Abstract
Rationale
Cocaine use not only depends on the reinforcing properties of the drug, but also on its pharmacological effects on alternative nondrug activities. In animal models investigating choice between cocaine and alternative sweet rewards, the latter influence can have a dramatic impact on choice outcomes. When choosing under cocaine influence is prevented by imposing sufficiently long intervals between choice trials, animals typically prefer the sweet reward. However, when choosing under the drug influence is permitted, animals shift their preference in favor of cocaine.
Objectives
We previously hypothesized that this preference shift is mainly due to a direct suppression of responding for sweet reward by cocaine pharmacological effects. Here we tested this hypothesis by making rats tolerant to this drug-induced behavioral suppression.
Results
Contrary to our expectation, tolerance did not prevent rats from shifting their preference to cocaine when choosing under the influence.
Conclusion
Thus, other mechanisms must be invoked to explain the influence of cocaine intoxication on choice outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Whether recreational or problematic, drug use not only depends on the inherent reinforcing properties of the drug but also depends on its pharmacological or intoxicating effects on alternative behavioral activities. Some of these effects can be beneficial for other nondrug-related behaviors (Müller 2020; Müller and Schumann 2011; Pickard 2020). For instance, psychostimulants are often consumed to improve social interactions, to enhance cognitive performance, or to counteract fatigue (Müller and Schumann 2011). Alternatively, other drug effects can be detrimental to alternative nondrug activities, notably in the context of substance use disorder (SUD). In addition to multiple processes involving, notably, incentive sensitization (Berridge and Robinson 2016), opponent affective processes (Koob and Le Moal 2001), and impaired inhibitory control (Goldstein and Volkow 2012), the interference of drug use on alternative activities may contribute, at least partly, to explain some diagnostic criteria of SUD in the Diagnostic and Statistical Manual of Mental Disorders (DSM, 5th edition), notably: (1) “Substance use has caused relationship problems or conflicts with others”; (2) “Failure to meet responsibilities at work, school, or home because of substance use”; and (3) “Activities are given up in order to use the substance” (American Psychiatric Association 2013). The suppression of nondrug-related alternatives by problematic drug use in SUD can create a vicious circle where the drug becomes the most valuable option available (Heyman 2010). On the other hand, drug interference with important nondrug-related activities can motivate the decision to abstain and could constitute a strong incentive for addiction recovery (Branch 2011; Heyman 2013, 2010). Thus, investigating how drug effects can influence nondrug-related behaviors is important when considering the transition to or recovery from SUD.
In animal models of SUD involving drug self-administration in the presence of alternative nondrug rewards, choosing under the influence of the drug is typically prevented by imposing sufficiently long inter-trial intervals (ITI), allowing for drug dissipation between choice trials (Cantin et al. 2010; Lenoir et al. 2013a, 2007). In this condition, the large majority of rats prefer the alternative nondrug reward. This finding, first discovered more than 10 years ago (Lenoir et al. 2007), was repeatedly reproduced in a large set of conditions, including different drug and nondrug rewards; various drug doses and history of drug self-administration; and different reward delays and costs (Augier et al. 2012; Cantin et al. 2010; Caprioli et al. 2015; Huynh et al. 2017; Kearns et al. 2017; Kerstetter et al. 2012; Lenoir et al. 2013b; Lenoir and Ahmed 2008; Madsen and Ahmed 2015; Pelloux and Baunez 2017; Russo et al. 2018; Schwartz et al. 2017; Venniro et al. 2018). However, in a free-operant choice schedule in which both options are continuously available and, thus, in which choosing under the drug influence is permitted, choice behavior dramatically differs (Bozarth and Wise 1985; Freese et al. 2018; Thomsen et al. 2013, 2008; Vandaele et al. 2016). Notably, rats offered a choice between cocaine and saccharin in these conditions first self-administer saccharin before switching to cocaine exclusively until the end of the session. A similar choice pattern was observed in a discrete trial schedule when the ITI is sufficiently shortened to permit choice under the influence (Kerstetter et al. 2012; Vandaele et al. 2016). Finally, when the drug influence is induced artificially before each choice trial by a non-contingent injection of cocaine, this is sufficient to bias choice toward cocaine (Freese et al. 2018; Guillem and Ahmed 2018; Vandaele et al. 2016). Thus, when rats are choosing between cocaine and saccharin under the influence of cocaine, they shift their preference to cocaine. The mechanisms underlying this drug-induced shift in preference are yet to be fully understood.
We have previously suggested that cocaine intoxication shifts choice to cocaine through direct suppression of the alternative nondrug-related behavior. First, as a psychostimulant, cocaine exerts potent anorexic effects, suppressing both feeding and drinking behaviors in rats (Balopole et al. 1979; Cooper and Francis 1993; Vandaele et al. 2016; Wolgin and Hertz 1995; Woolverton et al. 1978). Psychostimulants anorexic effects are partly mediated by stereotypy-induced interference with consummatory behavior (Wolgin 2000; Wolgin and Hertz 1995). Second, cocaine-induced suppression of responding for saccharin is strongly correlated with cocaine-induced shift to cocaine choice (Vandaele et al. 2016). Furthermore, cocaine-induced shift in preference is associated with a suppression of the activity of saccharin-coding neurons in the orbitofrontal cortex and no facilitation of the activity of cocaine-coding neurons (Guillem and Ahmed 2018). Finally, drug-induced shift in preference is not observed with heroin intoxication which is known to enhance rather than suppress eating and drinking behaviors (Cooper et al. 1985; Parker et al. 1992; Vandaele et al. 2016; but see Chow and Beckmann 2021; Townsend et al. 2021). Thus, the comparison of cocaine and heroin in a free-operant choice schedule or following pre-trial drug injections suggests that their opposite effect on preference is likely mediated by their opposite effects (suppressing versus enhancing) on the alternative nondrug reward rather than by their common priming effects on drug seeking, as seen in other behavioral paradigms (Ahmed and Cador 2006; de Wit and Stewart 1983).
The goal of this study is to test more directly the role of the suppressive (anorexic) effects of cocaine in biasing choice in favor of exclusive drug use in a free-operant setting. To this end, tolerance to these effects was induced before choice testing. Previous research showed that a tolerance to drug-suppressive effects can be learned in hungry rats when amphetamine or cocaine is administered immediately before access to food (Wolgin 2000; Wolgin and Hughes 1997; Woolverton et al. 1978). This tolerance is an active learning process called “contingent tolerance” because its development does not result from passive drug exposure but instead requires that animals experience the suppressive effects of the drug while they are eating or, at least, trying to (Wolgin 2000; Wolgin and Jakubow 2004). Indeed, if animals are no longer under the influence of amphetamine or cocaine when given access to food, all else being equal, they do not develop tolerance and drastically suppress food intake when tested under drug influence. In our conditions, contingent tolerance was induced by allowing hungry rats to self-administer cocaine immediately before a short access to sucrose-sweetened water. If cocaine intoxication shifts choice in favor of drug use by suppressing responding for sucrose, then tolerance to these suppressive effects should prevent or retard such preference shift.
Materials and methods
Subjects
Twenty-eight male Wistar rats weighting in average 275–300 g at the beginning of the experiment were used (Charles River, L’Arbresle, France). Rats were housed in groups of 2 in a temperature-and light-controlled vivarium (21 °C, reversed 12-h light–dark cycle). Rats were food-restricted and maintained at 80% of their estimated free-feeding weight. Water was freely available in the home cages during behavioral testing. Three rats were excluded due to failure in catheter patency, leaving a total of 25 rats for the analysis. All experiments were conducted in accordance with institutional and international standards of care and use of laboratory animals (UK Animals (Scientific Procedures) Act, 1986; and associated guidelines; the European Communities Council Directive (2010/63/UE, 22 September 2010) and the French Directives concerning the use of laboratory animals (décret 2013–118, 1 February 2013). The animal studies were reviewed and approved by the Committee of the Veterinary Services Gironde, agreement number B33-063–5.
Apparatus
Fourteen identical operant chambers (30 × 40 × 36 cm) described in detail elsewhere (Lenoir et al. 2013a) were used (Imetronic, Pessac, France). Chambers were equipped with two retractable levers, a commercially available lickometer circuit, two syringe pumps, a single-channel liquid swivel (Lomir Biomedical Inc., Quebec, Canada), and two pairs of infrared beams to measure locomotor activity.
Surgery
Rats received a surgery for the implantation of chronic silastic catheters (Dow Corning Corporation, MI, USA) in the right jugular vein, exiting the skin in the middle of the back about 2 cm below the scapulae, as described previously (Lenoir et al. 2013a).
Operant training
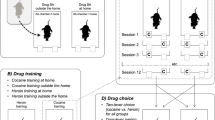
Animals were first trained to press on the left lever for a solution of 20% sucrose under a fixed ratio 1 (FR1 time out 20 s) schedule as described in detail elsewhere (Lenoir et al. 2013a). We chose to train hungry animals with a caloric solution of 20% sucrose to favor learning of contingent tolerance by increasing motivation for the nondrug reward. Discrete volumes of sucrose were delivered in the adjacent drinking cup by voluntary liking over the time out period of 20 s, signaled by the illumination of the cue light above the lever. The drinking cup was automatically filled with 2 volumes over the first 3-s, and additional volumes were obtained by licking, resulting in a maximum volume delivered of 0.32 mL. Responses during the 20-s time out were not rewarded. Sessions ended after rats had earned a maximum of 30 rewards, or 3 h had elapsed. Rats were trained under a FR1 schedule for 6 sessions followed by 3 sessions under a fixed ratio 2 (FR2) schedule. Rats were then trained to self-administer intravenous cocaine (0.25 mg delivered over 5 s) by pressing on the right lever under a FR2 schedule for 10 sessions (Fig. 1A). Sessions were limited to a maximum of 3 h or 50 injections. Importantly, rats were systematically tethered to the infusion line to equate training conditions across sucrose and cocaine rewards.
Schematic representation of the procedure. A Experimental timeline. Arrow heads indicate choice tests. B After initial sucrose and cocaine self-administration training, all rats are offered a 10-min sucrose access after a 2-h period with no reward available (OFF). C During tolerance training, one group of rats receive 1-h cocaine access followed by a 1-h break before the 10-min sucrose access (control group, 1-h condition). In the other group, cocaine self-administration occurs after 1-h and immediately before the 10-min sucrose access (tolerant group, 0-h condition). The gray box marked with a “T” represents a transition period during which rats can receive an extra cocaine injection before the next phase
Tolerance training procedure
After acquisition of lever pressing for sucrose and cocaine, rats were allowed to self-administer sucrose for 10 min under a FR2 schedule after an initial 2-h period with no reward available (Fig. 1B). The levers remained retracted during the first 2 h, and extension of the left lever signaled the onset of sucrose availability. During the first 3 sessions, half of the rats failed to notice the lever insertion. Thus, the onset of sucrose access was also signaled by the illumination of the house light and one free delivery of 0.08 mL of sucrose for three additional sessions. Upon correct acquisition of sucrose self-administration under these conditions, the house light cue and free sucrose delivery were removed, and training pursued for three more sessions.
One hour of cocaine self-administration was then introduced either immediately (0-h group; N = 14) or 1 h before sucrose access (1-h group; control condition; N = 11) (Fig. 1C). Thus, only rats in the 0-h group were under the influence of cocaine during sucrose self-administration. To minimize the delay between the last cocaine injection and subsequent access to sucrose in the 0-h group, we introduced a transition period (T) during which any infusion obtained resulted in the termination of the cocaine self-administration phase. The maximal duration of the transition period was 10 min (Fig. 1C). Importantly, only in two rare occasions did a rat missed the opportunity for this last cocaine injection. To equate training conditions, this transition period was also introduced at the end of cocaine self-administration in the 1-h group. Rats were randomly assigned to either group.
Free-operant choice procedure
After acquisition of contingent tolerance in the 0-h group, rats’ preference was tested in the free-operant choice procedure. Both levers were simultaneously presented throughout the duration of the 2-h session. Completion of the FR2 response requirement on either lever resulted in the delivery of the corresponding reward (i.e., intravenous cocaine injection or 20-s sucrose access), signaled by the illumination of the cue light above the selected lever during the 20-s time out. Two variants of this procedure were tested. In the first variant, rats were first allowed to self-administer cocaine for 20-min before initiation of the 2-h choice session (test 2). In the second variant, the duration of the session was extended to 5-h (test 4). Few baseline sessions with the tolerance training procedure were conducted between choice sessions. At the end of choice testing, tolerance expression was tested in all rats during a single tolerance test session conducted in the 0-h condition (Fig. 1A).
Data analysis
Licking efficiency was calculated by computing the ratio of the volume delivered divided by the volume available, during every sucrose accesses. The latency to initiate sucrose self-administration during tolerance training was log-transformed for statistical analysis to meet the normality assumption of parametric tests. All data were subjected to mixed analyses of variance (ANOVA), followed by post hoc comparisons using the Tukey’s honestly significant difference (HSD) test, when appropriate. Comparisons with a fixed theoretical level (e.g., 50%) were conducted using one sample t-test. Some behavioral variables did not follow a normal distribution as assessed by the Shapiro–Wilk test and were thus analyzed using non-parametric statistics (i.e., Wilcoxon’s test for paired comparisons; Mann–Whitney for group comparison).
Results
Development of a tolerance to cocaine suppressive effects in the 0-h group
During tolerance training, both groups of rats self-administered cocaine similarly during cocaine access at the beginning of the session, 1-h before sucrose access (1-h group) or immediately before (0-h group) (Fig. 2A; Mann–Whitney test, Z-values < 0.74, p-values > 0.4). As expected, during the first tolerance training session, responding for sucrose was drastically suppressed compared to baseline in rats from the 0-h group which were intoxicated with cocaine during sucrose access, (Fig. 2B; main effect of session, F1,19 = 260, p < 0.0001; main effect of group, F1,19 = 104.5, p < 0.0001; group by session interaction, F1,19 = 272.3, p < 0.0001). Post hoc analysis reveals that rats in this group earned significantly less sucrose rewards during the first tolerance training session compared to 1-h rats and baseline (p-values < 0.001). However, with repeated sessions in the tolerance training procedure, most 0-h rats (N = 10) learned to resist to the suppressive effects of cocaine and progressively increased their responding for sucrose despite cocaine intoxication (Fig. 2B). Repeated measure ANOVA revealed significant effects of sessions (F9,171 = 24.7, p < 0.0001), groups (F1,19 = 15.7, p < 0.001), and session by group interaction (F9,171 = 22.3, p < 0.0001). Post hoc analysis revealed that these 0-h rats significantly differed from 1-h rats on the first (p < 0.05), but not the following sessions (p > 0.8). However, sucrose self-administration remained significantly suppressed across tolerance training sessions in 4 rats from the 0-h group (< 80% of baseline responding over the last 3 sessions), compared to the 1-h group (Fig. 2B). These rats kept suppressing sucrose self-administration at 48.7 ± 5.2% of their baseline and thus did not develop reliable tolerance to the suppressive effects of cocaine.
The majority of rats in the 0-h group developed a tolerance to cocaine suppressive effects. A–D Mean (± SEM) number of cocaine injections (A), number of sucrose rewards (B), latency to initiate sucrose self-administration (C), and licking efficiency (D) across tolerance training sessions, in the 1-h group (white circles) and in tolerant rats (black circles) or non-tolerant rats (gray circles) of the 0-h group. Vertical dotted lines delimit tolerance training onset and mark the time of the first and second choice tests (choice T1 and T2)
Analysis of the latency to initiate sucrose self-administration reveals that the first sucrose access was significantly delayed in 0-h rats compared to 1-h rats during the first session relative to baseline (Fig. 2C; effect of sessions F1,19 = 17.39, p < 0.001; effect of groups F1,19 = 25.79, p < 0.0001; group by session interaction F1,19 = 27.47, p < 0.0001). Rats developing a tolerance to cocaine suppressive effects progressively learned to respond for sucrose from the onset of sucrose availability and progressively recovered a latency comparable to the 1-h group (Fig. 2C; effect of sessions F9,171 = 9.19, p < 0.0001; effect of groups F1,19 = 19.66, p < 0.001; group by session interaction F9,171 = 6.13, p < 0.0001). However, rats failing to develop tolerance maintained a significant post-cocaine delay to initiate sucrose self-administration compared to the 1-h group (Fig. 2C; last session: F1,14 = 19.43, p < 0.001).
Cocaine intoxication during sucrose access not only delayed initiation of sucrose self-administration, and consequently, the number of sucrose rewards, but also interfered with licking behavior as evidenced by a decrease in licking efficiency in the 0-h group compared to baseline (main effect of session: F1,19 = 8.92, p < 0.01) and compared to the 1-h group (Fig. 2D; main effect of groups F1,19 = 8.79, p < 0.01; group by session interaction F1,19 = 8.92, p < 0.01). This result indicates that rats in the 0-h group did not consume all the volumes available during sucrose accesses. However, rats developing a tolerance learned to overcome this suppressive effect on licking behavior and reached comparable licking efficiency as the 1-h group, as evidenced by significant effects of session (F9,171 = 5.55, p < 0.0001), group (F1,19 = 13.78, p < 0.01), and session by group interaction (F9,171 = 5.61, p < 0.0001). Licking efficiency also increased in 0-h non-tolerant rats but never reached the level of 1-h rats (Fig. 2D). Overall, the four 0-h non tolerant rats maintained a clear suppression of sucrose self-administration despite tolerance training. Since our approach is to assess the effect of tolerance to the suppressive effects of cocaine on preference, these rats were excluded from the group 0-h. Furthermore, the low number of 0-h non tolerant rats (N = 4) precludes their inclusion in statistical analyses. Thus, we analyzed individual choice patterns in subsequent tests for these rats, separately (supplemental Fig. 1).
Tolerance to cocaine suppressive effects did not prevent cocaine-biased shift in preference
Preference between cocaine and sucrose was first tested during a 2-h free-operant choice session. Surprisingly, the percentage of cocaine choice did not significantly differ between groups despite a trend toward lower preference for cocaine in 0-h rats (Fig. 3A; t19 = 2.05; p = 0.054). In fact, the main pattern of choice was overall similar between groups with subtle differences; all rats first began self-administering sucrose before shifting to cocaine. Although there was no group difference in the initial phase of sucrose self-administration (Fig. 3B; t19 = − 0.83; p > 0.4), two 0-h rats initiated the session by choosing cocaine. When these two rats were excluded, the group 0-h earned significantly more sucrose rewards before the first transition to cocaine (Fig. 3B; t17 = − 2.36, p < 0.05). Then, most rats continued to self-administer cocaine exclusively until the end of the session, but some occasionally sampled the sucrose option before switching back to cocaine (Fig. 3E–F). To quantify this behavior, we assessed the number of inter-reward transitions and found that tolerant rats in the group 0-h made significantly more transitions than 1-h rats (Fig. 3C; Mann–Whitney: Z = − 2.04; p < 0.05). However, analysis of the within-session time course of sucrose accesses revealed no group difference (F1,19 = 0.85, p > 0.3) nor group by time bin interaction (Fig. 3D; F11,209 = 0.38, p > 0.5). Rats that did not develop a tolerance in the 0-h group displayed a choice pattern similar to 1-h rats (supplemental Fig. 1A).
Tolerance to the suppressive effects of cocaine did not prevent cocaine-biased shift in preference. A–C Mean (± SEM) percentage of cocaine choice (A), number of sucrose rewards before transition to cocaine (B), and number of inter-reward transitions (C) in 1-h and 0-h rats. #p = 0.054, *p < 0.05. D Within-session time course of sucrose rewards in the 1-h and 0-h groups across 10-min time bins. E–F Choice patterns of representative non-tolerant rats in the 1-h group (E) and tolerant rats in the 0-h group (F). Vertical bars above or below the horizontal line represent sucrose (S) and cocaine (C) choices, respectively. For each rat, the number of inter-reward transitions is indicated
Tolerance to cocaine suppressive effects can favor sucrose preference in conditions of high motivation
The results above suggest that although tolerance to the suppressive effects of cocaine allowed some rats to sample sucrose once intoxicated, their usual pattern of sucrose self-administration was prevented. In fact, it seems that although possible, expressing a tolerance to cocaine suppressing effect is difficult. Thus, the initial loading period of sucrose self-administration could be sufficient to reduce sucrose value by sensory-specific satiety, thereby dampening motivation to overcome cocaine suppressive effects, once intoxicated. After three baseline tolerance training sessions, rats were tested in a modified choice session, comprising a 20-min period of exclusive cocaine self-administration, immediately before the 2-h choice session (choice test 2). We observed no group difference in the number of cocaine injections during the initial 20-min period (Fig. 4A; t19 = 0.24, p > 0.5). However, preventing the initial sucrose self-administration loading by intoxicating rats from the beginning of the session revealed a significant difference in preference between 0-h and 1-h rats (Fig. 4B; Mann–Whitney: Z = 2.46, p < 0.05). The high preference for cocaine in 1-h rats can be explained by the low number of inter-reward transitions in this group compared to 0-h rats (Fig. 4C and E, Mann–Whitney: Z = − 3.17, p < 0.01). In contrast, tolerant rats in the 0-h group succeeded to make at least one inter-reward transition to self-administer sucrose, generally at the beginning of the choice session (Fig. 4C–D and F). Thus, analysis of the within-session time course of sucrose accesses revealed a group difference (F1,19 = 12.20, p < 0.01), specifically during the first 10 min of the session (Fig. 4D; group by time interaction: F11,209 = 2.33, p < 0.05; post hoc 0–10 min, p < 0.05). Rats in the 0-h group that failed to develop a tolerance expressed choice patterns more comparable to the 1-h group (supplemental Fig. 1B).
An effect of tolerance to cocaine suppressive effects is revealed when motivation for sucrose is high. A Mean (± SEM) number of cocaine injections during pre-choice cocaine self-administration in 1-h and 0-h rats. B–C Mean (± SEM) percentage of cocaine choice (B) and number of inter-reward transitions (C) in 1-h and 0-h rats. *p < 0.05. D Within-session time course of sucrose rewards in the 1-h and 0-h rats across 10-min time bins. *p < 0.05. E–F Choice patterns of representative non-tolerant rats in the 1-h group (E) and tolerant rats in the 0-h group (F). Vertical bars above or below the horizontal line represent sucrose (S) and cocaine (C) choices, respectively. For each rat, the number of inter-reward transitions is indicated. In D–F, the gray area represents the 20-min period of pre-choice cocaine self-administration. The onset of the choice session is marked with a red vertical bar in E–F
These results suggest that when their motivation for sucrose was sufficient, 0-h rats expressed a tolerance to the suppressive effects of cocaine and maintained a preference for sucrose despite prior cocaine intoxication (t-test against indifference: t10 = − 3.55, p < 0.01). Yet, the expression of tolerance was not perfect and sucrose self-administration remained relatively suppressed in comparison to tolerance training sessions in which sucrose is the only reward available. Importantly, group differences in choice behavior disappeared when rats were tested for a second time in a 2-h choice session, after one baseline session, in the absence of prior cocaine self-administration as in the first choice session (supplemental Fig. 2; % cocaine choice; Mann–Whitney: Z = 1.26, p > 0.2).
Tolerance to cocaine suppressive effects did not favor sucrose choice during an extended 5-h choice session
We next asked whether given sufficient time, rats tolerant to cocaine suppressive effects would eventually switch back to sucrose after the shift to cocaine choices. Rats were tested in the final choice session for a duration of 5 h. Increasing the session duration had no effect on the expression of tolerance during choice behavior. There was no group difference in the percentage of cocaine choice (Fig. 5A; t19 = 1.59, p > 0.1) or in the number of sucrose access before the first transition (Fig. 5B; t19 = 0.71, p > 0.7). Although some rats in both groups made a high number of inter-reward transitions, the groups did not differ on this variable (Fig. 5C; Mann Whitney: Z = − 1.16, p > 0.2). Accordingly, we observed no group difference in the within-session time course of sucrose accesses (Fig. 5D; F1,19 = 2.58, p > 0.1).
Tolerance to cocaine suppressive effects had no effect on preference during an extended 5-h choice session. A–C Mean (± SEM) percentage of cocaine choice (A), number of sucrose rewards before transition to cocaine (B), and number of inter-reward transitions (C) in 1-h and 0-h rats. D Within-session time course of sucrose rewards in the 1-h and 0-h rats across 10-min time bins. E–F Choice patterns of representative non-tolerant rats in the 1-h group (E) and tolerant rats in the 0-h group (F). Vertical bars above or below the horizontal line represent sucrose (S) and cocaine (C) choices, respectively. For each rat, the number of inter-reward transitions is indicated
Few rats in the 1-h group made a high number of inter-reward transitions (i.e., 45 transitions, Fig. 5E, bottom panel). Alternatively, some tolerant rats made only a few numbers of inter-reward transitions (Fig. 5F, top panel). These results suggest that repeated choice testing may have favored the development or altered the expression of tolerance in 1-h and 0-h rats, respectively. To test this hypothesis, all rats were tested in a tolerance training session and allowed to self-administer cocaine for 1-h immediately before sucrose access (0-h condition). Cocaine and sucrose self-administration during this test was compared between groups and within-subject with respect to the last baseline session, conducted with the respective 0-h and 1-h tolerance training conditions.
Rats reliably self-administered cocaine with no difference between groups and compared to the baseline session (Fig. 6A; effect of group, F1,19 = 0.16, p > 0.6; effect of session, F1,19 = 0.07, p > 0.7). Prior cocaine self-administration significantly suppressed sucrose self-administration in both groups compared to baseline (Fig. 6B; effect of session, F1,19 = 75.4, p < 0.0001), suggesting that tolerance was partially lost in 0-h rats. However, there was a significant session by group interaction (Fig. 6B; F1,19 = 8.62, p < 0.01). Post-hoc analysis reveals that suppression of sucrose self-administration was stronger in the 1-h group compared to the 0-h group (Fig. 6B; p < 0.05). Analysis of the latency to initiate sucrose self-administration reveals a significant group by session interaction (Fig. 6C; F1,19 = 20.05, p < 0.001). Although the number of sucrose rewards earned by 0-h rats was lower than baseline, these rats initiated sucrose self-administration with a comparably short latency (Fig. 6C; post hoc p > 0.9). In sharp contrast, sucrose self-administration was considerably delayed in rats from the 1-h group compared to baseline (Fig. 6C; post hoc: p < 0.001). Cocaine intoxication altered licking efficiency in both groups of rats, with no significant group by session interaction (Fig. 6D; effect of session F1,19 = 13.31, p < 0.01). Together these results suggest that rats in the 1-h group did not develop a tolerance to cocaine suppressive effects with repeated choice testing. However, rats in the 0-h group partially lost their tolerance. Interestingly, the expression of tolerance during the test, assessed by the number of sucrose rewards earned under the influence of cocaine, was positively correlated with the number of inter-reward transitions during the preceding 5-h choice session (Fig. 6E; Spearman rank order correlation: r = 0.49, p < 0.05).
Partial loss of tolerance in 0-h rats with repeated choice sessions. A–D Mean (± SEM) number of cocaine injections (A), number of sucrose rewards (B), latency to initiate sucrose self-administration (C), and licking efficiency (D) during the tolerance test session (white bars) compared to baseline (black bars), in 1-h and 0-h rats. Baseline refers to the last tolerance training session. *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.0001. E Correlation between the number of sucrose rewards earned during the tolerance test and the number of inter-reward transitions during the preceding 5-h choice session. White and black circles represent rats from the 1-h and 0-h groups, respectively
Discussion
The majority of rats exposed to sucrose while intoxicated learned to tolerate the suppressive effects of cocaine on responding for sucrose, confirming and extending previous research (Wolgin 2000; Wolgin and Hertz 1995; Wolgin and Jakubow 2004). However, this tolerance only had a small effect on preference during subsequent choice under the influence. As reported previously, during free-operant choice, non-tolerant rats first chose sucrose before eventually switching to cocaine nearly exclusively until the end of the session. Overall, the same behavior was also observed in tolerant rats, except that once intoxicated, they tended to transition more between the two rewards. A significant effect of tolerance was only manifest when rats were under the influence of cocaine before onset of the choice session. Thus, contrary to our expectation, tolerance did not prevent rats from shifting their preference to cocaine when choosing under the influence. Other mechanisms must be invoked to explain the influence of cocaine intoxication on choice outcomes in free-operant choice schedule.
By the end of tolerance training, rats in the 0-h group expressed a robust tolerance to cocaine suppressive effects, self-administering sucrose with the same performance and efficiency as the control group. As predicted by Wolgin et al. (Wolgin 2000; Wolgin and Hertz 1995; Wolgin and Jakubow 2004), this tolerance only developed when rats had access to sucrose while intoxicated by cocaine. Few rats in the 0-h group failed to learn resisting cocaine anorexic effects. Further research is needed to explain this inter-individual variability. Although we did not observe tolerance learning in 1-h rats across repeated choice sessions, a partial extinction of tolerance occurred in some 0-h rats, in agreement with prior research (Woolverton et al. 1978). Importantly, the expression of tolerance at the end of choice testing was correlated with the number of inter-reward transitions during choice, suggesting that the suppressive effects of cocaine on responding for sucrose somehow influenced choice behavior. However, in most choice sessions, the learned tolerance to cocaine suppressive effects did not generalize well to the free-operant choice procedure.
In the present study, the lack of generalization of tolerance to the choice setting could be explained by several non-exclusive hypotheses. The contingent tolerance to cocaine suppressive effects is commonly described as a form of instrumental learning (Wolgin 2000). Indeed, it is hypothesized that rats learn to resist to psychostimulant-induced stereotypies, this behavior being reinforced by subsequent food consumption. Learning and expression of contingent tolerance is therefore context-dependent. Thus, it is possible that, although rats were trained and tested in the same conditioning chambers, the settings for tolerance training and choice testing were still considered as distinct instrumental contexts. Notably, during tolerance training, only one lever was presented at a time with a 1-h period without any reward whereas during choice testing, both levers were continuously presented throughout the session duration.
An alternative hypothesis directly supported by the data is that rats would be more motivated by sucrose during tolerance training compared to choice testing. Indeed, during tolerance training, the hungry rats only received 10-min access to sucrose at the end of the session, after a waiting time of 2 h. In contrast, during choice sessions, rats self-administered sucrose continuously from the very beginning of the session, for about 20–30 min. During this loading period, one should expect that motivation for sucrose progressively decreases, at least partly, by sensory-specific satiety. This process could increase the probability of initiating cocaine use and, thus, of the subsequent preference shift. Thus, although rats had learned to tolerate the suppressive effects of cocaine, the benefit for controlling drug-induced stereotypies may not be sufficient when the motivation for sucrose has decreased. Supporting this hypothesis, we showed that an effect of tolerance was only manifested when the initial loading period of sucrose self-administration was prevented by intoxicating rats before onset of the choice session. In these conditions, tolerant rats succeeded to resist cocaine suppressive effects and were able to respond for sucrose few times under the influence of cocaine. However, even in these conditions, the effect of tolerance was modest since the pattern of sucrose self-administration was significantly suppressed by cocaine self-administration during choice testing. It is worth noting that in contrast to tolerance training sessions in which cocaine and sucrose are presented sequentially, cumulating cocaine injections during choice sessions in the presence of the alternative sucrose reward likely reinforced further intoxication and sustained interference with the sucrose reward, thereby creating a vicious circle promoting drug preference.
Preference during free-operant choice not only depends on motivation for sucrose and expression of tolerance to the suppressive effects of cocaine, but can also depend on the motivation for the drug itself. Indeed, cocaine intoxication can prime responding for cocaine in behavioral paradigms such as drug-induced reinstatement (Ahmed and Cador 2006; de Wit and Stewart 1981; Shaham et al. 2003). Thus, in a free-operant setting, cocaine choice can transiently enhance motivation for cocaine by increasing cocaine incentive value (Robinson and Berridge 1993) or by inducing a negative affective state (i.e., withdrawal) that would be alleviated by another dose of cocaine (Ettenberg 2004; Koob and Le Moal 2001). We previously suggested that, in contrast to cocaine, heroin exerts orexigenic effects and that these effects would enhance, rather than suppress, responding for the alternative nondrug reward, when choosing under the influence is permitted (Vandaele et al. 2016). However, recent findings suggest that the processes controlling drug-vs-food choice under opioid influence cannot be limited to the drug orexigenic effects (Chow and Beckmann 2021; Townsend et al. 2021) indicating that, in agreement with the present study, the mechanisms controlling choice under drug influence are more complex than previously suggested.
Importantly, motivation for cocaine fluctuates within choice sessions. Indeed, at each cocaine injection, the priming effects of cocaine typically follow a period of lower satiated motivation (Freese et al. 2018; Norman and Tsibulsky 2006). This satiated motivation is thought to result from a satiating level of dopamine in the ventral striatum (Wise et al. 1995; Ahmed et al. 2003). Indeed, it was shown that a non-contingent injection of cocaine or heroin, which elevates dopamine in the ventral striatum, is sufficient to suppress intra-cerebral self-stimulation of dopamine neurons of the ventral tegmental area (Corre et al. 2018; Pascoli et al. 2015). How drug-induced changes in motivation for the drug and the nondrug rewards interact to dynamically influence preference during choice remains a challenging question deserving further research.
To conclude, our study shows that the cognitive processes underlying choice between drug and nondrug rewards under the influence of the drug are complex. A comparable complexity is likely at play when the drug exerts enhancing rather than suppressing effects on the alternative nondrug reward. The present study also reveals that a behavior learned during sequential presentation of the drug and the nondrug rewards (tolerance training) may not generalize well to a choice setting where the drug and nondrug alternatives directly compete with each other for the allocation of behavior. Finally, it is worth noting that although drug intoxication dynamically modulates motivations for both drug and nondrug rewards, individuals suffering from substance use disorders are not under drug influence when they make a lapse. However, after a lapse, they are under the influence, and this may precipitate under some circumstances further lapses and, eventually, a full-blown relapse. Thus, delineating the complex interactions between motivations for the drug and the alternative nondrug reward with or without drug influence is essential to progress our understanding of the maintenance of persistent drug use in substance use disorders.
References
Ahmed SH, Cador M (2006) Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology 31:563–571. https://doi.org/10.1038/sj.npp.1300834
Ahmed SH, Lin D, Koob GF, Parsons LH (2003) Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J Neurochem 86(1):102–113. https://doi.org/10.1046/j.1471-4159.2003.01833.x
American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Association. https://doi.org/10.1176/appi.books.9780890425596
Augier E, Vouillac C, Ahmed SH (2012) Diazepam promotes choice of abstinence in cocaine self-administering rats. Addict Biol 17:378–391. https://doi.org/10.1111/j.1369-1600.2011.00368.x
Balopole DC, Hansult CD, Dorph D (1979) Effect of cocaine on food intake in rats. Psychopharmacology 64:121–122. https://doi.org/10.1007/BF00427356
Berridge KC, Robinson TE (2016) Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol 71:670–679. https://doi.org/10.1037/amp0000059
Bozarth MA, Wise RA (1985) Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. JAMA J Am Med Assoc 254:81–83. https://doi.org/10.1001/jama.1985.03360010087032
Branch MN (2011) Drug addiction. Is it a disease or is it based on choice? A review of Gene Heyman’s Addiction: a disorder of choice. J Exp Anal Behav 95:263–267. https://doi.org/10.1901/jeab.2011.95-263
Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, Vouillac C, Ahmed SH (2010) Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS ONE 5. https://doi.org/10.1371/journal.pone.0011592
Caprioli D, Zeric T, Thorndike EB, Venniro M (2015) Persistent palatable food preference in rats with a history of limited and extended access to methamphetamine self-administration. Addict Biol 20:913–926. https://doi.org/10.1111/adb.12220
Chow JJ, Beckmann JS (2021) Remifentanil-food choice follows predictions of relative subjective value. Drug Alcohol Depend 218:108369. https://doi.org/10.1016/j.drugalcdep.2020.108369
Cooper SJ, Francis J (1993) A microstructural analysis of the effects of presatiation on feeding behavior in the rat. Physiol Behav 53:413–416. https://doi.org/10.1016/0031-9384(93)90227-7
Cooper SJ, Jackson A, Morgan R, Carter R (1985) Evidence for opiate receptor involvement in the consumption of a high palatability diet in nondeprived rats. Neuropeptides 5:345–348
Corre J, van Zessen R, Loureiro M, Patriarchi T, Tian L, Pascoli V, Lüscher C (2018) Dopamine neurons projecting to medial shell of the nucleus accumbens drive heroin reinforcement. Elife 7:1–22. https://doi.org/10.7554/eLife.39945
de Wit H, Stewart J (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 75:134–143. https://doi.org/10.1007/BF00432175
de Wit H, Stewart J (1983) Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology 79:29–31. https://doi.org/10.1007/BF00433012
Ettenberg A (2004) Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev 27:721–728. https://doi.org/10.1016/j.neubiorev.2003.11.009
Freese L, Durand A, Guillem K, Ahmed SH (2018) Pre-trial cocaine biases choice toward cocaine through suppression of the nondrug option. Pharmacol Biochem Behav 173:65–73. https://doi.org/10.1016/j.pbb.2018.07.010
Goldstein RZ, Volkow ND (2012) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669. https://doi.org/10.1038/nrn3119.Dysfunction
Guillem K, Ahmed SH (2018) Preference for cocaine is represented in the orbitofrontal cortex by an increased proportion of cocaine use-coding neurons. Cereb Cortex 28:819–832. https://doi.org/10.1093/cercor/bhw398
Heyman GM (2010) Addiction: a disorder of choice. Havard University Press
Heyman GM (2013) Addiction and choice: theory and new data. Front Psychiatry 4:1–5. https://doi.org/10.3389/fpsyt.2013.00031
Huynh C, Fam J, Ahmed SH, Clemens KJ (2017) Rats quit nicotine for a sweet reward following an extensive history of nicotine use. Addict Biol 22:142–151. https://doi.org/10.1111/adb.12306
Kearns DN, Kim JS, Tunstall BJ, Silberberg A (2017) Essential values of cocaine and non-drug alternatives predict the choice between them. Addict Biol 22:1501–1514. https://doi.org/10.1111/adb.12450
Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE (2012) Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology 37:2605–2614. https://doi.org/10.1038/npp.2012.99
Koob G, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24:97–129. https://doi.org/10.1016/S0893-133X(00)00195-0
Lenoir M, Ahmed SH (2008) Supply of a nondrug substitute reduces escalated heroin consumption. Neuropsychopharmacology 33:2272–2282. https://doi.org/10.1038/sj.npp.1301602
Lenoir M, Serre F, Cantin L, Ahmed SH (2007) Intense sweetness surpasses cocaine reward. PLoS ONE 2. https://doi.org/10.1371/journal.pone.0000698
Lenoir M, Augier E, Vouillac C, Ahmed SH (2013a) A choice-based screening method for compulsive drug users in rats. Curr Protoc Neurosci 1:1–17. https://doi.org/10.1002/0471142301.ns0944s64
Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH (2013b) Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology 38:1209–1220. https://doi.org/10.1038/npp.2013.17
Madsen HB, Ahmed SH (2015) Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues. Addict Biol 20:433–444. https://doi.org/10.1111/adb.12134
Müller CP (2020) Drug instrumentalization. Behav Brain Res 390:112672. https://doi.org/10.1016/j.bbr.2020.112672
Müller CP, Schumann G (2011) Drugs as instruments: a new framework for non-addictive psychoactive drug use. Behav Brain Sci 34:293–310. https://doi.org/10.1017/S0140525X11000057
Norman AB, Tsibulsky VL (2006) The compulsion zone: a pharmacological theory of acquired cocaine self-administration. Brain Res 1116:143–152. https://doi.org/10.1016/j.brainres.2006.07.092
Parker LA, Maier S, Rennie M, Crebolder J (1992) Morphine- and naltrexone-induced modification of palatability: analysis by the taste reactivity test. Behav Neurosci 106:999–1010. https://doi.org/10.1037//0735-7044.106.6.999
Pascoli V, Terrier J, Hiver A, Lüscher C (2015) Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron 88:1054–1066. https://doi.org/10.1016/j.neuron.2015.10.017
Pelloux Y, Baunez C (2017) Targeting the subthalamic nucleus in a preclinical model of alcohol use disorder. Psychopharmacology 234:2127–2137. https://doi.org/10.1007/s00213-017-4618-5
Pickard H (2020) What we’re not talking about when we talk about addiction. Hastings Cent Rep 50:37–46. https://doi.org/10.1002/hast.1172
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291
Russo M, Funk D, Loughlin A, Coen K, Lê AD (2018) Effects of alcohol dependence on discrete choice between alcohol and saccharin. Neuropsychopharmacology 43:1859–1866. https://doi.org/10.1038/s41386-018-0101-1
Schwartz LP, Kim JS, Silberberg A, Kearns DN (2017) Heroin and saccharin demand and preference in rats. Drug Alcohol Depend 178:87–93. https://doi.org/10.1016/j.drugalcdep.2017.04.031
Shaham Y, Shalev U, Lu L, de Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168:3–20. https://doi.org/10.1007/s00213-002-1224-x
Thomsen M, Fink-Jensen A, Woldbye DPD, Wörtwein G, Sager TN, Holm R, Pepe LM, Barak CS (2008) Effects of acute and chronic aripiprazole treatment on choice between cocaine self-administration and food under a concurrent schedule of reinforcement in rats. Psychopharmacology 201:43–53. https://doi.org/10.1007/s00213-008-1245-1
Thomsen M, Barrett AC, Negus SS, Caine SB (2013) Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J Exp Anal Behav 99:211–233. https://doi.org/10.1002/jeab.15
Townsend EA, Schwienteck KL, Robinson HL, Lawson ST, Banks ML (2021) A drug-vs-food “choice” self-administration procedure in rats to investigate pharmacological and environmental mechanisms of substance use disorders. J Neurosci Methods 354:109110. https://doi.org/10.1016/j.jneumeth.2021.109110
Vandaele Y, Ahmed SH (2021) Choosing between cocaine and sucrose under the influence: testing the effect of cocaine tolerance. bioRxiv 2021.04.02.438165. https://doi.org/10.1101/2021.04.02.438165
Vandaele Y, Cantin L, Serre F, Vouillac-Mendoza C, Ahmed SH (2016) Choosing under the influence: a drug-specific mechanism by which the setting controls drug choices in rats. Neuropsychopharmacology 41:646–657. https://doi.org/10.1038/npp.2015.195
Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, Morales M, Epstein DH, Shaham Y (2018) Volitional social interaction prevents drug addiction in rat models. Nat Neurosci 21:1520–1529
Wise RA, Leeb K, Pocock D, Newton P, Burnette B, Justice JB (1995) Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology 120(1):10–20. https://doi.org/10.1007/BF02246140
Wolgin DL (2000) Contingent tolerance to amphetamine hypophagia: new insights into the role of environmental context in the expression of stereotypy. Neurosci Biobehav Rev 24:279–294. https://doi.org/10.1016/S0149-7634(99)00070-6
Wolgin DL, Hertz JM (1995) Effects of acute and chronic cocaine on milk intake, body weight, and activity in bottle- and cannula-fed rats. Behav Pharmacol 6:746–753. https://doi.org/10.1097/00008877-199511000-00010
Wolgin DL, Hughes KM (1997) Role of behavioral and pharmacological variables in the loss of tolerance to amphetamine hypophagia. Psychopharmacology 132:342–349. https://doi.org/10.1007/s002130050354
Wolgin DL, Jakubow JJ (2004) Tolerance to amphetamine hypophagia: a real-time depiction of learning to suppress stereotyped movements in the rat. Behav Neurosci 118:470–478. https://doi.org/10.1037/0735-7044.118.3.470
Woolverton WL, Kandel D, Schuster CR (1978) Tolerance and cross-tolerance to cocaine and d-amphetamine. J Pharmacol Exp Ther 205:525–535
Funding
This work was supported by the French Research Council (CNRS), the Université de Bordeaux, the French National Agency (ANR-2010-BLAN-1404–01), the Ministère de l’Enseignement Supérieur et de la Recherche (MESR), the Fondation pour la Recherche Médicale (FRM DPA20140629788), and the Peter und Traudl Engelhorn foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization, SHA and YV; methodology, SHA and YV; investigation, YV; formal analysis, YV; supervision, SHA; visualization, YV; writing (original draft), YV; writing (review and editing), SHA and YV.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author comments
This manuscript has been posted as a preprint on bioRχiv: https://www.biorxiv.org/content/10.1101/2021.04.02.438165v1 (Vandaele and Ahmed 2021).
This article belongs to a Special Issue on Nature vs. Nurture in Addiction Research
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vandaele, Y., Ahmed, S.H. Choosing between cocaine and sucrose under the influence: testing the effect of cocaine tolerance. Psychopharmacology 239, 1053–1063 (2022). https://doi.org/10.1007/s00213-021-05987-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05987-5