, two-way ANOVA revealed a significant DBS effect (F [1,25] = 76.5, p < 0.0001), but no drug effect (F [1,25] = 0.90, p = 0.35), or a DBS × drug interaction (F [1,25] = 0.66, p = 0.42). A significant reduction in the latency to feed was observed between DBS stress vehicle animals (49.8 ± 9.2 s; n = 9) and both Stress vehicle (261.2 ± 49.3 s; n = 5; p < 0.0001) and Stress WAY groups (222.1 ± 25.1 s; n = 7; p < 0.0001). Similarly, a reduction in the latency to feed was found between DBS stress WAY mice (46.8 ± 7.8 s; n = 8) and both the Stress vehicle (p < 0.0001) and Stress WAY groups (p < 0.0001; Fig. 6D).
Forced swim test
Two-way ANOVA revealed a significant effect of DBS (F [1,27] = 37.5, p < 0.0001), but no drug effect (F [1,27] = 0.78, p = 0.39), or a DBS × drug interaction (F [1,27] = 1.0, p = 0.33). A significant reduction in immobility time was observed between the animals receiving DBS stress vehicle (63.0 ± 6.9 s) and both Stress vehicle (100.5 ± 11.3 s; p = 0.006) and Stress WAY groups (114.2 ± 6.6 s; p = 0.001). Similarly, a reduction in the latency to feed was found between DBS stress WAY mice (62.1 ± 3.8 s) and both Stress vehicle (p = 0.007) and Stress WAY groups (p = 0.002; Fig. 6E).
5-HT1B antagonism—GR-127935
Open field test
Two-way ANOVA revealed no effects of DBS (F [1,28] = 1.08, p = 0.31), drug (F [1,28] = 0.01, p = 0.92), or a DBS × drug interaction (F [1,28] = 0.61, p = 0.44) on locomotion (Fig. 7A). Similarly, no effects of DBS (F [1,28] = 0.13, p = 0.14), drug (F [1,28] = 0.30, p = 0.59), or a DBS × drug interaction (F [1,28] = 2.30, p = 0.14) were observed on the distance travelled (Fig. 7B). No significant differences were found for either variable when DBS stress GR (172.2 ± 13.7 s; 6.6 ± 0.7 m.; n = 8), DBS stress vehicle (180.6 ± 13.1 s; 7.3 ± 0.8 m.; n = 10), Stress GR (194.4 ± 7.4 s; 7.9 ± 0.3 m.; n = 7) and Stress vehicle groups were compared (183.8 ± 10.2 s; 6.5 ± 0.6 m.; n = 7).
The antidepressant- and anxiolytic-like effects of deep brain stimulation (DBS) are diminished by the 5-HT1B antagonist GR-127935 (GR). No significant differences were found when groups receiving Stress vehicle (Veh; n = 7), Stress GR (n = 7), DBS stress vehicle (n = 10), or DBS stress GR (n = 8) were compared in A, B the open field or C the defensive burying test (DBT). D In the novelty suppressed feeding test (NSF), a significant reduction in the latency to feed was observed between groups receiving DBS stress vehicle (n = 9) and Stress vehicle (n = 7). Values recorded in DBS stress GR animals (n = 6) and Stress GR mice (n = 7) were similar to those observed in Stress vehicle controls. E In the forced swim test (FST), significant differences in immobility were found between groups receiving DBS stress vehicle and either Stress GR or DBS stress GR. None of the groups differed significantly from Stress vehicle controls. Values represent mean and standard error. *Statistically significant
Defensive burying
No effects of DBS (F [1,28] = 0.14, p = 0.72), drug (F [1,28] = 0.01, p = 0.95), or a DBS × drug interaction (F [1,28] = 0.14, p = 0.72) were found in the DBT. The number of marbles buried was similar in groups receiving DBS stress GR (4.0 ± 0.8), DBS stress vehicle (3.6 ± 0.9), Stress GR (4.00 ± 1.0), or Stress vehicle (4.3 ± 0.9; Fig. 7C).
Novel suppressed feeding
Two-way ANOVA revealed a significant DBS effect (F [1,25] = 8.0, p = 0.009), but no drug effect (F [1,25] = 0.08, p = 0.78), or a DBS × drug interaction (F [1,25] = 2.2, p = 0.16). A significant reduction in the latency to feed was observed between groups receiving DBS stress vehicle (49.8 ± 9.2 s; n = 9) and Stress vehicle (213.0 ± 49.3 s; n = 7; p = 0.02). Values recorded in DBS stress GR animals (115.7 ± 38.6 s; n = 6) and Stress GR mice (168.1 ± 49.9 s; n = 7) were similar to stress vehicle controls (Fig. 7D).
Forced swim test
Two-way ANOVA revealed significant effects of DBS (F [1,28] = 4.4, p = 0.05) and drug (F [1,28] = 7.8, p = 0.01), but no DBS × drug interaction (F [1,28] = 1.4, p = 0.24). Significant differences in immobility were found between groups receiving DBS stress vehicle (63.0 ± 6.9 s), and either Stress GR (112.9 ± 9.8 s; p = 0.01), or DBS stress GR (103.8 ± 12.5 s; p = 0.03). None of the groups differed significantly from Stress vehicle controls (96.6 ± 11.7 s; Fig. 7E).
Discussion
Our study shows that chronic vmPFC stimulation induces anxiolytic and antidepressant-like effects in a chronic social defeat stress paradigm. Most importantly, the behavioural response of DBS was mitigated by the administration of the 5-HT1B but not 5-HT1A antagonists. This finding suggests that, in contrast to antidepressant medications (Lesch 1991; Lucki 1996; Lucki et al. 1994), the anxiolytic- and antidepressant-like effects of vmPFC stimulation may be mediated by 5-HT1B serotonin receptors.
Based on anatomical connections and cytoarchitectural features, the ventral aspect of the medial prefrontal cortex (vmPFC) has been suggested to be the anatomical correlate of the subgenual cingulum (Gabbott et al. 2003; Hamani et al. 2010b, 2011; Takagishi and Chiba 1991). Since the SCG is a commonly stimulated target in clinical trials for major depression (Holtzheimer et al. 2012, 2017; Lozano et al. 2012; Mayberg et al. 2005; Riva-Posse et al. 2018), we opted to stimulate the vmPFC in the current study.
The behavioural effects of chronic vmPFC stimulation have been previously demonstrated in rodent models induced by stress, neurobiological preparations, and in specific animal lines (Bambico et al. 2015; Gersner et al. 2010; Hamani et al. 2012b; Lim et al. 2015b; Moshe et al. 2016; Veerakumar et al. 2014). A commonly used rodent paradigm involves exposure to chronic unpredictable mild stress (Muscat et al. 1988; Willner et al. 1992, 1987). This model, however, is notoriously complex and difficult to replicate (Antoniuk et al. 2019; Willner 2017). Moreover, it is particularly cumbersome in mice, often requiring long timeframes and complex readouts to detect anxiety- and depressive-like behaviours (Maluach et al. 2017; Prevot et al. 2019). An alternative model is chronic social defeat stress, which has been shown to induce depressive- and anxiety-type behaviours in rodents (Bartolomucci et al. 2001; Berton et al. 1999, 2006; Hammels et al. 2015; Kudryavtseva et al. 1991; Raab et al. 1986). In the current study, we used a modified version of this paradigm. In contrast to a continuous interaction (Golden et al. 2011), intruders were exposed to residents in two 3-day sessions separated by a 4-day break. By inserting an interval between two runs of social defeat, we were able to deliver 1 week of DBS to stress-exposed animals before behavioural testing began. This timeline corresponds to a scenario in which patients would receive DBS after the development of the disease. In addition, in this modified version of the model, DBS could be administered for 1–2 weeks, a timeframe that would allow several neuroplastic phenomena to develop. In general, the expression of anxiety and depression-type responses following stress tends to decrease over time following stress exposure. By dividing the 6 days of social encounters into two segments of 3 days, we theoretically maximized the chances of detecting depressive- and anxiety-like behaviours. While our modified preparation was adequate for our purposes, we do not know if changes in the interval between exposure trials or the timeframe between stress and testing would yield different results.
In the clinical scenario, DBS has been shown to improve several symptoms associated with depression (Holtzheimer et al. 2012, 2017; Lozano et al. 2012; Mayberg et al. 2005; Riva-Posse et al. 2018). In the current study, we have selected a battery of behavioural tests to study the effects of DBS in multiple dimensions, including depressive-like behaviour (e.g. FST), anxiety (e.g. defensive burying, novelty supressed feeding), and memory function (e.g. novel object and location recognition). We found that vmPFC stimulation was most effective in improving depressive- and anxiety-like behaviour, exerting no effect on memory performance. Though anhedonic-like behaviour following vmPFC stimulation has been previously demonstrated by our group and others (Bambico et al. 2015; Hamani et al. 2012b; Lim et al. 2015b), this was not formally assessed in the current study. Whether DBS-induced improvements in sucrose preference, sucrose consumption, or the splash test are dependent on 5-HT1B receptors remains to be demonstrated.
The main findings of our initial behavioural experiments were that vmPFC DBS reduced the latency to feed in the NSF and immobility in the FST. In the latter, the effects of DBS could not exclusively be attributed to the electrical current delivered to the parenchyma but instead to a combination of stimulation plus electrode insertion since sham implanted animals also presented an antidepressant-like effect. In contrast, stimulation did not affect defensive burying in the DBT or memory performance in the NORT and NLRT. The former is an innate rodent behaviour that has routinely been used as a measure of anxiety (Reznikov et al. 2016). In our memory paradigm, no substantial interval was given between the initial item presentation and changes in either object or location. We investigated short-term memory because we were directly stimulating the vmPFC, a region largely involved in mechanisms of novel object recognition, novel location recognition, and working memory (Aggleton and Nelson 2020; Chao et al. 2020). Previous studies have shown that DBS delivered to stressed rats rescued CUMS-induced memory deficits (Papp et al. 2018, 2019). Future work in CSDS paradigms is still required to test whether vmPFC stimulation mitigates deficits in hippocampus- or amygdala-dependent memory tasks.
Similar to our previous work, one of the main tests used to measure depression-like behaviour in the current study was the FST (Hamani et al. 2010a, b, 2014). In addition to immobility, different types of behaviours may be assessed in this test, including swimming and climbing. Previous work has shown that the former is more sensitive to serotonergic compounds while the latter is more responsive to drugs that modulate catecholamine transmission (Cryan et al. 2002, 2005a, b; Detke et al. 1995). Our choice to predominantly focus on immobility time was based on three factors observed in our previous work. DBS had no significant effect on climbing (Hamani et al. 2010a, b, 2014). Reductions in immobility time were almost always the inverse representation of swimming (Hamani et al. 2010a, b, 2014). Immobility has been a reliable measure for the study of the antidepressant-like effects of DBS in mice (Bregman et al. 2018; Hamani and Nobrega 2012).
As described above, some DBS effects may be partially attributed to electrode implantation. This so-called insertional effect has been previously reported in rodents. In those studies, animals demonstrated behavioural or neurochemical changes following electrode insertion that resembled those recorded after DBS, albeit of smaller magnitude (Casquero-Veiga et al. 2018; Hamani and Nobrega 2012; Perez-Caballero et al. 2018). The mechanisms responsible for such an effect are still disputed. Perez-Caballero et al. have shown that an increase in astrocytic immunoreactivity temporally correlates with behavoural responses (Perez-Caballero et al. 2018). Of note, however, the consequences of electrode insertion may still be observed long after surgery in brain regions distant from the stimulated target (Chakravarty et al. 2016; Hamani et al. 2012b; Hamani and Nobrega 2012). Similar to rodent studies, the presence of an insertional effect has been documented in patients with tremor, pain, epilepsy, and even depression (Fenoy et al. 2018; Hamani et al. 2006, 2021; Lim et al. 2007; Tasker 1998). Clinically, this is characterized by symptomatic amelioration following electrode implantation, prior to the administration of current.
In our view, the consequences of DBS, at least in preclinical models, are a composite of electrode insertion and current delivery. As the effects of DBS often tend to be more robust than those of electrode insertion alone (Hamani et al. 2012b; Hamani and Nobrega 2012; Hammels et al. 2015), we opted to only include DBS-treated animals and not a separate sham implanted group during our pharmacological experiments. Previous studies have shown that the administration of anti-inflammatory drugs, but not analgesics mitigated the antidepressant-like effects of DBS in rodents (Perez-Caballero et al. 2014, 2018). This was particularly evident when the interval between surgery and testing was relatively short (Perez-Caballero et al. 2014). Though evidence for a focal inflammatory effect of electrode insertion is compelling, it is possible that additional mechanisms may play a role. For example, the behavioural effects of DBS have been mitigated by blocking serotonergic transmission (Hamani et al. 2010b; Perez-Caballero et al. 2014). In our current study, GR-127935 countered antidepressant- and anxiolytic-type responses.
At present, the mechanisms through which DBS exerts its antidepressant effects remain elusive (Dandekar et al. 2018; Hamani and Nobrega 2012; Hamani and Temel 2012). One possibility is the modulation of the serotonergic system (Dandekar et al. 2018; Hamani and Nobrega 2012; Hamani and Temel 2012). vmPFC stimulation increases serotonin levels in several brain regions and induces neuroplasticity of raphe nuclei and serotonin receptors (Bregman et al. 2018; Hamani et al. 2010b; Lim et al. 2015b; Srejic et al. 2015; Veerakumar et al. 2014; Volle et al. 2018). In addition, the antidepressant-like effects of DBS were not observed in rats bearing serotonin-depleting raphe lesions (Hamani et al. 2010b). We have recently measured 5-HT1A and 5-HT1B receptor binding in several areas receiving serotonergic projections, as well as their mRNA expression in the raphe following acute and chronic treatment with vmPFC DBS or fluoxetine (Volle et al. 2018). In general, chronic DBS increased 5-HT1B receptor binding in the dorsal raphe, prelimbic cortex, substantia nigra, and lateral globus pallidum, but did not alter the binding of the 5-HT1A receptor in any region (Volle et al. 2018). In contrast, chronic fluoxetine administration decreased 5-HT1A binding in the PFC and hippocampus but did not affect 5-HT1B binding (Volle et al. 2018). In the current study, we investigated whether chronic vmPFC DBS altered 5-HT1A or 5-HT1B protein expression in the vmPFC, hippocampus, nucleus accumbens, and raphe. As some of the structures showing significant binding in our previous report were too small to be dissected, they were not analysed in the current study. While no significant differences were found when DBS, sham stimulation, or non-implanted Stress controls were compared, DBS-treated mice had a non-significant increase in 5-HT1B expression in all investigated structures, but the raphe.
5-HT1B receptors can be categorized as autoreceptors or heteroreceptors (Sari 2004). In general, 5-HT1B autoreceptors inhibit the release of 5-HT into the synapse, whereas heteroreceptors modulate the transmission of glutamate, GABA, ACh, and DA (Maura and Raiteri 1986; Moore et al. 2000; Sari 2004; Tiger et al. 2018). In the raphe, 5-HT1B receptors are expressed in interneurons and principal cells, including collateral axonal projections (Bagdy et al. 2000; Davidson and Stamford 1995; Tao et al. 1996). The activation of raphe 5-HT1B autoreceptors reduces neuronal firing and serotonin release (Lim et al. 2015b; Srejic et al. 2015). From a behavioural perspective, mice with selective knockdown of raphe 5-HT1B autoreceptors present reduced depressive-like behaviours, while the overexpression of raphe 5-HT1B receptors is anxiogenic (Anthony et al. 2000; Nautiyal et al. 2016; Neumaier et al. 1996). In our study, vmPFC DBS induced antidepressant- and anxiolytic-like effects, while non-significantly reducing raphe 5-HT1B protein expression. Though these findings may help to explain the reduced firing of raphe cells following DBS, they are difficult to reconcile with the increase in serotonin release observed after vmPFC stimulation (Volle et al. 2018). Also difficult to reconcile are the disparities observed in the current study and the increased 5-HT1B binding observed in DBS-treated rats from our previous report. Potential explanations for these discrepancies include the use of different species, techniques to detect 5-HT1B expression, and the use of naïve versus stressed animals. Future studies need to be conducted to clarify the neurochemical effects of vmPFC stimulation in raphe serotonin receptors.
In contrast to the raphe, our current and previous findings suggest that 5-HT1B expression increased, albeit non-significantly, in regions expressing heteroreceptors (e.g. PFC). 5-HT1B heteroreceptors have been associated with the antidepressant-like effect of SSRIs in the FST (Chenu et al. 2008; Medrihan et al. 2017). Moreover, the pharmacological activation of these receptors induces antidepressant- and anxiolytic-like effects in different rodent models (Tatarczynska et al. 2004). To examine whether 5-HT1B and 5-HT1A receptors are involved in the antidepressant-like effects of vmPFC stimulation, we conducted pharmacological experiments antagonizing these receptors in animals receiving DBS. Based on our current and previous work, we predicted that blocking 5-HT1B but not 5-HT1A receptors would mitigate behavioural DBS responses. Confirming our hypothesis, the antidepressant- and anxiolytic-like effects of DBS were countered in animals given the 5-HT1B antagonist GR-127935, but not in those given the 5-HT1A antagonist WAY-100635. These effects could not be attributed to the simple administration of the drugs, as no differences were found between animals given GR-127935 or WAY-100635 alone and vehicle-treated controls.
In our study, 5-HT1B and 5-HT1A antagonists were administered 30 min prior to stimulation for the last 4 days of the study (i.e. 3.5 h before behavioural testing began). This timing was chosen to ensure that the drugs would peak during the onset of DBS. The dose of WAY was selected based on previous work showing that it could mitigate the neurochemical effects of several antidepressants, including SSRI-induced improvements in the FST (Castro et al. 2008; Cryan et al. 2005b; Kaster et al. 2005; O’Neill and Conway 2001; Takahashi et al. 2020; Zanelati et al. 2010). Moreover, at this dose range, WAY was observed to counter gepirone-mediated changes in aggressive behaviour (Lopez-Mendoza et al. 1998; Rogoz et al. 2012; Tatarczynska et al. 2002). Finally, 2.5 mg/kg of WAY administered to residents receiving DBS in our paradigm successfully decreased the anti-aggressive-like effects of this therapy (data not shown). Considering that vmPFC did not change 5-HT1A receptor binding or protein levels in our studies (Volle et al. 2018), it was not surprising that WAY administration did not block the anxiolytic- and antidepressant-like effects of DBS in the CSDS model. Despite not mitigating the antidepressant- or anxiolytic-like effects of vmPFC stimulation, we note that it is still possible that 5-HT1A might play a role in the behavioural effects of DBS. Different and sometimes antagonistic effects have been recorded with the focal administration of drugs that modulate 5-HT1A autoreceptors and heteroreceptors in different parts of the brain (Bambico et al. 2018; Blier et al. 1998; Gardier et al. 1996; Popova and Naumenko 2013). Future work taking these variables into account is still required.
To determine whether 5-HT1B receptors were involved in the DBS response, we administered GR-127935 at a 5.0-mg/kg dose 30 min prior to DBS onset. Corroborating our initial hypothesis, antagonism of the 5-HT1B receptor countered the effects of DBS in the NSF and FST. Previous studies suggest that this drug blocks 5-HT1B receptors at a wide dose range (0.056–10 mg/kg) (de Almeida et al. 2001; Hogg and Dalvi 2004; Mayorga et al. 2001). Though GR-127935 has primarily been studied in the context of aggressive behaviour (Bannai et al. 2007), it has also been shown to block the effects of antidepressants. At 10 mg/kg, GR-127935 diminished immobility induced by the agonist RU 24,969, imipramine, and paroxetine in the tail suspension test (O’Neill et al. 1996). Likewise, 4 mg/kg of GR-127935 reduced the antidepressant-like effects of paroxetine and citalopram in the FST (Chenu et al. 2008). Though GR-127935 was found to block the effects of some antidepressant treatments, the administration of this drug even at high doses (e.g. 10 mg/kg in mice and 20 mg/kg in rats) was not found to induce depressive-like behaviour (O’Neill and Conway 2001; Tatarczynska et al. 2002). In a pilot study, we have injected animals with 10 mg/kg and found this dose to be toxic, with the animals presenting a substantial decrease in locomotion, grooming, and shivering. Therefore, we have decided to lower the dose to 5 mg/kg, a threshold recommended in this compound’s safety sheet (Tocris Safety Data Sheet).
Conclusion
Our study shows that chronic vmPFC stimulation induces antidepressant- and anxiolytic-like responses in a modified CSDS paradigm. More importantly, we demonstrate that the behavioural effects of DBS were mitigated in animals given the 5-HT1B antagonist GR-127935. Future studies are still necessary to further dissect the molecular and neurochemical mechanisms through which DBS interacts with 5-HT1B receptors and modulates the serotonergic system.
References
Aggleton JP, Nelson AJD (2020) Distributed interactive brain circuits for object-in-place memory: a place for time? Brain Neurosci Adv 4:2398212820933471. https://doi.org/10.1177/2398212820933471
Anthony JP, Sexton TJ, Neumaier JF (2000) Antidepressant-induced regulation of 5-HT(1b) mRNA in rat dorsal raphe nucleus reverses rapidly after drug discontinuation. J Neurosci Res 61:82–87. https://doi.org/10.1002/1097-4547(20000701)61:1<82::AID-JNR10>3.0.CO;2-E
Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J (2019) Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci Biobehav Rev 99:101–116. https://doi.org/10.1016/j.neubiorev.2018.12.002
Awan NR, Lozano A, Hamani C (2009) Deep brain stimulation: current and future perspectives. Neurosurg Focus 27:E2. https://doi.org/10.3171/2009.4.FOCUS0982
Bagdy E, Kiraly I, Harsing LG Jr (2000) Reciprocal innervation between serotonergic and GABAergic neurons in raphe nuclei of the rat. Neurochem Res 25:1465–1473. https://doi.org/10.1023/a:1007672008297
Bambico FR, Bregman T, Diwan M, Li J, Darvish-Ghane S, Li Z, Laver B, Amorim BO, Covolan L, Nobrega JN, Hamani C (2015) Neuroplasticity-dependent and -independent mechanisms of chronic deep brain stimulation in stressed rats. Transl Psychiatry 5:e674. https://doi.org/10.1038/tp.2015.166
Bambico FR, Comai S, Diwan M, Hasan SMN, Conway JD, Darvish-Ghane S, Hamani C, Gobbi G, Nobrega JN (2018) High frequency stimulation of the anterior vermis modulates behavioural response to chronic stress: involvement of the prefrontal cortex and dorsal raphe? Neurobiol Dis 116:166–178. https://doi.org/10.1016/j.nbd.2018.03.011
Bannai M, Fish EW, Faccidomo S, Miczek KA (2007) Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology 193:295–304. https://doi.org/10.1007/s00213-007-0780-5
Bartolomucci A, Palanza P, Gaspani L, Limiroli E, Panerai AE, Ceresini G, Poli MD, Parmigiani S (2001) Social status in mice: behavioral, endocrine and immune changes are context dependent. Physiol Behav 73:401–410. https://doi.org/10.1016/s0031-9384(01)00453-x
Bergfeld IO, Mantione M, Hoogendoorn ML, Ruhe HG, Notten P, van Laarhoven J, Visser I, Figee M, de Kwaasteniet BP, Horst F, Schene AH, van den Munckhof P, Beute G, Schuurman R, Denys D (2016) Deep brain stimulation of the ventral anterior lof the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiat 73:456–464. https://doi.org/10.1001/jamapsychiatry.2016.0152
Berton O, Durand M, Aguerre S, Mormede P, Chaouloff F (1999) Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience 92:327–341. https://doi.org/10.1016/s0306-4522(98)00742-8
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868. https://doi.org/10.1126/science.1120972
Blier P, Pineyro G, el Mansari M, Bergeron R, de Montigny C (1998) Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann N Y Acad Sci 861:204–216. https://doi.org/10.1111/j.1749-6632.1998.tb10192.x
Bregman T, Nona C, Volle J, Diwan M, Raymond R, Fletcher PJ, Nobrega JN, Hamani C (2018) Deep brain stimulation induces antidepressant-like effects in serotonin transporter knockout mice. Brain Stimul 11:423–425. https://doi.org/10.1016/j.brs.2017.11.008
Brown EC, Clark DL, Forkert ND, Molnar CP, Kiss ZHT, Ramasubbu R (2020) Metabolic activity in subcallosal cingulate predicts response to deep brain stimulation for depression. Neuropsychopharmacology 45:1681–1688. https://doi.org/10.1038/s41386-020-0745-5
Bruchim-Samuel M, Lax E, Gazit T, Friedman A, Ahdoot H, Bairachnaya M, Pinhasov A, Yadid G (2016) Electrical stimulation of the vmPFC serves as a remote control to affect VTA activity and improve depressive-like behavior. Exp Neurol 283:255–263. https://doi.org/10.1016/j.expneurol.2016.05.016
Casquero-Veiga M, Garcia-Garcia D, Desco M, Soto-Montenegro ML (2018) Understanding deep brain stimulation: in vivo metabolic consequences of the electrode insertional effect. Biomed Res Int 2018:8560232. https://doi.org/10.1155/2018/8560232
Castro E, Diaz A, Rodriguez-Gaztelumendi A, Del Olmo E, Pazos A (2008) WAY100635 prevents the changes induced by fluoxetine upon the 5-HT1A receptor functionality. Neuropharmacology 55:1391–1396. https://doi.org/10.1016/j.neuropharm.2008.08.038
Chakravarty MM, Hamani C, Martinez-Canabal A, Ellegood J, Laliberte C, Nobrega JN, Sankar T, Lozano AM, Frankland PW, Lerch JP (2016) Deep brain stimulation of the ventromedial prefrontal cortex causes reorganization of neuronal processes and vasculature. Neuroimage 125:422–427. https://doi.org/10.1016/j.neuroimage.2015.10.049
Chao OY, de Souza Silva MA, Yang YM, Huston JP (2020) The medial prefrontal cortex - hippocampus circuit that integrates information of object, place and time to construct episodic memory in rodents: behavioral, anatomical and neurochemical properties. Neurosci Biobehav Rev 113:373–407. https://doi.org/10.1016/j.neubiorev.2020.04.007
Chenu F, David DJ, Leroux-Nicollet I, Le Maitre E, Gardier AM, Bourin M (2008) Serotonin1B heteroreceptor activation induces an antidepressant-like effect in mice with an alteration of the serotonergic system. J Psychiatry Neurosci 33:541–550
Christoffel DJ, Golden SA, Russo SJ (2011) Structural and synaptic plasticity in stress-related disorders. Rev Neurosci 22:535–549. https://doi.org/10.1515/RNS.2011.044
Coenen VA, Schlaepfer TE, Bewernick B, Kilian H, Kaller CP, Urbach H, Li M, Reisert M (2019) Frontal white matter architecture predicts efficacy of deep brain stimulation in major depression. Transl Psychiatry 9:197. https://doi.org/10.1038/s41398-019-0540-4
Coenen VA, Schlaepfer TE, Sajonz B, Dobrossy M, Kaller CP, Urbach H, Reisert M (2020) Tractographic description of major subcortical projection pathways passing the anterior limb of the internal capsule. Corticopetal organization of networks relevant for psychiatric disorders. Neuroimage Clin 25:102165. https://doi.org/10.1016/j.nicl.2020.102165
Cryan JF, Markou A, Lucki I (2002) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23:238–245
Cryan JF, Page ME, Lucki I (2005a) Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology 182:335–344. https://doi.org/10.1007/s00213-005-0093-5
Cryan JF, Valentino RJ, Lucki I (2005b) Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29:547–569. https://doi.org/10.1016/j.neubiorev.2005.03.008
Dallman MF, Akana SF, Bhatnagar S, Bell ME, Strack AM (2000) Bottomed out: metabolic significance of the circadian trough in glucocorticoid concentrations. Int J Obes Relat Metab Disord 24(Suppl 2):S40–S46. https://doi.org/10.1038/sj.ijo.0801276
Dandekar MP, Fenoy AJ, Carvalho AF, Soares JC, Quevedo J (2018) Deep brain stimulation for treatment-resistant depression: an integrative review of preclinical and clinical findings and translational implications. Mol Psychiatry 23:1094–1112. https://doi.org/10.1038/mp.2018.2
Dandekar MP, Saxena A, Scaini G, Shin JH, Migut A, Giridharan VV, Zhou Y, Barichello T, Soares JC, Quevedo J, Fenoy AJ (2019) Medial forebrain bundle deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: importance of BDNF and inflammatory cytokines. Mol Neurobiol 56:4364–4380. https://doi.org/10.1007/s12035-018-1381-5
Davidson C, Stamford JA (1995) Evidence that 5-hydroxytryptamine release in rat dorsal raphe nucleus is controlled by 5-HT1A, 5-HT1B and 5-HT1D autoreceptors. Br J Pharmacol 114:1107–1109. https://doi.org/10.1111/j.1476-5381.1995.tb13321.x
Davidson B, Suresh H, Goubran M, Rabin JS, Meng Y, Mithani K, Pople CB, Giacobbe P, Hamani C, Lipsman N (2020) Predicting response to psychiatric surgery: a systematic review of neuroimaging findings. J Psychiatry Neurosci 45:387–394. https://doi.org/10.1503/jpn.190208
de Almeida RM, Nikulina EM, Faccidomo S, Fish EW, Miczek KA (2001) Zolmitriptan–a 5-HT1B/D agonist, alcohol, and aggression in mice. Psychopharmacology 157:131–141. https://doi.org/10.1007/s002130100778
Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 121:66–72. https://doi.org/10.1007/BF02245592
Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O’Reardon JP, Eskandar EN, Baltuch GH, Machado AD, Kondziolka D, Cusin C, Evans KC, Price LH, Jacobs K, Pandya M, Denko T, Tyrka AR, Brelje T, Deckersbach T, Kubu C, Malone DA Jr (2015) A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry 78:240–248. https://doi.org/10.1016/j.biopsych.2014.11.023
Edemann-Callesen H, Voget M, Empl L, Vogel M, Wieske F, Rummel J, Heinz A, Mathe AA, Hadar R, Winter C (2015) Medial forebrain bundle deep brain stimulation has symptom-specific anti-depressant effects in rats and as opposed to ventromedial prefrontal cortex stimulation interacts with the reward system. Brain Stimul 8:714–723. https://doi.org/10.1016/j.brs.2015.02.009
Fenoy AJ, Schulz PE, Selvaraj S, Burrows CL, Zunta-Soares G, Durkin K, Zanotti-Fregonara P, Quevedo J, Soares JC (2018) A longitudinal study on deep brain stimulation of the medial forebrain bundle for treatment-resistant depression. Transl Psychiatry 8:111. https://doi.org/10.1038/s41398-018-0160-4
Frank AC, Scangos KW, Larson PS, Norbu T, Lee AT, Lee AM (2021) Identification of a personalized intracranial biomarker of depression and response to DBS therapy. Brain Stimul 14:1002–1004. https://doi.org/10.1016/j.brs.2021.06.009
Furlanetti LL, Coenen VA, Aranda IA, Dobrossy MD (2015) Chronic deep brain stimulation of the medial forebrain bundle reverses depressive-like behavior in a hemiparkinsonian rodent model. Exp Brain Res 233:3073–3085. https://doi.org/10.1007/s00221-015-4375-9
Gabbott PL, Warner TA, Jays PR, Bacon SJ (2003) Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res 993:59–71
Gardier AM, Malagie I, Trillat AC, Jacquot C, Artigas F (1996) Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: recent findings from in vivo microdialysis studies. Fundam Clin Pharmacol 10:16–27. https://doi.org/10.1111/j.1472-8206.1996.tb00145.x
Gersner R, Toth E, Isserles M, Zangen A (2010) Site-specific antidepressant effects of repeated subconvulsive electrical stimulation: potential role of brain-derived neurotrophic factor. Biol Psychiatry 67:125–132. https://doi.org/10.1016/j.biopsych.2009.09.015
Gidyk DC, Diwan M, Gouveia FV, Giacobbe P, Lipsman N, Hamani C (2021) Investigating the role of CB1 endocannabinoid transmission in the anti-fear and anxiolytic-like effects of ventromedial prefrontal cortex deep brain stimulation. J Psychiatr Res 135:264–269. https://doi.org/10.1016/j.jpsychires.2021.01.029
Golden SA, Covington HE 3rd, Berton O, Russo SJ (2011) A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6:1183–1191. https://doi.org/10.1038/nprot.2011.361
Gomez-Lazaro E, Arregi A, Beitia G, Vegas O, Azpiroz A, Garmendia L (2011) Individual differences in chronically defeated male mice: behavioral, endocrine, immune, and neurotrophic changes as markers of vulnerability to the effects of stress. Stress 14:537–548. https://doi.org/10.3109/10253890.2011.562939
Hamani C, Nobrega JN (2012) Preclinical studies modeling deep brain stimulation for depression. Biol Psychiatry 72:916–923. https://doi.org/10.1016/j.biopsych.2012.05.024
Hamani C, Schwalb JM, Rezai AR, Dostrovsky JO, Davis KD, Lozano AM (2006) Deep brain stimulation for chronic neuropathic pain: long-term outcome and the incidence of insertional effect. Pain 125:188–196
Hamani C, Diwan M, Isabella S, Lozano AM, Nobrega JN (2010a) Effects of different stimulation parameters on the antidepressant-like response of medial prefrontal cortex deep brain stimulation in rats. J Psychiatr Res 44:683–687. https://doi.org/10.1016/j.jpsychires.2009.12.010
Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, Raymond R, Lozano AM, Fletcher PJ, Nobrega JN (2010b) Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry 67:117–124. https://doi.org/10.1016/j.biopsych.2009.08.025
Hamani C, Nobrega JN, Lozano AM (2010c) Deep brain stimulation in clinical practice and in animal models. Clin Pharmacol Ther 88:559–562. https://doi.org/10.1038/clpt.2010.133
Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM (2011) The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry 69:301–308. https://doi.org/10.1016/j.biopsych.2010.09.034
Hamani C, Giacobbe P, Diwan M, Balbino ES, Tong J, Bridgman A, Lipsman N, Lozano AM, Kennedy SH, Nobrega JN (2012a) Monoamine oxidase inhibitors potentiate the effects of deep brain stimulation. Am J Psychiatry 169:1320–1321. https://doi.org/10.1176/appi.ajp.2012.12060754
Hamani C, Machado DC, Hipolide DC, Dubiela FP, Suchecki D, Macedo CE, Tescarollo F, Martins U, Covolan L, Nobrega JN (2012b) Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiatry 71:30–35. https://doi.org/10.1016/j.biopsych.2011.08.025
Hamani C, Amorim BO, Wheeler AL, Diwan M, Driesslein K, Covolan L, Butson CR, Nobrega JN (2014) Deep brain stimulation in rats: different targets induce similar antidepressant-like effects but influence different circuits. Neurobiol Dis 71:205–214. https://doi.org/10.1016/j.nbd.2014.08.007
Hamani C, Fonoff ET, Parravano DC, Silva VA, Galhardoni R, Monaco B, Navarro J, Yeng LT, Teixeira MJ, Ciampi de Andrade D (2021) Motor cortex stimulation for chronic neuropathic pain: results of a double-blind randomized study. Brain. https://doi.org/10.1093/brain/awab189
Hamani C, Temel Y (2012) Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med 4:142rv8. https://doi.org/10.1126/scitranslmed.3003722
Hammels C, Pishva E, De Vry J, van den Hove DL, Prickaerts J, van Winkel R, Selten JP, Lesch KP, Daskalakis NP, Steinbusch HW, van Os J, Kenis G, Rutten BP (2015) Defeat stress in rodents: from behavior to molecules. Neurosci Biobehav Rev 59:111–140. https://doi.org/10.1016/j.neubiorev.2015.10.006
Hogg S, Dalvi A (2004) Acceleration of onset of action in schedule-induced polydipsia: combinations of SSRI and 5-HT1A and 5-HT1B receptor antagonists. Pharmacol Biochem Behav 77:69–75. https://doi.org/10.1016/j.pbb.2003.09.020
Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Wint D, Craighead MC, Kozarsky J, Chismar R, Moreines JL, Mewes K, Posse PR, Gutman DA, Mayberg HS (2012) Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry 69:150–158. https://doi.org/10.1001/archgenpsychiatry.2011.1456
Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S, Slavin KV, Berman J, McKhann GM, Patil PG, Rittberg BR, Abosch A, Pandurangi AK, Holloway KL, Lam RW, Honey CR, Neimat JS, Henderson JM, DeBattista C, Rothschild AJ, Pilitsis JG, Espinoza RT, Petrides G, Mogilner AY, Matthews K, Peichel D, Gross RE, Hamani C, Lozano AM, Mayberg HS (2017) Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry 4:839–849. https://doi.org/10.1016/S2215-0366(17)30371-1
Iniguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, Cruz B, Warren BL (2014) Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress 17:247–255. https://doi.org/10.3109/10253890.2014.910650
Jimenez-Sanchez L, Castane A, Perez-Caballero L, Grifoll-Escoda M, Lopez-Gil X, Campa L, Galofre M, Berrocoso E, Adell A (2016a) Activation of AMPA receptors mediates the antidepressant action of deep brain stimulation of the infralimbic prefrontal cortex. Cereb Cortex 26:2778–2789. https://doi.org/10.1093/cercor/bhv133
Jimenez-Sanchez L, Linge R, Campa L, Valdizan EM, Pazos A, Diaz A, Adell A (2016b) Behavioral, neurochemical and molecular changes after acute deep brain stimulation of the infralimbic prefrontal cortex. Neuropharmacology 108:91–102. https://doi.org/10.1016/j.neuropharm.2016.04.020
Kaster MP, Santos AR, Rodrigues AL (2005) Involvement of 5-HT1A receptors in the antidepressant-like effect of adenosine in the mouse forced swimming test. Brain Res Bull 67:53–61. https://doi.org/10.1016/j.brainresbull.2005.05.025
Krishnan V, Nestler EJ (2011) Animal models of depression: molecular perspectives. Curr Top Behav Neurosci 7:121–147. https://doi.org/10.1007/7854_2010_108
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131:391–404. https://doi.org/10.1016/j.cell.2007.09.018
Kronfeld-Schor N, Einat H (2012) Circadian rhythms and depression: human psychopathology and animal models. Neuropharmacology 62:101–114. https://doi.org/10.1016/j.neuropharm.2011.08.020
Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA (1991) Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav 38:315–320. https://doi.org/10.1016/0091-3057(91)90284-9
Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ (2010) Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A 107:4436–4441. https://doi.org/10.1073/pnas.0910072107
Lesch KP (1991) 5-HT1A receptor responsivity in anxiety disorders and depression. Prog Neuropsychopharmacol Biol Psychiatry 15:723–733. https://doi.org/10.1016/0278-5846(91)90001-h
Lim SN, Lee ST, Tsai YT, Chen IA, Tu PH, Chen JL, Chang HW, Su YC, Wu T (2007) Electrical stimulation of the anterior nucleus of the thalamus for intractable epilepsy: a long-term follow-up study. Epilepsia 48:342–347. https://doi.org/10.1111/j.1528-1167.2006.00898.x
Lim LW, Janssen ML, Kocabicak E, Temel Y (2015a) The antidepressant effects of ventromedial prefrontal cortex stimulation is associated with neural activation in the medial part of the subthalamic nucleus. Behav Brain Res 279:17–21. https://doi.org/10.1016/j.bbr.2014.11.008
Lim LW, Prickaerts J, Huguet G, Kadar E, Hartung H, Sharp T, Temel Y (2015b) Electrical stimulation alleviates depressive-like behaviors of rats: investigation of brain targets and potential mechanisms. Transl Psychiatry 5:e535. https://doi.org/10.1038/tp.2015.24
Lopez-Mendoza D, Aguilar-Bravo H, Swanson HH (1998) Combined effects of Gepirone and (+)WAY 100135 on territorial aggression in mice. Pharmacol Biochem Behav 61:1–8. https://doi.org/10.1016/s0091-3057(97)00563-7
Lozano AM, Giacobbe P, Hamani C, Rizvi SJ, Kennedy SH, Kolivakis TT, Debonnel G, Sadikot AF, Lam RW, Howard AK, Ilcewicz-Klimek M, Honey CR, Mayberg HS (2012) A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg 116:315–322. https://doi.org/10.3171/2011.10.JNS102122
Lucki I (1996) Serotonin receptor specificity in anxiety disorders. J Clin Psychiatry 57(Suppl 6):5–10
Lucki I, Singh A, Kreiss DS (1994) Antidepressant-like behavioral effects of serotonin receptor agonists. Neurosci Biobehav Rev 18:85–95. https://doi.org/10.1016/0149-7634(94)90039-6
Lueptow LM (2017) Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp. https://doi.org/10.3791/55718
Malone DA Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, Tyrka AR, Price LH, Stypulkowski PH, Giftakis JE, Rise MT, Malloy PF, Salloway SP, Greenberg BD (2009) Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry 65:267–275. https://doi.org/10.1016/j.biopsych.2008.08.029
Maluach AM, Misquitta KA, Prevot TD, Fee C, Sibille E, Banasr M, Andreazza AC (2017) Increased neuronal DNA/RNA oxidation in the frontal cortex of mice subjected to unpredictable chronic mild stress. Chronic Stress (Thousand Oaks) 1.https://doi.org/10.1177/2470547017724744
Maura G, Raiteri M (1986) Cholinergic terminals in rat hippocampus possess 5-HT1B receptors mediating inhibition of acetylcholine release. Eur J Pharmacol 129:333–337. https://doi.org/10.1016/0014-2999(86)90443-7
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45:651–660. https://doi.org/10.1016/j.neuron.2005.02.014
Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I (2001) Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J Pharmacol Exp Ther 298:1101–1107
Medrihan L, Sagi Y, Inde Z, Krupa O, Daniels C, Peyrache A, Greengard P (2017) Initiation of behavioral response to antidepressants by cholecystokinin neurons of the dentate gyrus. Neuron 95(564–576):e4. https://doi.org/10.1016/j.neuron.2017.06.044
Moore P, Landolt HP, Seifritz E, Clark C, Bhatti T, Kelsoe J, Rapaport M, Gillin JC (2000) Clinical and physiological consequences of rapid tryptophan depletion. Neuropsychopharmacology 23:601–622. https://doi.org/10.1016/S0893-133X(00)00161-5
Moshe H, Gal R, Barnea-Ygael N, Gulevsky T, Alyagon U, Zangen A (2016) Prelimbic stimulation ameliorates depressive-like behaviors and increases regional BDNF expression in a novel drug-resistant animal model of depression. Brain Stimul 9:243–250. https://doi.org/10.1016/j.brs.2015.10.009
Muscat R, Towell A, Willner P (1988) Changes in dopamine autoreceptor sensitivity in an animal model of depression. Psychopharmacology 94:545–550. https://doi.org/10.1007/BF00212853
Nautiyal KM, Tritschler L, Ahmari SE, David DJ, Gardier AM, Hen R (2016) A lack of serotonin 1B autoreceptors results in decreased anxiety and depression-related behaviors. Neuropsychopharmacology 41:2941–2950. https://doi.org/10.1038/npp.2016.109
Neumaier JF, Root DC, Hamblin MW (1996) Chronic fluoxetine reduces serotonin transporter mRNA and 5-HT1B mRNA in a sequential manner in the rat dorsal raphe nucleus. Neuropsychopharmacology 15:515–522. https://doi.org/10.1016/S0893-133X(96)00095-4
O’Neill MF, Conway MW (2001) Role of 5-HT(1A) and 5-HT(1B) receptors in the mediation of behavior in the forced swim test in mice. Neuropsychopharmacology 24:391–398. https://doi.org/10.1016/S0893-133X(00)00196-2
O’Neill MF, Fernandez AG, Palacios JM (1996) GR 127935 blocks the locomotor and antidepressant-like effects of RU 24969 and the action of antidepressants in the mouse tail suspension test. Pharmacol Biochem Behav 53:535–539. https://doi.org/10.1016/0091-3057(95)02047-0
Papp M, Gruca P, Lason M, Tota-Glowczyk K, Niemczyk M, Litwa E, Willner P (2018) Rapid antidepressant effects of deep brain stimulation of the pre-frontal cortex in an animal model of treatment-resistant depression. J Psychopharmacol 32:1133–1140. https://doi.org/10.1177/0269881118791737
Papp M, Gruca P, Lason M, Niemczyk M, Willner P (2019) The role of prefrontal cortex dopamine D2 and D3 receptors in the mechanism of action of venlafaxine and deep brain stimulation in animal models of treatment-responsive and treatment-resistant depression. J Psychopharmacol 33:748–756. https://doi.org/10.1177/0269881119827889
Paxinos G, Franklin KBJ (2012) Paxinos and Franklin’s the mouse brain in stereotaxic coordinates, 4th edn. Academic Press
Perez-Caballero L, Perez-Egea R, Romero-Grimaldi C, Puigdemont D, Molet J, Caso JR, Mico JA, Perez V, Leza JC, Berrocoso E (2014) Early responses to deep brain stimulation in depression are modulated by anti-inflammatory drugs. Mol Psychiatry 19:607–614. https://doi.org/10.1038/mp.2013.63
Perez-Caballero L, Soto-Montenegro ML, Hidalgo-Figueroa M, Mico JA, Desco M, Berrocoso E (2018) Deep brain stimulation electrode insertion and depression: patterns of activity and modulation by analgesics. Brain Stimul 11:1348–1355. https://doi.org/10.1016/j.brs.2018.06.010
Popova NK, Naumenko VS (2013) 5-HT1A receptor as a key player in the brain 5-HT system. Rev Neurosci 24:191–204. https://doi.org/10.1515/revneuro-2012-0082
Prevot TD, Misquitta KA, Fee C, Newton DF, Chatterjee D, Nikolova YS, Sibille E, Banasr M (2019) Residual avoidance: a new, consistent and repeatable readout of chronic stress-induced conflict anxiety reversible by antidepressant treatment. Neuropharmacology 153:98–110. https://doi.org/10.1016/j.neuropharm.2019.05.005
Puigdemont D, Portella M, Perez-Egea R, Molet J, Gironell A, de Diego-Adelino J, Martin A, Rodriguez R, Alvarez E, Artigas F, Perez V (2015) A randomized double-blind crossover trial of deep brain stimulation of the subcallosal cingulate gyrus in patients with treatment-resistant depression: a pilot study of relapse prevention. J Psychiatry Neurosci 40:224–231. https://doi.org/10.1503/jpn.130295
Raab A, Dantzer R, Michaud B, Mormede P, Taghzouti K, Simon H, Le Moal M (1986) Behavioural, physiological and immunological consequences of social status and aggression in chronically coexisting resident-intruder dyads of male rats. Physiol Behav 36:223–228. https://doi.org/10.1016/0031-9384(86)90007-7
Reznikov R, Binko M, Nobrega JN, Hamani C (2016) Deep brain stimulation in animal models of fear, anxiety, and posttraumatic stress disorder. Neuropsychopharmacology 41:2810–2817. https://doi.org/10.1038/npp.2016.34
Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, Crowell AL, Garlow SJ, Rajendra JK, Mayberg HS (2014) Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry 76:963–969. https://doi.org/10.1016/j.biopsych.2014.03.029
Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, McIntyre CC, Gross RE, Mayberg HS (2018) A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry 23:843–849. https://doi.org/10.1038/mp.2017.59
Rogoz Z, Kabzinski M, Sadaj W, Rachwalska P, Gadek-Michalska A (2012) Effect of co-treatment with fluoxetine or mirtazapine and risperidone on the active behaviors and plasma corticosterone concentration in rats subjected to the forced swim test. Pharmacol Rep 64:1391–1399. https://doi.org/10.1016/s1734-1140(12)70936-2
Rummel J, Voget M, Hadar R, Ewing S, Sohr R, Klein J, Sartorius A, Heinz A, Mathe AA, Vollmayr B, Winter C (2016) Testing different paradigms to optimize antidepressant deep brain stimulation in different rat models of depression. J Psychiatr Res 81:36–45. https://doi.org/10.1016/j.jpsychires.2016.06.016
Sankar T, Chakravarty MM, Jawa N, Li SX, Giacobbe P, Kennedy SH, Rizvi SJ, Mayberg HS, Hamani C, Lozano AM (2020) Neuroanatomical predictors of response to subcallosal cingulate deep brain stimulation for treatment-resistant depression. J Psychiatry Neurosci 45:45–54. https://doi.org/10.1503/jpn.180207
Sari Y (2004) Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev 28:565–582. https://doi.org/10.1016/j.neubiorev.2004.08.008
Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V (2008) Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33:368–377. https://doi.org/10.1038/sj.npp.1301408
Schlaepfer TE, Bewernick BH, Kayser S, Madler B, Coenen VA (2013) Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry 73:1204–1212. https://doi.org/10.1016/j.biopsych.2013.01.034
Srejic LR, Hamani C, Hutchison WD (2015) High-frequency stimulation of the medial prefrontal cortex decreases cellular firing in the dorsal raphe. Eur J Neurosci 41:1219–1226. https://doi.org/10.1111/ejn.12856
Takagishi M, Chiba T (1991) Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res 566:26–39
Takahashi K, Kitamura Y, Ushio S, Sendo T (2020) Immobility-reducing effects of ketamine during the forced swim test on 5-HT1A receptor activity in the medial prefrontal cortex in an intractable depression model. Acta Med Okayama 74:301–306. https://doi.org/10.18926/AMO/60368
Tao R, Ma Z, Auerbach SB (1996) Differential regulation of 5-hydroxytryptamine release by GABAA and GABAB receptors in midbrain raphe nuclei and forebrain of rats. Br J Pharmacol 119:1375–1384. https://doi.org/10.1111/j.1476-5381.1996.tb16049.x
Tasker RR (1998) Deep brain stimulation is preferable to thalamotomy for tremor suppression. Surg Neurol 49: 145–53; discussion 153–4.
Tatarczynska E, Klodzinska A, Chojnacka-Wojcik E (2002) Effects of combined administration of 5-HT1A and/or 5-HT1B receptor antagonists and paroxetine or fluoxetine in the forced swimming test in rats. Pol J Pharmacol 54:615–623
Tatarczynska E, Klodzinska A, Stachowicz K, Chojnacka-Wojcik E (2004) Effects of a selective 5-HT1B receptor agonist and antagonists in animal models of anxiety and depression. Behav Pharmacol 15:523–534. https://doi.org/10.1097/00008877-200412000-00001
Thiele S, Furlanetti L, Pfeiffer LM, Coenen VA, Dobrossy MD (2018) The effects of bilateral, continuous, and chronic Deep Brain Stimulation of the medial forebrain bundle in a rodent model of depression. Exp Neurol 303:153–161. https://doi.org/10.1016/j.expneurol.2018.02.002
Tiger M, Varnas K, Okubo Y, Lundberg J (2018) The 5-HT1B receptor - a potential target for antidepressant treatment. Psychopharmacology 235:1317–1334. https://doi.org/10.1007/s00213-018-4872-1
Torres-Sanchez S, Perez-Caballero L, Berrocoso E (2017) Cellular and molecular mechanisms triggered by Deep Brain Stimulation in depression: a preclinical and clinical approach. Prog Neuropsychopharmacol Biol Psychiatry 73:1–10. https://doi.org/10.1016/j.pnpbp.2016.09.005
Torres-Sanchez S, Perez-Caballero L, Mico JA, Celada P, Berrocoso E (2018) Effect of Deep Brain Stimulation of the ventromedial prefrontal cortex on the noradrenergic system in rats. Brain Stimul 11:222–230. https://doi.org/10.1016/j.brs.2017.10.003
Veerakumar A, Challis C, Gupta P, Da J, Upadhyay A, Beck SG, Berton O (2014) Antidepressant-like effects of cortical deep brain stimulation coincide with pro-neuroplastic adaptations of serotonin systems. Biol Psychiatry 76:203–212. https://doi.org/10.1016/j.biopsych.2013.12.009
Volle J, Bregman T, Scott B, Diwan M, Raymond R, Fletcher PJ, Nobrega JN, Hamani C (2018) Deep brain stimulation and fluoxetine exert different long-term changes in the serotonergic system. Neuropharmacology 135:63–72. https://doi.org/10.1016/j.neuropharm.2018.03.005
Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolanos-Guzman CA (2013) Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry 73:7–14. https://doi.org/10.1016/j.biopsych.2012.06.006
Warren BL, Sial OK, Alcantara LF, Greenwood MA, Brewer JS, Rozofsky JP, Parise EM, Bolanos-Guzman CA (2014) Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Dev Neurosci 36:250–260. https://doi.org/10.1159/000362875
Willner P (2017) The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress 6:78–93. https://doi.org/10.1016/j.ynstr.2016.08.002
Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93:358–364. https://doi.org/10.1007/BF00187257
Willner P, Muscat R, Papp M (1992) Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 16:525–534. https://doi.org/10.1016/s0149-7634(05)80194-0
Zanelati TV, Biojone C, Moreira FA, Guimaraes FS, Joca SR (2010) Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br J Pharmacol 159:122–128. https://doi.org/10.1111/j.1476-5381.2009.00521.x
Funding
This work was supported in part with funds from the Harquail Centre for Neuromodulation and Veteran Affairs Canada.
Author information
Authors and Affiliations
Contributions
ES, MD, TR, HK, ACPC, and FVG conducted behavioural and neurochemical experiments. ES and CH analysed the results and wrote the manuscript. PG and NL critically revised the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Suppl. Fig 1.
Representative blots showing 5-HT1A and 5-HT1B expression in the ventromedial prefrontal cortex (vmPFC) and hippocampus (HPC) of stress exposed mice receiving deep brain stimulation (DBS), sham stimulation or controls with no implanted electrodes. (PNG 795 kb)

Suppl. Fig 2.
Representative blots showing 5-HT1A and 5-HT1B expression in the raphe and nucleus accumbens (NAcc) of stress exposed mice receiving deep brain stimulation (DBS), sham stimulation or controls with no implanted electrodes. (PNG 742 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silk, E., Diwan, M., Rabelo, T. et al. Serotonin 5-HT1B receptors mediate the antidepressant- and anxiolytic-like effects of ventromedial prefrontal cortex deep brain stimulation in a mouse model of social defeat. Psychopharmacology 239, 3875–3892 (2022). https://doi.org/10.1007/s00213-022-06259-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06259-6