Abstract
Rationale
The experience of reward entails both positive affect and motivation. While the brain regions responsible for these distinct aspects of reward are dissociable from each other, the paraventricular nucleus of the thalamus (PVT) may play a role in both.
Objectives
To investigate the role of the PVT in both affect and motivation, and to identify neuropeptides that might mediate these effects.
Methods
Male rats were tested for conditioned place preference following temporary inactivation of the anterior or posterior PVT with local injections of the GABAB and GABAA agonists, baclofen + muscimol. They were tested for sucrose seeking under a fixed ratio 3 (FR3) schedule of reinforcement and after extinction, following injection into the posterior PVT of baclofen + muscimol or saline vehicle. Finally, quantitative real-time PCR was used to examine local neuropeptide gene expression following injection into the posterior PVT of baclofen + muscimol or saline vehicle.
Results
Conditioned place preference was induced by temporary inactivation of the posterior but not anterior PVT. While sucrose seeking under an FR3 schedule of reinforcement was unaffected by inactivation of the posterior PVT, reinstatement of sucrose seeking was promoted by posterior PVT inactivation. Local gene expression of pituitary adenylate cyclase–activating polypeptide (PACAP), but not enkephalin or neurotensin, was reduced following inactivation of the posterior PVT.
Conclusions
Temporary inactivation of the posterior PVT affects both affect and motivation as well as local gene expression of PACAP. These results suggest that the posterior PVT is one brain region that may participate in both major aspects of reward.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The experience of reward entails both positive affect and motivation (Berridge and Robinson 2016; Morales and Berridge 2020). While the brain regions responsible for these distinct aspects of reward are dissociable from each other, regions that mediate positive affect have nevertheless been found to be embedded within the larger circuitry that mediates motivation (Morales and Berridge 2020), suggesting that some nuclei or subnuclei may participate in both the affective and motivational aspects of reward. One candidate region that may be involved in both positive affect and motivation is the paraventricular nucleus of the thalamus (PVT). This dorsal midline thalamic nucleus is a key node in the limbic system, with extensive interconnections with numerous other limbic nuclei (Dong et al. 2017; Li and Kirouac 2008; 2012; Vertes and Hoover 2008). Due to findings with behavior changes, anatomical connections, and expression of specific neuropeptides, the PVT has been proposed to play a major role in both affect and motivation (Barson et al. 2020; Curtis et al. 2020; Iglesias and Flagel 2021; Kelley et al. 2005; McGinty and Otis 2020).
Published results using behavioral testing support a role for the PVT in both positive affect and motivation, although the direction of this role has varied across experiments. For example, as a test of affect, real-time place aversion was found to be induced by photoactivation of rat PVT projections to the nucleus accumbens or amygdala (Do-Monte et al. 2017). In contrast, conditioned place preference for a high-fat diet-paired context was blocked by inhibition of the rat PVT with local injection of a glucagon-like peptide-1 receptor agonist (Ong et al. 2017), and the expression of conditioned place preference for a cocaine-paired chamber was prevented by temporary inactivation of the rat PVT with the GABAB and GABAA agonists, baclofen + muscimol (Browning et al. 2014). Similarly, as a test of motivation, cue-induced lever-pressing for sucrose during unexpected reward omission was found to be increased by inhibition with muscimol injected in the rat PVT or by photoinhibition of rat PVT projections to the nucleus accumbens (Do-Monte et al. 2017). In contrast, cue-induced lever-pressing for sucrose during a reinstatement test was decreased by inhibition with the glucagon-like peptide-1 receptor agonist injected in the rat PVT (Ong et al. 2017). While these disparate results may be due to differences in the experimental paradigms, it is also possible that different cell populations within the PVT make different contributions to the different aspects of reward.
The two major subregions of the PVT have been speculated to play different roles in affect and motivation. Recent research has found that specific anatomical projections and neuropeptide gene expression varies across the antero-posterior axis, with some showing a progressive decrease from the anterior to the posterior PVT or in the opposite direction. For example, while neurons across the rat PVT send robust projections to the nucleus accumbens, bed nucleus of the stria terminalis, and central amygdala, the anterior PVT has denser projections to the dorsomedial nucleus accumbens shell, while the posterior PVT has denser projections to the ventromedial shell, as well as the bed nucleus of the stria terminalis and central amygdala (Dong et al. 2017; Li and Kirouac 2008; Vertes and Hoover 2008). Similarly, while the neuropeptide galanin is most dense in cells of the mouse anterior PVT and becomes progressively less dense across the antero-posterior axis, tachykinin 2 is more dense in cells of the mouse posterior PVT (Curtis et al. 2020; Gao et al. 2020). This distribution of projections and gene expression has led some groups to speculate that the anterior PVT could play a greater role in positive affect and appetitive motivation, while the posterior PVT plays a greater role in negative affect and aversive motivation (Dong et al. 2017; Iglesias and Flagel 2021). Our own group has speculated that the anterior PVT may make a greater contribution to arousal, while the posterior PVT makes a greater contribution to valence and motivation (Curtis et al. 2020).
The purpose of this study was to investigate the role of the PVT in both affect and motivation, and to identify neuropeptides that might mediate these effects. With both metabotropic GABAB receptors and ionotropic GABAA receptors identified in the rat PVT (Bischoff et al. 1999; Gao et al. 1995; Margeta-Mitrovic et al. 1999), we temporarily inactivated the PVT subregions using local injections of baclofen + muscimol to determine the effects on conditioned place preference (as a measure of positive affect) and sucrose seeking behavior (as a measure of motivation), under a fixed ratio 3 (FR3) schedule of reinforcement and during reinstatement testing following extinction. Then, with the neuropeptides pituitary adenylate cyclase–activating polypeptide (PACAP), enkephalin, and neurotensin all identified in the rodent PVT (Curtis et al. 2020; Gupta et al. 2018; Hermanson et al. 1995; Pandey et al. 2019) and implicated in both affect and motivation (Guy et al. 2011; Hurley et al. 2019; Lopak and Erb 2005; Miles et al. 2018; Ollmann et al. 2015; Phillips et al. 1983), we used quantitative real-time PCR to measure the effects of local baclofen + muscimol on local gene expression of these neuropeptides. We hypothesized that inhibition of the posterior PVT would affect both affect and motivation and that this inhibition would also affect gene expression of one or more neuropeptides within the PVT.
Materials and methods
Animals and housing
Adult, male Long-Evans rats (N = 24; 251–275 g (Exps. 1 and 3) or 201–225 g (Exp. 2) at the start of the experiment, Charles River Laboratories International, Inc., Malvern, PA, USA) were housed in an AAALAC-accredited facility, on a 12-h light/dark cycle (lights off at 0900 h). Throughout the experiment, they were individually housed in polycarbonate cages, with Beta Chip® bedding, and one Bed-r’Nest nestlet (The Andersons, Inc., Maumee, OH). They were given 1 week to acclimate to the facility and were handled daily prior to the start of experiments. Animals received ad libitum chow (Laboratory Rodent Diet 5001, Lab Diet, St. Louis, MO) via a metal food hopper and water via one 16 oz Macrolon bottle (Ancare, Bellmore, NY) with a non-drip sipper tube. Experiments were approved by the Institutional Animal Care and Use Committee of Drexel University College of Medicine and followed the NIH Guide for the Care and Use of Laboratory Animals.
Drugs
The GABAB receptor agonist, baclofen, and the GABAA receptor agonist, muscimol, were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved together in 0.9% saline (Baxter International Inc., Deerfield, IL, USA) at a dose of 0.3 nmol baclofen and 0.03 nmol muscimol per 0.3 μl of solution. This dose is commonly used to inactivate limbic nuclei (Barson and Leibowitz 2015; Li et al. 2015; Sartor and Aston-Jones 2012).
Cannulations and microinjections
Rats were cannulated in the anterior PVT (1.7 mm posterior to bregma, ± 0.0 mm lateral to midline, 4.6 mm ventral to the level skull) or posterior PVT (3.4 mm posterior to bregma, ± 0.0 mm lateral to midline, 4.6 mm ventral to the level skull) as described (Barson and Leibowitz 2015; Pandey et al. 2019). They were initially anesthetized with 5% isoflurane in 2 L/min oxygen and were then maintained under continuous anesthesia with 2–3% isoflurane in 1 L/min oxygen. Warm saline (5 ml s.c., Baxter) was injected to prevent dehydration and bupivacaine (2 mg/kg s.c., Hospira Worldwide, Lake Forest, IL, USA) was injected into the scalp prior to incision. Four 1/8″ stainless steel screws (Small Parts at Amazon.com, Seattle, WA, USA) were threaded into the bone and a single 10 mm stainless steel guide shaft (21-gauge, Small Parts) was implanted perpendicularly, with the midsagittal sinus gently moved to the side prior to implantation. The cannula was anchored into the skull with dental acrylic (Yates Motloid, Chicago, IL, USA). To prevent occlusion, a 26-gauge stainless steel stylet was left in the guide shaft between injections. Buprenorphine hydrochloride (0.03 mg/kg s.c., Reckitt & Colman Inc., Slough, UK) was administered for post-operative analgesia. During the week of recovery from surgery, animals were handled daily, and their stylet was removed and replaced to acclimate them to the microinjection procedure.
For injection, similar to previous publications (Barson and Leibowitz 2015; Pandey et al. 2019), freshly prepared solutions were given through concentric microinjectors of 26-gauge stainless steel outside and fused-silica tubing inside (74 μm ID, 154 μm OD; Polymicro Technologies, Phoenix, AZ, USA) that extended 1.5 mm beyond the guide shafts to reach the target region. A syringe pump (Harvard Apparatus, Holliston, MA, USA) delivered 0.3 μl of solution over 30 s, and the microinjector was left in place for another 30–60 s to allow for diffusion. We have previously demonstrated that injections of methylene blue dye at this volume (0.3 μl) have a radial spread of approximately 0.5 mm, such that those made in the anterior PVT never reach the posterior PVT and vice versa (Barson et al. 2015; Barson and Leibowitz 2015). Similarly, injection of 0.3 μl of the neuropeptide, neurotensin, into the anterior or posterior PVT increases levels of c-Fos in a radius of approximately 0.5 mm (Pandey et al. 2019). Nevertheless, it is possible that the baclofen + muscimol also affected adjacent thalamic nuclei, which also contain GABA receptors (Churchill et al. 1996). For brain images of cannula placement in the anterior and posterior PVT, the reader is directed to some of our previous publications, which used the same approach (Barson et al. 2015; Pandey et al. 2019).
Statistical analyses
All data were analyzed using SPSS (Version 26, IBM, Armonk, NY, USA). Significance was determined at p < 0.05. Data are reported as mean ± standard error of the mean (SEM). Graphical representation of the data was created with Prism 8 (GraphPad Software, San Diego, CA). Schematic representations were created with BioRender.com.
Detailed methods
Experiment 1 — Effects of PVT subregion inactivation on conditioned place preference
Conditioned place preference apparatus
Conditioned place preference was assessed in an automated activity chamber with an area of 43.2 cm × 43.2 cm and 42 cm high walls with a two-chamber place preference insert (Med Associates, Inc., St. Albans, VT, USA). The insert contained distinct tactile environments, with a wire mesh floor in one chamber and a grid floor in the other, separated by a black wall with a manual guillotine door. As pilot experiments indicated that rats strongly preferred the mesh floor chamber when in complete darkness, a low level of light was provided to each chamber, to reduce this disparity in preference. The lighting of the wire mesh chamber was set at a lux of 10, while that of the grid chamber was at 3. Time in each chamber (s) and distance traveled (cm) were recorded by infrared beams.
Experimental protocol
To determine the effect of temporary inactivation of the anterior and posterior PVT on affective behavior, rats (n = 5/subregion) were tested for their place preference following conditioning with local injections of baclofen + muscimol (Fig. 1a). One week following their arrival in the facility, rats were cannulated in the anterior or posterior PVT and were then given 1 week of recovery prior to testing. Testing occurred in three phases over 5 days. For the initial, baseline pretest phase (day 1), starting 1 h into the dark cycle (1000 h), rats were allowed to explore both chambers of the apparatus for 15 min, with the manual door removed. For the conditioning phase (days 2–4), an unbiased design was used, where chambers with a wire mesh or grid floor were randomly assigned in a counterbalanced manner to be paired with injection of baclofen + muscimol or saline vehicle. Over 3 days, rats received one drug or vehicle injection 1 h into the dark cycle and were then, starting 5–10 min later, confined to its associated chamber for 30 min; they then received the opposite injection 5 h into the dark cycle and were confined to its associated chamber for 30 min. Thus, rats received a total of 6 injections — 3 pairings of baclofen + muscimol and its chamber and 3 pairings of saline vehicle and its chamber — with the order of injections counterbalanced across days for each subject. Baclofen + muscimol was paired with the chamber with the wire mesh floor for 2 of the 5 rats receiving injections in the anterior PVT and 3 of the 5 rats receiving injections in the posterior PVT, while baclofen + muscimol was paired with the grid floor for the remaining rats. For the final, posttest phase (day 5), 1 h into the dark cycle, the manual door was again removed and rats were again allowed to explore both chambers of the apparatus for 15 min, with initial placement into the wire mesh or grid floor randomized across subjects. The difference in seconds between the time spent in the drug-paired chamber during the posttest and the pretest is a measure of the degree of conditioning induced by baclofen + muscimol. A positive difference indicates that animals have developed a preference for the drug-paired chamber. To verify injection location, slide-mounted 30 μm coronal brain slices were examined under a microscope (for anterior PVT) or cannula placement was visually verified during brain dissection (for posterior PVT; see Experiment 3).
Experimental timelines. a In Experiment 1, after 1 week of acclimation, rats were cannulated in the anterior or posterior PVT. After 1 week of recovery, they underwent conditioned place preference testing: a baseline pretest, three test days involving injection with 0.3 μl of saline vehicle or 0.3 nmol baclofen + 0.03 nmol muscimol, and a posttest. b In Experiment 2, rats were trained for 15 days under a fixed ratio 1 (FR1) schedule for a sucrose reward, then for 3 days under an FR2 schedule, then for 2 days under an FR3 schedule, and they were subsequently cannulated in the posterior PVT. Following 1 week of recovery, they had five more FR3 sessions and were then tested for FR3 responding following injection with saline vehicle or baclofen + muscimol. After that, they underwent extinction training for 8 days and were then tested again following saline vehicle or baclofen + muscimol. c In Experiment 3, rats after 1 week of acclimation were cannulated in the posterior PVT and then given 1 week of recovery or were given 1 week of recovery after conditioned place preference testing (Experiment 1). They were then injected with saline vehicle or baclofen + muscimol and sacrificed 30 min later for analysis using qRT-PCR
Statistical analyses
To assess chamber preference during the pretest, a one sample two-tailed t-test was used, to determine if the preference for one chamber was different from 50%. To assess the development of preference for the drug-paired chamber and changes in locomotor activity, a mixed ANOVA was used, to determine if time in the drug-paired chamber or distance traveled was different between pretest and posttest (repeated measure) and between animals injected into the anterior PVT and posterior PVT. Significant interaction effects were followed up with a Sidak pairwise comparison test (using codes added to the SPSS program). In addition, a one sample two-tailed t-test was used, to determine if the difference in time in the chamber between pretest and posttest was different from 0.
Experiment 2 — Effects of posterior PVT inactivation on sucrose seeking behavior
Self-administration apparatus
Operant testing with 10% sucrose w/v was performed in a 29.5 × 23.5 × 27.3 cm chamber (Med Associates, Inc., St. Albans, VT) inside a sound-attenuating, ventilated cubicle (Med Associates, Inc., St. Albans, VT). Each chamber had a clear polycarbonate door, rear panel, and top, and stainless steel side panels. The floor was a stainless steel grid and below that was a stainless steel waste pan. One wall contained two retractable levers, 6.5 cm above the floor, with a single cup liquid receptacle between them, connected to a contact lickometer. A white stimulus light and 2,900 Hz sonalert module was positioned above the receptacle. The start of each session was signaled by the extension of both active and inactive levers, which were kept extended for the duration of the testing session. All testing was conducted with the house lights off. During fixed ratio (FR) testing, upon completion of a ratio requirement, 0.1 ml of sucrose was delivered into the receptacle over 3.6 s via an 18-gauge tube connected to a syringe pump and the white stimulus light and 2,900 Hz tone were activated during delivery. Consumption of the reinforcer was confirmed through comparison of the timestamp of the ratio requirement completion and the lickometer beam break. Active lever presses made during reward delivery, all inactive lever presses, and all lever presses made during extinction and reinstatement testing were recorded but had no consequences.
Experimental protocol
To determine if temporary inactivation of the posterior PVT also affects motivated behavior, rats (N = 9) were tested for their fixed ratio self-administration of sucrose and reinstatement of sucrose seeking following local injections of baclofen + muscimol (Fig. 1b). All self-administration sessions were conducted over a 1-h period, starting 1 h into the dark cycle (1000 h), Monday–Friday. One week following their arrival in the facility, rats underwent water deprivation for 17 h and were then put into the operant chambers for the first time, where they could self-administer sucrose under an FR1 schedule (one lever press for one reward). Over the next 14 days, they underwent daily 1-h FR1 sessions, until they reached stable responding over at least 2 sessions. Over the subsequent 3 days, they underwent daily FR2 sessions, until they reached stable responding over at least 2 sessions. They then underwent 2 sessions of daily FR3 sessions and were subsequently cannulated in the posterior PVT. Following 1 week of recovery, rats underwent 5 daily 1-h FR3 sessions, until they reached stable responding over at least 2 sessions. During the next week, rats were tested in FR3 sessions starting 5–10 min after injection of baclofen + muscimol or saline vehicle, which was given within-subject in counterbalanced order across days, with one injection-free day of self-administration in between, to re-establish responding. Extinction training, during which active lever-pressing resulted in no reward delivery, stimulus light, or tone, was then conducted over 8 sessions until responding on the active lever over at least 2 sessions was less than 5% of the group average of FR3 responding (< 25 active lever presses). Finally, rats were tested in reinstatement sessions starting 5–10 min after injection of baclofen + muscimol or saline vehicle, which was given within-subject in counterbalanced order across days, with one injection-free day of self-administration in between, to re-establish responding. No light or tone stimuli or sucrose was provided during these reinstatement tests. To verify injection location, slide-mounted 30 μm coronal brain slices were examined under a microscope.
Statistical analyses
To assess lever-pressing during FR1, FR2, FR3, and extinction training sessions, a two-way repeated-measures ANOVA was used, with lever type and session as repeated measures. Significant effects were followed up with pairwise comparisons using the least significant difference, to maximize the chances of detecting any differences. To assess effects of baclofen + muscimol on lever-pressing under an FR3 schedule of reinforcement or following extinction, a two-way repeated-measures ANOVA was used, with lever type and drug/session as repeated measures, and significant effects were followed up with Sidak pairwise comparison tests (using codes added to the SPSS program to test interaction effects).
Experiment 3 — Effects of posterior PVT inactivation on local neuropeptide gene expression
Quantitative real-time PCR (qRT-PCR)
To obtain samples for analysis with qRT-PCR, the brain immediately after sacrifice was placed with the ventral surface facing up in a matrix slicing guide set on ice. Three coronal cuts were made, starting with the middle optic chiasm (Bregma − 1.5 mm) (Paxinos and Watson 2005). The second cut was 1.0 mm caudal to this, yielding a slice (Bregma − 1.5 to − 2.5 mm) for microdissection of the anterior PVT, and the third cut was 1.0 mm caudal to that, yielding a slice (Bregma − 2.5 to − 3.5 mm) for the posterior PVT. Under a microscope, on a slide placed on a Petri dish filled with ice, these slices were first examined to determine cannula placement. See Fig. 4b for an example of a fresh brain slice from a rat that had previously received an injection in the PVT. Then, the anterior PVT and posterior PVT were dissected as inverted isosceles triangles directly ventral to the dorsal third ventricle, approximately 0.8 mm wide at the base. The sections were stored at − 20 °C in RNAlater (Qiagen Inc., Valencia, CA, USA) until extraction of RNA.
As previously described (Pandey et al. 2019), total RNA from each brain section was purified using an RNeasy Mini Kit (Qiagen Inc.) and DNA was removed using RNase-free DNase 1 (Qiagen Inc.). The yield was quantified with a NanoDrop Lite spectrophotometer (Thermo Electron North America LLC, Madison WI), with resulting A260/A280 ratios between 1.82 and 2.12, indicating high purity. Using 1 µg of RNA from each sample, cDNA was reverse transcribed using SuperScript® VILO™ Master Mix (Invitrogen, Grand Island, NY, USA) in a SimpliAmp™ Thermal Cycler (Applied Biosystems, Waltham, MA, USA). Minus RT controls were synthesized by denaturing the reverse transcriptase. For qRT-PCR, the SYBR Green PCR core reagents kit (Applied Biosystems, Grand Island, NY, USA) was used in MicroAmp® Fast Optical 96-Well Reaction Plates (Applied Biosystems), with 12.5 ng of cDNA template in a 25 µl reaction volume, and run on a StepOnePlus Real-Time PCR System (Applied Biosystems), under the conditions of 2 min at 50 °C (primer annealing), 10 min at 95 °C (polymerase activation and sequence extension), and 40 cycles of 15 s at 95 °C (denaturation) plus 1 min at 60 °C (annealing and extension). Each sample was run in triplicate, and each run included a no-template and negative RT control. Target gene expression was quantified relative to cyclophilin A using the relative quantification method (ΔΔCT). Primers were designed with the NCBI Primer design tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Ye et al. 2012) and acquired from Invitrogen at Thermo Fisher Scientific (Grand Island, NY, USA) (Table 1).
Experimental protocol
To determine if temporary inactivation of the posterior PVT also affects gene expression of local neuropeptides, rats (N = 10) were examined using qRT-PCR following local injection with baclofen + muscimol (Fig. 1c). One week following their arrival in the facility, rats (n = 5, “drug-naïve”) were cannulated in the posterior PVT and then given 1 week of recovery prior to injection and sacrifice. The remaining rats (n = 5, “drug-experienced”), from Experiment 1, were given 1 week of recovery after conditioned place preference testing prior to injection and sacrifice. Rats were selected in a counterbalanced order from the drug-naïve and drug-experienced groups to receive injection of baclofen + muscimol (n = 5) or saline vehicle (n = 5), starting 1 h into the dark cycle (1000 h). Thus, 3 of the 5 rats that received baclofen + muscimol were drug-experienced, while 2 of the 5 that received saline vehicle were drug-experienced, and the other subjects were drug-naïve. Thirty minutes later, they were sacrificed via rapid decapitation and the anterior PVT and posterior PVT were dissected for analysis using qRT-PCR. We have previously performed qRT-PCR experiments with brain regions that had received drug microinjections (Barson et al. 2017; Karatayev et al. 2009).
Statistical analyses
To compare gene expression of each neuropeptide following injection with baclofen + muscimol or saline vehicle, an unpaired two-tailed t-test was used.
Results
Experiment 1 — Effects of PVT subregion inactivation on conditioned place preference
Injection verification
To ensure that injections were made into the intended subregion, cannula placement was verified under a microscope (for anterior PVT) or visually verified during brain dissection (for posterior PVT; see Experiment 3). Injections into the anterior PVT were made between − 1.72 and − 2.04 mm while those into the posterior PVT were made between − 3.12 and − 3.36 mm, relative to Bregma (Paxinos and Watson 2005) (Fig. 2a).
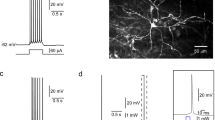
Effects of thalamic paraventricular nucleus (PVT) subregion inactivation on conditioned place preference. a Injection locations (indicated by black dots) in the anterior and posterior PVT (n = 5/subregion). Coordinates are relative to Bregma. Adapted from The Rat Brain, 5th edition, G. Paxinos and C. Watson, Copyright 2005, with permission from Elsevier. b Time spent in the baclofen + muscimol–paired chamber was significantly increased after injections in the posterior but not anterior PVT. The conditioned place preference (CPP) score is the difference in seconds between the time spent in the drug-paired chamber during the posttest and the pretest. +p < 0.05 vs. pretest; *p < 0.05 vs. anterior PVT. c Distance traveled during the test was significantly decreased following injections in the PVT. *p < 0.05 vs. acclimation (pretest). d Representative traces of posttest locomotor activity from rats injected in the anterior or posterior PVT. Injection of baclofen + muscimol was paired with the left chamber for this anterior PVT subject and with the right chamber for the posterior PVT subject. Values are mean ± SEM
Inactivation of the posterior PVT promotes conditioned place preference
To determine the effects of temporary inactivation of the PVT subregions on affective behavior, rats underwent conditioned place preference testing. Using an unbiased design, where chambers with a wire mesh or grid floor were randomly assigned in a counterbalanced manner to be paired with injection of baclofen + muscimol or saline vehicle, rats after an initial baseline pretest underwent a total of 6 drug-chamber pairings and were then allowed to freely explore both chambers during a final drug-free posttest. During the baseline pretest, rats overall showed a preference for the wire mesh floor, spending significantly more than 50% of their time in the associated chamber (49.39–66.86%; t9 = 3.621, p = 0.006); however, no rat spent more than 67% of their time in one chamber. Pairing of temporary inactivation of the PVT subregions with one chamber led to a significant change in the time spent in that chamber between pretest and posttest (t9 = 2.511, p = 0.033), although this differed between the subregions (Fig. 2b). With no main effect of PVT subregion on the time spent in the chambers (F1, 8 = 1.394, p = 0.272), there was a significant main effect of session (F1, 8 = 9.784, p = 0.014) and a significant interaction effect between subregion and session (F1, 8 = 5.964, p = 0.040). Specifically, while inactivation of the anterior PVT failed to significantly change time spent in the baclofen + muscimol–paired chamber (p = 0.641), inactivation of the posterior PVT significantly increased the time spent in the drug-paired chamber (p = 0.004). While distance traveled in the chambers differed significantly between the pretest and posttest (F1, 8 = 9.136, p = 0.016), there was no main effect of PVT subregion (F1, 8 = 0.035, p = 0.856), and this time there was no significant interaction effect between subregion and session (F1, 8 = 2.830, p = 0.131, Fig. 2c). Representative traces of posttest locomotor activity from rats injected in the anterior or posterior PVT are shown in Fig. 2d. Together, these data support the idea that inactivation of the PVT induces conditioned place preference, but this occurs specifically from inactivation of the posterior PVT.
Experiment 2 — Effects of posterior PVT inactivation on sucrose seeking behavior
Injection verification
To ensure that injections were made into the intended subregion, cannula placement was verified under a microscope. Two subjects were determined to have received injections outside of the target region and their data were thus removed from all analyses. All other subjects received injections in the posterior PVT, between − 3.24 and − 3.48 mm, relative to Bregma (Paxinos and Watson 2005) (Fig. 3a).
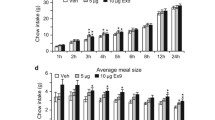
Effects of posterior thalamic paraventricular nucleus (PVT) inactivation on sucrose seeking behavior. a Injection locations (indicated by black dots) in the posterior PVT (N = 7). Coordinates are relative to Bregma. Adapted from The Rat Brain, 5th edition, G. Paxinos and C. Watson, Copyright 2005, with permission from Elsevier. b Self-administration of sucrose under a fixed ratio 1 schedule of reinforcement. *p < 0.05 vs. session 1. c Self-administration under a fixed ratio 2 schedule of reinforcement. *p < 0.05 vs. session 1. d Self-administration under a fixed ratio 3 schedule of reinforcement. *p < 0.05 vs. session 1. Dotted line indicates the time away from fixed ratio testing due to cannulation and recovery. e Self-administration under a fixed ratio 3 schedule of reinforcement was not significantly altered by injection with baclofen + muscimol into the posterior PVT. f Self-administration during extinction training. *p < 0.05 vs. session 1. g Reinstatement of extinguished sucrose seeking was promoted by injection with baclofen + muscimol into the posterior PVT. *p < 0.05 vs. last extinction and saline vehicle. Values are mean ± SEM
Inactivation of the posterior PVT promotes reinstatement of sucrose seeking
To determine if temporary inactivation of the posterior PVT also affects motivated behavior, rats were first tested for their self-administration of sucrose following local injection of baclofen + muscimol. They were trained to lever-press for sucrose under an FR1, FR2, and then FR3 schedule of reinforcement. During the FR1 training period, rats showed a significant increase in their lever-pressing across sessions (F14, 84 = 12.995, p < 0.001) and a significant preference for the active over the inactive lever (F1, 6 = 22.839, p = 0.003), and there was a significant interaction effect between session and lever (F14, 84 = 12.843, p < 0.001) (Fig. 3b). Across the 15 test sessions, overall lever-pressing became stable by session 11, such that pressing was no longer significantly different than subsequent sessions by session 11 (p = 0.117–0.931). Similarly, while inactive lever-pressing became stable by session 6 (p = 0.179–1.000), active lever-pressing became stable by session 11 (p = 0.266–0.865). During the FR2 training period, rats also showed a significant increase in their lever-pressing across sessions (F2, 12 = 5.511, p = 0.020) and a significant preference for the active over the inactive lever (F1, 6 = 236.216, p < 0.001), and there was a significant interaction effect between session and lever (F2, 12 = 6.047, p = 0.015) (Fig. 3c). Across the 3 test sessions, overall lever-pressing became stable by session 2 (p = 0.301). Similarly, while inactive lever-pressing was stable across all sessions (p = 0.118–1.000), active lever-pressing became stable by session 2 (p = 0.090–0.305). During the FR3 training period, rats also showed a significant increase in their lever-pressing across sessions (F6, 36 = 2.579, p = 0.035) and a significant preference for the active over the inactive lever (F1, 6 = 132.625, p < 0.001), and there was a significant interaction effect between session and lever (F6, 36 = 2.405, p = 0.047) (Fig. 3d). Across the 7 training sessions, overall lever-pressing was stable by session 3 (p = 0.065–0.803). Similarly, for both the active and inactive levers, pressing became stable by session 3 (p = 0.066–0.865). Following injection with baclofen + muscimol into the posterior PVT, while rats continued to show a significant preference for the active over the inactive lever (F1, 6 = 100.764, p < 0.001), they showed no difference compared to injection with saline vehicle in their overall lever-pressing under this FR3 testing (F1, 6 = 1.082, p = 0.388), and there was no significant interaction effect between drug and lever (F1, 6 = 1.041, p = 0.347) (Fig. 3e).
To test for reinstatement of sucrose seeking following local injection of baclofen + muscimol, rats next underwent extinction training. During this training, rats showed a significant decrease in their lever-pressing across sessions (F7, 42 = 26.716, p < 0.001) and a significant preference for the active over the inactive lever (F1, 6 = 40.024, p = 0.001), and there was a significant interaction effect between session and lever (F7, 42 = 21.209, p < 0.001) (Fig. 3f). Across the 8 test sessions, overall lever-pressing became stable by session 7 (p = 0.855). Similarly, both active and inactive lever-pressing was stable by session 7 (p = 0.411–0.724). With injection of baclofen + muscimol into the posterior PVT, compared to both the final extinction session and injection with saline vehicle, rats showed a significant difference in lever-pressing (F2, 12 = 9.162, p = 0.004) and a significant preference for the active over the inactive lever (F1, 6 = 16.763, p = 0.006), and there was a significant interaction effect between session and lever (F2, 12 = 12.304, p = 0.001) (Fig. 3g). Notably, while there was no significant difference in active lever-pressing between the final extinction session and the saline vehicle session (p = 0.171), lever-pressing during the baclofen + muscimol session was significantly greater than both of these control sessions (p = 0.040–0.045). Thus, while inactivation of the posterior PVT does not appear to affect self-administration of sucrose, it does promote reinstatement of extinguished sucrose seeking.
Experiment 3 — Effects of posterior PVT inactivation on local neuropeptide gene expression
Injection verification
To ensure that injections were made into the intended subregion, cannula placement was visually verified during brain dissection. Injections into the posterior PVT were made between − 3.12 and − 3.36 mm, relative to Bregma (Paxinos and Watson 2005) (Fig. 4a). See Fig. 4b for an example of a fresh brain slice from a rat that had previously received an injection in the PVT.
Effects of posterior thalamic paraventricular nucleus (PVT) inactivation on local neuropeptide gene expression. a Injection locations in the posterior PVT (N = 10, n = 5/injection). Red dots indicate locations from “drug-naïve” rats; black dots indicate locations from “drug-experienced” rats from Experiment 1. Coordinates are relative to Bregma. Adapted from The Rat Brain, 5th edition, G. Paxinos and C. Watson, Copyright 2005, with permission from Elsevier. b Example of a fresh brain slice from a rat that had previously received an injection in the PVT. Note that both the tract from the guide cannula and tissue damage from the injector can be seen in this image. c Local injection of baclofen + muscimol significantly reduced gene expression of pituitary adenylate cyclase–activating polypeptide (PACAP) but not enkephalin or neurotensin in the posterior PVT. *p < 0.05 vs. saline vehicle. d Injection of baclofen + muscimol in the posterior PVT did not significantly affect gene expression of PACAP, enkephalin, or neurotensin in the anterior PVT. Values are mean ± SEM
Inactivation of the posterior PVT reduces PACAP gene expression
To determine if temporary inactivation of the posterior PVT also affects gene expression of local neuropeptides, rats were locally injected with baclofen + muscimol or saline vehicle and their posterior as well as anterior PVT was examined using qRT-PCR. Examining neuropeptides in the posterior PVT, local injection of baclofen + muscimol was found to significantly reduce gene expression of PACAP (t8 = 4.863, p = 0.001) but not enkephalin (t8 = 0.262, p = 0.800) or neurotensin (t8 = 0.753, p = 0.473) (Fig. 4c). In the anterior PVT, which was not injected with baclofen + muscimol, there were no significant changes in the expression of PACAP (t8 = − 0.252, p = 0.807), enkephalin (t8 = 0.271, p = 0.793), or neurotensin (t8 = − 0.900, p = 0.394) (Fig. 4d). Thus, inactivation of the posterior PVT with baclofen + muscimol appears to selectively reduce local levels of PACAP mRNA, with no effects in the nearby anterior PVT.
Discussion
In the present study, we show that temporary inactivation of the posterior PVT affects both affect and motivation as well as local gene expression of PACAP. Specifically, local injection of baclofen + muscimol into the posterior but not anterior PVT induced conditioned place preference. Similarly, baclofen + muscimol in the posterior PVT promoted reinstatement of sucrose seeking but not sucrose seeking under an FR3 schedule of reinforcement. Finally, baclofen + muscimol in the posterior PVT specifically reduced local gene expression of PACAP but not enkephalin or neurotensin. While it cannot be ruled out that the baclofen + muscimol injections also affected adjacent thalamic nuclei, these results suggest that the posterior PVT is one brain region that may participate in both the affective and motivational aspects of reward.
The finding that inactivation of the posterior PVT induces conditioned place preference suggests that inhibition of this brain region can disinhibit positive affective behavior, or at the very least reduce aversion to the drug-paired side. This is consistent with the recent finding in a cell type largely restricted to the posterior PVT that calcium transients are decreased to purportedly rewarding stimuli (access to a female conspecific (for males) or a thermoneutral zone) (Gao et al. 2020). Interestingly, the finding that real-time place aversion was induced by photoactivation of rat PVT projections was found specifically for the anterior PVT, with the posterior PVT not tested (Do-Monte et al. 2017). This activation was accomplished using an AAV with a CaMKII promoter, which purportedly targets glutamatergic projections (Do-Monte et al. 2017), the main projection type of the PVT (Curtis et al. 2020; Huang et al. 2006; Parsons et al. 2007). In contrast, the baclofen + muscimol injections used in the present study would have acted on both soma and fibers (Gao et al. 1995; Margeta-Mitrovic et al. 1999), and thus could have affected both efferent and afferent signaling in the PVT. Moreover, the smaller subject number in the present study leaves open the possibility of a type II statistical error with the anterior PVT data, although the centering around 0 of the conditioned place preference score (the difference between the time spent in the drug-paired chamber during the posttest and the pretest) argues against this possibility. These changes in conditioned place preference are likely due, at least in part, to changes in the activity of PVT projections to the nucleus accumbens shell. Indeed, with both the anterior and posterior PVT sending projections to the shell, it is notable that conditioned place preference and positive hedonic reactions to sucrose have been observed following muscimol-induced inhibition of the rostral medial nucleus accumbens shell (Reynolds and Berridge 2002). Given the finding that baclofen + muscimol in the PVT prevented conditioned place preference for a cocaine-paired chamber (Browning et al. 2014), different results might be expected if a natural or drug reward were paired with the inactivation. The finding of reduced distance traveled during the conditioned place preference test may have reflected greater acclimation to the conditioned place preference apparatus. It is also consistent with our prior finding that locomotor activity is reduced after injection of baclofen + muscimol in the anterior or posterior PVT (Barson and Leibowitz 2015).
The finding that inactivation of the posterior PVT induces reinstatement of sucrose seeking but does not affect sucrose seeking under an FR3 schedule of reinforcement suggests that inhibition of this brain region can also disinhibit motivation, although not necessarily intake. Prior research had suggested that the posterior PVT was not involved in sucrose seeking, as muscimol-induced inhibition the rat posterior PVT was not found to affect cue-induced lever-pressing for sucrose during unexpected reward omission (Do-Monte et al. 2017). Instead, inhibition the rat anterior PVT, either by injection of muscimol or by photoinhibition of its projections to the nucleus accumbens, enhanced cue-induced sucrose seeking under this reward omission paradigm (Do-Monte et al. 2017). While the present study was not designed to rule out the possibility of involvement of the anterior PVT in reinstatement of sucrose seeking, it may be that the addition of the GABAB agonist, baclofen, was sufficient to bring on board the posterior PVT with this behavior. Indeed, injections of baclofen + muscimol into the rat posterior PVT, or both the anterior and posterior PVT, have been found to affect cue-induced reinstatement for another reinforcing substance, cocaine (Kuhn et al. 2018; Matzeu et al. 2015). Moreover, population calcium signaling in the rat posterior PVT is increased in response to a stimulus signaling the delivery of sucrose and in fact predicts behavioral approach to the magazine where sucrose is delivered, to an even greater extent than signaling in the rat anterior PVT (Choi et al. 2019). It is possible that activity in one population of neurons in the PVT drives approach behavior while activity in another instead opposes it. While the finding of a lack of effect of baclofen + muscimol on sucrose intake was unexpected, given that neuropeptide-specific stimulation of the posterior PVT has been found to stimulate home cage sucrose drinking (Barson et al. 2015, 2017), it is nevertheless consistent with a lack of effect of muscimol in the posterior PVT on lever-pressing for sucrose when sucrose reward is available (Do-Monte et al. 2017). It remains to be determined if this discrepancy in the findings is due to operant compared with home cage testing or if there are specific populations of neurons that are differentially affected by these different manipulations. Furthermore, it is possible that a history of baclofen + muscimol injections is required to affect lever-pressing for sucrose.
The finding that inactivation of the posterior PVT specifically reduced local gene expression of PACAP expands on the behavioral findings to suggest that a change in PACAP in the PVT could mediate the changes in affect and motivation, although this is purely speculative at this time. It is important to note that changes in PACAP in the PVT have not been directly linked here with the behavioral effects of baclofen + muscimol. Moreover, with some but not all of the baclofen + muscimol–injected animals in the experiment having previously received injections of this drug, it is not possible to extrapolate how neuropeptide levels may differ after single compared to multiple inactivations, or under different behavioral testing paradigms. While studies on the behavioral effects of PACAP in the PVT are currently lacking, several published studies have examined the effects of PACAP in PVT projection areas. Consistent with a role for a reduction in PVT PACAP in disinhibiting positive affective behavior, injection of PACAP into the nucleus accumbens core was found to reduce appetitive responses to oral sucrose (Hurley et al. 2019). Consistent with a role for a reduction in PVT PACAP in disinhibiting reinstatement of extinguished seeking, injection of PACAP into the bed nucleus of the stria terminalis was found to reinstate previously extinguished cocaine-seeking behavior (Miles et al. 2018). Moreover, injection of PACAP into the nucleus accumbens shell was found not to affect sucrose drinking behavior (Gargiulo et al. 2021). While the smaller subject number in the present study leaves open the possibility of a type II statistical error with the enkephalin and neurotensin results, and a finding of a change in gene expression does not necessarily indicate a change in peptide levels, the results nevertheless are consistent with the known role of PACAP in affect and motivation.
In summary, this study demonstrates that temporary inactivation of the posterior PVT affects both affect and motivation as well as local gene expression of PACAP. It shows that this inactivation induces conditioned place preference, promotes reinstatement of sucrose seeking, and reduces local gene expression of PACAP. Together, these results suggest that the posterior PVT participates in both the affective and motivational aspects of reward.
References
Barson JR, Leibowitz SF (2015) GABA-induced inactivation of dorsal midline thalamic subregions has distinct effects on emotional behaviors. Neurosci Lett 609:92–96. https://doi.org/10.1016/j.neulet.2015.10.029
Barson JR, Ho HT, Leibowitz SF (2015) Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol 20:469–481. https://doi.org/10.1111/adb.12139
Barson JR, Poon K, Ho HT, Alam MI, Sanzalone L, Leibowitz SF (2017) Substance P in the anterior thalamic paraventricular nucleus: promotion of ethanol drinking in response to orexin from the hypothalamus. Addict Biol 22:58–69. https://doi.org/10.1111/adb.12288
Barson JR, Mack NR, Gao WJ (2020) The paraventricular nucleus of the thalamus is an important node in the emotional processing network. Front Behav Neurosci 14:598469. https://doi.org/10.3389/fnbeh.2020.598469
Berridge KC, Robinson TE (2016) Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol 71:670–679. https://doi.org/10.1037/amp0000059
Bischoff S, Leonhard S, Reymann N, Schuler V, Shigemoto R, Kaupmann K, Bettler B (1999) Spatial distribution of GABA(B)R1 receptor mRNA and binding sites in the rat brain. J Comp Neurol 412:1–16
Browning JR, Jansen HT, Sorg BA (2014) Inactivation of the paraventricular thalamus abolishes the expression of cocaine conditioned place preference in rats. Drug Alcohol Depend 134:387–390. https://doi.org/10.1016/j.drugalcdep.2013.09.021
Choi EA, Jean-Richard-Dit-Bressel P, Clifford CWG, McNally GP (2019) Paraventricular thalamus controls behavior during motivational conflict. J Neurosci 39:4945–4958. https://doi.org/10.1523/JNEUROSCI.2480-18.2019
Churchill L, Zahm DS, Duffy P, Kalivas PW (1996) The mediodorsal nucleus of the thalamus in rats–II. Behavioral and neurochemical effects of GABA agonists. Neuroscience 70:103–112. https://doi.org/10.1016/0306-4522(95)00352-j
Curtis GR, Oakes K, Barson JR (2020) Expression and distribution of neuropeptide-expressing cells throughout the rodent paraventricular nucleus of the thalamus. Front Behav Neurosci 14:634163. https://doi.org/10.3389/fnbeh.2020.634163
Do-Monte FH, Minier-Toribio A, Quinones-Laracuente K, Medina-Colon EM, Quirk GJ (2017) Thalamic regulation of sucrose seeking during unexpected reward omission. Neuron 94:388-400 e4. https://doi.org/10.1016/j.neuron.2017.03.036
Dong X, Li S, Kirouac GJ (2017) Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Brain Struct Funct 222:3927–3943. https://doi.org/10.1007/s00429-017-1445-8
Gao B, Fritschy JM, Moore RY (1995) GABAA-receptor subunit composition in the circadian timing system. Brain Res 700:142–156. https://doi.org/10.1016/0006-8993(95)00944-l
Gao C, Leng Y, Ma J, Rooke V, Rodriguez-Gonzalez S, Ramakrishnan C, Deisseroth K, Penzo MA (2020) Two genetically, anatomically and functionally distinct cell types segregate across anteroposterior axis of paraventricular thalamus. Nat Neurosci 23:217–228. https://doi.org/10.1038/s41593-019-0572-3
Gargiulo AT, Pirino BE, Curtis GR, Barson JR (2021) Effects of pituitary adenylate cyclase-activating polypeptide isoforms in nucleus accumbens subregions on ethanol drinking. Addict Biol 26:e12972. https://doi.org/10.1111/adb.12972
Gupta A, Gargiulo AT, Curtis GR, Badve PS, Pandey S, Barson JR (2018) Pituitary adenylate cyclase-activating polypeptide-27 (PACAP-27) in the thalamic paraventricular nucleus is stimulated by ethanol drinking. Alcohol Clin Exp Res 42:1650–1660. https://doi.org/10.1111/acer.13826
Guy EG, Choi E, Pratt WE (2011) Nucleus accumbens dopamine and mu-opioid receptors modulate the reinstatement of food-seeking behavior by food-associated cues. Behav Brain Res 219:265–272. https://doi.org/10.1016/j.bbr.2011.01.024
Hermanson O, Hallbeck M, Blomqvist A (1995) Preproenkephalin mRNA-expressing neurones in the rat thalamus. NeuroReport 6:833–836. https://doi.org/10.1097/00001756-199504190-00002
Huang H, Ghosh P, van den Pol AN (2006) Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol 95:1656–1668. https://doi.org/10.1152/jn.00927.2005
Hurley MM, Robble MR, Callan G, Choi S, Wheeler RA (2019) Pituitary adenylate cyclase-activating polypeptide (PACAP) acts in the nucleus accumbens to reduce hedonic drive. Int J Obes (lond) 43:928–932. https://doi.org/10.1038/s41366-018-0154-6
Iglesias AG, Flagel SB (2021) The paraventricular thalamus as a critical node of motivated behavior via the hypothalamic-thalamic-striatal circuit. Front Integr Neurosci 15:706713. https://doi.org/10.3389/fnint.2021.706713
Karatayev O, Barson JR, Chang GQ, Leibowitz SF (2009) Hypothalamic injection of non-opioid peptides increases gene expression of the opioid enkephalin in hypothalamic and mesolimbic nuclei: Possible mechanism underlying their behavioral effects. Peptides 30:2423–2431. https://doi.org/10.1016/j.peptides.2009.09.025
Kelley AE, Baldo BA, Pratt WE (2005) A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol 493:72–85. https://doi.org/10.1002/cne.20769
Kuhn BN, Klumpner MS, Covelo IR, Campus P, Flagel SB (2018) Transient inactivation of the paraventricular nucleus of the thalamus enhances cue-induced reinstatement in goal-trackers, but not sign-trackers. Psychopharmacology 235:999–1014. https://doi.org/10.1007/s00213-017-4816-1
Li S, Kirouac GJ (2008) Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol 506:263–287. https://doi.org/10.1002/cne.21502
Li S, Kirouac GJ (2012) Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct 217:257–273. https://doi.org/10.1007/s00429-011-0360-7
Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y (2015) The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacology 40:1297–1306. https://doi.org/10.1038/npp.2014.320
Lopak V, Erb S (2005) Activation of central neurotensin receptors reinstates cocaine seeking in the rat: modulation by a D1/D5, but not D2/D3, receptor antagonist. Psychopharmacology 182:297–304. https://doi.org/10.1007/s00213-005-0089-1
Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI (1999) Immunohistochemical localization of GABA(B) receptors in the rat central nervous system. J Comp Neurol 405:299–321. https://doi.org/10.1002/(sici)1096-9861(19990315)405:3%3c299::aid-cne2%3e3.0.co;2-6
Matzeu A, Weiss F, Martin-Fardon R (2015) Transient inactivation of the posterior paraventricular nucleus of the thalamus blocks cocaine-seeking behavior. Neurosci Lett 608:34–39. https://doi.org/10.1016/j.neulet.2015.10.016
McGinty JF, Otis JM (2020) Heterogeneity in the paraventricular thalamus: the traffic light of motivated behaviors. Front Behav Neurosci 14:590528. https://doi.org/10.3389/fnbeh.2020.590528
Miles OW, Thrailkill EA, Linden AK, May V, Bouton ME, Hammack SE (2018) Pituitary adenylate cyclase-activating peptide in the bed nucleus of the stria terminalis mediates stress-induced reinstatement of cocaine seeking in rats. Neuropsychopharmacology 43:978–986. https://doi.org/10.1038/npp.2017.135
Morales I, Berridge KC (2020) “Liking” and “wanting” in eating and food reward: brain mechanisms and clinical implications. Physiol Behav 227:113152. https://doi.org/10.1016/j.physbeh.2020.113152
Ollmann T, Peczely L, Laszlo K, Kovacs A, Galosi R, Berente E, Karadi Z, Lenard L (2015) Positive reinforcing effect of neurotensin microinjection into the ventral pallidum in conditioned place preference test. Behav Brain Res 278:470–475. https://doi.org/10.1016/j.bbr.2014.10.021
Ong ZY, Liu JJ, Pang ZP, Grill HJ (2017) Paraventricular thalamic control of food intake and reward: Role of glucagon-like peptide-1 receptor signaling. Neuropsychopharmacology 42:2387–2397. https://doi.org/10.1038/npp.2017.150
Pandey S, Badve PS, Curtis GR, Leibowitz SF, Barson JR (2019) Neurotensin in the posterior thalamic paraventricular nucleus: inhibitor of pharmacologically relevant ethanol drinking. Addict Biol 24:3–16. https://doi.org/10.1111/adb.12546
Parsons MP, Li S, Kirouac GJ (2007) Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. J Comp Neurol 500:1050–1063. https://doi.org/10.1002/cne.21224
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates, 5th edn. Elsevier Academic Press, San Diego
Phillips AG, LePiane FG, Fibiger HC (1983) Dopaminergic mediation of reward produced by direct injection of enkephalin into the ventral tegmental area of the rat. Life Sci 33:2505–2511. https://doi.org/10.1016/0024-3205(83)90159-5
Reynolds SM, Berridge KC (2002) Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/"disliking" reactions, place preference/avoidance, and fear. J Neurosci 22:7308–20 (20026734: 20026734)
Sartor GC, Aston-Jones GS (2012) A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci 32:4623–4631. https://doi.org/10.1523/JNEUROSCI.4561-11.2012
Vertes RP, Hoover WB (2008) Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol 508:212–237. https://doi.org/10.1002/cne.21679
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL (2012) Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinf 13:134. https://doi.org/10.1186/1471-2105-13-134
Funding
This research was supported by the National Institute on Alcohol Abuse and Alcoholism under Award Number R01AA028218 (JRB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gargiulo, A., Badve, P., Curtis, G. et al. Inactivation of the thalamic paraventricular nucleus promotes place preference and sucrose seeking in male rats. Psychopharmacology 239, 2659–2671 (2022). https://doi.org/10.1007/s00213-022-06160-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06160-2