Abstract
Rationale
Current nicotine replacement products provide a much slower onset of nicotine delivery than cigarettes, and hence are only marginally effective at supplanting cigarette smoking. Therefore, more effective forms of nicotine replacement are needed.
Objectives
This initial investigation characterized the pharmacokinetic (PK) and subjective effects of a novel sublingual (SL) nicotine tablet designed to deliver nicotine more rapidly to the bloodstream of smokers.
Methods
Study 1 (N = 6) characterized the pharmacokinetics of a 2 mg nicotine SL tablet in comparison to an FDA-approved, marketed 2 mg nicotine lozenge. Study 2 (N = 24) assessed subjective responses of smokers to a single use of a 1 mg and 2 mg SL tablet.
Results
Study 1 found that the time to maximum blood nicotine concentrations was significantly shorter for the SL tablet (14 min) than for the lozenge (82 min), and the initial rate of nicotine absorption was higher (0.4 ng/mL*min vs. 0.0 ng/mL*min), supporting the hypothesis that the SL tablet delivered nicotine more rapidly. Study 2 found that participants reported immediate relief of nicotine withdrawal symptoms after tablet administration, and craving reduction after the 2 mg tablet approached the degree reported for their usual brands of cigarettes (4.2 vs. 4.6 on a 7-point scale). Other subjective responses showed the tablet to be an appealing alternative to smoking.
Conclusions
The novel SL tablet studied shows promise as a nicotine substitution strategy for tobacco harm reduction and smoking cessation treatment. Additional studies are warranted to further investigate the potential of this new approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is increasingly well accepted by experts and regulatory authorities that nicotine products which administer nicotine through the oral mucosa are considerably less harmful than combustible cigarettes (CC) (Abrams et al. 2018; Gottlieb and Zeller 2017; McNeill et al. 2001; Murray et al. 2009). For example, nicotine chewing gum and lozenges have been approved for over-the-counter use in the USA and many other countries (Amodei and Lamb 2008; Hartmann-Boyce et al. 2018). Some forms of smokeless tobacco have also received an official designation from the FDA as reduced risk relative to CC, and their marketing has been authorized as appropriate for the protection of public health (Morgan and Cappella 2021).
Although products delivering nicotine to the oral mucosa in the absence of other harmful tobacco constituents may reduce health risks associated with smoking, their potential impact on public health is limited because only a small fraction of smokers switch from cigarettes to alternative nicotine products (Shahab et al. 2016; Shiffman et al. 2003). Not only do most current products such as nicotine gum or lozenge lack the familiar behavioral and sensory feedback associated with smoking, but their pharmacokinetics are slow compared to inhalation (Benowitz et al. 2009). Hence, for an addicted smoker, the reinforcing effects pale in comparison with cigarettes (West et al. 2000).
Recently, however, a novel formulation of nicotine tablet has been developed which administers nicotine through the sublingual mucosa (McCarty 2015). This region of the oral mucosa is relatively thin and highly vascularized (Hua 2019), which facilitates nicotine absorption through the mucosa. Additionally, the product is formulated with a specific solvent/carrier system (described below) to provide faster release and absorption of nicotine than current nicotine replacement therapy (NRT) products. Such a product has the potential to compete more effectively with cigarettes and to help smokers switch to a less harmful alternative source of nicotine.
Below we describe two studies conducted with cigarette smokers: the first study evaluated the pharmacokinetics of a 2 mg nicotine tablet formulation; the second study evaluated the subjective responses to 1 mg and 2 mg doses.
Study 1: Pharmacokinetic study
It is widely recognized that more rapid delivery of nicotine to the bloodstream results in more rapid relief of craving, which in turn may aid smokers to switch from cigarette smoking to an alternative source of nicotine (West et al. 2000). Smoking provides a rapid bolus of nicotine with a time to maximum plasma concentration (Tmax) of 5 to 8 min (Benowitz et al. 2009). Nicotine chewing gums, lozenges, and currently marketed SL tablets all show Tmax values around 45–60 min (Choi et al. 2003; Dautzenberg et al. 2007; Molander and Lunell 2001). The only NRT approaching the Tmax of smoking a cigarette is the nasal spray, which has a Tmax of 11 to 18 min (Benowitz et al. 2009) but it is reported to be very irritating to the nasal mucosa, a factor that for many smokers makes its use intolerable (Blondal et al. 1999).
The primary aim of this initial study was to ascertain the pharmacokinetics of a novel NRT technology, a 2 mg nicotine SL tablet, in comparison to a commercially available 2 mg nicotine lozenge (Commit®). This SL tablet (BRST™) was composed of nicotine solubilized in oleic acid, which was adsorbed onto silica particles that were compressed into a small tablet. As water from saliva enters the tablet, it rapidly disintegrates, quickly releasing thousands of nicotine/oleic acid–laden silica particles under the tongue. The water from saliva then permeates into the silica particles and drives the lipophilic drug solution into the lipid environment of the sublingual mucosa. Pilot data indicated that the onset of nicotine delivery is within a few minutes and most of the nicotine is absorbed within 10 to 15 min.
Methods: Study 1
This project was a collaboration between Friends Research Institute (FRI) (a non-profit research institute) and Pharmaceutical Productions Inc. (PPI) (a for-profit small business). FRI scientists and physicians performed the pilot PK study at the FRI clinic in Torrance, CA, and the bioanalytical work was done by the laboratory of Neal Benowitz, MD, at the University of California, San Francisco. PPI was responsible for the development and manufacture of the clinical trial materials. Approval to conduct the study was obtained from the Friends Research Institute IRB. The FDA determined that an IND was not required according to 21 CFR 320.31.
Study design
This exploratory study was a randomized, 2-way cross-over pilot study comparing the nicotine pharmacokinetics of the investigational 2 mg SL tablet vs. the 2 mg lozenge. The study enrolled six healthy smokers, who were instructed to report to the study center after overnight smoking abstinence. A blood sample was taken just prior to product administration and an additional eleven blood samples were obtained over the following 3-h period.
Participants, screening, and eligibility criteria
Participants were 18–45 years old, free from any clinically significant pathology, smoked more than 15 cigarettes daily, smoked their first cigarette within 30 min of waking, and had a Fagerström Test for Cigarette Dependence (FTND) score of > 4. Volunteers were excluded if they used any other smoked substance other than tobacco, other nicotine-containing products or smoking cessation treatments within 30 days; BMI deviated more than 15% from Kettle’s weight-height index; blood pressure exceeded 150 mmHg systolic or 90 mmHg diastolic; they had severe allergies or recent infectious diseases, surgical operations or diseases of the GI tract, donated 450 mL of blood/plasma within 2 months, were currently involved in another clinical trial, or had used any investigational drug within 3 months of study entry; or they consumed more than 10 units of alcohol per week or had a history of alcohol or drug abuse. Women of childbearing potential, in addition to having a negative urine pregnancy test, had to agree to use an approved form of birth control during the study, and to avoid hormonal contraceptives within 2 months prior to study entry. Participants were compensated for their time.
Nicotine SL tablet
The BRST nicotine-containing dissolvable tablet is placed under the tongue where it disintegrates rapidly, releasing nicotine for absorption through the oral mucosa. The USP-grade nicotine in these tablets was extracted from tobacco, but the products contained no tobacco leaf material. Each SL tablet (60 mg by weight) contained nicotine (2 mg), oleic acid, silicon dioxide, diluent, disintegrant, and tablet lubricant. All non-nicotine ingredients are commonly used in the manufacturing of pharmaceutical tablets and meet current United States Pharmacopeia (USP) and National Formulary (NF) compendial specifications.
Nicotine lozenge
The 2 mg nicotine polacrilex Commit® lozenge, manufactured by GlaxoSmithKline, Inc., was used as the comparator product.
Procedure
After arrival at the laboratory, participants were under observation and not allowed to smoke for at least 4 h, which ensured that plasma nicotine levels would be low, and could not eat or drink for 15 min before drug administration. A single 2 mg dose of either of the SL nicotine tablet or the nicotine lozenge was administered in each session, with a washout between sessions of at least 48 h, but no more than 2 weeks.
With the SL tablet, participants were instructed to place one tablet under the tongue and keep it undisturbed under the tongue until it completely disintegrated (usually within a minute). With the nicotine lozenge, they were instructed to take the lozenge and to suck slowly until a sharp taste was perceived, then “park” the lozenge between the gingiva and cheek. When the taste disappeared, the suck-and-park procedure was repeated until the lozenge dissolved completely, usually within 30 min.
A venous catheter was used to collect 5 ml blood samples at times: 0 (prior to administration of the study drug), 4, 8, 10, 12, 16, 30, 45, 60, 90, 120, and 180 min after product administration. These timepoints were selected to capture the expected rapid increases in nicotine during the first minutes after product administration, and to capture the peak levels after nicotine absorption was expected to be complete.
Data analysis
Pharmacokinetic parameters were determined as follows:
-
Cmax — the observed maximum plasma concentration after dosing.
-
Tmax — the time at which Cmax was reached.
-
AUC0-180 — the truncated area under the plasma concentration–time curve from the beginning of dosing to 180 min (the time of last quantifiable concentration). The linear trapezoidal rule was used to calculate the area from the beginning of dosing to the last quantifiable concentration.
-
R0 — the initial rate of increase in nicotine concentration in the first 4 min (the first post-drug blood collection point).
Tmax and AUC0-180 were calculated using uncorrected nicotine concentrations because a correction would have required an estimate of each participant’s nicotine elimination rate, which was not measured. However, since Cmax generally occurred before substantial nicotine metabolism would have taken place, given that the average metabolic half-life of nicotine is 2 h (Benowitz et al. 2009), Cmax values were adjusted by subtracting each participant’s baseline nicotine concentration. Statistical analyses of differences in Tmax and R0 used one-tailed test procedures, considering that there was a clear directional hypothesis that the tablet would deliver nicotine more rapidly than the lozenge. Two-tailed tests were applied to Cmax and AUC0-180 where there were no directional hypotheses. Statistical interpretations were conducted using analysis of variance (ANOVA) for log-transformed AUC0-180, Tmax, and Cmax data, in accordance with FDA guidance (Chow and Liu 2009). R0 values were in some instances zero so a log transform was not used for this measure.
Results: Study 1
Participants
Five males and one female participated in the study, comprising four white (two Hispanic) and two African Americans. Their mean age was 28.7 years (SD = 5.8) and their mean body weight was 78.2 kg (SD = 15.8). Baseline smoking-abstinent nicotine concentrations were 3.5 ng/mL (SD = 1.7 ng/mL) and baseline cotinine concentrations averaged 211.6 ng/mL (SD = 84.0 ng/mL).
Pharmacokinetics
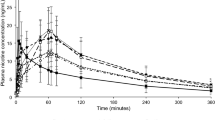
Figure 1 presents plasma nicotine results from the 2 mg SL tablet compared to the 2 mg lozenge for the first 45 min, the most relevant interval for comparing the initial rate of nicotine absorption. The summary of pharmacokinetic parameters is presented in Table 1. The Tmax for the SL tablet was significantly shorter than for the lozenge, a median of 14.0 min vs. 82.5 min (F(1,5) = 9.80, P = 0.01) (1-tailed). Correspondingly, there was a significantly higher initial rate of nicotine absorption with the tablet, with median values of 0.4 ng/mL*min vs. 0.0 ng/mL*min (F(1,5) = 7.13, P = 0.02) (1-tailed), reaching 50% of Cmax within 4 min. However, the median AUC0-180 value was higher for the lozenge, 1189.9 ng*min/mL vs. 949.5 ng*min/mL (F(1,5) = 19.02, P = 0.007, 2-tailed). There was no difference between products in Cmax (median of 4.4 ng/mL for the tablet vs. 5.3 ng/mL for the lozenge, F(1,5) = 0.04, P = 0.84 (2-tailed)).
Discussion: Study 1
The pharmacokinetic data presented above demonstrate that the novel SL tablet has a faster nicotine delivery than the lozenge, as reflected in the shorter Tmax as well as a faster rise in nicotine plasma concentration within the first minutes. To the extent that rapid onset of nicotine effects is important in alleviating craving and other nicotine withdrawal symptoms, these data are encouraging for the potential efficacy of the SL tablet formulation as a substitute for cigarettes.
There was a lower AUC0-180 for the SL tablet versus the lozenge, which may have been due to more nicotine being swallowed in the SL tablet condition. Nicotine that is swallowed is largely converted (70%) to cotinine on first pass through the liver (Benowitz et al. 2009; Olsson Gisleskog et al. 2021) and therefore does not contribute appreciably to the total nicotine AUC0-180.
Interestingly, despite having a lower AUC0-180, the Cmax of the tablet was very similar to that for the lozenge, which may have been due to the lower initial volume of distribution of nicotine in the first minutes after administration (Feyerabend et al. 1985), when the tablet achieved more rapid delivery of nicotine to the circulation.
Study 2: Subjective response study
The aim of this study was to characterize the subjective responses of the SL nicotine tablet, in two strengths, 1 mg and 2 mg, in a group of 24 smokers. Positive rewarding and aversive effects as well as craving reduction were measured over a 2-h period after product administration. This information would be useful in evaluating the potential of this product to satisfy tobacco consumers including smokers seeking alternative sources of nicotine without other harmful and potentially harmful constituents (HPHC) found in tobacco.
Methods: Study 2
This study was conducted by Rose Research Center in Raleigh and Charlotte, NC, in collaboration with Nicotine BRST LLC. Approval was obtained from the Advarra Institutional Review Board.
Study design
This study was a single-visit, repeated measures, open-label study (N = 24) that evaluated smokers’ responses to the nicotine SL tablet in 1 mg and 2 mg nicotine strengths (order not counterbalanced). The total duration of participation was approximately 8–9 h. Screening procedures required approximately 1 h and the on-site visit lasted 7–8 h.
Participants, screening, and eligibility criteria
Healthy cigarette smoking adults, 21–60 years old, with no restriction on gender, race and ethnicity, or social-economic status, who had smoked an average of at least 10 commercially available cigarettes per day for the last 6 months, were screened for enrollment in this study. To qualify, volunteers must have reported smoking their first cigarette within 30 min of waking and have an expired air carbon monoxide (CO) reading of ≥ 10 ppm.
Volunteers were excluded if they had a history of coronary heart disease, structural cardiac disease (including, but not limited to valvular heart disease or cardiac murmurs), cardiac dysrhythmias, syncope, cardiac chest pain, or history of heart attack or heart failure; BMI was less than 15.0 kg/m2 or greater than 40.0 kg/m2; blood pressure exceeded 150 mmHg systolic or 95 mmHg diastolic; urine drug screen was positive for cocaine, amphetamines, or opiates; or they were employed by a tobacco company or the study center. Women of childbearing potential were required to have a negative pregnancy test. Participants were compensated for their time.
Procedure
Following a 2-h nicotine deprivation period, participants were administered a 1 mg nicotine SL tablet and asked to complete subjective questionnaires over the following 2 h. During this time, vital signs were assessed at predetermined intervals. At the end of the 2 h, a 45-min lunch period ensued (30 min to eat and drink water followed by 15 min without any food or drink). After the lunch break, they were administered a 2 mg nicotine SL tablet and asked to complete subjective questionnaires over a second 2-h period. During this time, vital signs were also assessed at predetermined intervals.
Nicotine tablets
The 1 mg and 2 mg nicotine tablets used in this study were essentially identical to those in the PK study except they also contained sucralose as a sweetener and peppermint oil flavor. Each tablet was placed under a participant’s tongue by study staff. Participants were instructed not to swallow or chew the SL tablet but to let it disintegrate under the tongue undisturbed and if any residue remained after 15 min it could be swallowed. No drinks were given to the subject to aid in dissolving the study drug.
Outcome measures
The following outcome measures were assessed:
-
Expired Air CO Breath Test: Carbon monoxide (CO) in participants’ exhaled breath (expressed as ppm) was measured using a Vitalograph CO Monitor.
-
Fagerström Test for Nicotine Dependence (FTND) (Heatherton et al. 1991): This six-item questionnaire allowed for the classification of nicotine dependence in three different levels: mild (0–3 points), moderate (4–6 points), and severe (7–10 points).
-
Cigarette Evaluation Questionnaire-modified (mCEQ): This questionnaire, designed to assess the positive and negative subjective effects of cigarette smoking, was modified from the original version (Westman et al. 1992) by adding a single item on enjoyment of smoking. A validation study of this questionnaire was conducted by Cappelleri et al. (2007). Additionally, Bergeria et al. (2019) showed that several subscales predicted behavioral preference for cigarettes with varying levels of nicotine. The mCEQ provided five subscale scores: satisfaction (satisfying, tastes good, enjoy smoking), psychological reward (calms down, more awake, less irritable, helps concentrate, reduces hunger), aversion (dizziness, nauseated), enjoyment of respiratory tract sensations (single-item assessment), craving reduction (single-item assessment). Participants were asked to assess the 12 items of the questionnaire on a 7-point scale, ranging from “not at all” to “extremely”. These items were used to rate the last cigarette smoked prior to the session.
-
Minnesota Nicotine Withdrawal Scale-modified (mMNWS): This assessment was based on a widely used questionnaire (Hughes and Hatsukami 1998; Hughes 1986; Toll et al. 2007), and was given 5 min before, and 5, 15, 30, 60, 90, and 120 min after each product administration. Participants were asked to rate a subset of nicotine withdrawal symptoms that comprised “desire to smoke,” “anger,” “anxiety,” “difficulty concentrating,” and “depression”, using a 5-point rating scale ranging from 0 to 4, where 0 = none, 1 = slight, 2 = mild, 3 = moderate, and 4 = severe. A total score was computed.
-
Tobacco Product Evaluation Questionnaire (TPEQ): This questionnaire was given 30 min after each product administration and contained the 12 items of the mCEQ reworded to apply to the nicotine SL tablet. Additional items asked how likely it would be for participants to use the product again if it were available, how difficult it would be to use instead of smoking cigarettes, and how much more enjoyment would be experienced if it had more (or less) nicotine. Participants were also asked to assess these items on a 7-point scale, ranging from “not at all” to “extremely”. An additional open-ended question assessed any other positive or negative effects of product use.
-
Desire to Smoke Visual Analog Scale (VAS): Participants rated their desire to smoke on a 100-mm scale, 5 min before, and 5, 10, 15, 30, 45, 60, 90, and 120 min after each product administration.
-
Product Use Questionnaire: The first item of this questionnaire, given 15 min after each product administration, assessed whether participants were able to keep the tablet under the tongue until it dissolved. The second question, given 30 min after the second product administration, asked which of the two products (“first” or “second”) was preferred.
-
Replacing Cigarettes VAS: Participants used a 100-point visual analog scale to estimate the percentage of cigarettes they would replace with SL nicotine tablets if the product were available and shown to be a healthier alternative to smoking cigarettes. This questionnaire, given 30 min after each product administration, also assessed the influence of other factors (i.e., health effects on self and others, effectiveness in reducing cravings, enjoyment, ability for discreet use, and price) on the percentage of cigarettes participants would replace.
-
Product Price Questionnaire: This two-item questionnaire assessed what participants would expect to pay for the investigative product compared to a pack of cigarettes using a 5-point scale, ranging from “a lot less than your cigarettes” to “a lot more than your cigarettes.” It also assessed the influence of price on the percentage of cigarettes participants would replace. The questionnaire was given 5 min before, and 30 min after each product administration.
-
Heart rate and blood pressure: These measurements were assessed at baseline and at 30, 60, 90, and 120 min after each product administration.
-
Adverse events (AE): Any adverse effects were assessed using an open-ended inquiry and coded by the medical team for severity (mild, moderate, or severe) and relationship, if any, to tablet use.
Data analysis
As this study was exploratory in nature, a descriptive approach was taken to the data, characterizing means and standard deviations of outcome measures.
Results: Study 2
Participants
Participants (11 males, 13 females) had a mean age of 45.8 (SD = 10.72) years and comprised 19 whites, 5 African Americans. Baseline cigarette consumption averaged 19.0 cigarettes/day (SD = 5.23), FTND score was 4.5 (SD = 1.22), and expired air CO was 25.5 ppm (SD = 10.50).
Subjective responses
Figure 2 shows the total mMNWS withdrawal symptom score in the 1 mg and 2 mg tablet conditions, and Fig. 3 shows the craving item separately. In both conditions, there was a rapid drop in craving and total withdrawal ratings after tablet administration with a partial recovery over time. A post hoc analysis on the change in craving and withdrawal symptoms from − 5 min to 5 min showed a significant decrease in both dose conditions (for craving, F(1,23) = 23.00, P < 0.0001 in the 1 mg condition and F(1,23) = 38.74, P < 0.0001 in the 2 mg condition; for total withdrawal score, F(1,23) = 15.11, P = 0.0007 in the 1 mg condition and F(1,23) = 16.97, P = 0.0004 in the 2 mg condition). There appeared to be a more prolonged relief of craving and withdrawal symptoms for the 2 mg tablet.
Table 2 summarizes the subjective ratings for the 1 mg and 2 mg SL tablets as well as the baseline ratings of participants’ usual brands of cigarettes on the mCEQ. While ratings of satisfaction and psychological reward were somewhat lower than for participants’ usual brands of cigarettes, immediate craving reduction was similar in magnitude.
Product preference: Slightly more than half of participants, 58.3% (14 /24), reported preferring the 1 mg tablet dose.
Desire to Smoke Visual Analog Scale (VAS): The Desire to Smoke Scale showed similar results as the MNWS craving item. The mean value decreased from 48.8 (SD = 26.2) before tablet administration to 19.8 (SD = 21.4) at 15 min in the 1 mg condition and from 55.0 (SD = 28.0) to 22.0 (SD = 23.2) in the 2 mg condition, with a gradual recovery toward initial levels over time.
Replacing cigarettes VAS: Overall, participants estimated that they would replace 67.0% of cigarettes (SD = 33.7) with the 1 mg nicotine SL tablet, and 64.7% (SD = 35.6) with the 2 mg nicotine SL tablet. Those stating that they would be more than “moderately likely” (4 on a 7-point scale) to use the product if it were available (11/24 participants with the 1 mg SL tablet and 12/24 with the 2 mg SL tablet), estimated they would replace 87.1% (SD = 12.9) of cigarettes with the 1 mg SL tablet and 87.3% (SD = 16.6) with the 2 mg SL tablet.
Product Price Questionnaire: Participants rated the amount they would expect to pay for a 20-tablet pack compared to a pack of cigarettes as 2.8 (SD = 1.2) for the 1 mg tablet and 2.6 (SD = 1.1) for the 2 mg tablet (on a 5-point scale). These ratings fell between “a little less” (2) and “the same amount” (3) as their cigarettes.
Cardiovascular measures: Neither dose of the nicotine SL tablet affected systolic or diastolic blood pressure. Heart rate, however, showed an increase after the 2 mg dose, from 67.8 bpm (SD = 11.5 bpm) at 50 min pre-dosing, to 76.2 bpm (SD = 8.4 bpm) at 30 min post-dosing, a boost of 8.3 bpm (SD = 5.8 bpm). This increase in heart rate was sustained to a great extent through the post-dosing period; at 120 min, the mean heart rate was 73.8 (SD = 11.9). No effect on heart rate could be discerned in the 1 mg tablet condition.
Side effects: Adverse effects related to tablet usage were rated as mild (easily tolerated and not interfering with normal activities) and included excessive salivation (n = 4), tongue or throat irritation (n = 4), heartburn and nausea (n = 3), hiccups (n = 2), asymptomatic bradycardia (n = 2), burping (n = 1), heartburn (n = 1), and dizziness (n = 1).
Open-ended comments: There were numerous spontaneous positive comments (15 participants) about the product, which focused on the effectiveness in alleviating craving for cigarettes, that it was relaxing, took the edge off withdrawal, had good flavor, did not have a smoke smell, had potentially reduced harmful effects, could be used discretely and conveniently, and that it was an appealing alternative to smoking. A small number of negative comments (2 participants) focused mainly on the harsh taste reported by one participant and burping/hiccups experienced by another participant.
Discussion: Study 2
The findings of this study, focusing on subjective and physiological responses, complement those of the previous pharmacokinetic study. The subjective responses to the SL tablet were generally positive, with more than half of participants stating they would be likely to use the product and that they would use it to replace a large fraction of their cigarettes. Notably, subjective craving reduction was similar between 2 mg SL tablet (mean of 4.2) and participants’ retrospective ratings of the last cigarette they smoked (mean of 4.6). Additionally, the extent and duration of craving reduction after SL tablet use is similar to that reported in other studies assessing craving after cigarette smoking (Adriaens et al. 2018). The 2 mg dose, also seemed to produce a more prolonged relief of nicotine withdrawal symptoms (Fig. 1). In general, ratings for the SL tablets were comparable to cigarettes in terms of a high degree of craving relief and low ratings of aversion (nausea, dizziness). Satisfaction and psychological reward, however, were somewhat higher for the usual brands of cigarettes.
Notably, craving and withdrawal symptoms were rapidly alleviated following SL tablet administration. Other nicotine replacement products that deliver nicotine more slowly through the buccal mucosa do not appear to alleviate craving and withdrawal symptoms as rapidly (Kotlyar et al. 2017).
General discussion
The two studies described above suggest that the novel SL tablet achieves a favorable pharmacokinetic profile that delivers nicotine faster than the currently marketed nicotine lozenge. This rapid nicotine delivery was paralleled by a rapid relief of nicotine withdrawal symptoms and cigarette craving in a sample of dependent smokers. Indeed, it is remarkable that the tablet alleviated craving almost as effectively as participants’ usual brands of cigarettes. The increase in heart rate apparent after the 2 mg nicotine SL tablet dose (8 bpm) approached that of the heart rate boost after smoking, which is typically 8–15 bpm (Stiles et al. 2017; Ward et al. 1992). The initial rise in plasma nicotine concentrations, 0.4 ng/mL*min, while significantly faster than with the nicotine lozenge, was less than typically obtained with smoking, 1–3 ng/mL*min (typically a 10–15 ng/mL boost over 5–10 min (Ebajemito et al. 2020; Stiles et al. 2017)).
Prior studies suggested that the initial rate of drug delivery may be more important than Cmax in producing subjective effects for drugs of abuse (de Wit et al. 1993). Studies using intravenous (IV) nicotine administration have also reported that more rapid infusions were more effective at relieving craving for cigarettes (Rose et al. 2003). One reason rapid nicotine delivery is important is because it leads to the prompt restoration of a “normal” state due to relief of withdrawal symptoms. Additionally, rapid nicotine delivery can elicit positively reinforcing pharmacological effects (e.g., brain dopamine release) mediated by nicotinic receptors, before they undergo desensitization (Grady et al. 2008; Koranda et al. 2014; Picciotto et al. 2008). Thus, nicotinic receptors will respond maximally to rapidly increasing nicotine concentrations, rather than to slowly increasing concentrations.
The importance of providing immediate reinforcement for alternative nicotine products has been noted by some leading experts (Abrams et al. 2018), but has not played a major role in pharmaceutical development. For example, nicotine patches provide slow sustained nicotine levels. Nicotine gums and lozenges, while delivering nicotine more rapidly than the patch, are still relatively slow compared with cigarette smoking. Thus, the immediate reinforcement provided by current NRT products is modest at best and therefore are often underutilized by smokers (Shiffman et al. 2008). For example, while some clinical trials have found that 61% of smokers adhere to a course of NRT over 4–10 weeks, population-based studies have found only 16–35% adherence rates (Mersha et al. 2021). Moreover, in a study of UK stop-smoking services, only 6% of smokers who were abstinent at 4 weeks were still using NRT at 1 year (Shahab et al. 2016). Instead of seeking to replace the dangerous habit of smoking with an alternative noncombustible rapidly absorbed nicotine product, the approach in cessation treatment has generally been to provide a temporary treatment aimed at relieving smoking withdrawal symptoms in the short run. As a result, relapse rates are extremely high, with almost all smokers returning to the use of cigarettes within a year after a quit attempt using NRT (Etter 2006; Jackson et al. 2019). The public health impact and efficacy of NRT and other smoking cessation medications has been significantly undermined by long-term relapse (Rosen et al. 2021).
If instead, the strategy is to provide a long-term nicotine substitution with the goal of tobacco harm reduction, then it is crucial for a product to provide rapid nicotine delivery and immediate reinforcement. If an alternative nicotine product is not sufficiently reinforcing, it will not effectively supplant the smoking habit. The advantages of rapid nicotine delivery, however, need to be weighed against its potential abuse liability and appeal to nonsmokers. Nonetheless, long-term relapse to smoking currently remains such a serious problem for smokers trying to maintain abstinence that there is clear value to a more reinforcing, effective mode of nicotine substitution.
Limitations of the studies include the relatively small sample sizes used, and the absence of pharmacokinetic data in Study 2. Additional limitations were that there was no other active comparator (e.g., cigarettes) or placebo condition, and the order of dose administration was fixed. The results, however, were sufficiently robust that clear differences were seen between the pharmacokinetics of the SL nicotine tablet and the nicotine lozenge in Study 1, and the heart rate boosts and relief of craving and withdrawal symptoms in Study 2 provided corroboration of rapid nicotine absorption. An additional limitation was the absence of a placebo control in Study 2, such that expectancy effects could have contributed to the craving relief and other subjective effects. However, the difference in the time course of craving, with the 2 mg dose outlasting the 1 mg dose, suggests that participants were sensitive to the individual product characteristics.
In summary, the results of the studies described above support that this novel nicotine SL tablet formulation shows promise as a harm reduction nicotine substitution strategy for cigarette smokers, and has potential as a smoking cessation treatment. Further randomized placebo-controlled studies are clearly warranted to fully characterize the potential of this approach.
References
Abrams DB, Glasser AM, Pearson JL, Villanti AC, Collins LK, Niaura RS (2018) Harm minimization and tobacco control: reframing societal views of nicotine use to rapidly save lives. Annu Rev Public Health 39(1):193–213. https://doi.org/10.1146/annurev-publhealth-040617-013849
Adriaens K, Gucht DV, Baeyens F (2018) IQOSTM vs. e-cigarette vs. tobacco cigarette: a direct comparison of short-term effects after overnight-abstinence. Int J Environ Res Public Health 15(12):2902. https://doi.org/10.3390/ijerph15122902
Amodei N, Lamb RJ (2008) Over-the-counter nicotine replacement therapy: can its impact on smoking cessation be enhanced? Psychol Addict Behav 22(4):472–485. https://doi.org/10.1037/0893-164X.22.4.472
Benowitz NL, Hukkanen J, Jacob P (2009) Nicotine chemistry, metabolism, kinetics and biomarkers. In: Henningfield JE, London ED, Pogun S (eds) Nicotine psychopharmacology, vol 192. Springer Berlin, Heidelberg, pp 29–60. https://doi.org/10.1007/978-3-540-69248-5_2
Bergeria CL, Heil SH, Davis DR, Streck JM, Sigmon SC, Bunn JY, Tidey JW, Arger CA, Reed DD, Gallagher T, Hughes JR, Gaalema DE, Stitzer ML, Higgins ST (2019) Evaluating the utility of the modified cigarette evaluation questionnaire and cigarette purchase task for predicting acute relative reinforcing efficacy of cigarettes varying in nicotine content. Drug Alcohol Depend 197:56–64. https://doi.org/10.1016/j.drugalcdep.2019.01.004. Epub 2019 Feb 13
Blondal T, Gudmundsson LJ, Olafsdottir I, Gustavsson G, Westin A (1999) Nicotine nasal spray with nicotine patch for smoking cessation: randomised trial with six year follow up. BMJ (Clin Res Ed) 318(7179):285–288. https://doi.org/10.1136/bmj.318.7179.285
Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG (2007) Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav 32(5):912–923. https://doi.org/10.1016/j.addbeh.2006.06.028
Choi J, Dresler C, Norton M, Strahs K (2003) Pharmacokinetics of a nicotine polacrilex lozenge. Nicotine Tob Res 5(5):635–644. https://doi.org/10.1080/1462220031000158690
Chow S-C, Liu J (2009) Design and analysis of bioavailability and bioequivalence studies, 3rd edn. CRC Press
Dautzenberg B, Nides M, Kienzler J-L, Callens A (2007) Pharmacokinetics, safety and efficacy from randomized controlled trials of 1 and 2 mg nicotine bitartrate lozenges (Nicotinell®). BMC Clin Pharmacol 7(1):11. https://doi.org/10.1186/1472-6904-7-11
de Wit H, Dudish, S, Ambre, J (1993) Subjective and behavioral effects of diazepam depend on its rate of onset. Psychopharmacology 112:324–330. https://doi.org/10.1007/BF02244928
Ebajemito JK, McEwan M, Gale N, Camacho OM, Hardie G, Proctor CJ (2020) A randomised controlled single-centre open-label pharmacokinetic study to examine various approaches of nicotine delivery using electronic cigarettes. Sci Rep 10(1):19980. https://doi.org/10.1038/s41598-020-76610-4
Etter J-F (2006) Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control 15(4):280–285. https://doi.org/10.1136/tc.2005.015487
Feyerabend C, Ings R, Russel M (1985) Nicotine pharmacokinetics and its application to intake from smoking. Br J Clin Pharmacol 19(2):239–247. https://doi.org/10.1111/j.1365-2125.1985.tb02637.x
Gottlieb S, Zeller M (2017) A nicotine-focused framework for public health. N Engl J Med 377(12):1111–1114. https://doi.org/10.1056/NEJMp1707409
Grady SR, Marks MJ, Collins AC (2008) Desensitization of nicotine-stimulated [3H]dopamine release from mouse striatal synaptosomes. J Neurochem 62(4):1390–1398. https://doi.org/10.1046/j.1471-4159.1994.62041390.x
Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T (2018) Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev 2019(1). https://doi.org/10.1002/14651858.CD000146.pub5
Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO (1991) The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 86(9):1119–27. https://doi.org/10.1111/j.1360-0443.1991.tb01879.x
Hua S (2019) Advances in nanoparticulate drug delivery approaches for sublingual and buccal administration. Front Pharmacol 10:1328. https://doi.org/10.3389/fphar.2019.01328
Hughes JR (1986) Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43(3):289. https://doi.org/10.1001/archpsyc.1986.01800030107013
Hughes J, Hatsukami DK (1998) Errors in using tobacco withdrawal scale. Tob Control 7(1):92–93. https://doi.org/10.1136/tc.7.1.92a
Jackson SE, McGowan JA, Ubhi HK, Proudfoot H, Shahab L, Brown J, West R (2019) Modelling continuous abstinence rates over time from clinical trials of pharmacological interventions for smoking cessation. Addiction 114(5):787–797. https://doi.org/10.1111/add.14549
Koranda JL, Cone JJ, McGehee DS, Roitman MF, Beeler JA, Zhuang X (2014) Nicotinic receptors regulate the dynamic range of dopamine release in vivo. J Neurophysiol 111(1):103–111. https://doi.org/10.1152/jn.00269.2013
Kotlyar M, Lindgren BR, Vuchetich JP, Le C, Mills AM, Amiot E, Hatsukami DK (2017) Timing of nicotine lozenge administration to minimize trigger induced craving and withdrawal symptoms. Addict Behav 71:18–24. https://doi.org/10.1016/j.addbeh.2017.02.018
McCarty JA (2015) Transmucosal drug delivery system (Patent No. US 8,992,974)
McNeill A, Foulds J, Bates C (2001) Regulation of nicotine replacement therapies (NRT): a critique of current practice. Addiction (Abingdon, England) 96(12):1757–1768. https://doi.org/10.1080/09652140120089508
Mersha AG, Eftekhari P, Bovill M, Tollosa DN, Gould GS (2021) Evaluating level of adherence to nicotine replacement therapy and its impact on smoking cessation: a systematic review and meta-analysis. Arch Public Health 79(1):26. https://doi.org/10.1186/s13690-021-00550-2
Molander L, Lunell E (2001) Pharmacokinetic investigation of a nicotine sublingual tablet. Eur J Clin Pharmacol 56(11):813–819. https://doi.org/10.1007/s002280000223
Morgan JC, Cappella JN (2021) Harm perceptions and beliefs about potential modified risk tobacco products. Int J Environ Res Public Health 18(2):576. https://doi.org/10.3390/ijerph18020576
Murray RP, Connett JE, Zapawa LM (2009) Does nicotine replacement therapy cause cancer? Evidence from the Lung Health Study. Nicotine Tob Res 11(9):1076–1082. https://doi.org/10.1093/ntr/ntp104
Olsson Gisleskog PO, Perez Ruixo JJ, Westin Å, Hansson AC, Soons PA (2021) Nicotine population pharmacokinetics in healthy smokers after intravenous, oral, buccal and transdermal administration. Clin Pharmacokinet 60(4):541–561. https://doi.org/10.1007/s40262-020-00960-5
Picciotto MR, Addy NA, Mineur YS, Brunzell DH (2008) It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84(4):329–342. https://doi.org/10.1016/j.pneurobio.2007.12.005
Rose JE, Behm FM, Westman EC, Bates JE, Salley A (2003) Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacol Biochem Behav 76(2):243–250. https://doi.org/10.1016/j.pbb.2003.07.002
Rosen LJ, Galili T, Kott J, Rees V (2021) Beyond “safe and effective”: the urgent need for high-impact smoking cessation medications. Prev Med 150:106567. https://doi.org/10.1016/j.ypmed.2021.106567
Shahab L, Dobbie F, Hiscock R, McNeill A, Bauld L (2016) Prevalence and impact of long-term use of nicotine replacement therapy in UK Stop-Smoking Services: findings from the ELONS Study. Nicotine Tobacco Res ntw58. https://doi.org/10.1093/ntr/ntw258
Shiffman S, Hughes JR, Di Marino ME, Sweeney CT (2003) Patterns of over-the-counter nicotine gum use: persistent use and concurrent smoking: misuse of nicotine gum. Addiction 98(12):1747–1753. https://doi.org/10.1111/j.1360-0443.2003.00575.x
Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG (2008) Use of smoking-cessation treatments in the United States. Am J Prev Med 34(2):102–111. https://doi.org/10.1016/j.amepre.2007.09.033
Stiles MF, Campbell LR, Graff DW, Jones BA, Fant RV, Henningfield JE (2017) Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability. Psychopharmacology 234(17):2643–2655. https://doi.org/10.1007/s00213-017-4665-y
Toll BA, O’Malley SS, McKee SA, Salovey P, Krishnan-Sarin S (2007) Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychol Addict Behav 21(2):216–225. https://doi.org/10.1037/0893-164X.21.2.216
Ward KD, Garvey AJ, Bliss RE (1992) Evidence of transient heart rate change after smoking cessation. Psychopharmacology 106(3):337–340. https://doi.org/10.1007/BF02245414
West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A (2000) A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology 149:198–202
Westman EC, Levin ED, Rose JE (1992) Smoking while wearing the nicotine patch-is smoking satisfying or harmful. Clin Res 40(4):A871–A871
Funding
Study 1 was funded by the National Institute of Health via a National Institute on Drug Abuse STTR Grant 1R41DA033710-01A1, and Study 2 was funded by Nicotine BRST LLC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JER and FMB disclose research support from Foundation for a Smoke-Free World, Philip Morris International, Altria, Embera Neurotherapeutics, Inc., Otsuka Pharmaceutical, JUUL Labs, consulting with Revive pharmaceuticals, and consulting and patent purchase agreement with Philip Morris International.
JM is Nicotine BRST’s Chief Scientific Officer at which he holds an equity interest.
FV has consulted with Revive Therapeutics.
TLB, DRB, and PNW disclose research support from Foundation for a Smoke-Free World, Philip Morris International, Altria, Embera Neurotherapeutics, Inc., Otsuka Pharmaceutical, and JUUL Labs.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rose, J.E., Behm, F.M., Botts, T.L. et al. Novel rapid-acting sublingual nicotine tablet as a cigarette substitution strategy. Psychopharmacology 239, 2853–2862 (2022). https://doi.org/10.1007/s00213-022-06171-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06171-z