Abstract
Rationale
Basal forebrain cholinergic neurons modulate the activation of cortical neurons by several stimuli such as fear and anxiety. However, the role of the muscarinic receptor in the medial prefrontal cortex (MPFC) in the modulation of the conditioned emotional response (CER) evoked in the model contextual conditioned fear remains unclear.
Objectives
The objective of this study is to test the hypothesis that inhibition of the muscarinic receptor in ventral MPFC modulates CER observed during animal’s re-exposure to the aversive context.
Methods
Rats implanted with cannulae aimed at the prelimbic (PL) or the infralimbic (IL) were submitted to a high-intensity contextual fear conditioning protocol. Before the test session, they received microinjections of the hemicholinium (choline reuptake blocker), atropine (muscarinic antagonist), J104129 fumarate (M1-M3 muscarinic antagonists), pirenzepine (M1 muscarinic antagonist), neostigmine (inhibitor acetylcholinesterase enzyme), or the systemic administration of the FG7142 (inverse benzodiazepine agonist). Additional independent groups received the neostigmine or FG7142 before the ineffective doses of J104129 fumarate in the low-intensity protocol of contextual fear conditioning.
Results
In the high-intensity protocol, the administration of hemicholinium (1 nmol), atropine (0.06–6 nmol), J104129 fumarate (6 nmol), or pirenzepine (6 nmol) attenuated the expression of CER in rats. However, in the low-intensity protocol, only J10129 fumarate (0.06 nmol) reduced the expression of the CER. Finally, neostigmine (0.1–1 nmol) or FG7142 (8 mg/Kg) increased CER expression, an effect inhibited by the low dose of the J10129 fumarate.
Conclusions
These results indicated that the blockade of M3 muscarinic receptor in the vMPFC attenuates the CER expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ventral portion of the medial prefrontal cortex (vMPFC) is composed by the infralimbic (IL) and the prelimbic (PL) cortices. It influences both behavioral and autonomical responses associated with threat situations (Frysztak and Neafsey 1991, 1994; Radley et al. 2006; Resstel et al. 2008a, b; Resstel et al. 2006).
Pavlovian fear conditioning has been intensely used in the study of stress-related disorder such as posttraumatic stress disorder (PTSD) (Di Giacinto et al. 2014; Feng et al. 2014; Kwapis and Wood 2014). The contextual fear conditioning is evoked by re-exposing an animal to an environment (context) previously paired with an aversive or unpleasant stimulus such as an electric footshock (Fanselow 1980; Resstel et al. 2008a). In rats, the re-exposure to the aversive context evokes freezing behavior and autonomic changes, such as increase in both the mean arterial pressure (MAP) and heart rate (HR) and decrease in cutaneous temperature (CT) (Borelli et al. 2013; Fabri et al. 2014). These responses are defined as conditioned emotional responses (CER).
The CER expression is associated with increased vMPFC neuronal activity (Beck and Fibiger 1995). The IL and PL are important for the regulation of cardiovascular and behavioral components evoked by contextual fear conditioning (Lisboa et al. 2010a, b). However, the role of such areas in the modulation of these responses remains unclear, since interference on PL/IL functions have produced contradictory effects that may depend on factors such as the inactivation method and the animal model employed (Vidal-Gonzalez et al. 2006).
The use of the contextual fear conditioning model, which allows the analysis of freezing behavior and autonomic responses at the same time, allows the understanding of both cognitive and the autonomic changes during stress disorders (Resstel and Correa 2005, 2006a, b; Resstel et al. 2009; Gomes et al. 2012). In addition, the basal forebrain cortical cholinergic system seems to play a crucial role between the anxiety state and autonomic regulation (Acquas et al. 1996; Stock et al. 1978; Verberne and Owens 1998). These cholinergic neurons also appear to be involved in attention (Muir et al. 1994; Yu and Dayan 2002), memory (Beninger et al. 1992; DeSousa et al. 1994), and anxiety induction processes (Berntson et al. 1998). Cholinergic afferents to the vMPFC rise primarily from the basal forebrain nucleus basalis (Gaykema et al. 1991a, b). Specific lesions of cholinergic terminations in the vMPFC blocked increases in cardiovascular activity observed during defensive responses (Hart et al. 1999). Moreover, the blockade of muscarinic receptor with scopolamine impaired acquisition of auditory and contextual fear conditioning (Bang and Brown 2009; Pang et al. 2010).
In addition, the administration of a selective antagonist of M1 muscarinic receptor into the vMPFC induced anxiolytic-like effects, suggesting the participation of vMPFC M1 muscarinic receptor in the regulation of anxiety-like behavior (Hsu et al. 1996; Wall and Messier 2002). Corroborating the involvement of the muscarinic receptor in the vMPFC in the neurobiology of anxiety, it has been reported that M3 muscarinic receptor knockout mice show a deficit in contextual fear conditioning (Poulin et al. 2010).
Considering the above mentioned, the present study hypothesizes that vMPFC muscarinic receptors modulate behavioral and autonomic responses associated with contextual fear conditioning expression. Therefore, we examined the effects of bilateral microinjections of hemicholinium (choline reuptake blocker), atropine (muscarinic antagonist), J104129 fumarate (M1-M3 muscarinic receptor antagonist), pirenzepine (M1 muscarinic receptor antagonist), neostigmine (inhibitor acetylcholinesterase enzyme), or the systemic administration of FG7142 (inverse benzodiazepine agonist) into the vMPFC 10 min before re-exposure to the conditioning chamber.
Materials and methods
Ethical approval and animals
Experimental procedures followed protocols approved by the ethical review committee of the School of Medicine of Ribeirão Preto (Protocol number 125/2010), which complies with the guiding principles for research involving animals and human beings of the American Physiological Society. Male Wistar rats weighing 230–270 g were used in the present experiments. Animals were housed in plastic cages in a temperature-controlled room at 25 °C in the Animal Care Unit of the Department of Pharmacology, School of Medicine of Ribeirao Preto, University of São Paulo. They were kept under a 12:00-h light-dark cycle (lights on between 6:00 am and 6:00 pm) and had free access to water and rat chow.
Animal preparation
Seven days before the experiment, rats were anesthetized with tribromoethanol (250 mg/kg, i.p., Sigma, St. Louis, MO, USA). After local anesthesia with 2 % lidocaine, the skull was surgically exposed and stainless steel guide cannulae (26G) were bilaterally implanted in the vMPFC using a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). Stereotaxic coordinates for cannulae implantation in the vMPFC were selected from the rat brain atlas of Paxinos and Watson (1997): AP = +3.3 mm, L = 2.7 mm from the medial suture, and V = −3.2 mm from the skull, with a lateral inclination of 24°. Cannulae were fixed to the skull with dental cement and one metal screw. After the surgery, animals were treated with a polyantibiotic preparation of streptomycins and penicillins (i.m., Pentabiotico®, Fort Dodge, Campinas, São Paulo, Brazil) to prevent infection and with the nonsteroidal anti-inflammatory flunixin meglumine (s.c., Banamine®, Schering Plough, Cotia, São Paulo, Brazil) for postoperative analgesia.
One day before the test day, rats were again anesthetized with tribromoethanol (250 mg/kg, i.p.) and a catheter (a 4-cm segment of PE-10 that was heat-bound to a 13-cm segment of PE-50, Clay Adams, Parsippany, NJ, USA) was inserted into the abdominal aorta through the femoral artery, for blood pressure recording. The catheter was tunneled under the skin and exteriorized on the animal’s dorsum. After the surgery, treatment with polyantibiotic and anti-inflammatory drugs was repeated.
Fear conditioning and testing
Habituation, conditioning, and test sessions were carried out in 25 × 22 × 22-cm footshock chambers (context). Chambers are composed of a grid floor with 18 stainless steel rods (2 mm in diameter), spaced 1.5 cm apart, and wired to a shock generator (Insight, Ribeirão Preto, Brazil). Chambers were cleaned with 70 % ethanol, before and after use. In the habituation session, the animals were exposed to chamber for 10 min and no shock was delivered. In the conditioning shock session, performed 24 h after the habituation session, animals were divided in two experimental groups: nonconditioned and conditioned groups. The nonconditioned group was re-exposed to the footshock chamber for 10 min, but no shock was delivered. The conditioned group was submitted to a shock session consisting of six electric footshocks (1.5 mA/3 s) delivered randomly at 20-s to 1-min intervals (Resstel and Correa 2006b; Hott et al. 2012). The footshocks started 2 min after the animals were placed into the chamber. An additional group was submitted to a different conditioning protocol, less aversive, in which three electric footshocks (0.80 mA/2 s) were delivered randomly at 20-s to 1-min intervals, to obtain less intense conditioned fear responses as described previously (Lisboa et al. 2010a).
Autonomic and behavioral (freezing) responses evoked by context re-exposure were evaluated 2 days after the conditioning session. Animals were transferred from the animal room to the experimental room (a different room was used for conditioning) in their home cage. The test session consisted of a 10-min-long re-exposure to the footshock chamber without shock delivery. MAP and HR were recorded using an HP-7754A amplifier (Hewlett Packard, Palo Alto, CA, USA) connected to a signal acquisition board (Biopac M-100, Biopac Systems, Goleta, CA, USA) and computer-processed. Rats were tested one at a time. After 5 min of baseline recording, injections were performed bilaterally in the vMPFC. Changes in CT were recorded with a thermal camera: Multi-Purpose Thermal Imager IRI 4010 (InfraRed Integrated Systems Ltd Park Circle, Tithe Barn Way Swan Valley Northampton, USA) positioned 50 cm from the tail.
Freezing was evaluated during the test session by an experimenter sitting 30 cm away from the footshock chamber, and it was defined as the complete absence of movement while the animal assumed a characteristic tense posture.
Drugs administration
The following drugs were used: reuptake of choline inhibitor hemicholinium (Sigma, St. Louis, MO, USA), nonselective muscarinic antagonist atropine (Sigma, St. Louis, MO, USA), M1-M3 muscarinic receptor antagonist J104129 fumarate (Tocris, Westwoods Business Park Ellisville, MO, USA), selective M1 muscarinic receptor antagonist pirenzepine (Sigma, St. Louis, MO, USA), and acetylcholinesterase inhibitor neostigmine (Sigma, St. Louis, MO, USA). The inverse benzodiazepine receptor agonist N-methyl-β-carboline-3-carboxamide (FG7142) (Tocris, Westwoods Business Park Ellisville, MO, USA) was suspended in polyoxyethylenesorbitan monooleate (Tween 80, Sigma) 2 % in saline. All other drugs were dissolved in sterile saline (0.9 % NaCl). Solutions were prepared immediately before use and were kept on ice and protected from light during the experimental sessions. The injections into the vMPFC were made manually and over a 20-s period. Volume for central injection was 200 nl on each side.
Experimental protocols
Experiment 1
Involvement of vMPFC cholinergic neurotransmission in the expression of the high-intensity contextual fear conditioning.
Firstly, nonconditioned (n = 6) and conditioned animals (n = 6) received bilateral microinjections of saline or hemicholinium (1 nmol) (Fernandes et al. 2005) into the vMPFC 10 min before the test. Secondly, to investigate the role of muscarinic receptors, independent groups of nonconditioned and conditioned animals received bilateral microinjections of saline (n = 8) or atropine (nonconditioned group, 6 nmol, n = 6; conditioned group, 0.006–6 nmol, n = 5–12) (Resstel et al. 2005) into the vMPFC 10 min before the test. Additionally, it was investigated if the IL or PL atropine (n = 6) administration evoked similar effects when compared to the vehicle group (n = 5). Thirdly, based on obtained results from the previous experiment, we investigated the participation of M1-M3 muscarinic receptors. Therefore, independent groups of nonconditioned (n = 6–7) and conditioned (n = 7–10) received bilateral microinjections of saline, J104129 fumarate (6 nmol), or pirenzepine (6 nmol) into the vMPFC 10 min before the test. Additionally, it was investigated if the administration of either J104129 fumarate or pirenzepine into the IL or PL (6 nmol, n = 4) would evoke similar effects.
After the experiment with muscarinic antagonists described above, a complementary experiment was performed with an independent group of conditioned animals to investigate the participation of vMPFC M3 muscarinic receptors on expression of contextual fear conditioning. Despite pirenzepine’s affinity for M1 muscarinic receptors, this antagonist binds with low affinity to M3 muscarinic receptors. Thus, we investigated whether the effects observed with pirenzepine could be due to a loss of selectivity at 6 nmol. Moreover, considering the inhibition constants (Ki) of each drug for M3 muscarinic receptors, we selected a dose of J104129 fumarate that is equipotent to pirenzepine at 6 nmol (Ki values 4 and 400 nM, respectively, Lee et al. 1994; Mitsuya et al. 1999). Consequently, J104129 fumarate at 0.06 nmol should be able to block the same proportion of M3 muscarinic receptors as pirenzepine at 6 nmol. Moreover, as the affinity of these two drugs for M1 muscarinic receptors is very similar (pirenzepine, Ki 16 nM; J104129 fumarate, Ki 19 nM), the dose of J104129 fumarate (0.06 nmol) would be able to block the same proportion of M1 muscarinic receptors as pirenzepine at 0.06 nmol. To confirm this hypothesis, J104129 fumarate (0.06 nmol, n = 6) and pirenzepine (0.06 nmol, n = 7) were microinjected in the vMPFC of conditioned animals.
Experiment 2
Effects of vMPFC M1-M3 muscarinic receptor antagonism in the expression of the low-intensity contextual fear conditioning.
Finally, using a less aversive conditioning protocol (low-intensity), we evaluated the effects of vMPFC bilateral microinjection of a reversible acetylcholinesterase inhibitor neostigmine (0.1–1 nmol, n = 5–6) (Fernandes et al. 2005) into the vMPFC 10 min before the test. As a positive control for the possible anxiogenic-like effects induced by neostigmine, the inverse benzodiazepine agonist FG7142 (n = 6, 8 mg/kg) (Hart et al. 1999) was administrated systemically 20 min before the test. Additionally, we investigated the participation of vMPFC M3 muscarinic receptors on responses evoked by either neostigmine (0.1 nmol) or FG7142, using the vMPFC bilateral administration of J104129 fumarate (0.06 nmol, n = 6) 5 min before each treatment.
Histological procedures
At the end of the experiments, rats were anesthetized with urethane (1.25 g/kg, i.p.) and 200 nL of 1 % Evan’s Blue dye was bilaterally injected in the vMPFC as injection site marker. The chest was surgically opened, the descending aorta occluded, the right atrium severed, and the brain perfused with 10 % formalin through the left ventricle. Brains were postfixed for 24 h at 4 °C, and 40 μm sections were cut using a cryostat (CM1900, Leica, Wetzlar, Germany). Serial brain sections were stained with 1 % Neutral Red and injection sites according to the rat brain atlas of Paxinos and Watson (1997).
Data analysis
MAP, HR, and CT values were continuously recorded during the 5-min period before and the 10-min period during exposure to the footshock chamber. Data were expressed as means ± SEM of MAP, HR, and CT changes (respectively ΔMAP, ΔHR, and ΔCT) sampled at 60-s intervals. Points sampled during 300 s before exposure were used as control baseline value. MAP, HR, and CT changes were analyzed using three-way ANOVA with group (conditioned or nonconditioned) and treatment (drug or vehicle) as main factors and time as repeated measurement. When interaction between the factors was observed, groups were compared separately using the Bonferroni posttest to compare the variations at a given time. Freezing was expressed as percentage of the test period. Freezing time was analyzed using two-way ANOVA with condition (conditioned or nonconditioned) and treatment (drug or vehicle) as the two factors. When interaction between the factors was observed, specific one-way ANOVA followed by the Bonferroni posttest was performed or the groups were compared separately using the Student’s t test, assuming P < 0.05 as statistically significant. Finally, the graded falls in freezing behavior and autonomic responses evoked by rising doses of the atropine in vMPFC were used to generate dose-response curves.
Results
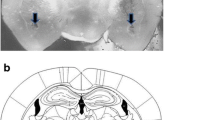
Figure 1 shows a representative photomicrograph of a vMPFC coronal section.
a Recordings of pulsatile arterial pressure (PAP), mean arterial pressure (MAP), and heart rate (HR) showing the cardiovascular changes observed before and during a re-exposure to the aversive context period of 10 min in control conditioned and nonconditioned rats. The onset of re-exposure was at t = 0 min. b Representative coronal brain sections showing bilateral microinjection sites in the vMPFC-IL or vMPFC-PL. Closed circles represent the injection sites into vMPFC and open circles represent the injection sites out of the vMPFC. c Photomicrographs of a representative of the bilateral injection sites into the vMPFC
Experiment 1
vMPFC cholinergic neurotransmission in the expression of the high-intensity contextual fear conditioning.
In the first set of experiments, vehicle-treated conditioned rats (n = 6) spent more time in freezing behavior than did nonconditioned controls (n = 6), during the test (F 1,20 = 48.56, P < 0.001, Fig. 2). Furthermore, conditioned animals presented increased autonomic responses (MAP F 14,280 = 30.97, P < 0.001; HR F 14,280 = 18.78, P < 0.001; and CT F 14,280 = 54.92, P < 0.001), whereas nonconditioned animals presented similar increase but in a smaller extent (MAP F 1,20 = 40.25, P < 0.001; HR F 1,20 = 20.65, P < 0.001; and CT F 1,20 = 29.92, P < 0.001).
a Effects of bilateral injection of 200 nL of vehicle or hemicholinium (1 nmol) into the vMPFC PL or IL, on the percentage of time spent in freezing behavior in nonconditioned (n = 6 each group) and conditioned (n = 6 each group) animals during chamber exposure. Values are represented by means ± SEM. *P < 0.05 compared to the nonconditioned vehicle group and # P < 0.05 compared to the conditioned vehicle group; Bonferroni post hoc test. b Time course of mean arterial pressure (ΔMAP) and heart rate (ΔHR) increases and cutaneous temperature (ΔCT) decreases in nonconditioned and conditioned animals. The symbols represent the mean and bars the SEM. *P < 0.05 compared to the nonconditioned vehicle group; Bonferroni post hoc test
The bilateral injection of hemicholinium into the vMPFC reduced the freezing behavior (F 1,20 = 52.72, P < 0.001). However, the effect of hemicholinium was only observed in conditioned animals (F 3,21 = 77.5, P < 0.001, n = 6, Fig. 2), but not in nonconditioned animals (P > 0.05, n = 6). Notwithstanding, hemicholinium reduced the magnitude of autonomic responses (MAP F 3,20 = 49.3, P < 0.001; HR F 3,20 = 29.1, P < 0.001; and CT F 3,20 = 15.3, P < 0.001) in both groups (Conditioned: MAP F 1,14 = 121.8, P < 0.001; HR F 1,14 = 115.18, P < 0.001; and CT F 1,14 = 158.2, P < 0.001. Nonconditioned: MAP F 1,14 = 56.17, P < 0.001; HR F 1,14 = 138.2, P < 0.001; and CT F 1,14 = 121.7, P > 0.001, Fig. 2).
In conditioned animals, microinjection of hemicholinium into areas surrounding the vMPFC (OUT; n = 6) did not affect the behavioral (P > 0.05) and autonomic responses (MAP P > 0.05; HR P > 0.05; and CT P > 0.05) observed during the test, when compared to vehicle-treated animals (n = 6, data not shown).
In the second set of experiments, the vehicle-treated conditioned group (n = 8) spent more time in freezing behavior than did the nonconditioned group (n = 8), during the test (F 1,14 = 43.14, P < 0.001). Conditioned rats treated with atropine showed a significant reduction in freezing behavior (F 3,29 = 56.8, P < 0.001, Fig. 3) in a dose-dependent manner, showing a significant correlation between atropine doses and attenuation of the freezing behavior (r 2 = 0.80, df = 17, P < 0.01, Fig. 3). This effect of atropine on conditioned animals was observed with the doses of 0.06 nmol (n = 5) and 6 nmol (n = 12), but not with the dose of 0.006 nmol (n = 5). In nonconditioned animals the dose of 6 nmol of atropine (n = 6) had no significant effect on freezing behavior (t = 0.3, P > 0.05, Fig. 3).
a Effects of bilateral injection of 200 nL of vehicle (n = 8 each group) or atropine (0.006, 0.06, or 6 nmol) into the vMPFC PL or IL, on the percentage of time spent in freezing behavior in nonconditioned (n = 6, only dose of 6 nmol) and conditioned animals (n = 5, 12, and 5, respectively) during chamber exposure. Values are represented by means ± SEM. *P < 0.05 compared to the nonconditioned vehicle group and # P < 0.05 compared to the conditioned vehicle group; Bonferroni post hoc test. b Time course of mean arterial pressure (ΔMAP) and heart rate (ΔHR) increases and cutaneous temperature (ΔCT) decreases in nonconditioned and conditioned animals. The symbols represent the mean and bars the SEM. *P < 0.05 compared to the nonconditioned vehicle group; Bonferroni post hoc test. c, d Percentage of time spent in freezing behavior and autonomic responses to the bilateral microinjection of increasing doses of atropine in conditioned rats. Dose-effect curves were generated by nonlinear regression analysis. Symbols represent the means ± SEM
The re-exposure to the context increased autonomic responses in both groups, but in a smaller extent in the nonconditioned animals (MAP F 1,180 = 77.8, P < 0.001; HR F 1,180 = 173.9, P < 0.001; and CT F 1,180 = 47.7, P < 0.001, Fig. 3). Additionally, atropine-treated conditioned rats showed significant reduction in the autonomic responses (MAP F 3,390 = 320.5, P < 0.001; HR F 3,390 = 122.1, P < .001; and CT F 3,390 = 91.92, P < 0.001, Fig. 3) in a dose-dependent manner. (MAP r 2 = 0.92, df = 17, P < 0.01; HR r 2 = 0.85, df = 17, P < 0.01; and CT r 2 = 0.90, df = 17, P < 0.01, Fig. 3). The effect of atropine on autonomic responses of conditioned animals was observed only with the two higher doses (0.06 and 6 nmol; P < 0.01) Finally, the higher dose of 6 nmol reduced the autonomic responses in nonconditioned animals (MAP F 1,180 = 47.7, P < 0.001; HR F 1,180 = 73.6, P < 0.001; and CT F 1,180 = 42.3, P < 0.001, Fig. 3). Finally, microinjection of atropine (6 nmol) into areas surrounding the vMPFC (OUT, n = 6) did not change behavior (P > 0.05) and autonomic responses (MAP P > 0.05; HR P > 0.05; and CT P > 0.05) observed during re-exposure to the chamber when comparing conditioned animals to vehicle-treated animals (n = 8, data not shown).
Furthermore, in order to verify possibly differences between PL or IL after the blockade of muscarinic receptor in conditioned animals, bilateral administration of atropine (6 nmol) into the PL or the IL was performed. Reduction in freezing time (F 3,118 = 0.179, P > 0.05, n = 6) and autonomic responses (MAP F 1,150 = 1.5, P > 0.05; HR F 1,150 = 0.37, P > 0.05; and CT F 1,150 = 1.4, P > 0.05, n = 6) were similar after atropine in both areas (data no shown).
In the third set of experiments, conditioned groups treated with vehicle (n = 7), J104129 fumarate (n = 10), and pirenzepine (n = 10) spent more time in freezing behavior than did nonconditioned animals of respective treatment (vehicle n = 6; J104129 fumarate F 1,23 = 48.92, P < 0.001, n = 7; pirenzepine F 1,28 = 74.57, P < 0.001, n = 7) during re-exposure to context.
The conditioned groups receiving J104129 fumarate (6 nmol, n = 7) or pirenzepine (6 nmol, n = 10) showed decreased freezing behavior (F 1,23 = 18.5, P < 0.01 and F 1,28 = 39.47, P < 0.001, respectively, compared to the vehicle-treated conditioned groups). No effect was observed on nonconditioned groups (J104129 fumarate, n = 6; P > 0.05; pirenzepine, n = 6, P > 0.05, Fig. 4).
a, c Effects of bilateral injection of 200 nL of vehicle, J104129 fumarate (6 nmol, n = 7 and 6, conditioned and nonconditioned group, respectively), or pirenzepine (6 nmol, n = 10 and 6, conditioned and nonconditioned group, respectively) into the vMPFC PL or IL, on the percentage of time spent in freezing behavior in nonconditioned (vehicle n = 6 and 7, J104129 fumarate and pirenzepine, respectively) and conditioned animals (vehicle n = 7 and 10, J104129 fumarate and pirenzepine, respectively) during chamber exposure. Values are represented by means ± SEM. *P < 0.05 compared to the nonconditioned vehicle group and # P < 0.05 compared to the conditioned vehicle group; Bonferroni post hoc test. b, d Time course of mean arterial pressure (ΔMAP) and heart rate (ΔHR) increases and cutaneous temperature (ΔCT) decreases in nonconditioned and conditioned animals. The symbols represent the mean and bars the SEM. *P < 0.05 compared to the nonconditioned vehicle group; Bonferroni post hoc test
Administration of either J104129 fumarate (n = 4) or pirenzepine (n = 4) in the PL or the IL caused similar reduction in freezing in conditioned animals (J104129 fumarate t = 0.92, P > 0.05; pirenzepine t = 0.57, P > 0.05, data no shown). Both conditioned and nonconditioned vehicle-treated groups presented a significant increase in MAP and HR response (J104129 fumarate: MAP F 14,342 = 42.91, P < 0.01, HR F 14,342 = 58.55, P < 0.01; pirenzepine: MAP F 14,683 = 41.97, P < 0.01, HR F 14,683 = 19.58, P < 0.01) associated with a decrease in CT (J104129 fumarate: CT F 14,342 = 25.71, P < 0.001; pirenzepine F 14,683 = 63.66, P < 0.001) during the test (Fig. 4). Also, autonomic responses were smaller in nonconditioned animals when compared with those in the conditioned group (J104129 fumarate: MAP F 1,165 = 31.23, P < 0.01, HR F 1,165 = 59.6, P < 0.01, and CT F 1,165 = 34.2, P < 0.01; pirenzepine: MAP F 1,210 = 15.93, P < 0.01, HR F 1,210 = 58.45, P < 0.01, and CT F 1,210 = 47.32, P < 0.01, Fig. 4).
Similarly to behavioral responses, J104129 fumarate or pirenzepine into the vMPFC significantly reduced the increase in both MAP (J104129 fumarate F 3,330 = 238.1, P < 0.001; pirenzepine F 3,420 = 122.8, P < 0.001) and HR (J104129 fumarate F 3,330 = 101, P < 0.001; pirenzepine F 3,420 = 93.1, P < 0.001), together with a decrease of CT (J104129 fumarate F 3,330 = 109.3, P < 0.001; pirenzepine F 3,420 = 114.3, P < 0.001) in nonconditioned (J104129 fumarate: MAP F 1,150 = 29.4, P < 0.001; FC F 1,150 = 115, P < 0.001; CT F 1,150 = 19.5, P < 0.001; pirenzepine: MAP F 1,150 = 95.3, P < 0.001; FC F 1,150 = 58, P < 0.001; CT F 1,150 = 19.6, P < 0.001, Fig. 4) and conditioned (J104129 fumarate: MAP F 1,800 = 295.5, P < 0.001; HR F 1,180 = 251.1, P < 0.001; CT F 1,180 = 260.6, P < 0.001; pirenzepine: MAP F 1,270 = 171.6, P < 0.001; HR F 1,270 = 184, P < 0.001; CT F 1,270 = 238.8, P < 0.001, Fig. 4) animals.
Finally, microinjection of 6 nmol of either J14129 fumarate or pirenzepine into areas surrounding the vMPFC (n = 4) during re-exposure to the chamber did not change either behavior (P > 0.05) or autonomic responses (MAP P > 0.05; HR P > 0.05, and CT P > 0.05 in conditioned animals) when compared to vehicle-treated animals (n = 6, data not shown).
Furthermore, to dissect the participation of M1-M3 muscarinic receptors with equipotent doses of J14129 fumarate (0.06 nmol) and pirenzepine (0.06 nmol), our results showed that bilateral administration of J14129 fumarate into the vMPFC significantly reduced freezing behavior (F 2,18 = 22.62, P < 0.001) in conditioned animals when compared to the vehicle-treated group (n = 6, Fig. 5). However, the bilateral administration of pirenzepine into the vMPFC had no significant effects on freezing behavior in conditioned animals (P > 0.05), when compared to the vehicle-treated group (Fig. 5). Likewise, administration of J14129 fumarate (6 nmol) into the vMPFC significantly reduced the magnitude of autonomic responses (MAP F 1,150 = 242.3, P < 0.001; HR F 1,150 = 381, P < 0.001; CT F 1,150 = 79.42, P < 0.001, Fig. 5) observed in conditioned animals, when compared to the vehicle-treated group (n = 6). However, the bilateral administration of pirenzepine (0.06 nmol) into the vMPFC had no significant effects on autonomic responses (MAP F 1,165 = 0.85, P > 0.05, HR F 1,165 = 0.67, P > 0.05, CT F 1,165 = 0.67, P > 0.05, Fig. 5) in conditioned animals when compared to the vehicle-treated group (n = 6).
a Effects of bilateral injection of 200 nL of vehicle (n = 6), J104129 fumarate (0.06 nmol, n = 6), or pirenzepine (0.06 nmol, n = 7) into the vMPFC PL or IL, on the percentage of time spent in freezing behavior in conditioned animals during chamber exposure. Values are represented by means ± SEM. *P < 0.05 compared to the nonconditioned vehicle group; Bonferroni post hoc test. b Time course of mean arterial pressure (ΔMAP) and heart rate (ΔHR) increases and cutaneous temperature (ΔCT) decreases in nonconditioned and conditioned animals. The symbols represent the mean and bars the SEM. *P < 0.05 compared to the nonconditioned vehicle group; Bonferroni post hoc test
Experiment 2
Potential anxiogenic effects of muscarinic receptor activation in the vMPFC in the low-intensity contextual fear conditioning.
In the less aversive conditioning protocol, the conditioned group (n = 6) spent less time in freezing behavior (45.6 ± 2 vs 81 ± 4 %, t = 5.8, P < 0.05) and presented significant but weaker increase in autonomic responses (n = 8; MAP F 1,180 = 129.2, P < 0.01; HR F 1,180 = 113.25, P < 0.01; and CT F 1,180 = 32.41, P < 0.001; Fig 6) when compared to animals conditioned to the more aversive conditioning protocol.
a Effects of bilateral injection of 200 nL of vehicle (n = 6), neostigmine (NEO, 0.1 or 1 nmol, n = 5), ineffective dose of the J104129 fumarate, in the low-intensity protocol (0.06 nmol, n = 6) or neostigmine (1 nmol) after J104129 fumarate (NEO + J104129, n = 6) into the vMPFC PL or IL, on the percentage of time spent in freezing behavior in conditioned animals during chamber exposure. Values are represented by means ± SEM. *P < 0.05 compared to the vehicle group and # P < 0.05 compared to the NEO 1 nmol-treated group; Bonferroni post hoc test. b Time course of mean arterial pressure (ΔMAP) and heart rate (ΔHR) increases and cutaneous temperature (ΔCT) decreases in conditioned animals. The symbols represent the mean and bars the SEM. *P < 0.05 compared to the nonconditioned vehicle group; Bonferroni post hoc test. c Effects of bilateral injection of 200 nL of vehicle (n = 6) or J104129 fumarate (J104129 0.06 nmol, n = 6) into the vMPFC PL or IL, systemic injection of FG7142 (n = 6) and systemic injection of FG7142 after bilateral injection of J104129 fumarate in vMPFC (FG7142 + J104129, n = 5) on the percentage of time spent in freezing behavior in conditioned animals during chamber exposure in the low-intensity protocol. Values are represented by means ± SEM. *P < 0.05 compared to the vehicle group and # P < 0.05 compared to the FG7142-treated group; Bonferroni post hoc test. d Time course of mean arterial pressure (ΔMAP) and heart rate (ΔHR) increases and cutaneous temperature (ΔCT) decreases in conditioned animals. The symbols represent the mean and bars the SEM. *P < 0.05 compared to the nonconditioned vehicle group; Bonferroni post hoc test
Still in the less aversive conditioning protocol, bilateral injection of neostigmine (0.1 nmol, n = 5, P < 0.05 or 1 nmol, n = 6, P < 0.05) into the vMPFC increased the time spent in freezing behavior (F 4,23 = 49.92, P < 0.001) when compared to the vehicle-treated group (n = 6, Fig. 6). These same doses of neostigmine (0.1 nmol, n = 5 and 1 nmol, n = 6) into the vMPFC increased autonomic responses (MAP F 4,345 = 97.47, P < 0.01; HR F 4,345 = 116.6, P < 0.01; and CT F 4,345 = 27.54, P < 0.001) when compared to the vehicle-treated group (n = 6, Fig. 6).
Similarly, the vehicle group conditioned in the less aversive conditioning protocol (n = 6) presented a significant increase, but in a smaller extent, in MAP (F 14,270 = 49.58, P < 0.01) and HR (F 14,270 = 118.3, P < 0.01), together with smaller decrease in CT (F 14,270 = 37.55, P < 0.001) during the re-exposure to the context, when compared to animals exposed to a more aversive conditioning protocol (n = 8) (MAP F 1,180 = 137, P < 0.01; HR F 1,180 = 115.9, P < 0.01; and CT F 1,180 = 30.1, P < 0.001, Fig. 6).
Continuing with the less aversive conditioning protocol, systemic administration of FG7142 (8 mg/kg; n = 6) increased the time spent in freezing behavior (F 3,18 = 16.73, P < 0.001) when compared to the vehicle-treated group (n = 6, Fig. 6). This drug was also able to increase the autonomic responses (MAP F 3,270 = 164.6, P < 0.01; HR F 3,270 = 272, P < 0.01; and CT F 3,270 = 57.65, P < 0.001) when compared with the vehicle-treated group (n = 6, Fig. 6).
The increase in freezing evoked by both neostigmine (0.1 nmol, n = 6) and FG7142 (8 mg/kg, n = 6) were inhibited by the pre-treatment with 0.06 nmol of J104129 fumarate injected into the vMPFC (n = 6, P > 0.05 and n = 5, P > 0.05, respectively, Fig. 6) when compared to the vehicle-treated animals. Finally, neostigmine (1 nmol) was unable to increase the freezing behavior when injected into structures surrounding the vMPFC (n = 5, P > 0.05).
Discussion
The present study showed the cholinergic modulation of the expression of behavior and receptors. In nonconditioned animals, only autonomic responses were attenuated. Contextual fear conditioning responses were potentiated by increases in vMPFC endogenous acetylcholine or by systemic administration of an anxiogenic drug—an effect prevented by the blockade of muscarinic M3 receptor vMPFC.
To verify the possible involvement of the cholinergic system present in the vMPFC in the modulation of contextual fear conditioning responses, the content of vMPFC acetylcholine was reduced by administration of hemicholinium, a choline reuptake inhibitor (Guyenet et al. 1973). The treatment with hemicholinium reduced the CER in conditioned animals, but only the autonomic responses in nonconditioned animals, suggesting that a cholinergic neurotransmission present in vMPFC is involved in the modulation of contextual fear responses.
Anxiolytic effects have been reported after administration of the muscarinic antagonist (Li et al. 2014). Also, vMPFC treatment with a nonselective muscarinic antagonist atropine blocks the cardiovascular activity on defensive responses (Hart et al. 1999). Systemic administration of scopolamine, a nonselective muscarinic antagonist, impairs both acquisition and consolidation of contextual fear conditioning (Anagnostaras et al. 1995; Young et al. 1995). Indeed, in the present study, microinjection of atropine into the vMPFC attenuated the expression of contextual fear conditioning responses in a dose-dependent manner.
Previous evidence indicates that vMPFC subregions may play either similar or distinct roles at different times with respect to stress exposure (Lisboa et al. 2010a, b; Scopinho et al. 2009, 2010; Terzian et al. 2014). Other studies showed that PL inactivation impaired fear expression in the auditory and contextual fear (Vidal-Gonzalez et al. 2006). On the other hand, IL activation plays an important role in extinction of auditory fear conditioning (Milad and Quirk 2002). Interestingly, the opposite roles of these subregions can be explained for your efferent projections.
Therefore, to clarify this issue, animals were subdivided into a group that received microinjection of atropine into the PL or IL. There was no significant difference between effects caused by PL or IL muscarinic receptor blockade on the expression of contextual fear conditioning. Our findings are in agreement with other studies, showing that reversible inhibition of these areas evoked similar reduction in the expression of contextual fear conditioning (Resstel et al. 2006). These results demonstrate the crucial role of cholinergic system in the IL and PL regions in the modulation of fear conditioning responses.
Neuroanatomical studies evidenced distinct patterns of expression of muscarinic receptor subtypes in forebrain and limbic structures (Levey et al. 1994, 1995a, b). M1 muscarinic receptors are the predominant subtypes in the central nervous system, involved in learning, memory, and anxiety functions (Hagan et al. 1987; Terzioglu et al. 2013). However, the role of neuronal M3 receptors (Tayebati et al. 2001) is not completely elucidated.
Thus, we investigated which subtypes of muscarinic receptors presented in the vMPFC could modulate the expression of contextual fear conditioning. Both J104129 fumarate (6 nmol) and pirenzepine (6 nmol) attenuated the CER in conditioned animals and reduced the autonomic responses in nonconditioned rats suggesting the participation of the M1-M3 muscarinic receptors in the expression of the CER. Nonetheless, the relative low ratio of selectivity between these compounds for M1-M3 muscarinic receptor subtypes does not allow us to fully characterize which specific receptor is involved in the modulation of expression of contextual fear conditioning in the vMPFC.
Given the intrinsic limitation of these drugs, we selected equipotent doses of J104129 fumarate to block approximately the same number of M3 muscarinic receptors blocked by pirenzepine. Consequently, only the dose of 0.06 nmol of J104129 fumarate attenuated both behavioral and autonomic response in conditioned animals to a similar extent observed with 6 nmol of J104129 fumarate or pirenzepine (Lee et al. 1994; Mitsuya et al. 1999). This means that equimolar doses of pirenzepine and J104129 fumarate could occupy a similar amount of M1 muscarinic receptors, whereas J104129 fumarate could bind to ∼100 times more M3 muscarinic receptors than pirenzepine could. Accordingly, all effects observed with 6 nmol of both drugs or 0.06 nmol of only J104129 fumarate depends on M3 muscarinic receptor antagonism, and not M1 muscarinic receptors.
Thus, the observation that antagonism of muscarinic receptors present in vMPFC which almost abolished the fear-conditioned responses clearly indicates a vMPFC contribution in expressing fear-related responses. Moreover, it modulates cardiovascular responses, not only the fear conditioned but also those evoked by simple re-exposure to context, which is probably associated with a greater motor activity and the stress induced by manipulation to this re-exposition.
In order to confirm the modulation of the cholinergic neurotransmission in the vMPFC in the expression of contextual fear conditioning responses, the effects of increased vMPFC endogenous acetylcholine level were also evaluated, using the acetylcholinesterase inhibitor neostigmine. For that, a low-intensity stress protocol was used, once the conditioned responses associated with a high intensity aversive protocol could present a “ceiling effect” (Baldi et al. 2004). In this situation, animals received microinjection of neostigmine or FG7142 (as a positive control) before re-exposure to an aversive context. Both drugs were able to increase freezing time and autonomic responses during re-exposure to the aversive environment. Previous studies demonstrated that FG7142 can potentiate autonomic defensive-like responses during threatening situations, which could involve the basal forebrain cholinergic projections in the potentiation of CER (Berntson et al. 1996). Moreover, anxiogenic stimuli have been reported to increase acetylcholine efflux in the vMPFC (Mark et al. 1996; Moore et al. 1995).
Finally, we reaffirmed the participation of the M3 muscarinic receptors in the expression of contextual fear conditioning. A low dose of the J104129 fumarate (0.06 nmol) into the vMPFC was used, which alone did not affect the expression of contextual fear conditioning when using the low-intensity protocol. Interestingly, this same dose was able to attenuate the expression of contextual fear conditioning in the high-intensity protocol. In addition, the injection of J104129 fumarate (0.06 nmol) into the vMPFC blocked the effect of both the neostigmine and the FG7142 in the expression of contextual fear conditioning. These results suggest that the number of muscarinic receptors occupied by acetylcholine in the vMPFC could have a direct relationship with stress intensity.
In conclusion, our results suggest that a cholinergic innervation, as well as activation of M3 muscarinic receptors, in the vMPFC is involved in the potentiation of the defensive response, particularly in the expression of contextual fear conditioning. Also, it could be part of a central pathway involved in the mediation of anxiogenic-like responses.
References
Acquas E, Wilson C, Fibiger HC (1996) Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: effects of novelty, habituation, and fear. J Neurosci Off J Soc Neurosci 16:3089–3096
Anagnostaras SG, Maren S, Fanselow MS (1995) Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiol Learn Mem 64:191–194
Baldi E, Lorenzini CA, Bucherelli C (2004) Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol Learn Mem 81:162–166
Bang SJ, Brown TH (2009) Muscarinic receptors in perirhinal cortex control trace conditioning. J Neurosci Off J Soc Neurosci 29:4346–4350
Beck CH, Fibiger HC (1995) Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci Off J Soc Neurosci 15:709–720
Beninger RJ, Ingles JL, Mackenzie PJ, Jhamandas K, Boegman RJ (1992) Muscimol injections into the nucleus basalis magnocellularis of rats: selective impairment of working memory in the double Y-maze. Brain Res 597:66–73
Berntson GG, Hart S, Ruland S, Sarter M (1996) A central cholinergic link in the cardiovascular effects of the benzodiazepine receptor partial inverse agonist FG 7142. Behav Brain Res 74:91–103
Berntson GG, Sarter M, Cacioppo JT (1998) Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behav Brain Res 94:225–248
Borelli KG, Albrechet-Souza L, Fedoce AG, Fabri DS, Resstel LB, Brandao ML (2013) Conditioned fear is modulated by CRF mechanisms in the periaqueductal gray columns. Horm Behav 63:791–799
DeSousa NJ, Beninger RJ, Jhamandas K, Boegman RJ (1994) Stimulation of GABAB receptors in the basal forebrain selectively impairs working memory of rats in the double Y-maze. Brain Res 641:29–38
Di Giacinto A, Brunetti M, Sepede G, Ferretti A, Merla A (2014) Thermal signature of fear conditioning in mild post traumatic stress disorder. Neuroscience 266:216–223
Fabri DR, Hott SC, Reis DG, Biojone C, Correa FM, Resstel LB (2014) The expression of contextual fear conditioning involves activation of a NMDA receptor-nitric oxide-cGMP pathway in the dorsal hippocampus of rats. Eur Neuropsychopharmacol 24:1676–1686
Fanselow MS (1980) Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 15:177–182
Feng T, Yang S, Wen D, Sun Q, Li Y, Ma C, Cong B (2014) Stress-induced enhancement of fear conditioning activates the amygdalar cholecystokinin system in a rat model of post-traumatic stress disorder. Neuroreport 25:1085–1090
Fernandes KB, Tavares RF, Correa FM (2005) The lateral septal area is involved in the pressor pathway activated by microinjection of norepinephrine into the rat brain cingulate cortex. Neuropharmacology 49:564–571
Frysztak RJ, Neafsey EJ (1991) The effect of medial frontal cortex lesions on respiration, “freezing,” and ultrasonic vocalizations during conditioned emotional responses in rats. Cereb Cortex 1:418–425
Frysztak RJ, Neafsey EJ (1994) The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res 643:181–193
Gaykema RP, Gaal G, Traber J, Hersh LB, Luiten PG (1991a) The basal forebrain cholinergic system: efferent and afferent connectivity and long-term effects of lesions. Acta Psychiatr Scand Suppl 366:14–26
Gaykema RP, van Weeghel R, Hersh LB, Luiten PG (1991b) Prefrontal cortical projections to the cholinergic neurons in the basal forebrain. J Comp Neurol 303:563–583
Gomes FV, Reis DG, Alves FH, Correa FM, Guimaraes FS, Resstel LB (2012) Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT1A receptors. J Psychopharmacol 26:104–113
Guyenet P, Lefresne P, Rossier J, Beaujouan JC, Glowinski J (1973) Inhibition by hemicholinium-3 of (14C)acetylcholine synthesis and (3H)choline high-affinity uptake in rat striatal synaptosomes. Mol Pharmacol 9:630–639
Hagan JJ, Jansen JH, Broekkamp CL (1987) Blockade of spatial learning by the M1 muscarinic antagonist pirenzepine. Psychopharmacol (Berl) 93:470–476
Hart S, Sarter M, Berntson GG (1999) Cholinergic inputs to the rat medial prefrontal cortex mediate potentiation of the cardiovascular defensive response by the anxiogenic benzodiazephine receptor partial inverse agonist FG 7142. Neuroscience 94:1029–1038
Hott SC, Gomes FV, Fabri DR, Reis DG, Crestani CC, Correa FM, Resstel LB (2012) Both alpha1- and beta1-adrenoceptors in the bed nucleus of the stria terminalis are involved in the expression of conditioned contextual fear. Br J Pharmacol 167:207–221
Hsu JC, Zhang L, Wallace MC, Eubanks JH (1996) Cerebral ischemia alters the regional hippocampal expression of the rat m1 muscarinic acetylcholine receptor gene. Neurosci Lett 219:87–90
Kwapis JL, Wood MA (2014) Epigenetic mechanisms in fear conditioning: implications for treating post-traumatic stress disorder. Trends Neurosci 37:706–720
Lee SW, Woo CW, Kim JG (1994) Selectivity of oxomemazine for the M1 muscarinic receptors. Arch Pharm Res 17:443–451
Levey AI, Edmunds SM, Heilman CJ, Desmond TJ, Frey KA (1994) Localization of M3 muscarinic receptor protein and M3 receptor binding in rat brain. Neuroscience 63:207–221
Levey AI, Edmunds SM, Hersch SM, Wiley RG, Heilman CJ (1995a) Light and electron microscopic study of m2 muscarinic acetylcholine receptor in the basal forebrain of the rat. J Comp Neurol 351:339–356
Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ (1995b) Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci 15:4077–4092
Li H, Chen L, Li P, Wang X, Zhai H (2014) Insular muscarinic signaling regulates anxiety-like behaviors in rats on the elevated plus-maze. Behav Brain Res 270:256–260
Lisboa SF, Reis DG, da Silva AL, Correa FM, Guimaraes FS, Resstel LB (2010a) Cannabinoid CB1 receptors in the medial prefrontal cortex modulate the expression of contextual fear conditioning. Int J Neuropsychopharmacol 13:1163–1173
Lisboa SF, Stecchini MF, Correa FM, Guimaraes FS, Resstel LB (2010b) Different role of the ventral medial prefrontal cortex on modulation of innate and associative learned fear. Neuroscience 171:760–768
Mark GP, Rada PV, Shors TJ (1996) Inescapable stress enhances extracellular acetylcholine in the rat hippocampus and prefrontal cortex but not the nucleus accumbens or amygdala. Neuroscience 74:767–774
Milad MR, Quirk GJ (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420:70–74
Mitsuya M, Mase T, Tsuchiya Y, Kawakami K, Hattori H, Kobayashi K, Ogino Y, Fujikawa T, Satoh A, Kimura T, Noguchi K, Ohtake N, Tomimoto K (1999) J-104129, a novel M3 muscarinic receptor antagonist with high selectivity for M3 over M2 receptors. Bioorg Med Chem 7:2555–2567
Moore H, Stuckman S, Sarter M, Bruno JP (1995) Stimulation of cortical acetylcholine efflux by FG 7142 measured with repeated microdialysis sampling. Synapse 21:324–331
Muir JL, Everitt BJ, Robbins TW (1994) AMPA-induced excitotoxic lesions of the basal forebrain: a significant role for the cortical cholinergic system in attentional function. J Neurosci Off J Soc Neurosci 14:2313–2326
Pang MH, Kim NS, Kim IH, Kim H, Kim HT, Choi JS (2010) Cholinergic transmission in the dorsal hippocampus modulates trace but not delay fear conditioning. Neurobiol Learn Mem 94:206–213
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, Sydney
Poulin B, Butcher A, McWilliams P, Bourgognon JM, Pawlak R, Kong KC, Bottrill A, Mistry S, Wess J, Rosethorne EM, Charlton SJ, Tobin AB (2010) The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc Natl Acad Sci U S A 107:9440–9445
Radley JJ, Arias CM, Sawchenko PE (2006) Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci Off J Soc Neurosci 26:12967–12976
Resstel LB, Correa FM (2005) Pressor and tachycardic responses evoked by microinjections of L-glutamate into the medial prefrontal cortex of unanaesthetized rats. Eur J Neurosci 21:2513–2520
Resstel LB, Correa FM (2006a) Involvement of the medial prefrontal cortex in central cardiovascular modulation in the rat. Auton Neurosci Basic Clin 126–127:130–138
Resstel LB, Correa FM (2006b) Medial prefrontal cortex NMDA receptors and nitric oxide modulate the parasympathetic component of the baroreflex. Eur J Neurosci 23:481–488
Resstel LB, Fernandes KB, Correa FM (2005) alpha-Adrenergic and muscarinic cholinergic receptors are not involved in the modulation of the parasympathetic baroreflex by the medial prefrontal cortex in rats. Life Sci 77:1441–1451
Resstel LB, Joca SR, Guimaraes FG, Correa FM (2006) Involvement of medial prefrontal cortex neurons in behavioral and cardiovascular responses to contextual fear conditioning. Neuroscience 143:377–385
Resstel LB, Correa FM, Guimaraes FS (2008a) The expression of contextual fear conditioning involves activation of an NMDA receptor-nitric oxide pathway in the medial prefrontal cortex. Cereb Cortex 18:2027–2035
Resstel LB, Joca SR, Correa FM, Guimaraes FS (2008b) Effects of reversible inactivation of the dorsal hippocampus on the behavioral and cardiovascular responses to an aversive conditioned context. Behav Pharmacol 19:137–144
Resstel LB, Moreira FA, Guimaraes FS (2009) Endocannabinoid system and fear conditioning. Vitam Horm 81:421–440
Scopinho AA, Tavares RF, Correa FM (2009) The medial forebrain bundle mediates cardiovascular responses to electrical stimulation of the medial prefrontal cortex. Auton Neurosci Basic Clin 147:38–47
Scopinho AA, Scopinho M, Lisboa SF, Correa FM, Guimaraes FS, Joca SR (2010) Acute reversible inactivation of the ventral medial prefrontal cortex induces antidepressant-like effects in rats. Behav Brain Res 214:437–442
Stock G, Schlor KH, Heidt H, Buss J (1978) Psychomotor behaviour and cardiovascular patterns during stimulation of the amygdala. Pflugers Arch - Eur J Physiol 376:177–184
Tayebati SK, Vitali D, Scordella S, Amenta F (2001) Muscarinic cholinergic receptors subtypes in rat cerebellar cortex: light microscope autoradiography of age-related changes. Brain Res 889:256–259
Terzian AL, dos Reis DG, Guimaraes FS, Correa FM, Resstel LB (2014) Medial prefrontal cortex Transient Receptor Potential Vanilloid Type 1 (TRPV1) in the expression of contextual fear conditioning in Wistar rats. Psychopharmacology 231:149–157
Terzioglu B, Kaleli M, Aydin B, Ketenci S, Cabadak H, Goren MZ (2013) Increased noradrenaline levels in the rostral pons can be reversed by M1 antagonist in a rat model of post-traumatic stress disorder. Neurochem Res 38:1726–1733
Verberne AJ, Owens NC (1998) Cortical modulation of the cardiovascular system. Prog Neurobiol 54:149–168
Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ (2006) Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem 13:728–733
Wall PM, Messier C (2002) Infralimbic kappa opioid and M1 muscarinic receptor interactions in the concurrent modulation of anxiety and memory. Psychopharmacology 160:233–244
Young SL, Bohenek DL, Fanselow MS (1995) Scopolamine impairs acquisition and facilitates consolidation of fear conditioning: differential effects for tone vs context conditioning. Neurobiol Learn Mem 63:174–180
Yu AJ, Dayan P (2002) Acetylcholine in cortical inference. Neural Netw 15:719–730
Acknowledgments
The authors wish to thank Argentin, L.H.C., Fortunato, I.A.C. and Guilhaume, S.S. for technical help. This research was supported by grants from FAPESP (2012/09300-4 and 2012/17626-7), CNPq, and FAEPA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fedoce, A.G., Ferreira-Junior, N.C., Reis, D.G. et al. M3 muscarinic receptor in the ventral medial prefrontal cortex modulating the expression of contextual fear conditioning in rats. Psychopharmacology 233, 267–280 (2016). https://doi.org/10.1007/s00213-015-4109-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4109-5